Abstract
Multichannel recording technologies have revealed travelling waves of neural activity in multiple sensory, motor and cognitive systems. These waves can be spontaneously generated by recurrent circuits or evoked by external stimuli. They travel along brain networks at multiple scales, transiently modulating spiking and excitability as they pass. Here, we review recent experimental findings that have found evidence for travelling waves at single-area (mesoscopic) and whole-brain (macroscopic) scales. We place these findings in the context of the current theoretical understanding of wave generation and propagation in recurrent networks. During the large low-frequency rhythms of sleep or the relatively desynchronized state of the awake cortex, travelling waves may serve a variety of functions, from long-term memory consolidation to processing of dynamic visual stimuli. We explore new avenues for experimental and computational understanding of the role of spatiotemporal activity patterns in the cortex.
Synchrony in the activity of neuronal populations has long been of interest in neuroscience1,2, but its function has remained elusive. Neural oscillations are rhythmic fluctuations of spiking activity within neuronal populations. It is proposed that synchrony of neural oscillations in different neural populations might be able to shape input gain and aid information transfer between these populations3,4 because the synchronous occurrence of action potentials in many neurons is known to increase their ability to drive spikes in shared target neurons5,6. Indeed, experimental evidence suggests that oscillation synchrony modulates the effective connectivity of populations in the cortex7,8, shapes plasticity between neurons9 and drives processing of stimulus features between visual cortex areas V1 and V4 (REF. 10).
These proposed functions have traditionally been thought to involve precise zero-lag synchrony11,12, in which oscillations across single areas or distant regions in the cortex are aligned perfectly in time. However, with the advent of simultaneous multichannel recording technologies, we have begun to appreciate that the relative timing of neural population activity is constantly changing. Instead of precise zero-lag synchrony, a range of flexible phase offsets is possible. These flexible phase relationships can, in their simplest form, be travelling waves of various shapes (including plane, radial and spiral waves). The combination of multiple travelling waves can form complex spatiotemporal patterns.
Stimulus-driven and internally generated travelling waves occur across different brain states and sensory conditions. Brain state and synaptic activity strongly shape these waves so that they differ profoundly from sleep13 to wake14. In turn, these waves transiently modulate neuronal excitability15–19 and shape responses to external input20 over a wide range of spatial and temporal scales. They travel over spatial scales that range from the mesoscopic (single cortical areas and millimetres of cortex) to the macroscopic (global patterns of activity over several centimetres) and extend over temporal scales from tens to hundreds of milliseconds. Although, in initial studies, mesoscopic travelling waves were pronounced only under conditions of low visual luminance contrast21,22 and were mostly reported in anaesthetized states23–28, recent work has shown that stimulus-evoked mesoscopic travelling waves robustly appear in the awake visual cortex14,29 and can influence intracortical dynamics during the viewing of natural stimuli30.
In vitro and in vivo cortical travelling waves have been reviewed previously22,31,32. In this article, we cover a series of recent studies that have led to important advances in our understanding of travelling waves and the generality of their role in cortical processing. We first summarize experimental observations of travelling waves in the cortex, focusing on advances in recording and analysis techniques that have enabled the detection of waves in single cortical regions. Next, we briefly review the broad computational and mathematical literature on travelling waves in neural networks, focusing on theoretical mechanisms for wave generation and possible experimental tests of these predictions. Finally, we discuss emerging computational roles for cortical travelling waves, from sensory processing to memory consolidation. As we describe, at the macroscopic scale, these waves allow the formation of the large-scale, distributed cell assemblies thought to underlie long-term memory33,34. At the mesoscopic scale, multiple lines of experimental evidence suggest that evoked waves are a way for neural circuits to flexibly store and use information from the recent past. In closing, we highlight open questions for new experiments and theoretical work to further elucidate the biological mechanisms underlying cortical travelling waves and their role in neural computation.
Travelling waves in neural systems
Travelling waves were first observed at the macroscopic, whole-brain scale. More recently, new high-resolution optical and electrophysiological recording technologies have led to the observation of travelling waves at the mesoscopic scale of single regions in the cortex. Distinct axonal fibre tracts carry waves at the two scales, and there are observable differences in the resulting dynamics — most notably, spatial extent and propagation speed — that make clear distinctions between the two possible. Macroscopic waves are generally recorded using electroencephalography (EEG) or electrocorticography (ECoG), in which arrays of electrodes either outside the skull (EEG) or directly on the cortical surface (ECoG) record potentials from neural populations with high temporal (millisecond) resolution but low spatial (centimetre) resolution. These waves generally have propagation speeds from 1 to 10 metres per second, falling within the range of the axonal conduction speeds of myelinated white matter fibres in the cortex35. Mesoscopic waves are generally observed in the local field potential (LFP) using multielectrode arrays (MEAs) or in optical imaging signals recorded with voltage-sensitive dyes (VSDs). These techniques record from single regions in the cortex with high temporal and spatial resolution36,37. Stimulus-evoked mesoscopic waves can appear in the sensory cortex during the fast onset transient that follows the presentation of a stimulus; these waves are thus time-locked to the stimulus. Alternatively, they may appear during sustained responses in a manner that is not necessarily locked to stimulus onset (often called induced waves). Mesoscopic waves generally have propagation speeds from 0.1 to 0.8 metres per second, consistent with the axonal conduction speed of the unmyelinated long-range horizontal fibres within the superficial layers of the cortex38.
Macroscopic travelling waves
Neuroscientists first observed oscillations in EEG through the work of clinicians in the late nineteenth and early twentieth centuries39. In 1929, Hans Berger reported the 10 Hz alpha oscillation in EEG recordings of human occipital lobes40. Shortly thereafter, there was much interest in understanding the spatial organization of alpha oscillations through multielectrode recordings, which often appeared to show a wave travelling from occipital to frontal regions41. Alpha-band travelling waves in EEG recordings under both spontaneous and stimulus-driven conditions continue to be a subject of active interest42,43. In subsequent studies, it also has been reported that the 0.1-1.0 Hz slow oscillation that occurs during sleep is an anterior-to-posterior travelling wave44 (FIG. 1a) and that auditory-induced 30–80 Hz gamma oscillations make a coherent anterior-to-posterior sweep across the cortex (observed using magnetic field tomography)45. Signal distortion by cranial tissues46 and volume conduction, however, pose substantial possible confounds for detection of travelling waves in EEG. Understanding the generation of electric and magnetic fields by neural activity remains an active pursuit for experimental and theoretical research46,47; however, in the absence of a general physical theory48,49, the origin and interpretation of travelling waves observed solely in EEG is problematic (see REF. 50 for an initial comparison).
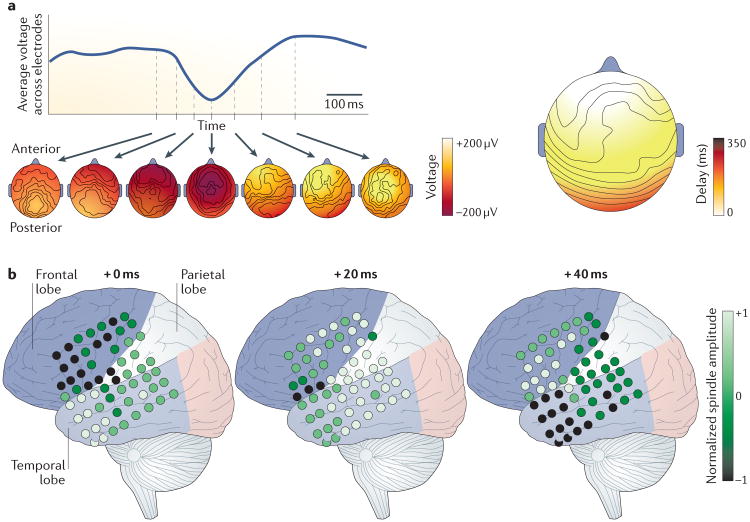
Figure 1. Macroscopic waves during human sleep.
a | The slow oscillation of deep non-rapid-eye-movement (non-REM) sleep has been reported to be a travelling wave that moves globally from anterior to posterior regions44. The upper left panel represents the time course of one slow oscillation averaged across electroencephalogram (EEG) channels. The lower left panels illustrate the evolution of voltages across the scalp at different times during the slow wave. At the start of the slow oscillation, negative EEG potentials begin in anterior sections of the scalp, and as the oscillation progresses, these travel to posterior regions. The negative peak of the slow oscillation is delayed in posterior regions by hundreds of milliseconds, reflecting the propagation time of the travelling wave (right panel). b | Sleep ‘spindles’ are 11–15 Hz oscillations that occur during stage 2 non-REM sleep and have long been known to be important for learning and memory. These spindles systematically travel as global rotating waves from the temporal to the parietal to the frontal cortex (and are therefore called TPF waves) in intracranial electrocorticography (ECoG) recordings13. The panels illustrate the normalized spindle amplitude at each ECoG electrode (small circles) at successive points in the spindle cycle. From the start of an oscillation cycle (+0 ms), spindle amplitudes peak successively in the temporal (+0 ms), parietal (+20 ms) and frontal (+40 ms) lobes. The amplitudes recorded for each channel are normalized to their maximum amplitude. Part a is republished with permission of the Society of Neuroscience, from The sleep slow oscillation as a traveling wave. Massimini, M et al. J. Neurosci. 24 (31), 6872–6870 (2004); permission conveyed through Copyright Clearance Center, Inc. (REF. 44). Part b is adapted with permission from REF 13, eLife.
In recent years, intracranial ECoG recordings in clinical patients have made detailed analyses of human cortical dynamics possible. Following a technique developed by Penfield and Jasper for mapping the cortical sites involved in generating epileptic seizures51, surgeons typically implant two-dimensional ECoG electrode arrays on the surface of one cortical hemisphere, with coverage of temporal, parietal and frontal lobes supplemented by additional linear strips of electrodes on the same or the contralateral hemisphere. ECoG recordings have a high signal-to-noise ratio and spatial localization, reflecting neural activity spanning less than 1 centimetre of the cortex52,53. Furthermore, by capturing power in the 80-150 Hz high-gamma band, which serves as an index for neuronal firing near the electrode54, ECoG recordings can link oscillatory patterns with local population spiking activity.
Analysis of ECoG recordings has revealed travelling waves during both awake and sleep states. The spatial range of these oscillatory patterns can vary from relatively small patches of cortex to the global, whole-brain scale. In the awake state, travelling alpha waves in ECoG recordings have a dorsoventral direction of propagation that is markedly different from waves observed in EEG recordings55. Similarly, the sleep slow oscillation56 and the related K-complex57 have complicated and variable patterns of propagation in ECoG recordings, in contrast to the smooth anterior-to-posterior propagation observed in EEG. Thus, at the higher spatial resolution afforded by intracranial recordings, oscillatory activity generally has different and more complicated dynamical patterns than those observed with EEG. However, ECoG recordings have shown that sleep spindles in humans exhibit robust spatiotemporal travelling waves that either radiate out from a point source or rotate across the cortex in a preferred direction, travelling from the temporal cortex to the parietal cortex, on to the frontal lobe and back again to the temporal cortex13 (FIG. 1b). These rotating waves represent a large proportion (50.8%) of all spindle cycles, with radial waves constituting a smaller fraction (15.6%) and the other cycles likely reflecting only local activity (33.6%)58,59. The observed wavelengths of these waves are similar to the size of the brain, blurring out any effects of the folds of cortical sulci and gyri; however, with higher-resolution imaging techniques, a more detailed impact of the cortical folds may become apparent. The speeds at which these waves travel are concentrated between 2 and 5 metres per second, consistent with the conduction speed of short-range38 and long-range35,60 association fibres in the cortical white matter. These association fibres thus provide a possible substrate for propagation of ECoG travelling waves, suggesting that the waves have a functional role in building distributed cell assemblies in the cortex13.
Mesoscopic travelling waves
Advances in multichannel recording technologies now allow single-trial recordings in individual cortical areas with very high spatial (up to 20 micrometres) and temporal (up to 1 millisecond) resolution. For the first time, the responses of distributed neuronal populations in the cortex to sensory stimuli can be monitored through both optical and electrophysiological approaches. These new recordings can give important clues about the neural code and the role of topographic maps in sensory systems. Furthermore, the analysis of single trials gives new insights into sources of variability in cortical responses to repeated presentations of the same sensory stimulus.
To understand results from these recordings in single regions, an important distinction must be made between two theoretical models of the cortical sensory response: stationary bumps of neural activity and cortical travelling waves14. A stationary bump of neural activity would remain centred on the point of strongest input to the cortex, perhaps increasing in amplitude and expanding in range over time (FIG. 2). In V1, the point of strongest input is the retinotopic representation in the cortex of the stimulus in the visual field. Here, spikes carried by thalamocortical fibres enter layer 4, which then projects to layer 2/3 of V1. Thus, the spatiotemporal pattern of activation of a stationary bump is mainly driven by the input and shaped to a lesser degree by intracortical axons61–63. In a stimulus-evoked cortical travelling wave, on the other hand, the cells at the point of strongest input would emit a peak of activity that expands progressively across the retinotopic map (FIG. 2). This pattern would indicate the presence of dominant recurrent interactions in the cortex, which are largely attributed to propagation along intracortical axons64,65. This distinction leads to quite different hypothesized downstream effects: in a cortical area receiving input from V1, cells would receive their strongest input at any point in time either from the central point (stationary bump) or from a progressively expanding ring of activation (cortical travelling wave)14. We expand on the computational consequences of each model for the sensory response below.
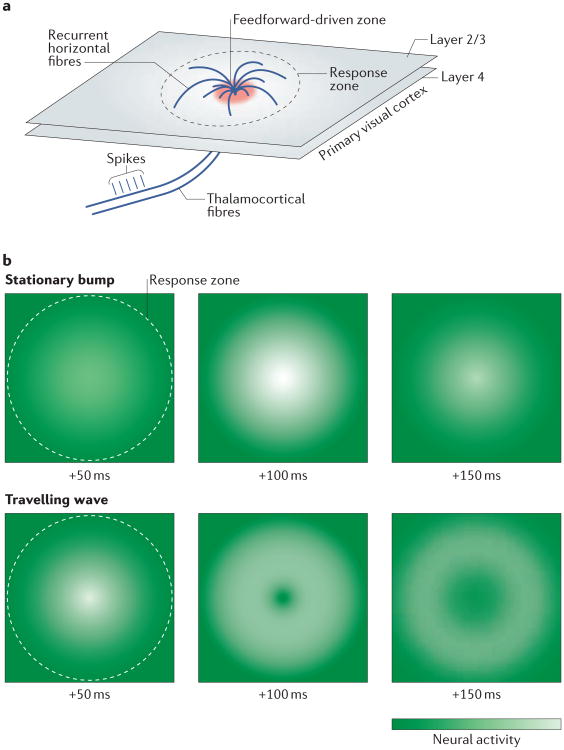
Figure 2. Two models for the stimulus-evoked response in the visual cortex.
a | Small visual stimuli evoke spikes in a set of thalamocortical fibres targeting layer 4 of the primary visual cortex, which, in turn, synapses onto a small segment of layer 2/3 (the feedforward-driven zone). Recurrent horizontal fibres synapse widely across layer 2/3, spanning up to 8 millimetres of distance in the cortex (response zone)107. b | Within the response zone, the activity pattern is proposed to take two main forms: a stationary bump of activity, suggesting a relatively input-driven system, or a travelling wave, suggesting a circuit dominated by recurrent activity14. Schematic diagrams indicate these hypothesized activity patterns and are based on results reported in REF. 14.
Whether the stimulus-evoked response in the awake sensory cortex is a stationary bump or a travelling wave is debated. Initial evidence that visual stimuli evoke travelling waves came from optical imaging during sustained responses in semi-isolated turtle cortex66. This was followed by intracellular recordings in V1 of anaesthetized cats, in which it was shown that the visually evoked response became progressively more delayed with increasing distance from the receptive field of the neuron67 (FIG. 3a), with the relationship between the distance and delay being consistent with the axonal conduction speeds of the unmyelinated horizontal fibres in layer 2/3 (0.1–0.8 metres per second38). By contrast, the initial VSD studies in V1 of awake behaving monkeys found a stationary bump of activation in the evoked response68 (FIG. 3b). These results, however, required averaging over many trials to increase the signal-to-noise ratio, which may have masked travelling waves. The first VSD studies able to image cortical dynamics at the single-trial level were in V1 of anaesthetized rodents69,70. These studies found an active travelling wave propagating out from the stimulus location in the cortex. A later study used a spike- triggered averaging (STA) approach in multielectrode array recordings to reveal spontaneous and stimulus-evoked waves in V1 of anaesthetized cats and macaques21. The validity of these findings, however, was later called into question because STA is open to artefacts caused by low-pass filtering71 (see REF 72 for a rebuttal) or by not fully accounting for the point process statistics of spike trains73. Furthermore, in another VSD study performed in V1 of awake monkeys, again at the level of trial- averaged data, no delay across cortical space (2.5 millimetres) in the initial rise of the neural response was found, suggesting that the delays of the response peak are explained by intracortical gain- control mechanisms rather than by horizontal-fibre travelling waves74.
Figure 3. Mesoscopic waves in the visual cortex.
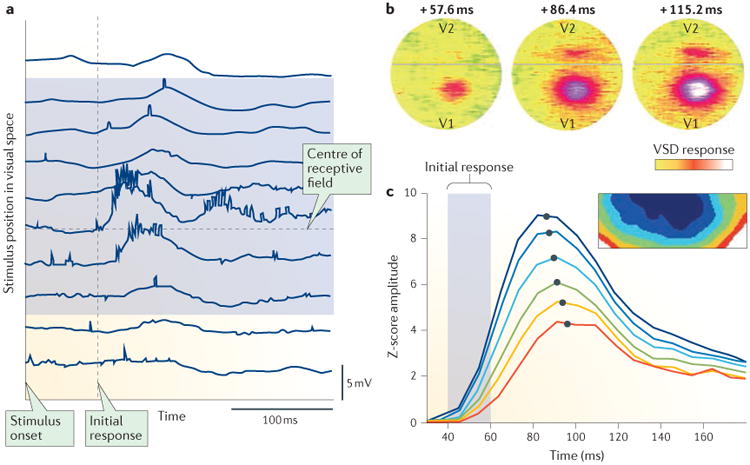
a | These intracellular recordings of a complex cell in the cat visual cortex area V1 during anaesthesia show responses to an optimally oriented bar stimulus67. The middle row is the response to stimulation in the centre of the receptive field of the cell. Response deflections become weaker and more delayed as the stimulus is presented farther away in visual space (top and bottom traces). This delay suggests that individual small visual stimuli evoke waves travelling across the retinotopic map of visual space. b | The first voltage-sensitive dye (VSD) studies in area V1 of an awake monkey exhibited only stationary bump responses to small visual stimuli, questioning the existence of travelling waves in V1 during waking states68. These plots show time from stimulus onset (above) and the VSD response in V1 and V2 (coloured in intensity from yellow to red to white). c | Single-trial analysis of VSD data in V1 of an awake monkey demonstrated that the stimulus onset transient (which occurs 40-140 ms after the start of stimulus presentation) is indeed a travelling wave, which is masked in trial-averaged data. Plotted are the average amplitudes of the VSD response in regions of interest located at increasing distances from the point of afferent input (indicated by colours from blue to red, as shown in inset box). The dark blue region contains the afferent input zone. Evoked amplitudes show increasing temporal offset in both the initial (40-60 ms) and peak (80-100 ms) response. Grey dots represent interpolated maxima illustrating the temporal offset in the peak response. Part a is adapted with permission from REF. 67, AAAS. Part b is adapted with permission from Slovin, H. et al. Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys. J. Neurophysiol. 88, 3421–3438 (2002) (REF 68). Part c is adapted from REF 14, Macmillan Publishers Limited.
New methods for the analysis of single-trial recordings have clarified this issue. A general phase-based algorithm was introduced that can detect arbitrarily shaped waves in high-noise multichannel data14. Applied to single- trial VSD recordings in awake behaving monkeys, this approach revealed that a small, high-contrast visual stimulus consistently evoked a single-cycle wave travelling across several millimetres of V1 (FIG. 3c), which was previously obscured by trial averaging. The isolated waves occurred during the fast stimulus onset response and exhibited consistent delays in initial rise times across the cortex (FIG. 3c), providing evidence against the gain-control mechanism previously suggested74. Furthermore, the waves were shown to possess an intrinsic reliability: although wave onset was variable across trials, their cross-trial variability did not increase as they travelled across the cortex, indicating a propagation substrate with a highly consistent speed. These shifts in wave onset time relative to stimulus onset strongly contribute to attenuation of the waves in trial-averaged data. Together with the measured propagation speeds and shift in initial rises, this result implicates the horizontal fibres in layer 2/3 as the physical substrate for a wave of neural activity evoked by sensory input.
Importantly, these evoked waves in awake monkeys appear not to cross anatomical boundaries in the cortex (that is, between V1 and V2)14, unlike those observed in anaesthetized rodents69. In V1 and V2, the waves evoked in rapid succession by feedforward input are aligned so that they travel simultaneously across their respective retinotopic maps. This alignment is important because it could allow integration of retinotopic information in parallel across multiple regions of the visual system. Whether this stimulus-evoked alignment of travelling waves indeed occurs in multiple visual areas, in addition to whether spontaneous waves exhibit such alignment, remains an open question. However, consistent phase relationships have been observed both within individual visual areas (for example, in LFP recorded from V4 (REF 75) and across different visual areas (in magnetoencephalography43), indicating that such spatiotemporal patterning may be apparent in visual cortical dynamics during the awake state76.
Previous work noted that the phase of gamma oscillations changed across retinotopic domains in V1 of awake monkeys77 and rabbits78 as well as across orientation domains in V1 of awake monkeys79. This suggests that these multicycle high-frequency oscillations ride on top of a broader stimulus-evoked travelling wave and form sustained complex patterns that reflect features of the input. Furthermore, using a measure of directed information transfer across recording sites, a recent study found that shifts of the phase of gamma oscillations between pairs of electrodes in V1 of anaesthetized primates during stimulation with Hollywood movies are consistent with the presence of complex travelling waves propagating in the direction of maximal information transfer30. Importantly, these interactions were consistent with propagation along the horizontal-fibre network and not with input changes, illustrating that the effect of the horizontal-fibre networks in the visual cortex (and their intrinsic time delays) does not disappear in more naturalistic high-input situations. Taken together, these results indicate that stimulus-evoked travelling waves are a general and important feature of dynamics in the visual system that can affect neuronal processing. The functional importance of these waves, however, remains unclear.
The importance of finding travelling waves evoked by high-contrast visual stimuli lies in what it implies about cortical dynamics during normal sensory processing. It has previously been noted that stimulus-evoked travelling waves, which modulate the excitability of neurons as they travel across a topographic sensory map, appear inconsistent with the precise retinotopy and orientation topography described by Hubel and Wiesel at the columnar level22,80,81. If the visual system works to localize activity through retinotopic projections at each stage of processing, then why would it distribute activity across the map through travelling waves? Although initial results suggested that stimulus-evoked travelling waves were pronounced only at low luminance contrast21, thus minimizing consideration of their impact during normal visual processing22, the results reviewed above demonstrate that they are indeed an important feature of cortical dynamics that must be taken into account in describing the stimulus-evoked visual response at normal levels of contrast. These results indicate that activity evoked at one point in the visual cortex can, through the generation of a travelling wave, impact neurons quite far across the retinotopic map, and this affects how subsequent inputs are processed across visual space82. Rather than being a detriment to precise neuronal selectivity, however, these dynamic activity patterns may in fact be mediating previously unappreciated computations at the single-trial level. Theoreticians have postulated that a travelling wave with total phase offset between 0 and 2π could mark locations in the cortex with a unique phase that is useful for visual coding83; however, it remains unclear how this can be used for neural computations with relevant ensembles of visual stimuli. We consider emerging ideas for computation with travelling waves below. Before this, however, we briefly consider the network-level mechanisms responsible for generating travelling waves at the mesoscopic and macroscopic scales.
Network mechanisms for travelling waves
As a paradigmatic example of a neural dynamic requiring the participation of millions of neurons and the recurrent connectivity of the cortex, the question of how travelling waves are generated and propagate has long attracted the attention of mathematicians and physicists. Computational and theoretical neuroscientists study cortical dynamics with a range of techniques, spanning from the mathematical continuum approximation of Wilson and Cowan84 to the simulation of millions of neurons and billions of synapses on multicore and parallel computers85. With recent advances in neural recording and optogenetic stimulation technologies, experimental tests of predictions generated by these approaches are now being realized. The results of mathematical and computational studies on travelling waves in neural networks have been reviewed previously32,83,86,87; here, we focus briefly on results that concern the waves and regime most pertinent to the studies reviewed above — that is, the sparse, weakly modulating waves that occur during the asynchronous and irregular state of neocortex in awake, behaving animals. We do not address the strongly modulating travelling waves that occur during pathological states, such as seizures and spreading depression; furthermore, we note that sparse travelling waves in the awake adult entail fundamentally different mechanisms than the slowly propagating waves observed during development88,89.
Macroscopic network mechanisms
At the whole-brain scale, neural recordings capture the effects of interaction among many billions of neurons across the cortex. Here, phase relationships among oscillations in individual cortical regions constitute the travelling wave, and interactions among regions can be effectively modelled by large-scale population descriptions, such as the Wilson-Cowan equations or coupled oscillator networks. Connections are mediated by cortical white matter fibres, which can traverse just below the grey matter when connecting two nearby regions or can run in the deep, long-range white matter tracts60,90. These connections have axonal conduction delays up to tens of milliseconds35,60 and, as with mesoscopic waves, these distance-dependent delays can strongly influence the emergence of spatiotemporal patterns at the macroscopic level. It has been shown that distance- dependent delays lead to a rich repertoire of spatiotemporal dynamics, including radial and spiral waves, in networks of coupled oscillators91. Furthermore, in simplified networks, mathematical analysis has established that highly structured, geometric connection patterns for networks arranged on a sphere can lead to rotating waves92. Further work is required, however, to understand how alpha band and spindle macroscale travelling waves emerge through the dynamics and time delays of the corticothalamic system.
Mesoscopic network mechanisms
In the cortex, neurons are embedded in a sea of so-called spontaneous activity. Pyramidal cells in V1 have dense recurrent synaptic connectivity, each receiving from 6,000 to 13,000 inputs per cell93. Roughly 80% of these connections come from within the same cortical area, 95% of which arise from neurons within 2 millimetres of the receiving cell94. Long-range horizontal fibres can span several milli metres across the supragranular layers of the cortex95. These axons thus connect neurons spanning multiple functional hypercolumns within a cortical map. During the awake state, pyramidal cells in V1 of monkeys exhibit a median spontaneous firing rate of 7 Hz (REF. 96). This indicates that neurons in V1 of primates receive tens of thousands of synaptic inputs per second, with most arriving from the mesoscopic, intracortical circuit. This synaptic noise has well-known effects on the integration properties of cortical neurons97, allowing them to respond quickly to transient input fluctuations98, and forms the basis for the difference in network state between anaesthetized to awake99.
Travelling waves transiently modulate this ongoing synaptic noise as they pass. For example, there is evidence that, depending on the specific cell-type recruitment, oscillations in the hippocampus may either excite or inhibit neurons in the local circuit at various points during the cycle100. Similarly, during normal cortical function in awake animals, travelling waves weakly modulate neural spiking activity in the motor cortex19. This is consistent with observations at single points in the visual cortex, where alpha and gamma oscillations weakly entrain spiking activity101–103, increasing and decreasing spike probability over the oscillation cycle. Here, we refer to this type of wave (in which the probability of any individual neuron participating in the wave is low) as a sparse wave (FIG. 4). This stands in contrast to the travelling waves observed in in vitro models of epilepsy104,105, where dense waves dominate the spiking activity of the local circuit as they pass. Under these in vitro conditions, networks lack a large fraction of the spontaneous synaptic input that is present in vivo and furthermore are often studied in disinhibited conditions
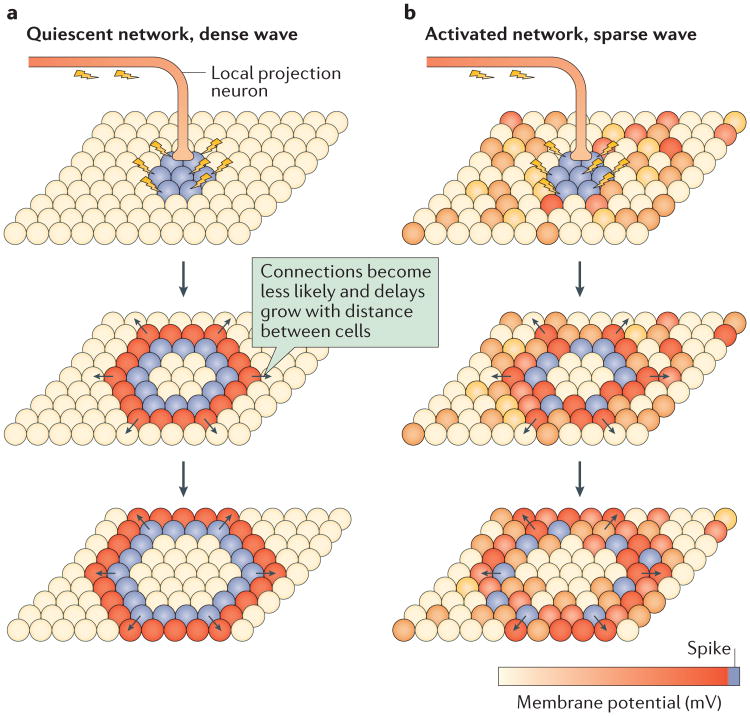
Figure 4. Two models for the generation of mesoscopic travelling waves.
Two schematic models for wave generation in topographic networks of neurons with local, random connections and linearly increasing axonal conduction delays are illustrated. Spheres represent neurons whose membrane potential is indicated by colour. a | In a cortical network with no background activity (as observed in deep anaesthesia or often in cortical slices in vitro), we hypothesize that local stimulation will elicit strong travelling waves, which recruit nearly all cells as they pass. Arrows indicate directions of wave propagation. b | In a cortical network with strong background activity (as observed in normal waking states), we hypothesize that local stimulation can elicit waves that weakly entrain neuronal spiking as they travel across the network.
When a burst of spikes arrives at a local patch of the cortex, it evokes a brief increase in spiking that can propagate out across the network24,67,69. As outlined above, while most studies have focused on waves during the stimulus onset response, travelling waves may be induced during sustained stimulation responses and during the brief increase of spiking activity at stimulus offset in the sensory cortex of awake, behaving animals, and these waves may not be time-locked to the stimulus. Bursts during the fast onset transient arise through feedforward input; however, during spontaneous and induced waves, these bursts can result from recurrent fluctuations or feedback connections26,106. Nevertheless, initial evidence indicates that induced waves travel over similar fibre networks as waves evoked during stimulus onset66,77.
The key factor governing the generation and propagation of stimulus-evoked, induced and spontaneous travelling waves at the mesoscale is the axonal conduction delay of the horizontal fibres of superficial layers in the cortex14,23,67. These fibres project over long distances across the cortical sheet95,107, providing the substrate for travelling waves and governing their amplitude decay. Computational and mathematical studies have demonstrated the role of distance-dependent axonal conduction delays in generating stimulus-evoked waves in recurrent networks87,108,109. Specifically, it has been shown that distance- dependent delays can destabilize synchrony in coupled oscillator networks, leading to travelling waves110. However, it remains unknown whether and how spontaneous and stimulus-evoked waves can be generated in large-scale spiking networks with biologically realistic synaptic noise, in which model neurons exhibit the irregular asynchronous (IA) state that is the hallmark of cortical dynamics111. Stochastic neural field theory has studied the effect of noise on propagation of travelling waves in neural systems112; however, because the noise generated by networks in the IA state has a specific and nonstationary signature113, the dynamics of large-scale spiking networks may differ substantially from those of stochastic neural fields, and few detailed comparisons have been done.
In computational models of spiking networks, the degree to which neurons are entrained by travelling waves is a key factor in determining the dynamics and interactions of waves. In small models, in which synaptic weights are strong and correlations are increased114 (a rough analogue of biological networks in vitro), travelling waves are dense and strongly entrain neurons in the local circuit as they pass (FIG. 4a). Waves in such networks generally exhibit repelling interactions115,116, in which two colliding waves will either disappear or deflect each other onto new paths. In larger networks, however, with network states matching those in the cortex and sparse (weakly entraining) waves (FIG. 4b), these interactions could be much different, with waves weakly interacting while passing through each other as they travel. These differences in wave interaction suggest vastly different general computational principles, to which we turn in the next section.
Emerging computational principles
As introduced above and noted previously22, spontaneous and stimulus-evoked travelling waves add a spatiotemporal context to the orderly response properties of the visual cortex first described by Hubel and Wiesel80,81. In the canonical model of visual processing (and, indeed, of sensory processing in general), cortical neurons are independent feature detectors that build progressively more complex representations through successive elaboration of receptive field selectivity in each cortical region117. Stimulus-evoked travelling waves in the visual cortex introduce strong contextual influences into this model of cortical dynamics: for example, if two nearby sensory stimuli are presented in succession, the first may generate a travelling wave that has an effect on the response to the second, transiently modulating the receptive field properties of neurons whose receptive fields contain the second stimulus. Such contextual modulations have been discussed previously in connection with the notion of ‘non-classical’ receptive fields118–121, which can implement spatial interactions that are well captured by the theory of association fields (REFS 122–124). Evidence that travelling waves actively influence visual processing, for example, by producing cortical correlates of low-level visual percepts such as the line-motion illusion24,125 or the perceived speed of apparent motion stimuli126,127, has been reported by linking cortical dynamics during anaesthetized states to human psychophysical data. An overview of observed cortical travelling waves and their potential computational roles is given in TABLE 1. A comprehensive conceptual framework for travelling waves and dominant recurrent interactions during normal awake sensory processing is, however, lacking.
Table 1. Observed cortical travelling waves and potential computational roles.
| Neural system | Specific regions | Appearance in single electrode recordings | Proposed role | Refs |
|---|---|---|---|---|
| Visual | V1 and V2 | Stimulus-evoked onset response (40–140 ms after stimulus onset) | Distributed computations with spatiotemporal patterns in sensory maps | 14,21,67 |
| V1 | Gamma oscillation (30–80 Hz) | Distributed computations with spatiotemporal patterns in sensory maps | 30,77,79 | |
| Sensorimotor | V4 | Saccade-evoked LFP transient | Perisaccadic suppression of visual processing or motor guidance for the following saccade | 29 |
| Motor | M1 and PMd | Beta oscillation (10–45 Hz) during movement planning | Sequencing activation in proximal-to-distal muscle representations in the motor cortex | 19,131–134 |
| Hippocampus | CA1 | Theta oscillation (6–12 Hz) | Patterning pyramidal spiking from small to large place fields in each theta cycle | 17,18,140 |
| Ripple oscillation (140–200 Hz) | Patterning spiking in either direction of the dorsoventral axis in hippocampus | 146 | ||
| Macroscopic | NA | Slow oscillation (0.1-1.0 Hz) | Orchestrating large-scale neural spiking in slow-wave sleep | 44,56 |
| Spindle oscillation (11–15 Hz) | Building distributed neuronal assemblies in the cortex during memory consolidation in sleep | 13 |
LFP, local field potential; M1, primary motor cortex; NA, not applicable; PMd, dorsal premotor cortex; V1, primary visual cortex; V2, secondary visual cortex; V4, visual area V4.
Converging evidence shows that the canonical model of visual processing is excellent for coding stationary images but has difficulty explaining processing of dynamic, nonstationary naturalistic stimuli128. For example, standard models of cortical neurons based on this framework capture only a fraction of the response variance observed under natural sensory conditions129. Below, we first review specific examples of functional roles for waves recently found in sensorimotor conditions and the hippocampus and then, inspired by a physical process for information storage by dynamic wave fields, introduce a general conceptual framework for cortical travelling waves in the processing of visual scenes.
Waves and sensorimotor coordination
Beta-frequency (15–30 Hz) oscillations have a strong connection to movement preparation and motor coordination130, and their spatiotemporal properties have been a subject of much interest in recent years. In the motor cortex of monkeys, beta oscillations before movement initiation during an instructed-delay reach task are waves travelling in a consistent anatomical direction131. Similar dynamics were found in the motor cortex of humans132. It was later confirmed that these LFP travelling waves modulate spiking activity at the single-unit level19. In the context of the instructed-delay reach task, the travelling waves in the motor cortex have a plausible proposed functional role: they have been suggested to facilitate sequences of activation in proximal-to-distal muscle representations in preparation for reaching behaviour133,134. While detailed future studies could illustrate the effect of recorded cortical activity on specific resulting patterns of muscle activation19, these observations provided a first link between cortical travelling waves and physiologically relevant neural outputs, suggesting that they play distinct functional roles.
In similar sensorimotor conditions in the visual system, saccades have been found to induce beta frequency travelling LFP waves in visual area V4 (REF. 29). These waves travel from foveal to peripheral regions of the V4 retinotopic map, creating a spatiotemporal pattern of post-saccadic excitability. Various functional roles are possible for this pattern, including saccadic suppression (useful in eliminating blurred visual scenes during eye movements)135 and motor guidance in planning the following saccade. While their exact functional role remains unclear, these saccade-induced travelling waves in the visual system represent a second sensorimotor condition that may be probed by future work for a clear link between cortical travelling waves and physiological function.
Travelling waves in the hippocampus
Hippocampal population rhythms are some of the most well-studied oscillations in the brain and are of particular interest because of their important connections to learning and long-term memory136. The 6–12 Hz theta oscillation modulates hippocampal LFP and pyramidal cell spiking during active exploration and rapid-eye-movement sleep15,137, organizing the firing of hippocampal place cells in a crucial manner138. Long thought to be approximately synchronous throughout the hippo campal formation139, theta oscillations have recently been shown to be waves travelling along the dorsoventral axis of CA1 (REFS 17,18,140) (FIG. 5a). Because hippocampal place fields become progressively larger along this axis141,142 (FIG. 5b), these travelling theta waves organize CA1 place cell spikes into a sweep from the finest to the coarsest scales of spatial selectivity17,18 (FIG. 5c), possibly linking high-precision spatial information with broader contextual content. In addtion, the specific spatiotemporal structure of the LFP during these travelling theta waves has been shown to contain precise information about an animal's location143
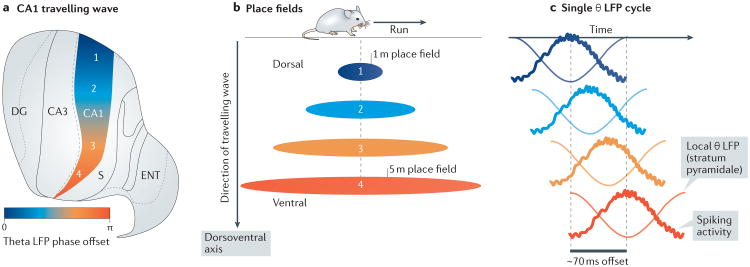
Figure 5. Patterning of place field firing by hippocampal travelling waves.
a | The 6–12 Hz hippocampal theta oscillation, which appears during active behavioural exploration and rapid-eye-movement sleep, is a wave travelling from dorsal to ventral hippocampal CA1 (REFS 17,18). This flattened map view of the rodent hippocampus shows the positions of the dentate gyrus (DG), CA3, CA1, subiculum (S) and entorhinal cortex (ENT). The phase offset of the theta oscillation is plotted in colour along the dorsoventral axis of CA1 (colour axis, bottom left). b | As the rodent runs on a linear track, pyramidal neurons in CA1 fire. These neurons exhibit place fields that become progressively larger as their location in CA1 changes from dorsal to ventral regions141,142. The schematic illustrates a typical example of the place fields of neurons that fire when the rodent is at a particular spatial location during the run and is based on data in REF 17. The place fields are arranged according to their location in CA1 and with their colour illustrating the phase offset of the theta oscillation at that location on the dorsoventral axis. c | Because of the phase offset of the theta local field potential (LFP; as recorded in stratum pyramidale18; shown as light transparent lines) owing to the travelling wave (represented by the distance between the dotted lines) and the modulation of neuronal firing by theta (solid lines), neurons with CA1 place fields centred at the rodent's position fire in a temporal sweep from those with the smallest scales of spatial selectivity to those with the largest scales of spatial selectivity. Part a is reproduced from REF. 17, Macmillan Publishers Limited. Part c is adapted with permission from REF. 18, Elsevier.
High-frequency 140-200 Hz ripple oscillations, which appear during awake resting and slow-wave sleep144 and are involved in replay of waking hippocampal spike sequences during sleep145, have also been shown to be travelling waves, starting at discrete points along the dorsoventral axis and propagating in either the dorsal or ventral direction146. The computational consequences of this exquisite mesoscopic organization of firing patterns by theta and ripple travelling waves in the hippocampus have not yet been investigated in detail; however, they have implications for theories involving synaptic plasticity as a mechanism for linking neurons across the hippocampus147,148.
Potential computational roles for stimulus-evoked travelling waves
In the specific examples presented above, the existence of travelling waves is not in conflict with what is known about the underlying physiology: in both cases, travelling waves may organize population spiking along some behaviourally relevant axis (in a sweep from proximal to distal muscle representations, foveal to peripheral visual fields or small to large place fields). As outlined at the beginning of this section, however, a coherent account of computational roles for stimulus- evoked waves in the sensory cortex is lacking. At present, we cannot exclude the possibility that no specific computational role exists for spontaneous and stimulus-evoked waves and that they may be an epiphenomenon of the massive recurrent connectivity in the cortex. A direct demonstration of the function of these waves in sensory processing is lacking, most importantly, in the awake animal. However, it is clear that the information carried by these waves could play important computational roles. Stimulus-evoked travelling waves in V1 extend well beyond the locus of the initial stimulus- evoked response, broadly influencing neurons over several millimetres of the retinotopic map14,24,25,67. Furthermore, it is important to consider that these waves travel over other functional maps such as ocular dominance and orientation maps in V1 and have been shown to distribute orientation specific information across the cortex (though this specificity decreases over a moderately short distance in the cortex)25,149,150. This suggests that waves will be useful in visual processing on scales beyond the imprint of feedforward activation, opening up interesting new computational capacities. To explore this further, we now consider general models for computation with evoked waves travelling over sensory maps in the cortex.
Computation with waves has been a subject of continued interest in reaction-diffusion systems151, with some applications to spiking neural networks116. In these models, a localized wave state (generally a small travelling excitation) is considered a unit (or packet) of information, with computations implemented by interactions between localized wave states. For example, the merging of two waves might serve to compute an AND logic operation, while the annihilation of two waves may represent a dynamic XOR gate. These collision-based computations152, however, require dense wave interactions of the kind observed in small spiking networks with low numbers of synaptic connections per cell (see above). In the weakly entrained regime consistent with the low-correlated firing in physiologically normal cortex153,154, sparse waves may interact much less strongly as they travel than dense waves. In this situation, multiple evoked waves could lead to a global spatiotemporal pattern of activation whose usefulness in computation must be considered within a different context.
What computations can be done with evoked, weakly interacting travelling waves in single cortical regions? For inspiration, we can look to a recently described physical process as an analogue. A recent study examined the dynamics of a silicone droplet bouncing on a vibrating oil bath155. The vibrations of this medium propel the droplet upward and cause it to bounce continually, creating a pattern of successive impacts across the surface (FIG. 6a). With each bounce, the droplet creates a set of waves in the oil bath. In our analogy, these droplet waves may be similar to those created in the sensory cortex by the impact of afferent, stimulus-driven action potentials. The waves evoked by each impact of the droplet then create a pattern across the oil bath that leaves a trace of the path of the droplet. With sufficient knowledge of the dynamic properties of the medium, one could decode the history of the path of the droplet simply from the observed spatial pattern at each point in time. Furthermore, temporal reversibility is preserved for waves even in chaotic systems; this property, along with a clever manipulation of the experimental apparatus, can be used to cause the droplet to exactly reverse its trajectory (FIG. 6a). It is argued that this last manipulation shows that the global wave field stores information about the trajectory of the droplet with sufficient precision that it can be read back out. While the authors did not have neuroscience in mind and, indeed, note that they have not yet considered practical implementations, they posit that this system implements the basic elements for general computations that could find application in future work.
Figure 6. Computation with cortical waves.
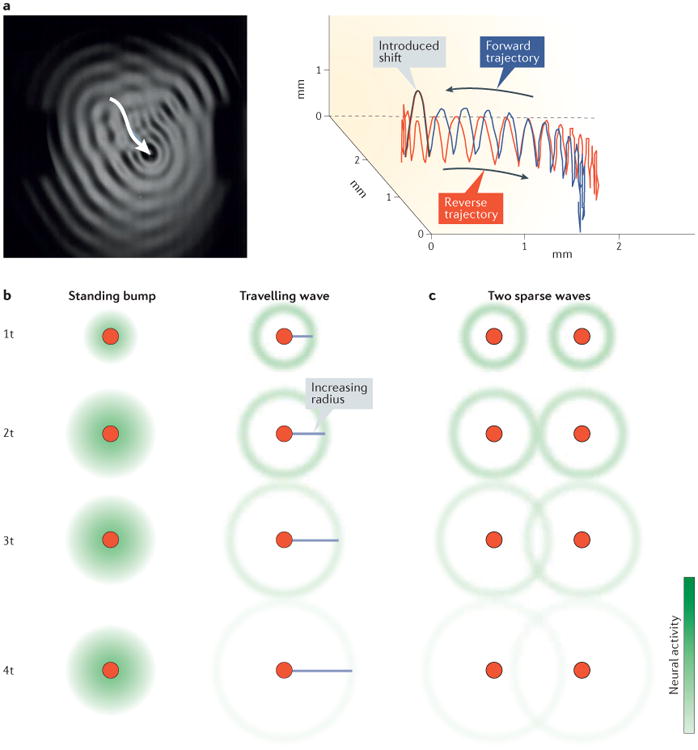
a | A droplet emits travelling radial waves as it bounces and creates a trajectory (white arrow) across the surface of a silicone oil bath (left panel)155. As it bounces, the droplet creates a forward trajectory (blue line, right panel). The x axis, y axis and z axis represent the position of the droplet in space. With a simple manipulation, or introduced shift (a higher bounce, black line), the droplet can reverse its trajectory (red line), illustrating that the evoked wave field stores information about the recent past. b | Schematic models of standing bumps and travelling waves evoked by an external stimulus (red dot) are shown. As time increases (from 1 to 4 time units (t)), the standing bump reaches a steady pattern, whereas the travelling wave continues to expand across the network. It is therefore hypothesized that time from stimulus onset can be decoded in the case of travelling waves but not standing bumps. c | It is hypothesized that two sparse waves interacting in the cortex will weakly interact, passing through each other (unlike dense waves) and creating a global wave field with excitations that are symmetric about each stimulated point. Part a is adapted with permission from REF. 155. Copyrighted by the American Physical Society.
This physical system, though in many ways an imperfect analogy to the sensory cortex, provides a suggestive example for possible computations that could be performed by waves radiating from the retinotopic loci of stimulus-evoked input. The key difference between stimulus-driven stationary bumps of activity and active travelling waves is that the latter contain information about time from stimulus onset (FIG. 6b). While stationary bumps contain information about stimulus position in a retinotopic map, in a network with active stimulus-evoked travelling waves, one could decode both stimulus position and onset time. During the lifetime of waves travelling over a retinotopic map, they could therefore encode both stimulus position and the recent past in a distributed spatiotemporal pattern of activation (FIG. 6c). The availability of information about the recent past may be useful in processing dynamic, non-stationary sensory scenes, which present difficulties for the static feedforward framework128,129. It is important to note that, though other activity patterns (such as expanding bumps) could encode both stimulus position and onset time, such activity patterns will be less robust than travelling waves arising from multiple points of stimulation. Similar ideas for computation have been discussed previously156 but never before in the context of sensory systems and cortical topographic maps. Finally, because of their timing relative to sensory stimuli, induced waves during sustained responses may exhibit a much richer interaction between feedforward and feedback pathways in the cortex; thus, they may serve different computational roles than waves evoked by the initial feedforward input. However, because induced waves will propagate similarly across topographic maps in the sensory cortex, the framework discussed here can serve as a guiding example for further investigations.
This framework could potentially provide a general outline for computation with travelling waves in the sensory cortex, helping to resolve the difficulties posed by stimulus-evoked travelling waves to the canonical feedforward model of visual processing. Further experimental and theoretical work will be needed to further refine our understanding of travelling waves in these regions. Here, however, we have highlighted the importance of multi-wave interactions and suggest that they provide fertile ground for future experimental and theoretical work. Analysis of multichannel recordings will make it possible to test whether stimulus position and timing can be reliably decoded from responses at the single-trial level. While it has been noted that cortical travelling waves may structure visual responses to dynamic natural scenes30, it remains unclear how the spatiotemporal interactions revealed following simple sensory stimuli14 generalize to multi-stimulus paradigms and natural scenes. Nevertheless, the effect of the abundant horizontal connections highlighted in this work (and their intrinsic axonal time delays) is not expected to disappear. Furthermore, it remains unknown how spontaneous and stimulus-evoked mesoscopic waves may influence computations in downstream cortical areas. Improved imaging techniques52,157 and new optogenetic approaches in awake, behaving primates158,159 may allow future studies to directly test the functional roles for cortical travelling waves discussed here. Central to this framework is the recognition that the fine-scale dynamics of neural populations can carry important additional information over sensory topographic maps. Even more complex interactions in higher-order association regions and in prefrontal cortex could be involved in cognitive functions such as planning and decision making.
Conclusions
In this Review, we have covered recent advances in travelling waves in neural systems. While initial evidence indicated that travelling waves were pronounced only in weak input regimes21,22, recent studies have shown that travelling waves are evoked following small, high-contrast visual stimuli14, and they appear to play a role in cortical dynamics driven by dynamic sensory input and sensorimotor planning30. It has previously been noted that the existence of stimulus-evoked travelling waves in the sensory cortex presents a challenge to the orderly topographic arrangement of selectivity first described by Hubel and Wiesel at the trial-averaged level22. Here, we have outlined an initial conceptual framework for computations with travelling waves in the sensory cortex. This regime, in which activity patterns generated by intracortical circuits actively shape responses to external stimuli, is well matched to the basic anatomy of the cortex, where neurons receive a large fraction of their input from a local patch of cells in the same region94. Much theoretical and experimental work remains to test the feasibility of computations with travelling waves in the cortex; however, the development of large-scale, high-precision recording technologies is a priority in neuroscience, which will drive advances in these fields.
Acknowledgments
The authors thank Z. Davis, T. Bartol, G. Pao, A. Destexhe, Y Frégnac and C. F. Stevens for helpful discussions and J. Ogawa for helpful discussions and help with illustrations. L.M. acknowledges support from the US National Institute of Mental Health (5T32MH020002-17). F.C. acknowledges support from Agence Nationale de la Recherche (ANR) projects BalaV1 (ANR-13-BSV4-0014-02) and Trajectory (ANR- 15-CE37-0011-01). J.R. acknowledges support from the Fiona and Sanjay Jha Chair in Neuroscience at the Salk Institute. T.J.S. acknowledges support from Howard Hughes Medical Institute, Swartz Foundation and the Office of Naval Research (N000141210299).
Glossary
- Phase offsets
The differences in phase (an amplitude-invariant measure of position in an oscillation cycle) between two (or more) oscillations
- Travelling waves
A disturbance that travels through a physical medium that may be water, air or a neural network
- Complex spatiotemporal patterns
Patterns that can result from the summation of many individual waves. Depending on the properties of the medium the pattern resulting from these interactions can differ greatly
- Mesoscopic
A scale between microscopic and macroscopic. In neuroscience, the mesoscopic scale describes single regions (such as cortical areas or subcortical nuclei) spanning millimetres to centimetres. Cortical networks at this scale can be imaged through recently developed recording technologies
- Macroscopic
The scale of the whole brain traditionally recorded with extracranial techniques (electroencephalography and magnetoencephalography) and more recently recorded with intracranial methods (electrocorticography)
- Electroencephalography
(EEG). A neural recording technique in which electrodes are placed on the scalp outside the skull (extracranial) that is of great use in studying the sensory and cognitive processes of normal human subjects
- Electrocorticography
(ECoG). A recording technique in which electrodes are placed directly on the cortical surface, offering both high spatial (up to 2 millimetres or greater) and high temporal resolution
- Local field potential
(LFP). The electric potential recorded in the extracellular space of the cortex. The LFP is thought to reflect the synaptic currents from neurons within a few hundred micrometres around the electrode
- Multielectrode arrays
(MEAs). One-dimensional or two-dimensional grids of electrodes, which offer the ability to sample local field potential and spiking activity at the mesoscopic scale
- Voltage-sensitive dyes
(VSDs). Fluorescent dyes applied directly to the surface of the cortex that allow the subthreshold membrane potential of neural populations to be recorded. The resulting signals are linearly related to the average membrane potential of neurons at each point in the cortex. This technique captures neural activity over a large field of view with very high spatial (up to 20 micrometres) and temporal (up to 1 millisecond) resolution
- Slow oscillation
The large, 0.1-1.0 Hz rhythm of deep non-rapid-eye-movement sleep
- Volume conduction
Passive transmission of an electric field through biological tissue. The fields can be created from a single source of neural activity and will appear as identical, highly synchronous waveforms across electrodes; a cause of spatial smoothing (blurring) in scalp electroencephalography
- K-Complex
A brief (∼1 second), biphasic waveform composed of a strong negative potential followed by a positive deflection. K-Complexes occur predominantly during stage 2 non-rapid-eye-movement sleep and are driven by transitions from cortical down to up states
- Sleep spindles
Thalamocortical 11–15 Hz oscillations prevalent in stage 2 non-rapid-eye-movement sleep. These oscillatory periods have long been associated with learning and memory, including sleep-dependent consolidation of long-term memory
- Cell assemblies
A group of interconnected, repeatedly co-activated neurons whose signature spike pattern is thought to collectively represent a specific sensory stimulus or memory
- Coupled oscillator networks
Models of emergent collective behaviour in large ensembles. In these networks, individual units are characterized by a state (or phase) between 0 and 2π. Interactions among units are typically modelled as attractive, such that units with different states tend to synchronize depending on the coupling strength of the interaction
- Irregular asynchronous (IA) state
A state of asynchronous, highly irregular firing in spiking network models. This state exhibits the low-correlated firing that is the hallmark of cortical dynamics under general conditions of excitatory and inhibitory balance
- Stochastic neural field theory
An extension of the neural field model of Wilson and Cowan to include the effects of neural and synaptic noise
- Dorsoventral axis
In rodents, the long axis of the hippocampus, running from a dorsal, medial position to a ventral, lateral position; synonymous with septotemporal axis
- Reaction–diffusion systems
Models of chemical dynamics that take into account local reactions and diffusion across space. These reactions exhibit complex dynamics, including travelling waves and emergent patterns
- Temporal reversibility
A property of a system whose dynamics remain the same when time is reversed. This feature implies important mathematical properties for the system under study
Footnotes
Author contributions: L.M., F.C, J.R. and T.J.S. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Competing interests: The authors declare no competing interests
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information: Nature Reviews Neuroscience thanks Gustavo Deco, Pascal Fries and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.von der Malsburg C. The Correlation Theory of Brain Function. Max-Planck-Institute for Biophysical Chemistry; Gottingen, Germany: 1981. [Google Scholar]
- 2.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akam T, Kullmann DM. Oscillations and filtering networks support flexible routing of information. Neuron. 2010;67:308–320. doi: 10.1016/j.neuron.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swadlow HA, Beloozerova IN, Sirota MG. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J Neurophysiol. 1998;79:567–582. doi: 10.1152/jn.1998.79.2.567. [DOI] [PubMed] [Google Scholar]
- 6.Usrey WM, Reid RC. Synchronous activity in the visual system. Annu Rev Physiol. 1999;61:435–456. doi: 10.1146/annurev.physiol.61.1.435. [DOI] [PubMed] [Google Scholar]
- 7.Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of ‘effective connectivity’. J Neurophysiol. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- 8.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 10.Ni J, et al. Gamma-rhythmic gain modulation. Neuron. 2016;92:240–251. doi: 10.1016/j.neuron.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray CM, König P, Engel AK, Singer W. W Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 12.Uhlhaas PJ, et al. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller L, et al. Rotating waves during human sleep spindles organize global patterns of activity that repeat precisely through the night. eLife. 2016;5:e17267. doi: 10.7554/eLife.17267. This analysis of ECoG recordings from human clinical patients reveals that the 11–15 Hz sleep spindle, long studied for its connection to learning and memory, is robustly organized into rotating waves travelling in a specific direction (from temporal to parietal to frontal cortex) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller L, Reynaud A, Chavane F, Destexhe A. The stimulus-evoked population response in visual cortex of awake monkey is a propagating wave. Nat Commun. 2014;5:3675. doi: 10.1038/ncomms4675. This paper showed that, during the awake state, small visual stimuli systematically evoke travelling waves extending far beyond the feedforward input in V1 and V2 of macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- 17.Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. This study showed that theta oscillations, long thought to be approximately synchronous throughout the hippocampus, are in fact travelling waves. [DOI] [PubMed] [Google Scholar]
- 18.Patel J, Fujisawa S, Berényi A, Royer S, Buzsáki G. Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron. 2012;75:410–417. doi: 10.1016/j.neuron.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, et al. Large-scale spatiotemporal spike patterning consistent with wave propagation in motor cortex. Nat Commun. 2015;6:7169. doi: 10.1038/ncomms8169. This paper specifically demonstrates that travelling waves modulate spiking activity in the motor cortex during normal waking behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 21.Nauhaus I, Busse L, Carandini M, Ringach DL. Stimulus contrast modulates functional connectivity in visual cortex. Nat Neurosci. 2009;12:70–76. doi: 10.1038/nn.2232. This study in anaesthetized cats and monkeys suggests that evoked travelling waves detected with spike-triggered LFP become less visible in high-input regimes; however, recent work has shown that travelling waves persist during high-input regimes and the awake state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato TK, Nauhaus I, Carandini M. Traveling waves in visual cortex. Neuron. 2012;75:218–229. doi: 10.1016/j.neuron.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Grinvald A, Lieke EE, Frostig RD, Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci. 1994;14:2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. This demonstrated the application of VSD in anaesthetized monkeys; using local stimuli, this study demonstrates that the cortical point spread function extends beyond the imprint of the feedforward input, with gradually increasing latency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jancke D, Chavane F, Naaman S, Grinvald A. Imaging cortical correlates of illusion in early visual cortex. Nature. 2004;428:423–426. doi: 10.1038/nature02396. This study showed that intracortical propagation can give rise to a coherently moving pattern of cortical activity from a sequence of two static stimuli. [DOI] [PubMed] [Google Scholar]
- 25.Chavane F, et al. Lateral spread of orientation selectivity in V1 is controlled by intracortical cooperativity. Front Syst Neurosci. 2011;5:4. doi: 10.3389/fnsys.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roland PE, et al. Cortical feedback depolarization waves: a mechanism of top-down influence on early visual areas. Proc Natl Acad Sci USA. 2006;103:12586–12591. doi: 10.1073/pnas.0604925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen CCH, Hahn TTG, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferezou I, Bolea S, Petersen CCH. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Zanos TP, Mineault PJ, Nasiotis KT, Guitton D, Pack CC. A sensorimotor role for traveling waves in primate visual cortex. Neuron. 2015;85:615–627. doi: 10.1016/j.neuron.2014.12.043. This study tied travelling waves in the sensory cortex to a sensorimotor behaviour — visual saccades. [DOI] [PubMed] [Google Scholar]
- 30.Besserve M, Lowe SC, Logothetis NK, Schölkopf B, Panzeri S. Shifts of gamma phase across primary visual cortical sites reflect dynamic stimulus-modulated information transfer. PLoS Biol. 2015;13:e1002257. doi: 10.1371/journal.pbio.1002257. Using an advanced information-theoretic analysis in recordings from anaesthetized monkeys, this study demonstrates that movie stimuli evoke complex spatiotemporal patterns consistent with propagation of activity along the horizontal-fibre network in V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JY, Huang X, Zhang C. Propagating waves of activity in the neocortex: what they are, what they do. Neuroscientist. 2008;14:487–502. doi: 10.1177/1073858408317066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller L, Destexhe A. Propagating waves in thalamus, cortex and the thalamocortical system: Experiments and models. J Physiol. 2012;106:222–238. doi: 10.1016/j.jphysparis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Hebb DO. The Organization of Behavior: A Neuropsychological Theory. Psychology Press; 2005. [Google Scholar]
- 34.Sejnowski TJ. The book of Hebb. Neuron. 1999;24:773–776. doi: 10.1016/s0896-6273(00)81025-9. [DOI] [PubMed] [Google Scholar]
- 35.Swadlow HA, Waxman SG. Axonal conduction delays. Scholarpedia. 2012;7:1451. [Google Scholar]
- 36.Maynard EM, Nordhausen CT, Normann RA. The Utah intracortical electrode array: a recording structure for potential brain-computer interfaces. Electroencephalogr Clin Neurophysiol. 1997;102:228–239. doi: 10.1016/s0013-4694(96)95176-0. [DOI] [PubMed] [Google Scholar]
- 37.Shoham D, et al. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron. 1999;24:791–802. doi: 10.1016/s0896-6273(00)81027-2. [DOI] [PubMed] [Google Scholar]
- 38.Girard P, Hupé JM, Bullier J. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. J Neurophysiol. 2001;85:1328–1331. doi: 10.1152/jn.2001.85.3.1328. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed OJ, Cash SS. Finding synchrony in the desynchronized EEG: the history and interpretation of gamma rhythms. Front Integr Neurosci. 2013;7:58. doi: 10.3389/fnint.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger H. Uber das Elektrenkephalogramm des Menschen. 1929 [Google Scholar]
- 41.Hughes JR. The phenomenon of travelling waves: a review. Clin Electroencephalogr. 1995;26:1–6. doi: 10.1177/155005949502600103. [DOI] [PubMed] [Google Scholar]
- 42.Hindriks R, van Putten MJAM, Deco G. Intra-cortical propagation of EEG alpha oscillations. Neuroimage. 2014;103:444–453. doi: 10.1016/j.neuroimage.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 43.van Ede F, van Pelt S, Fries P, Maris E. Both ongoing alpha and visually induced gamma oscillations show reliable diversity in their across-site phase-relations. J Neurophysiol. 2015;113:1556–1563. doi: 10.1152/jn.00788.2014. [DOI] [PubMed] [Google Scholar]
- 44.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribary U, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. With a novel technique for analysing data from magnetoencephalography, this study reports a unique, global anterior-to-posterior phase shift in gamma oscillations in the human cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. Oxford Univ. Press; 2006. [Google Scholar]
- 48.Bédard C, Destexhe A. Mean-field formulation of Maxwell equations to model electrically inhomogeneous and isotropic media. JEMAA. 2014;06:296–302. [Google Scholar]
- 49.Bedard C, Gomes JM, Bal T, Destexhe A. A framework to reconcile frequency scaling measurements, from intracellular recordings, local-field potentials, up to EEG and MEG signals. J Integr Neurosci. 2017;16:3–18. doi: 10.3233/JIN-160001. [DOI] [PubMed] [Google Scholar]
- 50.Alexander DM, et al. Traveling waves and trial averaging: the nature of single-trial and averaged brain responses in large-scale cortical signals. Neuroimage. 2013;73:95–112. doi: 10.1016/j.neuroimage.2013.01.016. This study details how trial averaging can shape evoked responses and observed spatiotemporal dynamics in macroscopic recordings of the human brain. [DOI] [PubMed] [Google Scholar]
- 51.Jasper H, Penfield W. Electrocorticograms in man: Effect of voluntary movement upon the electrical activity of the precentral gyrus. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1949;183:163–174. [Google Scholar]
- 52.Khodagholy D, et al. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 2015;18:310–315. doi: 10.1038/nn.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller L, Hamilton LS, Edwards E, Bouchard KE, Chang EF. Spatial resolution dependence on spectral frequency in human speech cortex electrocorticography. J Neural Eng. 2016;13:056013. doi: 10.1088/1741-2560/13/5/056013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray S, Maunsell JHR. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bahramisharif A, et al. Propagating neocortical gamma bursts are coordinated by traveling alpha waves. J Neurosci. 2013;33:18849–18854. doi: 10.1523/JNEUROSCI.2455-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hangya B, et al. Complex propagation patterns characterize human cortical activity during slow-wave sleep. J Neurosci. 2011;31:8770–8779. doi: 10.1523/JNEUROSCI.1498-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mak-McCully RA, et al. Distribution, amplitude, incidence, co-occurrence, and propagation of human K-complexes in focal transcortical recordings. eNeuro. 2015 doi: 10.1523/ENEURO.0028-15.2015. https://doi.org/10.1523/ENEURO.0028-15.2015. [DOI] [PMC free article] [PubMed]
- 58.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrillon T, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–17834. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schüz A, Braitenberg V. In: Cortical Areas: Unity and Diversity. Schüz A, Miller R, editors. Taylor Francis; London: 2002. pp. 377–385. [Google Scholar]
- 61.Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- 62.Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- 63.Chung S, Ferster D. Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron. 1998;20:1177–1189. doi: 10.1016/s0896-6273(00)80498-5. [DOI] [PubMed] [Google Scholar]
- 64.Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 65.Sompolinsky H, Shapley R. New perspectives on the mechanisms for orientation selectivity. Curr Opin Neurobiol. 1997;7:514–522. doi: 10.1016/s0959-4388(97)80031-1. [DOI] [PubMed] [Google Scholar]
- 66.Prechtl JC, Cohen LB, Pesaran B, Mitra PP, Kleinfeld D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc Natl Acad Sci USA. 1997;94:7621–7626. doi: 10.1073/pnas.94.14.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bringuier V, Chavane F, Glaeser L, Frégnac Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science. 1999;283:695–699. doi: 10.1126/science.283.5402.695. This study provided experimental evidence for evoked travelling waves in the mammalian cortex and uses intracellular recordings in which subthreshold depolarizing responses outside the spiking receptive field become more delayed for stimuli presented farther from the receptive field centre. [DOI] [PubMed] [Google Scholar]
- 68.Slovin H, Arieli A, Hildesheim R, Grinvald A. Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys. J Neurophysiol. 2002;88:3421–3438. doi: 10.1152/jn.00194.2002. [DOI] [PubMed] [Google Scholar]
- 69.Xu W, Huang X, Takagaki K, Wu JY. Compression and reflection of visually evoked cortical waves. Neuron. 2007;55:119–129. doi: 10.1016/j.neuron.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han F, Caporale N, Dan Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron. 2008;60:321–327. doi: 10.1016/j.neuron.2008.08.026. This is the first study to show dynamic changes in the pattern of spontaneous travelling waves in the cortex following repeated sensory stimulation, which is shown in anaesthetized rodents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ray S, Maunsell JHR. Network rhythms influence the relationship between spike-triggered local field potential and functional connectivity. J Neurosci. 2011;31:12674–12682. doi: 10.1523/JNEUROSCI.1856-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nauhaus I, Busse L, Ringach DL, Carandini M. Robustness of traveling waves in ongoing activity of visual cortex. J Neurosci. 2012;32:3088–3094. doi: 10.1523/JNEUROSCI.5827-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Telenczuk B, Destexhe A. How neuronal correlations affect the LFP signal? BMC Neurosci. 2015;16:60. [Google Scholar]
- 74.Sit YF, Chen Y, Geisler WS, Miikkulainen R, Seidemann E. Complex dynamics of V1 population responses explained by a simple gain-control model. Neuron. 2009;64:943–956. doi: 10.1016/j.neuron.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maris E, Womelsdorf T, Desimone R, Fries P. Rhythmic neuronal synchronization in visual cortex entails spatial phase relation diversity that is modulated by stimulation and attention. Neuroimage. 2013;74:99–116. doi: 10.1016/j.neuroimage.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maris E, Fries P, van Ede F. Diverse phase relations among neuronal rhythms and their potential function. Trends Neurosci. 2016;39:86–99. doi: 10.1016/j.tins.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Gabriel A, Eckhorn R. A multi-channel correlation method detects traveling γ-waves in monkey visual cortex. J Neurosci Methods. 2003;131:171–184. doi: 10.1016/j.jneumeth.2003.08.008. This study reported that gamma oscillations in V1 can be organized into travelling waves in awake macaques. [DOI] [PubMed] [Google Scholar]
- 78.Freeman WJ, Barrie JM. Analysis of spatial patterns of phase in neocortical gamma EEGs in rabbit. J Neurophysiol. 2000;84:1266–1278. doi: 10.1152/jn.2000.84.3.1266. [DOI] [PubMed] [Google Scholar]
- 79.Vinck M, et al. Gamma-phase shifting in awake monkey visual cortex. J Neurosci. 2010;30:1250–1257. doi: 10.1523/JNEUROSCI.1623-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reynaud A, Masson GS, Chavane F. Dynamics of local input normalization result from balanced short-and long-range intracortical interactions in area V1. J Neurosci. 2012;32:12558–12569. doi: 10.1523/JNEUROSCI.1618-12.2012. This study proposes that horizontal propagation interacts with feedforward input through a common gain-control mechanism, implementing a dynamic surround suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ermentrout GB, Kleinfeld D. Traveling electrical waves in cortex: insights from phase dynamics and speculation on a computational role. Neuron. 2001;29:33–44. doi: 10.1016/s0896-6273(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 84.Wilson HR, Cowan JD. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972;12:1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Helias M, et al. Supercomputers ready for use as discovery machines for neuroscience. Front Neuroinform. 2012;6:26. doi: 10.3389/fninf.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bressloff PC. Spatiotemporal dynamics of continuum neural fields. J Phys A Math Theor. 2011;45:033001. [Google Scholar]
- 87.Ermentrout B. Neural networks as spatio-temporal pattern-forming systems. Rep Prog Phys. 1998;61:353–430. [Google Scholar]
- 88.Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9:363–371. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- 89.Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Braitenberg V, Schüz A. Cortex: Statistics and Geometry of Neuronal Connectivity. Springer; 1998. [Google Scholar]
- 91.Jeong SO, Ko TW, Moon HT. Time-delayed spatial patterns in a two-dimensional array of coupled oscillators. Phys Rev Lett. 2002;89:154104. doi: 10.1103/PhysRevLett.89.154104. [DOI] [PubMed] [Google Scholar]
- 92.Udeigwe L, Ermentrout G. Waves and patterns on regular graphs. SIAM J Appl Dyn Syst. 2015;14:1102–1129. [Google Scholar]
- 93.Binzegger T, Douglas RJ, Martin KAC. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markov NT, et al. Weight consistency specifies regularities of macaque cortical networks. Cereb Cortex. 2011;21:1254–1272. doi: 10.1093/cercor/bhq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Angelucci A, et al. Circuits for local and global signal integration in primary visual cortex. J Neurosci. 2002;22:8633–8646. doi: 10.1523/JNEUROSCI.22-19-08633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y, et al. The linearity and selectivity of neuronal responses in awake visual cortex. J Vis. 2009;9:12. doi: 10.1167/9.9.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Destexhe A, Rudolph-Lilith M. Neuronal Noise. Springer; US: 2012. [Google Scholar]
- 98.Hô N, Destexhe A. Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J Neurophysiol. 2000;84:1488–1496. doi: 10.1152/jn.2000.84.3.1488. [DOI] [PubMed] [Google Scholar]
- 99.Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;27:5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 102.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Kerkoerle T, et al. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci USA. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pinto DJ, Patrick SL, Huang WC, Connors BW. Initiation, propagation, and termination of epileptiform activity in rodent neocortex in vitro involve distinct mechanisms. J Neurosci. 2005;25:8131–8140. doi: 10.1523/JNEUROSCI.2278-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trevelyan AJ, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci. 2007;27:3383–3387. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klink PC, Dagnino B, Gariel-Mathis MA, Roelfsema PR. Distinct feedforward and feedback effects of microstimulation in visual cortex reveal neural mechanisms of texture segregation. Neuron. 2017;95:209–220.e3. doi: 10.1016/j.neuron.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 107.Stettler DD, Das A, Bennett J, Gilbert CD. Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron. 2002;36:739–750. doi: 10.1016/s0896-6273(02)01029-2. [DOI] [PubMed] [Google Scholar]
- 108.Osan R, Ermentrout B. Two dimensional synaptically generated traveling waves in a theta-neuron neural network. Neurocomputing. 2001;38:789–795. [Google Scholar]
- 109.Veltz R, Faugeras O. Stability of the stationary solutions of neural field equations with propagation delays. J Math Neurosci. 2011;1:1. doi: 10.1186/2190-8567-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ermentrout B, Ko TW. Delays and weakly coupled neuronal oscillators. Phil Trans A Math Phys Eng Sci. 2009;367:1097–1115. doi: 10.1098/rsta.2008.0259. [DOI] [PubMed] [Google Scholar]
- 111.Brunel N. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J Comput Neurosci. 2000;8:183–208. doi: 10.1023/a:1008925309027. [DOI] [PubMed] [Google Scholar]
- 112.Bressloff PC. In: Neural Fields: Theory and Applications. Coombes S, beim Graben P, Potthast R, Wright J, editors. Springer; Berlin, Heidelberg: 2014. pp. 235–268. [Google Scholar]
- 113.Destexhe A, Contreras D. Neuronal computations with stochastic network states. Science. 2006;314:85–90. doi: 10.1126/science.1127241. [DOI] [PubMed] [Google Scholar]
- 114.Morrison A, Aertsen A, Diesmann M. Spike-timing-dependent plasticity in balanced random networks. Neural Comput. 2007;19:1437–1467. doi: 10.1162/neco.2007.19.6.1437. [DOI] [PubMed] [Google Scholar]
- 115.Keane A, Gong P. Propagating waves can explain irregular neural dynamics. J Neurosci. 2015;35:1591–1605. doi: 10.1523/JNEUROSCI.1669-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gong P, van Leeuwen C. Distributed dynamical computation in neural circuits with propagating coherent activity patterns. PLoS Comput Biol. 2009;5:e1000611. doi: 10.1371/journal.pcbi.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat Neurosci. 1999;2:1019–1025. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- 118.Blakemore C, Tobin EA. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15:439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- 119.Gilbert CD. Adult cortical dynamics. Physiol Rev. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- 120.Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- 121.Albright TD, Stoner GR. Contextual influences on visual processing. Annu Rev Neurosci. 2002;25:339–379. doi: 10.1146/annurev.neuro.25.112701.142900. [DOI] [PubMed] [Google Scholar]
- 122.Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local ‘association field’. Vision Res. 1993;33:173–193. doi: 10.1016/0042-6989(93)90156-q. [DOI] [PubMed] [Google Scholar]
- 123.Polat U, Sagi D. Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vision Res. 1993;33:993–999. doi: 10.1016/0042-6989(93)90081-7. [DOI] [PubMed] [Google Scholar]
- 124.Hess RF, Dakin SC. Contour integration in the peripheral field. Vision Res. 1999;39:947–959. doi: 10.1016/s0042-6989(98)00152-7. [DOI] [PubMed] [Google Scholar]
- 125.Ahmed B, et al. Cortical dynamics subserving visual apparent motion. Cereb Cortex. 2008;18:2796–2810. doi: 10.1093/cercor/bhn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Georges S, Seriès P, Frégnac Y, Lorenceau J. Orientation dependent modulation of apparent speed: psychophysical evidence. Vision Res. 2002;42:2757–2772. doi: 10.1016/s0042-6989(02)00303-6. [DOI] [PubMed] [Google Scholar]
- 127.Gerard-Mercier F, Carelli PV, Pananceau M, Troncoso XG, Frégnac Y. Synaptic correlates of low-level perception in V1. J Neurosci. 2016;36:3925–3942. doi: 10.1523/JNEUROSCI.4492-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carandini M, et al. Do we know what the early visual system does? J Neurosci. 2005;25:10577–10597. doi: 10.1523/JNEUROSCI.3726-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Olshausen BA, Field DJ. How close are we to understanding v1? Neural Comput. 2005;17:1665–1699. doi: 10.1162/0899766054026639. [DOI] [PubMed] [Google Scholar]
- 130.Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA. 1993;90:4470–4474. doi: 10.1073/pnas.90.10.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rubino D, Robbins KA, Hatsopoulos NG. Propagating waves mediate information transfer in the motor cortex. Nat Neurosci. 2006;9:1549–1557. doi: 10.1038/nn1802. This is one of the first studies to uncover travelling waves in the awake state and shows that these waves can carry task-relevant information during reaching behaviour in macaques. [DOI] [PubMed] [Google Scholar]
- 132.Takahashi K, Saleh M, Penn RD, Hatsopoulos NG. Propagating waves in human motor cortex. Front Hum Neurosci. 2011;5:40. doi: 10.3389/fnhum.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hatsopoulos NG, Olmedo L, Takahashi K. In: Motor Control. Danion F, Latash M, editors. Oxford Univ. Press; 2010. pp. 159–176. [Google Scholar]
- 134.Riehle A, Wirtssohn S, Grün S, Brochier T. Mapping the spatio-temporal structure of motor cortical LFP and spiking activities during reach-to-grasp movements. Front Neural Circuits. 2013;7:48. doi: 10.3389/fncir.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kleiser R, Seitz RJ, Krekelberg B. Neural correlates of saccadic suppression in humans. Curr Biol. 2004;14:386–390. doi: 10.1016/j.cub.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 136.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 138.Colgin LL. Mechanisms and functions of theta rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- 139.Bland BH, Anderson P, Ganes T. Two generators of hippocampal theta activity in rabbits. Brain Res. 1975;94:199–218. doi: 10.1016/0006-8993(75)90057-8. [DOI] [PubMed] [Google Scholar]
- 140.Zhang H, Jacobs J. Traveling theta waves in the human hippocampus. J Neurosci. 2015;35:12477–12487. doi: 10.1523/JNEUROSCI.5102-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung MW, Wiener SI, McNaughton B. L Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kjelstrup KB, et al. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- 143.Agarwal G, et al. Spatially distributed local fields in the hippocampus encode rat position. Science. 2014;344:626–630. doi: 10.1126/science.1250444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 145.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 146.Patel J, Schomburg EW, Berényi A, Fujisawa S, Buzsáki G. Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J Neurosci. 2013;33:17029–17041. doi: 10.1523/JNEUROSCI.2036-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sejnowski TJ. Storing covariance with nonlinearly interacting neurons. J Math Biol. 1977;4:303–321. doi: 10.1007/BF00275079. [DOI] [PubMed] [Google Scholar]
- 148.Paulsen O, Sejnowski TJ. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol. 2000;10:172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang X, Elyada YM, Bosking WH, Walker T, Fitzpatrick D. Optogenetic assessment of horizontal interactions in primary visual cortex. J Neurosci. 2014;34:4976–4990. doi: 10.1523/JNEUROSCI.4116-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rankin J, Chavane F. Neural field model to reconcile structure with function in primary visual cortex. PLoS Comput Biol. 2017;13:e1005821. doi: 10.1371/journal.pcbi.1005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adamatzky A, De Lacy Costello B, Asai T. Reaction-Diffusion Computers. Elsevier; 2005. [Google Scholar]
- 152.Adamatzky A, Durand-Lose J. In: Handbook of Natural Computing. Rozenberg G, Bäck T, Kok JN, editors. Springer; Berlin, Heidelberg: 2012. pp. 1949–1978. [Google Scholar]
- 153.Poulet JFA, Petersen CCH. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 154.Ecker AS, et al. Decorrelated neuronal firing in cortical microcircuits. Science. 2010;327:584–587. doi: 10.1126/science.1179867. [DOI] [PubMed] [Google Scholar]
- 155.Perrard S, Fort E, Couder Y. Wave-based Turing machine: time reversal and information erasing. Phys Rev Lett. 2016;117:094502. doi: 10.1103/PhysRevLett.117.094502. [DOI] [PubMed] [Google Scholar]
- 156.Izhikevich EM, Hoppensteadt FC. Polychronous wavefront computations. Int J Bifurcat Chaos. 2009;19:1733–1739. [Google Scholar]
- 157.Chemla S, et al. Improving voltage-sensitive dye imaging: with a little help from computational approaches. Neurophotonics. 2017;4:031215. doi: 10.1117/1.NPh.4.3.031215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Han X, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Diester I, et al. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]