Abstract
During pregnancy, abnormal proteinuria is defined as urine protein excretion greater than 300 mg/24 h. Although widely accepted, this definition is not based on clinical outcomes. Our study aimed to longitudinally examine proteinuria in healthy women prior to, and in late pregnancy and to compare inpatient and outpatient 24-hour urine collections. Nulliparous women planning to conceive were recruited and completed a 24-hour urinary collection. Those who subsequently conceived completed a second 24-hour urinary collection in late pregnancy. In the first 5 years of the study, urinary collections were completed during an inpatient admission; all collections during the latter part of the study were performed as outpatients. Urine protein was measured using the VITROS UPRO Slide kit. Wilcoxon signed rank tests were used for paired comparisons of prepregnancy and late pregnancy proteinuria and Wilcoxon rank sum tests were used to compare inpatient and outpatient collections. Among 134 women completing a prepregnancy collection, median urinary protein excretion was 188 mg/24 h (IQR 103-280). Sixty-five women subsequently conceived and completed a late pregnancy collection. In healthy women, urinary protein increased to 254 mg/24 h during pregnancy (IQR 166-396). Forty-five percent of women exceeded the defined normal threshold of proteinuria in 24 hours in the absence of disease. Inpatient collections resulted in higher levels of urinary protein than outpatient at both time points. Our data suggest that significant proteinuria is present in healthy nonpregnant women. Even in the absence of disease, proteinuria increases during pregnancy. Outpatient collections may underestimate proteinuria, especially in late pregnancy.
Keywords: pregnancy, proteinuria, 24-hour urinary protein
Introduction
Preeclampsia affects 5% to 8% of all pregnancies and is a significant contributor to maternal and fetal morbidity and mortality. In the presence of hypertension, urinary excretion of 300 mg of protein in 24 hours meets one current diagnostic criteria for preeclampsia.1 Although this threshold is widely accepted for defining abnormal proteinuria, its origin does not appear to be based on clinical outcomes but rather on expert opinion and small studies that have attempted to establish statistically normative values for pregnancy.
To our knowledge, the first definition of proteinuria as it relates to the diagnosis of preeclampsia appeared in the 1972 American College of Obstetricians and Gynecologists (ACOG) Committee on Terminology publication.2 Preeclampsia was defined as the development of hypertension with proteinuria, edema, or both due to pregnancy, and proteinuria as “the presence of urinary protein in concentrations greater than 0.3 gm per liter in a 24-hour urine collection.” Although 500 mg/24 h was subsequently proposed as a dividing line between normal and abnormal levels of excretion,3 the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy upheld the ACOG classification, with the exception that proteinuria was no longer defined as a concentration but simply as 0.3 gm or greater in a 24-hour specimen.4 Although the definition further evolved in 2000 when the working group included proteinuria in the required diagnostic criteria for preeclampsia,5 the most recent ACOG guidelines note that proteinuria is not a requirement for the diagnosis if high-risk features are present but, if used, the threshold remains 300 mg/24 h.1
The primary objectives of the present study were to (1) longitudinally examine proteinuria in a cohort of healthy women prior to pregnancy and in late pregnancy and (2) compare inpatient versus outpatient 24-hour urinary collections. We hypothesized that proteinuria increases as a result of gestation and often exceeds established thresholds in the absence of clinically apparent disease.
Materials and Methods
The University of Vermont Institutional Review Board approved this study. From May 2004 to July 2014, healthy nulligravid women were recruited as part of 2 consecutive prospective clinical studies characterizing the association of prepregnancy and late pregnancy maternal physiology. Eligible participants were nulligravid women planning to conceive within the next year. Women were included if they were 18 to 40 years of age, with regular menstrual cycles (every 26-35 days) as documented by the regularity of menses, presence of moliminal symptoms, or normal serum levels of follicle-stimulating hormone (<15 mlU/mL) and estradiol (<100 pg/mL) on cycle day 2. Women were excluded if they were active tobacco users; had hypertension, diabetes mellitus, autoimmune disease, or other major medical conditions; or if they had a multiple gestation. Written informed consent was obtained from each participant. Data for the current analysis were abstracted from patients evaluated at 2 time points: during the follicular phase of their menstrual cycle preconception and again at 30 to 32 weeks of pregnancy. Twenty-four-hour urine collections were completed at both time periods following a 3-day calorie- (based on weight and pregnancy status), sodium- (3500 mg), and potassium- (3500 mg) controlled meal plan that was supplied to the participants. Additionally, participants were instructed to refrain from the use of nonsteroidal anti-inflammatory medications and decongestants for 48 hours, and caffeine and alcohol for 24 hours, prior to the study. This was confirmed at the time of evaluation. There was no evidence of bacteriuria on urinalysis at the time of the urinary collection. The 2 trials were similar in design, with the exception of inpatient versus outpatient collections of the 24-hour urine sample. The inpatient collections were performed in the Clinical Research Center at the University of Vermont. Due to the cost and availability of staff, those patients enrolled in 2009 and beyond completed 24-hour urine collections in an outpatient setting. Collection was otherwise performed similarly and as follows: The first morning void was discarded, and this began the timing of collection. Each void within the next 24 hours was collected in a supplied container and kept refrigerated until collection was complete and the sample returned to the laboratory. Urine protein was measured using the VITROS UPRO Slide kit (Ortho Diagnostics, Raritan, New Jersey) in the clinical laboratory at our institution. This test recovers 98% of urinary albumin and 35% of urinary globulins, and, according to the manufacturer, up to 225 mg protein/24 h is considered normal in their nonpregnant test population. The clinical laboratory performs monthly reference range validation of the test. The 24-hour urinary collections were considered adequate if the urine creatinine was >1 g/24 h. Clinical outcomes including gestational age at delivery, birth weight, and presence or absence of preeclampsia were abstracted from the electronic medical record following delivery. Preeclampsia was defined as the presence of new-onset hypertension (blood pressure ≥140/90 on 2 occasions at least 6 hours apart after 20 weeks gestation) and the presence of ≥300 mg protein in a 24-hour urinary collection.
Two-sample t tests were used to compare participant characteristics between women who had inpatient and outpatient urine collections. Wilcoxon rank-sum tests were used to compare urinary outcomes between the 2 groups, as their distributions were nonnormal. The percentage of women exceeding specific thresholds for protein/24 h were compared using chi-square tests. Wilcoxon signed-rank tests were used to evaluate the changes in urinary outcomes from prepregnancy to late pregnancy, and McNemar test was used to compare correlated proportions. Because urinary outcomes were not normally distributed, results are presented as medians with interquartile range (IQR), and all correlations presented are Spearman rank correlation (r s). Statistical analyses were performed using SAS statistical software version 9.3 (SAS Institute, Cary, North Carolina). Statistical significance was determined using α = .05.
Results
One hundred and thirty-four women were consented, enrolled, and completed the preconception 24-hour urinary collection. Sixty-six women either did not conceive or miscarried. Sixty-eight women ultimately maintained singleton pregnancies and continued participation in the study; complete data are available for 65 women. Nineteen women completed a third-trimester urinary collection as an inpatient and 46 as an outpatient. Delivery data were available for all 65 completed pregnancies.
Patient characteristics of the study cohort and results of prepregnancy, follicular phase 24-hour urinary collections are displayed in Table 1. The median amount of proteinuria in 24 hours in our healthy nulliparous population was 187.6 mg (IQR 102.8-280.0), with 63% of participants having levels greater than the 150 mg/24 h threshold considered to be abnormal in the nonpregnant population. Using the VITROS UPRO assay threshold of 225 mg/24 h, 40% of the samples were classified as abnormal in the absence of additional evidence of disease. There was a strong correlation between urine protein to creatinine ratio (P:C) and 24-hour urine protein (r s = .92, P < .001) as well as urine volume and urine protein (r s = .89, P <.001). There was no correlation between prepregnancy body mass index and preconception proteinuria (r s = .01, P = .87).
Table 1.
Maternal Characteristics and Results of Prepregnancy 24-Hour Urine Collections.
| Valuea | |
|---|---|
| Maternal age, years | 30.3 (4.6) |
| Race | |
| Caucasian | 108 |
| Asian | 8 |
| Hispanic | 6 |
| African American | 5 |
| American Indian | 3 |
| Other | 4 |
| BMI, kg/m2 | 23.7 (4.7) |
| Cycle day, visit 1 | 9.2 (3.4) |
| Urine volume, mL | 2350 (1800-3075) |
| Urine creatinine, mg/dL | 47.2 (38.1-70.5) |
| Urine protein, mg/dL | 8 (6-10) |
| Urine protein: creatinine | 0.17 (0.08-0.26) |
| Urine protein, mg/24 h | 187.6 (102.8-280.0) |
| Above threshold (150 mg/24 h) | 63 |
| Above threshold (225 mg/24 h) | 40 |
Abbreviations: BMI, body mass index; SD, standard deviation.
aTabled values are mean (SD) or median (interquartile range [IQR]) unless otherwise specified.
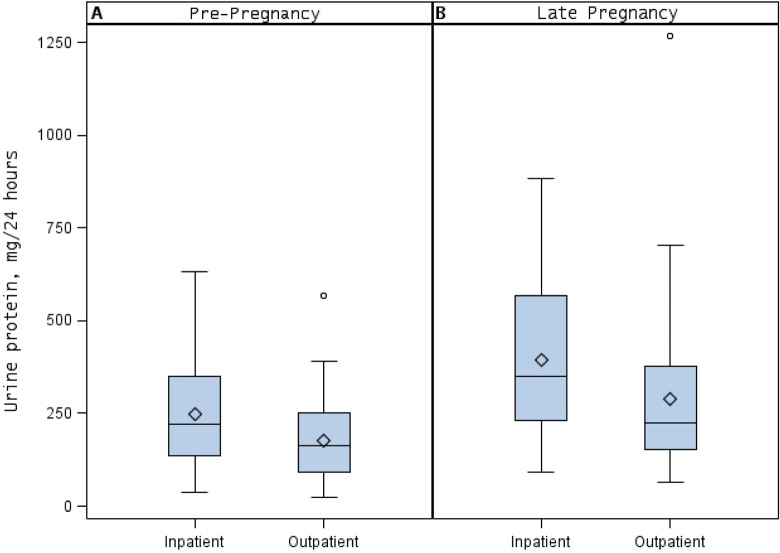
Comparisons of inpatient and outpatient prepregnancy 24-hour urinary collections are summarized in Table 2. There were no significant differences in mean age, body mass index, or cycle day of collection in women performing inpatient versus outpatient collections. The 24-hour proteinuria was significantly higher in the inpatient compared to the outpatient collections (222.5 mg/24 h [IQR 137.2-349] vs 162.5 mg/24 h [IQR 92-252]; P = .005; Figure 1, panel A). Consistent with a higher level of proteinuria, inpatient samples were more likely to exceed the threshold for abnormal proteinuria in the absence of disease compared to outpatient samples (72% vs 55%; P = .05). Urinary volumes and urine creatinine did not differ between nonpregnant inpatient and outpatient collections.
Table 2.
Comparison of Inpatient and Outpatient 24-Hour Urine Collections in Nonpregnant Healthy Nulligravid Women.a
| Inpatient, n = 60 | Outpatient, n = 74 | P Value | |
|---|---|---|---|
| Age, years | 29.6 (5.0) | 30.8 (4.2) | .13 |
| BMI, kg/m2 | 22.9 (3.6) | 24.3 (5.3) | .11 |
| Cycle day | 8.6 (3.4) | 9.7 (3.4) | .07 |
| Urine volume, mL | 2282.5 (1817-2925) | 2522.5 (1760-3150) | .52 |
| Urine creatinine, mg/dL | 51.1 (41.3-68.4) | 46.6 (34.9-73.8) | .34 |
| Urine protein, mg/dL | 9.5 (7.5-11.5) | 6 (5-8) | <.001 |
| Urine protein: creatinine | 0.19 (0.13-0.28) | 0.13 (0.06-0.21) | <.001 |
| Urine creatinine, mg/24 h | 1257.5 (1098.0-1354.8) | 1146.6 (994.1-1321.4) | .06 |
| Urine protein, mg/24 h | 222.5 (137.2-349.0) | 162.5 (92-252) | .005 |
| >150 mg urine protein/24 h, % | 72 | 55 | .05 |
| >225 mg urine protein/24 h, % | 50 | 31 | .03 |
Abbreviations: BMI, body mass index; IQR, interquartile range; SD, standard deviation.
aTabled values are median (IQR) unless otherwise specified. Age, BMI, and cycle day are reported as mean (SD), and significance levels are based on 2-sample t tests. Comparisons on continuous urinary outcome variables based on Wilcoxon rank-sum tests. Chi-square tests were used for dichotomous outcomes.
Figure 1.
Mean, median, and 25th and 75th percentiles of (A) prepregnancy and (B) late pregnancy 24-hour urine protein levels in healthy women with uncomplicated pregnancies, in both inpatient or outpatient collection.
Sixty-five women subsequently conceived and also completed a late pregnancy urinary collection. Results of paired comparisons of 24-hour urinary collections between prepregnancy and late pregnancy for this subgroup are shown in Table 3. Urinary volume increased in late pregnancy compared to prepregnancy (3010 mL [IQR 2110-3625] vs 2550 mL [IQR 1935-3235]; P = .003). There was a significant increase in proteinuria from prepregnancy to the third trimester (prepregnancy median 190 mg/24 h [IQR 106-306] vs third trimester median 254 mg/24 h [IQR 166-396]; P < .001), with no change in 24-hour urinary creatinine (median 1231.2 mg [IQR 1035.5-1371.8] vs 1225.8 mg [IQR 1030-1380.9]; P = .57). We observed a moderate correlation between 24-hour urinary protein levels prior to pregnancy and during pregnancy (r = .59, P < .001). The positive and negative predictive values of an abnormal preconception 24-hour urinary protein level (>150 mg/24 h) for abnormal third-trimester proteinuria (>300 mg/24 h) were 63% and 91%, respectively.
Table 3.
Comparison of Prepregnancy and Late Pregnancy Urinary Characteristics in Subgroup of Healthy Nulliparous Women.a
| Prepregnancy | Late Pregnancy | P Value | |
|---|---|---|---|
| Urine volume, mL | 2550 (1935-3235) | 3010 (2110-3625) | .003 |
| Urine creatinine, mg/dL | 45.8 (34.9-69.9) | 39.3 (32.4-60.8) | .002 |
| Urine protein, mg/dL | 8 (6-9) | 9 (7-12) | < .001 |
| Urine protein: creatinine | 0.18 (0.07-0.24) | 0.21 (0.13-0.33) | < .001 |
| Urine creatinine, mg/24 h | 1231.2 (1035.5-1371.8) | 1225.8 (1030.0-1380.9) | .57 |
| Urine protein, mg/24 h | 190 (106-306) | 254 (166-396) | < .001 |
| Above threshold (≥150 mg prepregnancy; ≥300 mg late pregnancy), % | 66 | 45 | .001 |
aValues are median (interquartile range [IQR]) unless otherwise specified. Significance levels based on Wilcoxon Signed-Rank test for continuous urinary outcomes and McNemar test for percentages.
Table 4 compares inpatient and outpatient 24-hour urinary collections among the 65 women completing late pregnancy collections. Similar to the prepregnancy collections, there were higher levels of proteinuria in the late pregnancy samples collected as an inpatient compared to those collected as an outpatient (median 349 mg/24 h [IQR 231-569] vs. 224 mg/24 h [IQR 152-377]; P = .04; Figure 1, panel B). Urinary volumes and urine creatinine were not significantly different between the inpatient and the outpatient collections. Additionally, we examined the 24-hour urinary creatinine within participants comparing prepregnant and third-trimester collections and found no significant difference in those with inpatient compared to outpatient collection (P = .26), suggesting similar efficacy in sample collection. In all, 68% and 35% of late pregnancy inpatient and outpatient collections, respectively, exceeded the diagnostic threshold of proteinuria for preeclampsia, in the absence of disease.
Table 4.
Comparison of Inpatient and Outpatient 24-Hour Urine Collections During Late Pregnancy in Healthy Primiparous Women.a
| Inpatient, n = 19 | Outpatient, n = 46 | P Value | |
|---|---|---|---|
| Gestational age at collection, days | 225 (11) | 216 (5) | < .001 |
| Urine volume, mL | 3210 (2150-4025) | 2910 (1910-3575) | .29 |
| Urine creatinine, mg/dL | 37.7 (29.3-58.0) | 39.8 (32.7-61.0) | .44 |
| Urine protein, mg/dL | 11 (10-14) | 9 (7-10) | .02 |
| Urine protein: creatinine | 0.32 (0.18-0.46) | 0.19 (0.13-0.31) | .10 |
| Urine creatinine, mg/24 h | 1235.9 (1142.8-1392.3) | 1160.9 (1014.8-1378.6) | .17 |
| Urine protein, mg/24 h | 349 (231-569) | 224 (152-377) | .04 |
| >300 mg urine protein/24 h, % | 68 | 35 | .01 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
aTabled values are median (IQR) unless otherwise specified. Gestational age is reported as mean (SD) and significance level is based on 2-sample t test. Comparisons on continuous urinary outcome variables based on Wilcoxon rank-sum tests. Chi-square tests were used for dichotomous outcome.
Six (9.2%) of 65 participants developed preeclampsia. Each developed preeclampsia after the third-trimester 24-hour urine collection was completed for study purposes, and all delivered at term. Of the 6 developing preeclampsia, 3 had greater than 300 mg proteinuria at the third-trimester collection. Two of these 3 also had greater than 300 mg proteinuria in the nonpregnant collection. Conversely, 26 women had greater than 300 mg proteinuria in the third trimester without developing preeclampsia.
Comment
Our data challenge the current definition of abnormal proteinuria prior to and during pregnancy. In our population, we have shown that a significant number of healthy women with uncomplicated pregnancies exceed the diagnostic threshold for abnormal proteinuria.
Prior attempts to quantify normal amounts of proteinuria in pregnancy were limited by small sample sizes, the inclusion of patients with hypertension, and the use of various methodologies for determining proteinuria.6-9 More rigorous attempts at defining pregnancy-associated proteinuria followed when Kuo et al measured proteinuria in normal pregnant women.10 They found the 99th percentile for proteinuria was 300 mg/24 h at 17 to 20 weeks and just below 200 mg/24 h at 33 to 36 weeks. Higby et al subsequently evaluated 270 healthy pregnant women and reported the 95th percentile for 24-hour urinary protein excretion to be 259.4 mg.11 Finally, a recent prospective study evaluated third-trimester proteinuria in singleton and twin gestations. Although both inpatient and outpatient collections were combined, the average amount of proteinuria in the third trimester in singleton gestations was 204 mg.12 Although the results of these studies support the 300 mg threshold of proteinuria, that threshold was not specifically linked to perinatal outcomes.
Despite acknowledging an increased perinatal risk with hypertension in the presence of proteinuria13 and with increasing amounts of proteinuria,14 a discriminatory value of proteinuria for adverse outcome has not been widely accepted. Waugh et al studied 197 hypertensive pregnancies and compared the cutoff values of 300 and 500 mg/24 h of proteinuria to adverse perinatal outcome.15 While 300 mg proteinuria in 24 hours was not associated with adverse outcome, 500 mg conferred a 50% increased risk of severe hypertension and a 72% increased risk of birth weight <10%. Although a retrospective study suggested that up to 50% of women with isolated proteinuria during pregnancy go on to develop preeclampsia,16 our results do not support that finding. Indeed, the low positive predictive value of 10.3% (3 of 29) of abnormal proteinuria for the development of preeclampsia indicates that in the absence of hypertension, many women have proteinuria greater than diagnostic thresholds but never develop preeclampsia.
Urinary protein excretion increases throughout pregnancy due to an increased glomerular filtration rate,17 increased permeability of the glomerular basement membrane,18 and an impairment of tubular absorption.19,20 In normal pregnancy, albumin excretion is thought to remain relatively stable20 or increase slightly11 but likely does not account for the total increase in proteinuria during pregnancy. In glomerular diseases, including preeclampsia, the glomerular basement membrane becomes more permeable, thus more albumin is filtered into the urine and contributes to the majority of pathologic proteinuria.19 We measured total urinary protein in our study, as this is the current clinical standard for assessing proteinuria.
Although the gold standard for quantitative analysis of total urine protein excretion is a 24-hour urine collection, multiple different assays exist to measure the total urinary protein within a sample, and there is no uniformity in the type of protein measured. These assays vary in their sensitivity (with most having poor sensitivity) and in their ability to detect albumin fragments. It is unclear whether or not these assays have been validated in pregnancy. Thus, there is significant variability in the assays used to measure proteinuria, particularly in their ability to identify albumin. Prior studies have likely underestimated the true amount of proteinuria by not adequately measuring urinary albumin, making comparison of proteinuria across studies using different assays difficult.
Our results highlight significant differences in inpatient and outpatient urinary collections. Both prior to pregnancy and in late pregnancy, inpatient urinary collections resulted in greater concentrations of urine protein as well as 24-hour proteinuria. As individual matched urine collections were similar in creatinine excretion, suggesting adequate sample collections, the differences in proteinuria may be a result of collection technique. Methods for optimizing 24-hour urinary collections attempt to minimize residual urine in the urinary system and promote diuresis. These include ensuring the patient is well hydrated and positioned in the lateral recumbent position for up to 1 hour prior to the first and last void collection.21 Additional factors including diet, volume intake, and physical activity likely differed between those completing an inpatient versus outpatient collection and may have influenced the recovered proteinuria. Therefore, results of urinary collections should be interpreted with caution, especially if comparing an inpatient to an outpatient collection. Although those participants completing an inpatient collection had a gestational age 9 days older than those performing outpatient collections, this is unlikely to be clinically significant.
Strengths of this study include its prospective and longitudinal design with matched urinary collections from prepregnancy to late pregnancy in healthy nulliparous women. Our study is limited by a relatively small sample size and a homogeneous population of mostly caucasian women, which may affect the generalizability of our results. For example, non-Hispanic blacks have been shown to have higher levels of urinary albumin, urine creatinine, and albumin–creatinine ratio. In one study, the frequency of microalbuminuria (when evaluated by albumin–creatinine) was higher in non-Hispanic blacks than whites.22,23 Additionally, we measured the total urine protein, but directly assessing albumin could also be useful.
In conclusion, significant proteinuria is present in healthy nonpregnant women and, even in the absence of disease, proteinuria increases during pregnancy. Outpatient collections may underestimate proteinuria, especially in late pregnancy. Timing of collection, inpatient versus outpatient collection, and type of assay used may all affect proteinuria results and may make comparisons of multiple collections difficult. Given the uncertainty in the origin of diagnostic thresholds, variability in the measurement of proteinuria, and the absence of outcome data, the current threshold criteria for proteinuria should be interpreted with caution.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [RO1 HL071944(IRB)]. JKP was supported in part by NIH 1P20GM103644.
References
- 1. Hypertension in Pregnancy: Executive Summary: Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 2. Hughes EC. Obstetric-Gynecologic Terminology. Philadelphia, PA: Davis Company, F.A.; 1972. [Google Scholar]
- 3. Chesley LC. Hypertensive Disorders in Pregnancy. New York, NY: Appleton-Century-Crofts; 1978. [Google Scholar]
- 4. National high blood pressure education program working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 1990;163(5 pt 1):1689–1712. [DOI] [PubMed] [Google Scholar]
- 5. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 6. Wearing MP. Protein in the urine during pregnancy: a simple quantitative method of estimation. Obstet Gynecol. 1957;9(5):549–553. [PubMed] [Google Scholar]
- 7. Elden CA, Cooney JW. The addis sediment count and blood urea clearance test in normal pregnant women. J Clin Invest. 1935;14(6):889–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lorincz AB, McCartney CP, Pottinger RE, Li KH. Protein excretion patterns in pregnancy. Am J Obstet Gynecol. 1961;82(2):252–259. [DOI] [PubMed] [Google Scholar]
- 9. Lopez-Espinoza I, Dhar H, Humphreys S, Redman CWG. Urinary albumin excretion in pregnancy. Br J Obstet Gynecol. 1986;93(2):176–181. [DOI] [PubMed] [Google Scholar]
- 10. Kuo VS, Koumantakis G, Gallery EDM. Proteinuria and its assessment in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1992;167(3):723–728. [DOI] [PubMed] [Google Scholar]
- 11. Higby K, Suiter CR, Phelps JY, Siler-Khodr T, Langer O. Normal values of urinary albumin and total protein excretion during pregnancy. Am J Obstet Gynecol. 1994;171(4):984–989. [DOI] [PubMed] [Google Scholar]
- 12. Osmundson SS, Lafayette RA, Bowen RA, Roque VC, Garabedian MJ, Aziz N. Maternal proteinuria in twin compared with singleton pregnancies. Obstet Gynecol. 2014;124(2 pt 1):332–336. [DOI] [PubMed] [Google Scholar]
- 13. Homer CSE, Brown MA, Mangos G, Davis GK. Non-proteinuric pre-eclampsia: a novel risk indicator in women with gestational hypertension. J Hypertens. 2008;26(2):295–302. [DOI] [PubMed] [Google Scholar]
- 14. Chan P, Brown M, Simpson JM, Davis G. Proteinuria in pre-eclampsia: how much matters? BJOG. 2005;112(3):280–285. [DOI] [PubMed] [Google Scholar]
- 15. Waugh J, Bell SC, Kilby MD, Lambert P, Shennan A, Halligan A. Urine protein estimation in hypertensive pregnancy: which thresholds and laboratory assay best predict clinical outcome? Hypertens Pregnancy. 2005;24(3):291–302. [DOI] [PubMed] [Google Scholar]
- 16. Morikawa M, Yamada T, Minakami H. Outcome of pregnancy in patients with isolated proteinuria. Curr Opin Obstet Gynecol. 2009;21(6):491–495. [DOI] [PubMed] [Google Scholar]
- 17. Davison JM. The physiology of the renal tract in pregnancy. Clin Obstet Gynecol. 1985;28(2):257–265. [DOI] [PubMed] [Google Scholar]
- 18. Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol. 1996;270(2 pt 2):F338. [DOI] [PubMed] [Google Scholar]
- 19. Hladunewich MA, Odutayo A, Thadhani R. The Normal and Diseased Kidney in Pregnancy In: Coffman TM, Falk RJ, Molitoris BA, Neilson EG, eds. Schrier’s Diseases of the Kidney. 9th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2013:1676–1709. [Google Scholar]
- 20. Beetham R, Dawnay A, Menabawy M, Silver A. Urinary excretion of albumin and retinol-binding protein during normal pregnancy. J Clin Pathol. 1988;41(10):1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindheimer MD, Kanter D. Interpreting abnormal proteinuria in pregnancy. Obstet Gynecol. 2010;115(2 pt 1):365–375. [DOI] [PubMed] [Google Scholar]
- 22. Mattix HJ, Chi-Yuan H, Shimon S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13(4):1034–1039. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens. Am J Epidemiol. 2002;155(12)1114–1119. [DOI] [PubMed] [Google Scholar]