Abstract
Maternal immune tolerance of fetal engraftment is critical for the establishment and maintenance of pregnancy, but the exact mechanisms permitting this semi-allograft in the maternal host are not completely understood. Further, failure of the embryo to implant in the uterus accounts for at least 30% of the best prognosis in vitro fertilization cycles when a perfect embryo is transferred to a normal uterus. We hypothesized that T regulatory cells (Tregs), defined by CD4+CD25hi surface expression and the FoxP3+ transcription factor, play an important role in the initiation of the earliest stages of pregnancy, specifically implantation of the embryo. In this study, we evaluated the role of Tregs in the establishment of pregnancy using a conditional depletion of Treg transgenic mouse model. We found that embryo implantation in the syngeneic mating was defective as evidenced by smaller litter sizes after Treg depletion and that embryo implantation could be restored by adoptively transferring Tregs into the mating mice. In allogeneic mating, litter sizes were not different but breeding efficiency was significantly decreased. These data reveal that Tregs are important for the establishment of the earliest stages of pregnancy and may be a potential cause of infertility due to recurrent implantation failure, which may be amenable to cellular or pharmacologic therapy to improve maternal immune tolerance of embryo implantation.
Keywords: implantation, T regulatory cells, pregnancy establishment, murine model
Introduction
Understanding the mechanisms of immune tolerance is central to many areas of medicine, including organ transplantation, cancer therapies, and human reproduction. Although advancements have been made since Sir Peter Medawar first described acquired immune tolerance in the early 1950s,1 understanding of maternal immune tolerance to the fetus continues to puzzle clinicians and researchers. It is known that successful embryo implantation requires a receptive endometrium with a state of maternal immunological tolerance of the fetal semi-allograft; however, the specific mechanisms responsible for endometrial receptivity and maternal tolerance remain incompletely elucidated.
Evidence points to the importance of T regulatory cell (Treg) function in both abnormal and normal implantation, early pregnancy, and even initiation of labor.2–8 T regulatory cells, defined by CD4+CD25hi FoxP3+ expression, are a subpopulation of T cells, which control and suppress a range of immune responses. FoxP3, a member of the forkhead-box/winged-helix transcription factor family, is a unique marker of Tregs and an essential gene controlling the function and development of naturally occurring Treg populations.9,10 T regulatory cells provide a strong suppressive effect on T-helper type 1 (Th1) cells in pregnancy, allowing for immune tolerance and normal pregnancy.3 In human pregnancies, when this suppressive mechanism is weak, a spectrum of adverse reproductive conditions, such as infertility (early defect) to miscarriage (mid gestation defect) to preeclampsia and intrauterine growth restriction (late defect), associated with abnormal placentation are thought to occur.3
Prior studies have experimentally targeted Tregs during the peri-implantation period, which demonstrated that Tregs were associated with miscarriage in murine models.11–15 These earlier studies used a less specific anti-CD25 antibody depleting approach to knock down or abolish Tregs. These results suggested an importance for CD25+ T cells in embryo implantation and successful early pregnancy. However, this approach is less specific to true Treg function due to the expression of CD25 on activated conventional T cells, which confounds interpretation of the role of Tregs specifically. A more targeted depletion of Tregs using a transgenic mouse with an inducible, conditional depletion of T regulatory cell (DEREGs) was developed by Lahl et al.16,17 This transgenic mouse is termed DEREG (C57BL/6-Tg(FoxP3-DTR/EDFP)23.2Spar/Mmjax; Jackson Labs, Bar Harbor, Maine). The DEREG mice express a diphtheria toxin (DT) receptor-enhanced green fluorescent protein (GFP) fusion protein under the control of the FoxP3 promoter, allowing for both detection and inducible depletion of FoxP3+ T regulatory cell with the administration of DT.
We sought to delineate more precisely to the reproductive mechanisms involving Tregs at the time of implantation. Through these studies, we have elucidated a role for Tregs in implantation, where we found lower pregnancy rates and smaller litters due to deficits in embryo implantation. The importance of Tregs in implantation was supported further by our findings that replacement of Tregs by adoptive transfer (ie, Treg transfusions) corrected the defective embryo implantation in this model.
Materials and Methods
Study Approval
Institutional review board approval was given through the National Institutes of Health (NIH) Animal Care and Use Committee protocol number H-0216. The care and use procedures for the mice were in accordance with the Institutional Guide for Laboratory Animals. Mice were housed under a 12/12-hour light/dark cycle at 25°C and 30% humidity and were fed ad libitum with a standard diet and water. Nulligravid, 6- to 8-week-old mice were used for the experiments. Wild-type (WT) mice were obtained through colony breeding as described below. The DEREG mice were originally provided previously to our research laboratory by Ethan Shevach at the NIH under a material transfer agreement (MTA) agreement. The BALB/c males (Jackson Laboratory, Sacramento, California) were used in allogeneic mating experiments.
The DEREG Genotyping
Mating pairs were set up using either DEREG females with a WT male or vice versa. Pups were weaned on day of life 21. Mice were analyzed for the presence of the DEREG transgene at around 5 to 6 weeks of age. Candidate mice were ear tagged and blood was collected via tail vein bleeding. The blood sample was red blood cell depleted via an ammonium chloride potassium (ACK) lysis protocol. Analysis for FoxP3 using GFP expression was performed using flow cytometry (Becton Dickinson Biosciences, San Jose, California). Those mice found to contain the transgene were identified as DEREG positive and used in subsequent experiments. Those mice without the transgene were identified as DEREG negative and used as WT controls.
Blood and Uterine Tissue T Regulatory Cell Depletion Analysis
Wild-type or DEREG mice were given 1, 2, or 3 continuous days of intraperitoneal (IP) injections of either 1 μg DT or phosphate-buffered saline (PBS). The first day of injections was marked as day 0. Injections were given once a day on day 0, days 0 and 1, of days 0, 1, and 2. Intraperitoneal injections were administered via an insulin syringe and needle. Blood and tissue levels were then analyzed on days 3, 10, 21, and 28 after the initial injection on day 0. Whole blood was collected via tail vein bleeding and red cell depleted. To obtain uterine tissue, mice were euthanized by CO2 gas with confirmation by cervical dislocation. The abdomen was opened, the mullerian tract identified, dissected out, and placed in 10 mL of media (dulbecco’s modified eagle medium [DMEM] + fetal bovine serum [FBS]). Excess fat, connective tissue, and the oviductal complex were dissected off. The uterine tissue was placed in 10 mL of fresh media and each uterine horn was transected into 4 to 5 donut-shaped pieces. The uterine tissue was then placed into a gentle magnetic antibody cell sorting (MACS) C tube (Miltenyi Biotech, San Diego, California) containing 10 mL of digest media (DMEM + FBS, 1 mg/mL collagenase, 1 mg/mL dispase, and 0.5 mg/mL of deoxyribonuclease [DNase]). The tissue was minced using a tissue dissociator (Miltenyi Biotech). The tissue was then placed in an incubator at 37°C with gentle agitation for 45 minutes. The tissue was centrifuged, the supernatant removed, and 10 mL of fresh digestion media added. The tissue was minced again and placed in an incubator at 37°C for an additional 30 minutes. The cells were centrifuged, the supernatant removed, and 1 mL of 0.05% trypsin was added with manual pipetting for 5 minutes. Trypsinization was stopped with 2 mL of fresh media (DMEM+FBS). The cells were then washed through a 40-μL strainer. The solution was centrifuged, the supernatant removed, and the resultant cell pellet was taken for cell staining. All experiments were performed in triplicate.
Cell Staining and Analysis
Cell pellets from whole blood and uterine tissue preparations were washed and resuspended in 1000 μL of PBS. Cell staining was performed using a mouse T regulatory cell staining kit (eBioscience, San Diego, California). Cells were divided equally into the control and experimental tubes. A volume of 2 μL of the following antibodies were added to (1) antigen presenting cell (APC) mouse IgG2 (isotype control), (2) APC anti-mouse CD45 (positive control), and (3) APC anti-mouse CD4 (experimental control) and incubated in the dark at 4°C for 4 to 6 hours per manufacturer’s recommendations. The cells were then fixed and permeabilized per manufacturer’s recommendations. Intracellular staining was performed by overnight incubation in the dark at 4°C with 5 μL of (1) CD45 PE antibody (positive control) and (2) FoxP3 antibody. Compared to CD25 or CD4, FoxP3 staining is a more specific marker for Tregs. The following morning the cells were washed and analysis was performed via flow cytometry. Ten thousand cells were counted and the proportion of Tregs to Th1 cells was used for comparison.
Mating Experiments
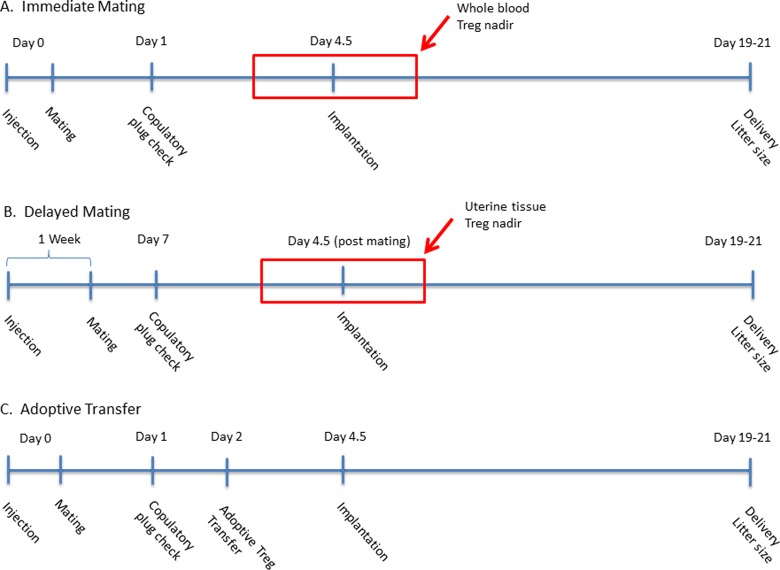
Injection and mating schedules were adjusted to have the window of implantation occur during the blood Treg nadir, which we have termed “immediate mating” (Figure 1). Females were caged together prior to mating to sync estrous. Soiled bedding from the male housing cages was placed in the breeding cage at the time of male placement. Wild-type or DEREG mice were given either 1 μL IP injection of saline or DT for 2 consecutive days. The day of first injection was marked as day 0. Harem mating (1:2, male and female) with age-matched, WT males was done in the afternoon of the first day of injection. Examination for a vaginal copulation plug was performed daily. Visualization of a copulatory plug was noted as day 1 (e+1). Those mice not demonstrating a copulatory plug after 2 checks were removed. Mice were either sacrificed at e+14 to 18 and implantation sacs counted or were allowed to deliver. Those mice allowed to deliver were checked early each morning to assess for overnight delivery and the number of live-born pups counted.
Figure 1.
Time line demonstrating injection and mating schedules. Injections were given once a day on day 0; days 0 and 1; or days 0, 1, and 2. Alterations in mating timing allow for implantation to occur during T regulatory cell (Treg) nadir in the peripheral blood (A) or uterine tissue (B). (C) Time line of injection, mating, and adoptive transfer of Tregs.
Next we sought to determine if implantation was impaired during the time of maximal uterine tissue Treg depletion. The injection and mating schedules were adjusted in order to time implantation during the uterine tissue Treg nadir, which we termed “delayed mating” (Figure 1). The first of 2 consecutive days of injections was counted as day 0, consistent with the prior mating protocol. Mice were mated with WT males on postinjection day 7. Mice were checked daily for evidence of a copulatory plug and allowed to deliver as described above.
Previous studies have observed even more dramatic differences in miscarriage/embryo resorption, when breeding with an allogeneic male. Reasoning that allogeneic mating would pose a stronger immunologic barrier and require a larger Treg suppressive effect, we hypothesized implantation defects would be more pronounced in the setting of allogeneic mating after Treg depletion. To test this, DEREG females were mated with age-matched WT BALB/c (Jackson Labs) males following the same Treg depletion and immediate mating procedures as previously described.
Superovulation
Next we sought to determine if decreased litter sizes and pregnancy rates were due to preimplantation defects. Therefore, oocyte numbers and fertilization rates were assessed between the experimental groups. Mice underwent a superovulation protocol as previously described.18 On day 0, both DEREG and WT mice were given a 5-IU IP injection of pregnant mare serum gonadotropin (PMSG). At 38 to 40 hours post-PMSG injection, mice were given 1 μL of either saline or DT as described earlier as a placebo or to induce Treg knock down respectively, followed by an IP injection of 5 IU human chorionic gonadotropin (HCG) 8 hours later to trigger ovulation. The females were then immediately placed in a cage with an age-matched WT male. The following morning, 24 hours post-DT injection, mice were evaluated for evidence of a copulation plug. Those mice exhibiting a plug were then sacrificed by cervical dislocation; the ovarian–tubal–fat pad complex was harvested and placed in media. The oviduct was gently opened, flushed with media, and the oocytes obtained. The oocytes were placed in M2 media (GlobalStem, Rockville, Maryland) and the cumulus oocyte complex was removed using 5% hyaluronidase (Irvine Scientific, Santa Ana, California). All embryos were then cultured in a 1 μL microdrop of M16 EmbryoMax media (EMD Millipore, Billerica, Massachusetts) and placed in an incubator at 37°C with 5% CO2 overnight. Percentage of fertilization was calculated by dividing the number of 2-cell embryos by the number of oocytes obtained.
Murine Ultrasound
To evaluate whether postimplantation pregnancy reabsorption might account for the reduction in pregnancy rates and litter sizes, murine uterine ultrasound was performed to compare implanted pregnancy sacs to delivered litter sizes. Injection and mating schedules were performed as previously described. Transabdominal ultrasound was performed 7 to 10 days after identification of copulatory plug. Pregnant mice were induced and maintained under anesthesia with 1% to 5% isoflurane. The abdominal hair was removed using Nair (Church and Dwight, Ewing, New Jersey). The mice were continuously monitored using electrodes and rectal temperature probe. Uterine images were captured using the VoluSonic 2100 ultrasound (Visualsonics, Toronto, Canada) with a 30 to 40 mHz abdominal probe. Each uterine horn was imaged from the cervix to the fundus and the number of implantations recorded. The mice were then removed from anesthesia, recovered, and transferred back to the housing facility where they were cared for and observed until delivery. Litter sizes were compared to ultrasound-visualized implantations.
T Regulatory Cell Adoptive Transfer
We next evaluated if the induced reproductive defect could be reversed by adoptively transferring Tregs from sex-matched, nonpregnant, litter mate mice lacking the bacterial artificial chromosome (BAC) transgene. The DEREG female mice were given IP DT injections for 2 days, consistent with previous experiments and mated with WT males in the afternoon of the first DT injection. On day 2 postinjection, nonpregnant sex- and litter-matched WT mice were selected as donors and lethally bled. Tail vein bleeding was performed and spleen was harvested from donor mice. Splenic tissue was pulverized through a 40-μm filter, combined with the whole-blood samples, refiltered, and red cell depleted. T regulatory cell isolation was carried out with Treg isolation kit (Miltenyi Biotech) and MACS through an MS MACS column (Miltenyi) per manufacturer’s recommendations and the cells were counted. The purified Tregs were then resuspended in no more than 0.3 mL of 0.01% bovine serum albumin in sterile saline and injected back into recipient mating females via tail vein injection. Mice were then allowed to deliver and pups counted per previous experiments.
Statistical Analysis
We determined 7 mating per experimental group would be needed to detect a 50% decrease in littler size with an α of 0.05 and a β of 0.8. Statistical analysis was performed using Microsoft Excel (Microsoft Office 2010) and VassarStats (www.vassarstats.net). Student t or Mann-Whitney rank-sum tests were used to compare continuous variables between groups. Paired t test was used for paired comparisons within each group. Differences in dichotomous outcomes between the 2 groups were analyzed using χ2 or Fisher exact test. Analysis of variance testing with Tukey correction for multiple comparisons was used to analyze comparisons between multiple groups. P value <.05 was considered statistically significant.
Results
Diphtheria Toxin Injection Results in Treg Knockdown in Whole Blood and Uterine Tissue
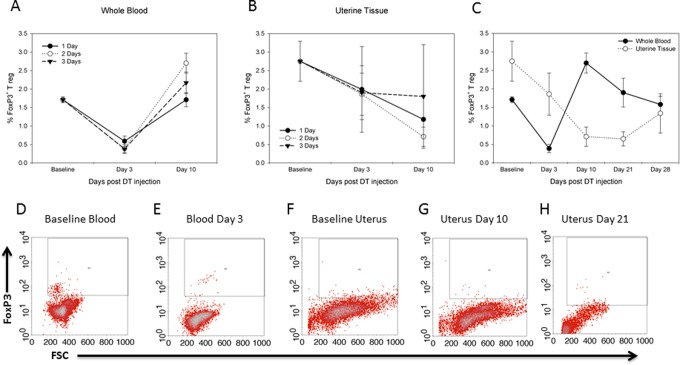
Depletion of Tregs following IP DT injections was confirmed as previously described,19 where 1, 2, or 3 days of DT provided equivalent depletion of Tregs in peripheral blood, spleen, and bone marrow. Baseline (day 0) levels of Tregs in whole blood were not significantly different between 1, 2, and 3 days of DT injection (Figure 2A). Representative plots of Treg percentages following IP injection of DT for 2 consecutive days are shown in Figure 2D and E, which were significantly decreased in whole blood on day 3 compared to day 0 (1.71% ± 0.08% vs 0.4% ± 0.1%, P < .05). Depletion was transient as levels returned to day 0 levels by dayd 21 and 28 postinjection (Figure 2C).
Figure 2.
T regulatory cell (Treg) depletion in peripheral blood and uterine tissue is equivalent between 1, 2, and 3 days of injection. Whole-blood Tregs nadir occurs earlier compared to uterine tissue nadir. (A) Comparison of whole-blood FoxP3 percentages after 1, 2, or 3 days of 1 μg intraperitoneal (IP) diphtheria toxin (DT) injection at days 0 to 3 and 10 days post day 1 injection. (B) Comparison of uterine tissue percentages of FoxP3 after 1, 2, or 3 days of IP DT injection at days 0 to 3 and 10 days postinjection. (C) Comparison of whole-blood and uterine percentages after 2 days of IP DT injection at days 0 to 3, 10, 21, and 28 days postinjection. Significant differences (P < .05) were noted between day 0 and day 3 in whole-blood percentages and between day 0 and day 10 and day 21 uterine tissue percentages. (D-H) Flow cytometry analysis of FoxP3 antibody staining. (D) Day 0—whole blood, (E) whole blood postinjection day 3, (F) day 0—uterine tissue, (G) uterine tissue postinjection day 10, and (H) uterine tissue post injection day 21. Student t test was used for analysis. Experiments were performed in triplicate.
In uterine tissue, baseline levels (day 0) of Tregs were 2.7% ± 0.05%. Similar to whole-blood analysis, there was no difference in Treg percentages between 1, 2, and 3 days of DT injection (Figure 2B). Intraperitoneal injection of DT for 2 consecutive days decreased uterine tissue Treg percentages maximally at days 10 (0.65% ± 0.05% vs 2.7% ± 0.05%, P < .05) and 21 (0.7% ± 0.2% vs 2.7% ± 0.05%, P < .05) compared to day 0 levels (Figure 2F–H). After 21 days, Treg levels trended back to baseline, which fully recovered by 28 days postinjection (Figure 2C). These results reconfirmed reliable and consistent depletion in our model system in both peripheral blood and the organ of interest. As a result of these findings, all future knockdown experiments were performed using 2 days of IP DT injections.
Decreased Embryo Implantation With Treg Depletion
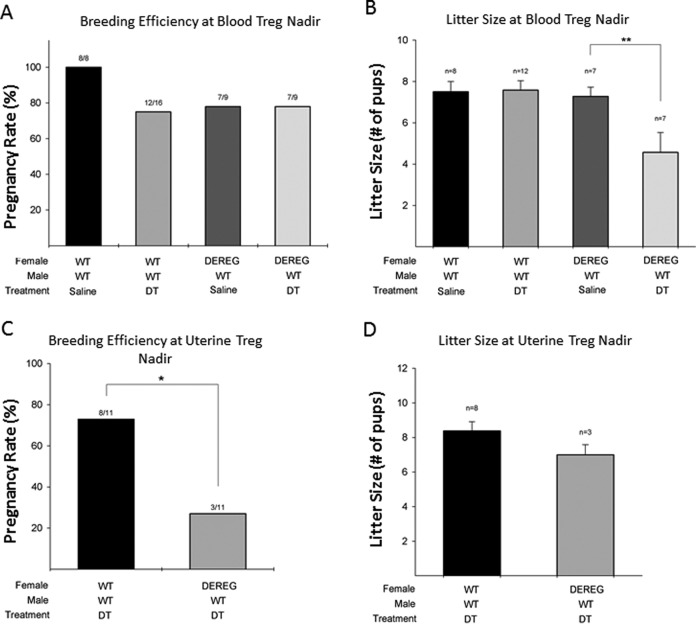
Implantation timed to occur during the blood Treg nadir (immediate mating) exhibited a subtle implantation defect, where the overall pregnancy rates (percent of mice which became pregnant) were similar among experimental groups (Figure 3A), but the number of embryos able to implant per pregnancy were statistically lower (Figure 3B). Wild-type mice given saline injections were used to determine the baseline number of embryos that implant per pregnancy in these mice, as evidenced by the number of pups that are born per litter (Figure 3B). Next we sought to test if the DT injection itself was associated with smaller litter sizes; WT mice given DT had similar number of pups per litter as WT mice given saline (7.6 vs 7.5, respectively; not significant). Similarly, we sought to test if there was an implantation defect associated with the transgenic model; DEREG mice given saline injections were also found to have similar numbers of pups per litter as WT mice (7.3 vs 7.5, respectively, not significant). In contrast, DEREG mice mated immediately after Treg depletion via DT injections had significantly smaller litter sizes compared to WT animals given saline (7.5 vs 4.5 pups per litter, respectively, P < .01; Figure 3B). Analysis was done to compare M-F sex ratios. No difference in M-F sex ratios of live-born pups was seen when comparing experimental mating groups to either control mating groups or historical colony breeding ratios (data not shown).
Figure 3.
Conditional knockdown of T regulatory cells (Tregs) followed by immediate mating timed to whole-blood Treg nadir leads to a subtle implantation defect, where fewer embryos are able to implant (as evidenced by smaller litter sizes) but showed similar pregnancy rates (breeding efficiency) overall. Tregs knockdown with delayed mating timed to uterine Treg nadir results in a more pronounced reproductive phenotype, where the overall breeding efficiency was significantly decreased. Breeding efficiency (A) and litter size (B) comparison after conditional Tregs knockdown and immediate mating timed to blood Treg nadir. Breeding efficiency (C) and litter sizes (D) after conditional Tregs knockdown and delayed mating timed to uterine Treg nadir. Analysis of variance with Tukey test for multiple comparisons was used for analysis. *P < .05, **P < .01.
Decreased Pregnancy Rates With Uterine Treg Nadir
Implantation timed with uterine Treg nadir exhibited an all-or-nothing effect, where significantly fewer DEREG mice given DT became pregnant compared to WT mice given DT (27% vs 72%, respectively, P = .04; Figure 3C), but the litter sizes were similar among mice which did become pregnant (8.4 vs 7.0, respectively, P = .5; Figure 3D).
Ovulation and Fertilization Are Not Affected by Treg Depletion
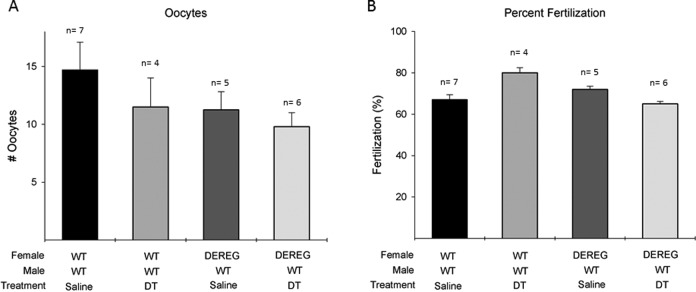
Oocyte numbers were not different between the 4 groups (P = .6; Figure 4A). Similarly, there was no difference in the fertilization rates between the groups, suggesting no preimplantation defects associated with the DT treatment or with the transgenic mouse (P = 0.5; Figure 4B). These results demonstrated that each of our experimental groups were starting with a statistically similar number of oocytes and embryos, isolating the reproductive defect to implantation or later.
Figure 4.
Equivalent numbers of oocytes and fertilization percentage demonstrate no preimplantation defects. (A) Comparison of oocytes between experimental groups. (B) Comparison of fertilization percentage between experimental groups. Analysis of variance with Tukey test for multiple comparisons was used for analysis.
T Regulatory Cell Depletion Is Not Associated With Pregnancy Loss
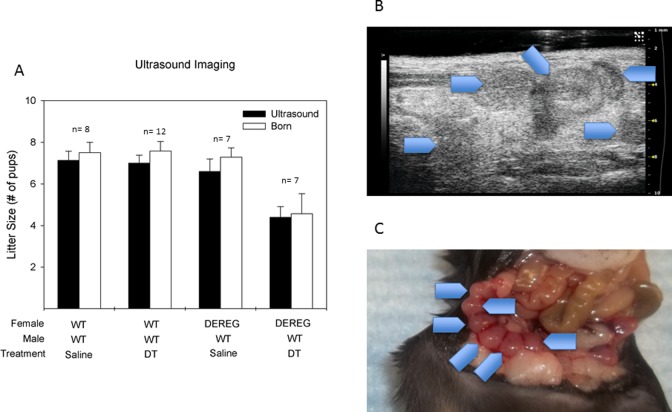
Ultrasound evidence of implanted pregnancy sacs at 7 to 10 days of gestation correlated well with observed delivered litter sizes in each of the 4 cohorts (Figure 5A). Wild-type females given saline had an average of 7.1 gestational sacs observed implantations on ultrasound and 7.5 pups delivered per litter (P = .47). Wild-type mice given DT had 7.0 implanted sacs on ultrasound and 7.6 pups delivered per litter (P = 0.65). The DEREG mice given saline had 6.6 visualized implanted sacs and 7.2 pups delivered per litter (P = 0.39). The DEREG mice given DT had 4.4 visualized implantations and 4.5 pups delivered per litter (P = 0.18).
Figure 5.
Equivalent ultrasound visualized implantations to delivered pups demonstrated no postimplantation defects. (A) Comparison of ultrasound-visualized implanted pups and delivered pups. (B) Representative ultrasound picture of implanted embryos. Gestational age e+7. Blue arrowheads depict ultrasound-visualized implantations. (C) Representative picture of implanted embryos from same mouse depicted in Figure 4B. Blue arrowheads depict gestational sacs in the right uterine horn. Paired t test was used for analysis.
Implantation Defects Associated With Treg Depletion Are Correctable With Treg Adoptive Transfer
The DEREG mice with conditional depletion of Tregs followed by adoptive transfer of Tregs had significantly increased litter sizes compared to DEREG mice with conditional depletion not adoptively transferred with Tregs (6.7 vs 4.5 pups per litter, respectively, P < .05; Figure 6A). No relationship between the number of cells transferred (5-32 million) and delivered pups was evident (Figure 6B).
Figure 6.
(A) Number of embryos that implant as evidenced by pups per litter in wild-type (WT) mice, depletion of T regulatory cell (DEREG) mice given diphtheria toxin (DT), and DEREG mice which were adoptively transferred (ie, transfused) with T regulatory cells (Tregs) after DT-induced Tregs depletion. (B) No correlation between the number of adoptively transferred Tregs and litter size. Analysis of variance with Tukey test for multiple comparisons was used for analysis. *P < .05, **P < .01.
Decreased Breeding Efficiency With Allogeneic Mating
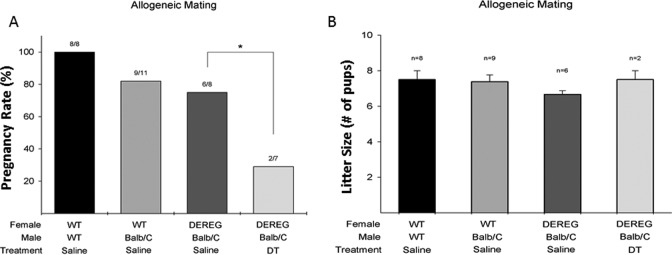
The DEREG mice with conditional depletion of Tregs bred with an age-matched, WT BALB/c male had significantly decreased pregnancy rates compared to WT mice given saline (29% vs 82%, respectively, P = 0.04; Figure 7A). An all-or-nothing effect was observed, although the litter sizes of mice achieving pregnancy were similar, the overall pregnancy rates were markedly lower. The DEREG mice given DT had similar litter sizes compared to WT mice given saline (7.5 vs 7.4 pups per litter, respectively, P = 0.4; Figure 7B).
Figure 7.
Allogeneic mating timed to blood T regulatory cells (Tregs) knockdown significantly decreased breeding efficiency. (A) Litter size comparison after conditional knockdown and immediate mating with BALB/c wild-type (WT) male. (B) Breeding efficiency (pregnancy rates) after conditional knockdown and immediate mating with BALB/c WT male. Analysis of variance with Tukey test for multiple comparisons was used for analysis. *P < .05.
Discussion
Human reproduction is inefficient. Each month, the most fertile couples only have up to a 25% chance of pregnancy and up to 75% of failed human conceptions are attributed to failure of implantation.20 Understanding the complex and unique nature of human pregnancy has challenged clinicians and researchers for decades. In 1956, Medawar first published a proposed relationship of regulatory mechanisms in the maternal immune system.1 We now understand that successful organ transplantation requires long-term immune tolerance in order to allow indefinite allograft engraftment. Similarly, establishment of pregnancy requires immune tolerance to allow engraftment of the semi-allogeneic (or allogeneic for pregnancies resulting from donor oocytes) fetus during gestation. Our understanding of the immune system in pregnancy has improved over the subsequent decades, however, much remains unknown. How does a semi-allogeneic fetus, which expresses paternal major histocompatibility antigens, evade maternal immune rejection and develop in the mother’s uterus? A delicate balance between effector and regulatory cells has been demonstrated to be essential for the establishment and maintenance of pregnancy.
Previous murine studies have suggested the importance of subpopulations of T cells for fetal implantation. In 1 study, in the absence of CD4+CD25+ T cells, allogeneic fetuses were uniformly rejected, while there was no difference in syngeneic pregnancies.11 Chen et al, depleted CD25+ cells the day prior to mating and demonstrated equivalent number of implantations between control and depleted mice but increased fetal resorption in the CD25+-depleted cohort.12 Both studies used an anti-CD25 antibody to deplete Tregs, which is not as specific as currently available techniques and models used in this study such as the conditional FoxP3-specific knockdown DEREG mouse. Samstein et al recently demonstrated that Treg depletion led to increased fetal resorption in semi-allogeneic matings.21 These findings correlate well with our data and continue to strengthen the evidence for the importance of Tregs in embryo implantation and establishment of pregnancy. Teles et al published a study that used DT knockdown of Tregs.22 Their study employed a different injection schedule and duration of injections. When evaluating the percentage of females with decreased implantations, it was demonstrated that DT mice had about a 50% decrease in the percentage of observed implantations. However, the P value was reported to be <0.1. The significance level that was set for this study is not clearly stated in the section on statistical methods. Regardless, in our study we used a P value of <0.05 for statistical significance. Our results correspond to those in the Teles et al study, but at a lower P value.
These data are different from prior reports, which show that Tregs are important for maternal tolerance of the embryo during midgestation after expression of the paternal antigens occurs. However, our data clearly show that prior reports of midgestation absorption may not have detected an earlier effect of Tregs important for implantation. First, we do see a subtle implantation defect, even with syngeneic mating, suggesting the effect of Tregs is not tolerance of the paternal antigens. While we know that even autoimmunity can occur in a single individual, our data suggest that Tregs are also critical for early implantation events. Our decreased litter size data support prior work suggesting a conventional role of Treg in midgestation after expression of the paternal antigens occurs, where we confirmed prior evidence of decreased litter sizes in allogenic matings. We were able to isolate the reproductive defect to an earlier time point suggested by prior data. Regarding circulating versus uterine Tregs in our model, we only found a reproductive phenotype when implantation was timed to occur during the serum nadir, but not the uterine nadir, suggesting that circulating Tregs are more important for implantation.
However, our timing experiments of the uterine nadir (which are not previously carefully described) show that uterine Tregs are maximally suppressed at days 10 to 21 but not depleted around the time of implantation. When implantation occurred at the time of whole-blood Tregs nadir, a subfertile reproductive phenotype was observed where fewer embryos were able to implant in syngeneic matings. In contrast, when implantation occurred at the time of uterine tissue Treg nadir, a more pronounced phenotype was observed where pregnancy rates were decreased. The uterine Treg depletion phenotype appeared to be an all-or-nothing effect, where the mice that did become pregnant had litter sizes similar to controls, although statistical analysis was limited by the small number of mice which did become pregnant. Similarly, when the immunologic challenge was increased by mating females with allogeneic males, a difference in pregnancy rates was observed during whole-blood nadir. It is possible that litter sizes could also be smaller in the small group of mice that did become pregnant at the time of uterine Treg nadir, but these experiments would require significantly greater numbers of mice in order to be powered appropriately. Alternatively, it is also possible that Tregs were incompletely depleted in the mice that did become pregnant or had reached some critical threshold for implantation as they were returning to normal, which the nonpregnant mice had not yet reached.
To further implicate the importance of Tregs in implantation, we demonstrated that pregnancy rates and litter sizes could be corrected by replacing Tregs through adoptive transfer. Adoptive transfer of Tregs has been shown to treat and even prevent disease along with preventing graft rejection and graft versus host disease (GVHD) in animal models.23–25 Zenclussen et al previously demonstrated, using an abortion-prone CBA/J mouse model, adoptively transferred purified Tregs from pregnant mice prevented fetal loss.15 In this study, we did not identify a specific number of cells or a threshold level of Treg percentage needed for implantation. Improvements in litter sizes were seen with levels as low as 5 million and as high as 32 million transferred cells, but the small numbers of cases prohibit adequate statistical analysis of critical cell numbers. These results, however, provide a conceptual base for further targeted studies investigating adoptive Treg transfer studies or other therapies (drugs) to increase Tregs in vivo.
In this study, we confirmed reliable and consistent blood Treg depletion in the DEREG mouse model system that are congruent with those results reported by Litzinger et al. The previous study used a slightly different method of Treg knockdown with denileukin toxin, a fusion protein of IL-2 and DT, but the results are consistent with the timing and reliability of Treg knockdown between our 2 studies. In addition, we were also able to determine the kinetics of Treg depletion in uterine tissue, which has not previously been reported. Knowing the precise time of maximal Treg depletion in the blood and uterus allowed us to test the effects of each on pregnancy establishment.
A strength of our study is that we carefully narrowed the reproductive defect by assessing for pre- or postimplantation defects associated with this model. By demonstrating nonstatistically different numbers of oocytes/embryos and no postimplantation fetal resorption, the time period for the observed reproductive defect was narrowed to the window of implantation. We also used a novel approach of murine ultrasound uterine imaging of implantation sites during early gestation to assess gestational sac number in order to compare it to the number of delivered pup numbers. During these experiments, it was noted that the accuracy of gestational sac counts was best with smaller sac numbers. As the number of implanted gestational sacs increased, the uterine horn became more tortuous, thereby increasing the difficulty of accurate sac counts. However, as the goal of ultrasound was to detect resorption, underestimation of gestational sacs compared to pups born makes fetal resorption in this model less likely. Although we cannot demonstrate exactly if the defect was lack of implantation or implantation and then rejection, in either case, there is an underlying defect in embryo–uterine interaction.
A potential weakness of this model is that it induced a transient, as opposed to a permanent, knockdown of Tregs, which likely resulted in a more subtle implantation defect rather than complete infertility. In addition, this is a conditional knockdown instead of knockout model of Treg depletion. However, using a conditional knockdown model is arguably more appropriate than a complete knockout for 2 reasons. First, complete knockout of Treg creates a “scurfy-like” condition16,17 in which the mice are extremely immunologically challenged, leading to death within the first 25 days of life. A human correlate condition of Tregs knockout, which is similar to mice, exists as a fatal autoimmune disease called immune dysregulation polyendocrinopathy, entropathy, X-linked. This disease is characterized by a mutation in the FoxP3 gene and can be rapidly fatal.26 Second, it is not likely that reproductive conditions, if they are related to Treg function, are complete deficiencies. It is more likely that Tregs are decreased or in other ways altered, thus leading to observed reproductive complications.
In this study, we characterized a reproductive model of whole-blood and uterine tissue Treg depletion, where we demonstrated that Tregs are critical for embryo implantation. Further, we have demonstrated that Treg transfusions are able to correct impaired implantation in this model. Advancements in assisted reproductive technologies to optimize embryo production have allowed clinicians to overcome many deficiencies in human reproduction, allowing many previously infertile couples to have children. Despite these advances in assisted reproductive technology, one large void still remains to be filled: specific treatments to improve the embryo implantation. In in vitro fertilization, even the healthiest blastocyst embryos fail to implant in a normal uterus 30% of the time. Treatments to increase Tregs may be an important therapy to help couples having infertility due to defective implantation. These types of approaches are a promising area of future research to address the so-called “endometrial factor” infertility, for which there are currently no effective treatments.
Acknowledgments
The authors would like to thank Lauren Libfraind and Nima Vahidi for their assistance with coordinating tissue sample shipping, Daryl Despres for his assistance with murine ultrasound, Celenia Ondeck for her assistance with the murine tail vein injections, and Assefa Davis for his assistance and care of the mice in the housing facility.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This intramural research was supported, in part, by the Program in Reproductive and Adult Endocrinology, NICHD, NIH, and the hematology branch of NHLBI, NIH.
References
- 1. Medawar PB. The immunology of transplantation. Harvey Lect. 1956;(series 52):144–176. [PubMed] [Google Scholar]
- 2. Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+ and FOXP3+ T regulatory cell during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178(4):2572–2578. [DOI] [PubMed] [Google Scholar]
- 3. Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15(5):517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006;12(5):301–308. [DOI] [PubMed] [Google Scholar]
- 5. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10(5):347–353. [DOI] [PubMed] [Google Scholar]
- 6. Wang WJ, Hao CF, Lin QD. Dysregulation of macrophage activation by decidual regulatory T cells in unexplained recurrent miscarriage patients. J Reprod Immunol. 2011;92(1-2):97–102. [DOI] [PubMed] [Google Scholar]
- 7. Yang H, Qiu L, Di W, et al. Proportional change of CD4+CD25+ regulatory T cells after lymphocyte therapy in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2009;92(1):301–305. [DOI] [PubMed] [Google Scholar]
- 8. Wang WJ, Hao CF, Yi L, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84(2):164–170. [DOI] [PubMed] [Google Scholar]
- 9. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. [DOI] [PubMed] [Google Scholar]
- 10. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. [DOI] [PubMed] [Google Scholar]
- 11. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. [DOI] [PubMed] [Google Scholar]
- 12. Chen T, Darrasse-Jeze G, Bergot AS, et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191(5):2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. 2010;107(20):9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shima T, Sasaki Y, Itoh M, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85(2):121–129. [DOI] [PubMed] [Google Scholar]
- 15. Zenclussen AC, Gerlof K, Zenclussen ML, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol Biol. 2011;707:157–172. [DOI] [PubMed] [Google Scholar]
- 18. Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology. 2006;65(9):1716–1726. [DOI] [PubMed] [Google Scholar]
- 19. Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110(9):3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400–1408. [DOI] [PubMed] [Google Scholar]
- 21. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teles A, Thuere C, Wafula PO, El-Mousleh T, Zenclussen ML, Zenclussen AC. Origin of Foxp3(+) cells during pregnancy. Am J Clin Exp Immunol. 2013;2(3):222–233. [PMC free article] [PubMed] [Google Scholar]
- 23. Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008;28(6):677–684. [DOI] [PubMed] [Google Scholar]
- 24. Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. [DOI] [PMC free article] [PubMed] [Google Scholar]