Abstract
Tubal ligation keeps the fimbriated end of the fallopian tube intact while interrupting the conduit for sperm and egg between the uterus and ovary. Tubal ligation is associated with an approximately 20% decreased risk of high-grade serous ovarian cancers, which mounting evidence suggests arise from the distal fallopian tube epithelium. We postulated that biological changes at the epithelial cellular level of the distal fallopian tube may account for the surgical procedure’s observed risk reduction. We compared the histology, presence of epithelial progenitors (basally located CD44-positive cells), and degree of epithelial proliferation (Ki67-positive cells) of distal fallopian tube from 10 patients with previous tubal ligation and 10 age-matched patients with uncut fallopian tubes. A significantly reduced population of proliferating epithelial progenitors (basally located CD44/Ki67 dual-positive cells) was detected in the tubal ligated specimens (P = .0002). To functionally assess the effect of tubal ligation, a murine model was utilized to compare the growth capacity of distal fallopian tube epithelial cells isolated from either ligated or sham-operated tubal epithelia. Murine fallopian tube epithelial cells isolated after tubal ligation showed a significantly reduced capacity to grow organoids in culture compared to sham-operated controls (P = .002). The findings of this study show that tubal ligation is associated with a reduced presence and decreased proliferation of progenitor cells in the distal fallopian tube epithelium. These compositional and functional changes suggest that tubal ligation induces quiescence of distal fallopian tube epithelial cells.
Keywords: fallopian epithelial progenitors, tubal ligation, fallopian tube epithelial proliferation
Introduction
Tubal ligation has sustained popularity as one of the most common methods of contraception for women in the United States since its peak usage in the 1970s.1,2 Initially a surgical procedure that only involved laparotomy incisions, tubal ligation has evolved to be less invasive with the utilization of laparoscopic methods.2 A laparoscopic tubal ligation may include cauterization or the application of clips or rings to the fallopian tube in order to block blood supply and cause scarring that blocks the passage of sperm or egg.3 The fallopian tube may also be partially removed, such as with the Pomeroy technique, or fully removed via a salpingectomy.3,4 Despite differences in approach, all these tubal ligation methods lead to gross morphologic changes in the fallopian tube to render the organ no longer functional. However, potential microscopic changes that may occur in the fallopian tube epithelium following a tubal ligation have not been widely investigated.
The fallopian tube is not just a passive conduit between the ovary and uterus to facilitate the passage of sperm and egg. The fallopian tube is an active organ that may undergo structural changes in response to the menstrual cycle as well as to menopause.5 The epithelium has the capacity to proliferate and change its cellular composition in preparation for ovulation.6 As a further demonstration of the organ’s dynamic nature, surgical reversal of tubal ligation via reanastomosis may be effective in restoring a woman’s ability to become pregnant by rehabilitating the function of the fallopian tube.7–9 Depending on a multitude of factors including the remaining length of the fallopian tube and type of tubal ligation performed, surgical reanastomosis has pregnancy success rates ranging from 25% to 83%.7,8 The success of surgical reanastomosis to recover function following tubal ligation associated scarring and blockage of blood flow indicates that the fallopian tube has regenerative capacity at the cellular level.10 As early descendants of stem cells that can differentiate into different cell types, progenitor cells enable a tissue to repair itself and may thus likely account for the fallopian tube’s regenerative function.11 Previous work in our laboratory has shown that progenitor cells reside along the fallopian tube from the proximal to distal end and are concentrated in the fimbriated epithelium.11 The presence of progenitor cells throughout the fallopian tube may account for the organ’s regenerative capacity following a surgical reanastomosis performed at any site.
Recent evidence strongly suggests that many serous ovarian carcinomas arise from the distal fallopian tube rather than from the ovarian surface epithelium.12–16 High-grade serous ovarian tumors are the most prevalent and deadly histotype of epithelial ovarian cancers, which are among the most aggressive and deadly gynecologic malignancies.17–19 In a pooled analysis of case–control studies, women with a history of tubal ligation had a reduced risk of invasive serous cancer by 19%.20 This procedure is also inversely associated with the development of endometrioid, clear cell, and mucinous carcinomas with a risk reduction of 32% to 52%.20 Although several mechanisms have been proposed to explain tubal ligation’s observed risk reduction of serous tumors, the existing literature does not specifically examine the possibility that changes at the tissue level of the fallopian tube epithelium may contribute to this risk reduction.
Mechanisms that have been proposed to explain risk reduction of epithelial ovarian cancers by tubal ligation include the prevention of ascending carcinogens (eg, talc) through the Müllerian system toward the ovaries, possible changes in ovarian function, and potential development of protective antibodies. Two population-based analyses have not demonstrated any clear association between exposure to talc use and ovarian cancer development.21,22 These studies suggest that interruption of the ascent of carcinogens by tubal ligation is unlikely to be a significant contributing factor to the procedure’s risk reduction. Moreover, although existing studies have reported lower hormone levels in women postligation compared to women without tubal ligation, they did not examine preligation hormone levels (reviewed in23). This makes it difficult to draw any conclusion regarding the effect of tubal ligation on ovarian function as a possible explanation for the risk reduction of ovarian cancer. Another proposed theory is that increased anti-Mucin-1 (MUC1) antibodies seen in women with tubal ligation may have a protective effect against ovarian cancer in younger women.24,25 Although this is an interesting population-based observation, the functional role of anti-MUC1 antibodies in the prevention of ovarian cancer remains to be investigated.
The greatest risk reduction following tubal ligation among the various epithelial ovarian cancers is observed for endometrioid and clear cell ovarian tumors.20 A suggested theory for the risk reduction in these 2 histologic subtypes is that tubal ligation prevents the migration of ascending cells originating in the endometrium from reaching and implanting onto the ovary by interrupting the conduit.14,23 However, this theory does not shed light on the mechanism underlying the risk reduction of high-grade serous cancers that are thought to arise from the distal fallopian tube, which remains intact following tubal ligation.
We hypothesized that changes in the cellular composition and activity of the distal fallopian tube epithelium following tubal ligation may explain why there is a risk reduction of serous cancers associated with this surgical procedure. Since the fimbriated end of the fallopian tube is left intact following tubal ligation and is the proposed site for serous cancer initiation, our study focused on analyzing the presence and activity of distal fallopian tube epithelial progenitor cells. Through analyses of human patient samples and in vitro growth assays using a murine model, here we investigate whether tubal ligation leads to quiescence of distal fallopian tube epithelium.
Materials and Methods
Patient Cohorts
This retrospective study was approved by the University of California Los Angeles (UCLA) Office for the Protection of Research Subjects (IRB 12-001213). Of approximately 600 reviewed medical records of patients that had undergone salpingectomy at UCLA in the past 10 years, 580 were excluded from our study due to either (1) history of any malignancy including gynecologic cancers, (2) any hormone replacement therapy including oral contraceptives since exogenous hormones may affect cellular composition of the fallopian tube, or (3) unavailability of histologic sections containing distal fallopian tube. This study consists of 2 cohorts with equal age distributions: 10 patients with a history of tubal ligation and 10 age-matched controls (Table 1). Both pre- and postmenopausal patients were selected for this study. An expert gynecologic pathologist (PS) reviewed all samples and determined them to be normal and without any evidence of pathology.
Table 1.
Patient Cohorts.
| Patient | Age | Indication for Surgery |
|---|---|---|
| A. Tubal ligation cohort | ||
| 1 | 31 | Pelvic pain, menorrhagia, ruptured luteal cyst |
| 2 | 54 | Benign ovarian cyst |
| 3 | 35 | Benign cystadenoma |
| 4 | 50 | Benign fibrothecoma |
| 5 | 62 | Benign cystadenoma |
| 6 | 53 | Benign cystadenoma |
| 7 | 37 | Benign sclerosing stromal tumor |
| 8 | 38 | Benign hemorrhagic corpus luteum cyst, BRCA+ |
| 9 | 50 | Hemorrhagic luteinized cyst |
| 10 | 62 | Risk-reducing bilateral salpingo-oophorectomy |
| B. Control cohort | ||
| 1 | 34 | Teratoma |
| 2 | 54 | Adenomyosis |
| 3 | 35 | Benign cystic follicles |
| 4 | 50 | Benign cystadenoma |
| 5 | 62 | Benign mucinous cystadenoma |
| 6 | 53 | Prolapse, stress urinary incontinence |
| 7 | 34 | Simple serous ovarian cyst |
| 8 | 40 | Risk-reducing salpingo-oophorectomy, BRCA+ |
| 9 | 50 | Adenomyosis |
| 10 | 62 | Benign fibrothecoma |
Immunohistochemistry
Formalin-fixed and paraffin-embedded histologic sections were stained with hematoxylin and eosin to observe tissue morphology. Paraffin sections were prepared for immunohistochemistry through a process of heat-induced epitope retrieval as previously described.11 Both the control and tubal ligation cohorts were immunostained for CD44 and Ki67 with antibodies and substrates listed in Supplementary Table 1. Staining quality and specificity for CD44 and Ki67 were evaluated against serous ovarian tumor as a positive control (high CD44 and Ki67 expression) and endometrial myometrium as a negative control (minimal CD44 and Ki67 expression). Clonal epithelial organoids regenerated from mouse fallopian tube epithelia were prepared in a similar fashion and immunostained for 2 antigens that mark the differentiated cells of the fallopian tube: Pax8, a transcription factor marking secretory cells,26,27 and β-tubulin, a structural protein that marks ciliated cells.28 Endometrial stroma was used as a negative control for Pax8 and β-tubulin staining. Human fallopian tube was used as a positive control for the Pax8 and β-tubulin stains.
Stained slides were submitted to the Translational Pathology Core Laboratory, a research facility in the UCLA Department of Pathology and Laboratory Medicine, for digital imaging. Using Aperio ScanScope linear-array scanning technology, high-quality scans of each slide were obtained at 20-fold magnification.29 Scanned samples were then analyzed using Definiens Tissue Studio, in which unique solutions were constructed for each stain in order to identify positively stained cells and to obtain a total cell count.30 For the single and dual stains, the total numbers of CD44-positive, Ki67-positive, and CD44/Ki67 dual-positive basal epithelial cells were manually verified independently by 2 researchers.
Images of the stains seen in the figures were produced on an Olympus BX51 upright microscope (Olympus, Melville, New York, http://www.olympusamerica.com) equipped with Optronics macrofire charge-coupled device camera and Optronics PictureFrame software.
Animals
Wild-type (C57BL/6), green transgenic (C57BL/6-Tg[ACTbEGFP]1Osb), and red transgenic (C57BL/6-Tg[ACTB-DsRed.MST]1Nagy/J) mice were obtained from Jackson Laboratory. Color-marked mice were only used for clonal assays related to the formation of fallopian tube organoids. For all other assays, wild-type mice were utilized. Mice were maintained in accordance with Division of Laboratory Animal Medicine guidelines at UCLA. All animal experiments were approved by the Animal Research Committee at UCLA.
In Vitro 3-Dimensional Organoid Assay of Fallopian Tube Epithelia
Microscopic tubal ligations mimicking a modified Pomeroy method and sham operations were performed on 6- to 8-week-old reproductive wild-type mice, followed by a 4-week recovery period. Distal fallopian tubes of 5 mm were harvested from both the sham and tubal ligation cohorts. This measurement ensured that an equal amount of fallopian tube was utilized in both experimental groups. These tubes were cut into fragments and suspended in Dulbecco modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS). They were then washed in phosphate-buffered saline and 5 mmol/L EDTA, incubated at 4°C for 45 minutes in 1% trypsin, then stopped with DMEM plus 10% FBS. Distal fallopian tube epithelial cell sheets were mechanically isolated from fragments using two 25-gauge needles and were then incubated at 37°C in 0.8 mg/mL collagenase in DMEM and 5 µg/mL insulin for 1 hour 30 minutes. Cells were harvested, washed, and resuspended in 0.5% trypsin-EDTA for 5 minutes before being dissociated into single cells using a syringe. Dissociated cells were incubated on ice for 15 minutes with antibodies in preparation for sorting (Supplementary Table 2). Fluorescence activated cell sorting was utilized to isolate all epithelial cells that expressed the epithelial cell marker epithelial cell adhesion molecule (EpCAM). Cells expressing lineage markers TER119 (red blood cells), CD45 (hematopoietic cells), and CD31 (endothelial cells) were excluded. Five thousand cells were then plated in 1:1 PREGM Matrigel and cultured for 11 days in an organoid assay as previously described.11
Statistical Methods
Median percentages of positive cells per antigen (CD44 and Ki67) were calculated from the total number of distal fallopian tube epithelial cells. For the CD44/Ki67 dual stains, median percentages of CD44/Ki67 dual-positive cells were calculated from the total number of CD44-positive basally located distal fallopian tube epithelial cells. Because the distribution of the antigens did not necessarily follow the normal distribution with constant variance, P values were computed using the nonparametric Wilcoxon rank sum test. Mean values of the number of organoids were compared between ligation and no ligation groups using a two-by-two repeated measure analysis of variance model. The criterion for statistical significance among all comparisons was set at an α of .05.
Results
Epithelia of Ligated Fallopian Tubes had a Lower Percentage of Basal Progenitors in the Fimbriated End Compared to Nonligated Samples
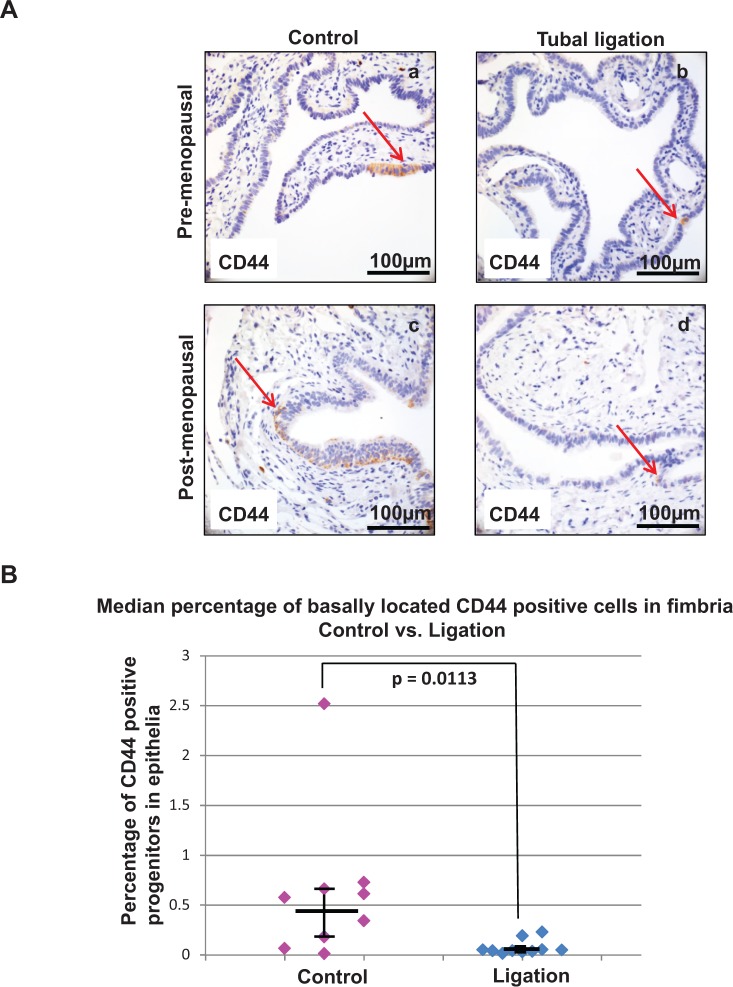
Previous work by this laboratory has shown that CD44 is expressed by a population of basally located epithelial cells with progenitor activity present throughout the fallopian tube and concentrated in the distal fimbria.11 Here we examine whether tubal ligation is associated with a change in the number of these progenitor cells specifically in the fimbria. Sections of distal fallopian tube epithelia from patients that had undergone tubal ligation and aged-matched controls were stained for CD44 (Figure 1A). Although no obvious histologic differences were observed between the ligated and nonligated human fallopian tube samples, the fimbriated fallopian tube epithelium of patients with previous tubal ligation had an approximately 9-fold decrease in the median percentage of progenitor epithelial cells compared to that of patients without tubal ligation. The epithelial lining of the tubal ligation cohort contained 0.05 median percent basal CD44-positive progenitors compared to the 0.46 median percent seen in control samples (P = .0113; Figure 1B). This suggests a significant reduction in progenitors in the distal fallopian tube epithelium with tubal ligation.
Figure 1.
A lower number of progenitors was detected in the distal fallopian tube epithelium of patients who underwent tubal ligation. (A) Immunohistochemistry demonstrated the representative distribution of CD44 expression in the fimbria of intact fallopian tubes (a and c) versus ligated patient samples (b and d). A lower number of basally located CD44-positive cells was seen in both pre- (a vs b) and postmenopausal (c vs d) tubal ligation patient samples. Arrows point to individual CD44-positive basal epithelial cells. (B) The median percentage of distal fallopian tube epithelial progenitors (basally located CD44-positive epithelial cells) was reduced with tubal ligation. Dot plot summarizes and compares data points of all clinical samples, confirming a statistically significant difference at P = .0113. Horizontal bars represent the median for each cohort and the vertical bars denote interquartile range.
Tubal Ligation is Associated With Decreased Proliferation in the Progenitor Cells of the Fimbriated Fallopian Tube
Increased proliferation as measured by Ki67 expression has been associated with the progression from normal tissue to dysplasia to malignancy in the Müllerian duct epithelium.31 It has also been shown that the expression of Ki67 can be a biomarker of aggressive behavior in tumors and may impact prognosis of disease.32,33 Even in preneoplastic tissue, a high level of Ki67 expression may portend an increased risk of developing malignancy at a later time.34 For example, a study of breast tissue found that a higher Ki67 index correlated with a significantly increased risk of developing invasive breast cancer in women with a diagnosis of atypical hyperplasia.34 These observations imply that the Ki67 index may be used as a surrogate measure of a tissue’s risk for becoming dysplastic.
Distal fallopian tube specimens from the tubal ligation and control cohorts were immunostained for Ki67 (Supplementary Figure 1A). Although the control group had a median Ki67 index of 0.44%, patients with tubal ligation had a median index of 0.14% (P = .0140; Supplementary Figure 1B). Decreased Ki67 expression indicated that the proliferation in the distal fallopian tube epithelium was significantly reduced in patients with tubal ligation compared to normal controls.
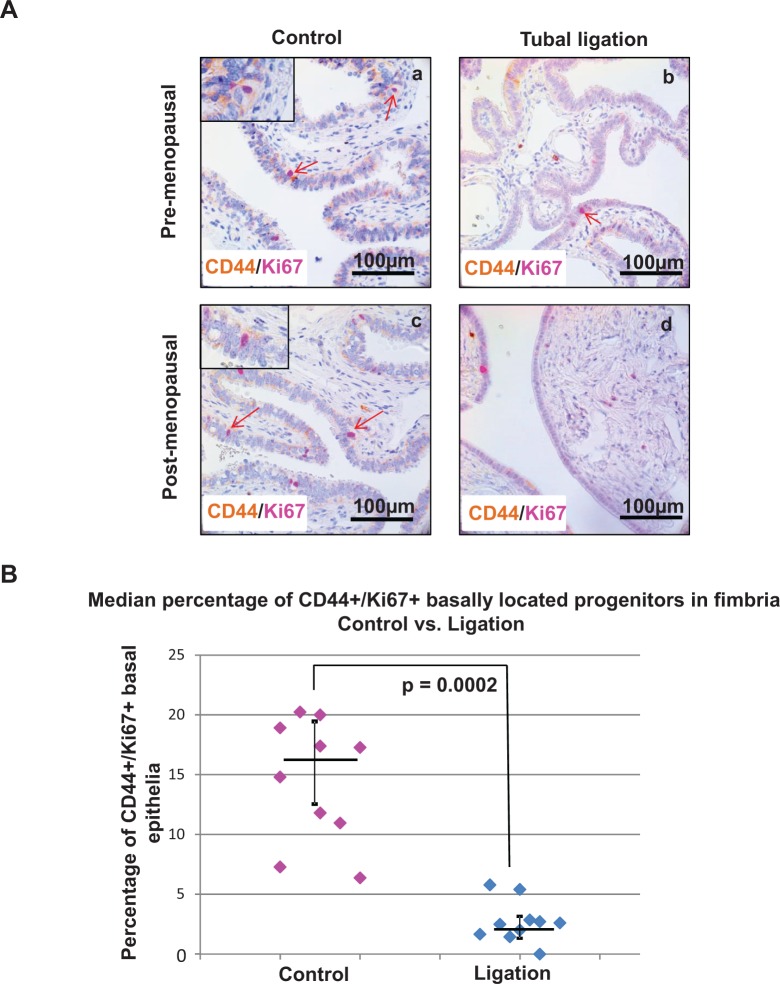
To investigate whether tubal ligation specifically affected the proliferation of the progenitor cells, histologic sections of distal fallopian tubes from patients with previous tubal ligation and their age-matched controls were dual stained for CD44 and Ki67 expression (Figure 2A). Although 16% of basally located CD44 cells stained positive for Ki67 in the control group, only 3% of basal CD44 cells were dual positive for Ki67 in the tubal ligation cohort (median values; P = .0002; Figure 2B). This indicated a 5-fold reduction in the proliferative progenitor cells of the distal fallopian tube epithelium following tubal ligation. Within both control and ligation cohorts in our study, the median percentages of basal CD44/Ki67 dual-positive epithelial cells was not significantly different between the pre- and postmenopausal patients (P > .05; Supplementary Figure 2). Collectively, we observed changes in both the composition and the proliferative index of epithelial progenitor cells in the distal fallopian tube when comparing age-matched cohorts of patients who had intact or ligated fallopian tubes.
Figure 2.
The percentage of proliferating epithelial progenitors was diminished in the distal fallopian tube of patient samples with tubal ligation. (A) Immunohistochemistry revealed a lower expression of proliferating progenitors (basally located CD44/Ki67 dual-positive epithelial cells) in the distal fallopian tube of ligated patient samples (b and d) compared to intact fallopian tubes (a and c). Light brown staining corresponds to CD44 expression and magenta staining indicates Ki67 nuclear expression. Tubal ligation samples from both pre- (b) or postmenopausal (d) patients showed a reduction in CD44/Ki67 dual expression compared to the control cohort. (B) The median percentage of proliferating progenitors was significantly lowered in the fimbria of tubal ligated patient specimens compared to age-matched controls at P = .0002. Each dot on the chart represents the percentage of basally located CD44-positive epithelial cells that also expressed Ki67. Horizontal bars represent the median for each cohort and the vertical bars denote interquartile range.
Tubal Ligation Reduced In Vitro Growth Capacity of Murine Distal Fallopian Tube Epithelia
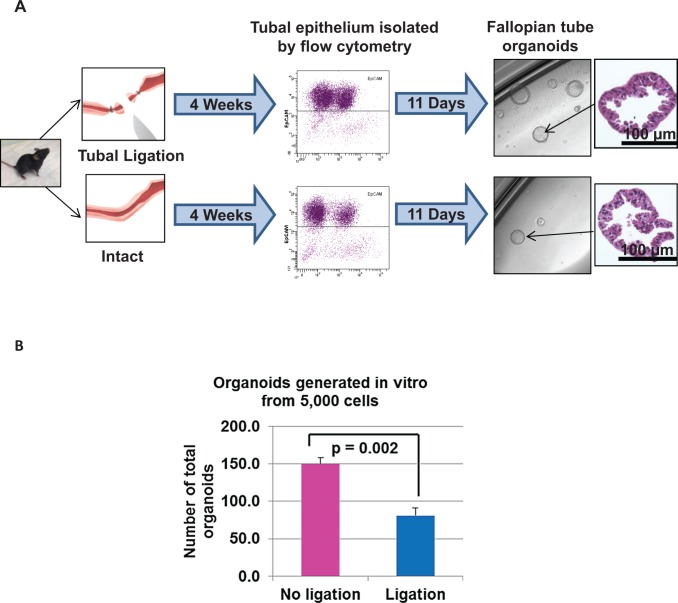
The decreased number and lower proliferation of progenitor cells detected in the human distal fallopian tube epithelia was correlational and may have been confounded by small sample size. Work by other investigators demonstrated that similar to the human tubal epithelium, mouse fallopian tubes also contain a progenitor stem-like population.35,36 Therefore, here a murine model was utilized to functionally test the effects of tubal ligation on the growth capacity of distal fallopian tube epithelia to determine whether the observations seen in the human samples correspond to biological changes in the tubal epithelium. Two cohorts of 10 reproductive mice (aged 6-8 weeks) each underwent either tubal ligation using a surgical technique that mimics a modified Pomeroy method or sham operation. In the modified Pomeroy method, 2 independent knots were placed in the ampullary region of the fallopian tube to allow excision of an approximately 5 mm portion while leaving the proximal and distal regions of the fallopian tube intact. After 4 weeks of recovery time, 5 mm sections of the distal fallopian tube were harvested from both cohorts. Using methods previously established in our laboratory,11 fallopian tube epithelium was isolated based on expression of EpCAM through a cell sorter. These epithelial cells were then plated in an in vitro 3-dimensional organoid growth assay that allowed for the quantification of individual epithelial cells with the capacity to give rise to fallopian tube epithelial organoids (Supplementary Figure 3A).11 Fallopian tube organoids were clonal in origin (arose from a single epithelial cell) and histologically resembled normal fallopian tube epithelium based on expression of epithelial markers Pax8 (secretory cells) and β-tubulin (ciliated cells; Supplementary Figure 3B and C). Equal numbers of enumerated epithelia isolated from mice with tubal ligation or sham operation were plated in the in vitro organoid assay. After 11 days, growth capacity was assessed as percentage of epithelial cells that gave rise to organoids (Figure 3A).
Figure 3.
Epithelial cells residing in the fimbriated end of ligated fallopian tubes had a decreased capacity for forming in vitro organoids in a murine model. (A) Experimental schema for isolation and in vitro 3-dimensional growth of fallopian tube epithelia from both ligated and intact murine fallopian tubes. (B) Epithelia obtained from ligated murine fallopian tubes showed a significant decrease in number of cells capable of organoid formation compared to intact tubes. Cumulative results from 2 independent experiments are shown with mean ± standard deviation (SD).
In 2 independent repeats of this experiment, an approximately 2-fold reduction in the number of tubal epithelial cells with regenerative capacity was detected upon tubal ligation with 1.6% of epithelial cells giving rise to organoids in ligated mice versus 3% in the sham-operated cohort (P = .002; Figure 3B). This functional analysis mirrors the findings seen in the human specimens where a reduction in epithelial progenitors of fimbriated fallopian tube was seen in patients with tubal ligation compared to controls. Findings here using the mouse model demonstrated that tubal ligation decreased the overall growth capacity of fimbriated epithelium.
Discussion
Pathologic changes in the fallopian tube following tubal ligation have been documented.10 Distortion and loss of musculature, thickening of epithelial folds, and dilation of the lumen of the remaining fallopian tube contribute to the organ’s loss of function following the surgical procedure.10 To our knowledge, however, it has not been greatly investigated how tubal ligation may lead to changes at the cellular level of the fallopian tube epithelium. We found that tubal ligation is associated with both a decreased number and reduced proliferative index of epithelial progenitors in the distal fallopian tube. Our observations of a decreased number and proliferation of progenitor cells in human distal fallopian tube specimens were corroborated by in vitro growth assays in a murine model that showed that distal fallopian tube epithelial cells have a decreased growth capacity following tubal ligation. Similarly, work by others has demonstrated that following a surgical procedure, changes do occur in the composition and proliferation in the epithelia of the colon, a tubular organ containing an intraepithelial lining similar to the fallopian tube.37,38 In these studies, a substantial decrease in number and mitotic activity of cells in the progenitor region of the colonic crypts was detected when comparing rats that had underwent a colostomy procedure to those with sham operations.37,38
Since our findings suggest that the epithelium is less active in proliferation following tubal ligation, we think the surgical procedure causes the distal fallopian tube epithelial progenitors to become quiescent. Given that high-grade serous tumors can initiate in the distal fallopian tube epithelium,12–16 quiescence of the epithelial progenitors in the fimbriated end of the fallopian tube may be one mechanism accounting for the risk reduction of serous cancer following tubal ligation. Recent evidence investigating squamous carcinomas demonstrated that tumorigenesis only begins when hair follicle stem cells are released from quiescence.39 Work from these researchers suggests that mechanisms that maintain the quiescent state prevent these cells from being susceptible to loss of tumor suppressor genes or gain of oncogenes.39 One can imagine that less active cell cycling of fallopian tube epithelial progenitors may play a protective role by reducing the frequency of cumulative genetic changes that can initiate disease. Such genetic changes may include functional inactivation of tumor suppressor genes BRCA1, BRCA2, and p53 that are seen in many serous cancers.
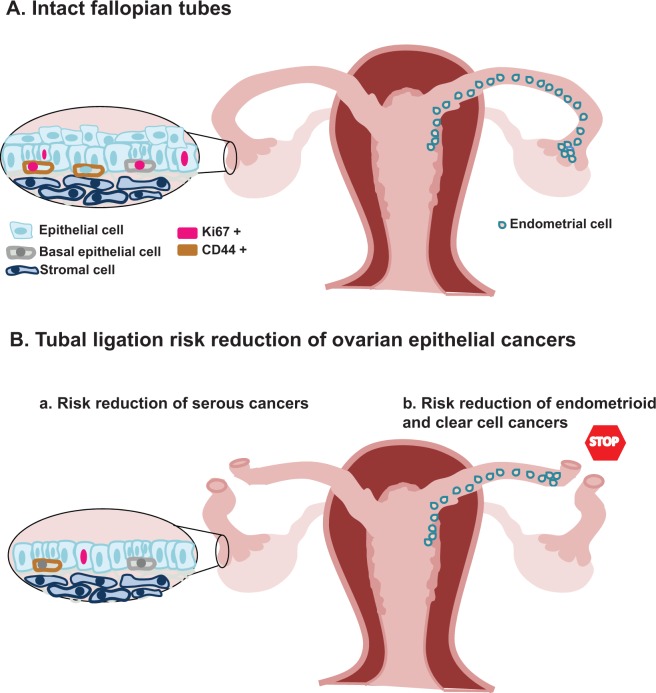
We think tubal ligation protects against epithelial ovarian cancers in a potential 2-pronged mechanism: (1) it interrupts the conduit and halts the migration of ascending endometrial cells from implanting on the ovary and developing into endometrioid and clear cell cancers as others have described23 and (2) work in this article suggests that tubal ligation leads to compositional and functional changes in the distal fallopian tube epithelium that renders it quiescent and possibly less likely to initiate serous carcinomas (Figure 4). Our findings reported here provide the basis for further validating these observations in a larger cohort of patients, which may require a multicentered analysis.
Figure 4.
A model for risk reduction in epithelial ovarian tumors following tubal ligation. (A) We propose that the progenitors of the distal fallopian tube are normally cycling. This may allow for accumulation of genetic mutations that lead to serous cancer initiation, such as inactivation of tumor suppressors BRCA1, BRCA2, and p53. It has been proposed by other studies that when the fallopian tube is left intact, ascending endometrial cells are able to migrate and implant onto the ovary, which may lead to the development of endometrioid and clear cell cancers. (B) Our findings suggest that tubal ligation induces a state of quiescence in epithelia of distal fimbria by leading to a decreased population of progenitor cells with a lower proliferative index. A diminished number of proliferating progenitors may lower the frequency of cumulative genetic changes associated with serous cancer. As others have previously proposed, tubal ligation may prevent the migration of endometrial cells from initiating endometrioid and clear cell cancers on the ovary by interrupting the conduit between the uterus and ovary.
Although our study did not specifically explore mechanisms that can lead to quiescence in the distal fallopian tube epithelia, we think that 2 possible mechanisms based on review of literature may include changes in oxygenation of the fallopian tissue due to alterations in microvasculature40–42 and/or decreased exposure to soluble cytokines and growth factors following tubal ligation.43 A continuation of this study focused on mechanisms that regulate growth and regression of fallopian tube epithelial progenitor cells could shed insight on the factors that regulate their proliferation versus quiescence.
Supplementary Material
Footnotes
Authors’ Note: Ekaterina Tiourin and Victor S. Velasco contributed equally to the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SM is supported by a VA CDA-2 Award, the CDU/UCLA NIH/NCI Grant #U54-CA-143931 award, a Sidney Kimmel Foundation award, the Concern foundation, The Jonsson Comprehensive Cancer Center Seed grant, the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124, the Leath L. and Marcia L. Millen Family Fund, the Ovarian Cancer Circle Inspired by Robin Babbini, and the Gynecologic Oncology (GO) Discovery Lab Foundation.
Supplemental Material: The online data supplements are available at http://rs.sagepub.com/supplemental.
References
- 1. Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1–6. [DOI] [PubMed] [Google Scholar]
- 2. Westhoff C, Davis A. Tubal sterilization: focus on the U.S. experience. Fertil Steril. 2000;73(5):913–922. [DOI] [PubMed] [Google Scholar]
- 3. Creinin MD, Zite N. Female tubal sterilization: the time has come to routinely consider removal. Obstet Gynecol. 2014;124(3):596–599. [DOI] [PubMed] [Google Scholar]
- 4. Siegle JC, Bishop LJ, Rayburn WF. Randomized comparison between two microlaparoscopic techniques for partial salpingectomy. JSLS. 2005;9(1):30–34. [PMC free article] [PubMed] [Google Scholar]
- 5. Crow J, Amso NN, Lewin J, Shaw RW. Morphology and ultrastructure of fallopian tube epithelium at different stages of the menstrual cycle and menopause. Hum Reprod. 1994;9(12):2224–2233. [DOI] [PubMed] [Google Scholar]
- 6. Donnez J, Casanas-Roux F, Caprasse J, Ferin J, Thomas K. Cyclic changes in ciliation, cell height, and mitotic activity in human tubal epithelium during reproductive life. Fertil Steril. 1985;43(4):554–559. [DOI] [PubMed] [Google Scholar]
- 7. Ribeiro SC, Tormena RA, Giribela CG, Izzo CR, Santos NC, Pinotti JA. Laparoscopic tubal anastomosis. Int J Gynaecol Obstet. 2004;84(2):142–146. [DOI] [PubMed] [Google Scholar]
- 8. Schepens JJ, Mol BW, Wiegerinck MA, Houterman S, Koks CA. Pregnancy outcomes and prognostic factors from tubal sterilization reversal by sutureless laparoscopical re-anastomosis: a retrospective cohort study. Hum Reprod. 2011;26(2):354–359. [DOI] [PubMed] [Google Scholar]
- 9. Lavy G, Diamond MP, DeCherney AH. Pregnancy following tubocornual anastomosis. Fertil Steril. 1986;46(1):21–25. [DOI] [PubMed] [Google Scholar]
- 10. Stock RJ. Histopathologic changes in fallopian tubes subsequent to sterilization procedures. Int J Gynecol Pathol. 1983;2(1):13–27. [DOI] [PubMed] [Google Scholar]
- 11. Paik DY, Janzen DM, Schafenacker AM, et al. Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: a site for injury and serous cancer initiation. Stem cells. 2012;30(11):2487–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–169. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA. 2012;109(10):3921–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. [DOI] [PubMed] [Google Scholar]
- 16. Perets R, Wyant GA, Muto KW, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24(6):751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 18. Seidman JD, Kurman RJ. Pathology of ovarian carcinoma. Hematol Oncol Clin North Am. 2003;17(4):909–925, vii. [DOI] [PubMed] [Google Scholar]
- 19. Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2012;119(suppl 2):S118–S129. [DOI] [PubMed] [Google Scholar]
- 20. Sieh W, Salvador S, McGuire V, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42(2):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huncharek M, Muscat J, Onitilo A, Kupelnick B. Use of cosmetic talc on contraceptive diaphragms and risk of ovarian cancer: a meta-analysis of nine observational studies. Eur J Cancer Prev. 2007;16(5):422–429. [DOI] [PubMed] [Google Scholar]
- 22. Whittemore AS, Wu ML, Paffenbarger RS, Jr, et al. Personal and environmental characteristics related to epithelial ovarian cancer. II. Exposures to talcum powder, tobacco, alcohol, and coffee. Am J Epidemiol. 1988;128(6):1228–1240. [DOI] [PubMed] [Google Scholar]
- 23. Cibula D, Widschwendter M, Zikan M, Dusek L. Underlying mechanisms of ovarian cancer risk reduction after tubal ligation. Acta Obstet Gynecol Scand. 2011;90(6):559–563. [DOI] [PubMed] [Google Scholar]
- 24. Pinheiro SP, Hankinson SE, Tworoger SS, et al. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rice MS, Murphy MA, Vitonis AF, et al. Tubal ligation, hysterectomy and epithelial ovarian cancer in the New England case-control study. Int J Cancer. 2013;133(10):2415–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73(1):1–14. [DOI] [PubMed] [Google Scholar]
- 27. Bowen NJ, Logani S, Dickerson EB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104(2):331–337. [DOI] [PubMed] [Google Scholar]
- 28. Roach MC, Boucher VL, Walss C, Ravdin PM, Luduena RF. Preparation of a monoclonal antibody specific for the class I isotype of beta-tubulin: the beta isotypes of tubulin differ in their cellular distributions within human tissues. Cell Motil Cytoskeleton. 1998;39(4):273–285. [DOI] [PubMed] [Google Scholar]
- 29. Staniszewski W. Virtual microscopy, data management and image analysis in Aperio ScanScope system. Folia Histochem Cytobiol. 2009;47(4):699–701. [DOI] [PubMed] [Google Scholar]
- 30. Braun M, Kirsten R, Rupp NJ, et al. Quantification of protein expression in cells and cellular subcompartments on immunohistochemical sections using a computer supported image analysis system. Histol Histopathol. 2013;28(5):605–610. [DOI] [PubMed] [Google Scholar]
- 31. Calil LN, Edelweiss MI, Meurer L, Igansi CN, Bozzetti MC. p16(INK4a) and Ki67 expression in normal, dysplastic and neoplastic uterine cervical epithelium and human papillomavirus (HPV) infection. Pathol Res Pract. 2014;210(8):482–487. [DOI] [PubMed] [Google Scholar]
- 32. Yoshioka T, Hosoda M, Yamamoto M, et al. Prognostic significance of pathologic complete response and Ki67 expression after neoadjuvant chemotherapy in breast cancer[published online May 5, 2013.]. Breast Cancer. 2013. [DOI] [PubMed] [Google Scholar]
- 33. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santisteban M, Reynolds C, Barr Fritcher EG, et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat. 2010;121(2):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y, Sacchetti A, van Dijk MR, et al. Identification of quiescent, stem-like cells in the distal female reproductive tract. PLoS One. 2012;7(7):e40691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Snegovskikh V, Mutlu L, Massasa E, Taylor HS. Identification of putative fallopian tube stem cells. Reprod Sci. 2014;21(12):1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Delvaux G, Caes F, Willems G. Influence of a diverting colostomy on epithelial cell proliferation in the colon of rats. Eur Surg Res. 1983;15(4):223–239. [DOI] [PubMed] [Google Scholar]
- 38. Kissmeyer-Nielsen P, Christensen H, Laurberg S. Diverting colostomy induces mucosal and muscular atrophy in rat distal colon. Gut. 1994;35(9):1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White AC, Khuu JK, Dang CY, et al. Stem cell quiescence acts as a tumour suppressor in squamous tumours. Nature Cell Biol. 2014;16(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. [DOI] [PubMed] [Google Scholar]
- 41. Wierenga AT, Vellenga E, Schuringa JJ. Convergence of hypoxia and TGFbeta pathways on cell cycle regulation in human hematopoietic stem/progenitor cells. PLoS One. 2014;9(3):e93494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kilic S, Tasdemir N, Lortlar N, Yuksel B, Budak G, Batioglu S. Vascular endothelial growth factor (VEGF) and inducible nitric oxide synthase (iNOS) immunoreactivities in rat ovaries and uterine tubes after tubal ligation: a controlled immunohistochemical study. Eur J Contracept Reprod Health Care. 2008;13(4):431–437. [DOI] [PubMed] [Google Scholar]
- 43. Valcourt JR, Lemons JM, Haley EM, Kojima M, Demuren OO, Coller HA. Staying alive: metabolic adaptations to quiescence. Cell Cycle. 2012;11(9):1680–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.