Abstract
Purpose:
Pain in patients with endometriosis is considered a significant source of stress but does not always correlate with severity of the condition. We have demonstrated that stress can worsen endometriosis in an animal model. Here, we tested the impact of a psychological stress protocol on pain thresholds and pain receptors.
Methods:
Endometriosis was induced in female rats by suturing uterine horn tissue next to the intestinal mesentery. Sham rats had sutures only. Rats were exposed to water avoidance stress for 7 consecutive days or handled for 5 minutes (no stress). Fecal pellets and serum corticosterone (CORT) levels were measured as an index of anxiety. Pain perception was assessed using hot plate and Von Frey tests. Substance P, enkephalin, endomorphin-2, Mu opioid receptor (MOR), and neurokinin-1 receptor expression in the spinal cord were measured by immunohistochemistry.
Results:
Fecal pellets and CORT were significantly higher in the endo-stress (ES) group than endo-no stress (ENS; P < .01) and sham-no stress groups (SNS; P < .01). The ES rats had more colonic damage (P < .001 vs SNS; P < .05 vs ENS), vesicle mast cell infiltration (P < .01 vs ENS), and more severe vesicles than ENS. The ES developed significant hyperalgesia (P < .05) but stress reversed the allodynic effect caused by endo (P < .001). The MOR expression was significantly reduced in ENS versus SNS (P < .05) and more enkephalin expression was found in endo groups.
Conclusion:
Animals subjected to stress develop more severe symptoms but interestingly stress seems to have beneficial effects on abdominal allodynia, which could be a consequence of the stress-induced analgesia phenomenon.
Keywords: endometriosis, stress, opioids, pain
Introduction
Endometriosis is a gynecological disorder that affects 5% to 10% of women of reproductive age in the United States.1–3 Although some women may be asymptomatic, the main clinical features are chronic pelvic pain, pain during intercourse, and infertility,1,4 as well as peritoneal inflammation, fibrosis, adhesions, and ovarian cysts.
One of the major complaints from women who have endometriosis is the severe pain they feel during menses, which prompts them to actively seek pain relief.5 It has been found that women with endometriosis compared to women without endometriosis have generalized hyperalgesia in body areas beyond the site of ectopic implants and that this is mediated by central and peripheral sensitization mechanisms.6–9 Some of the clinical manifestations include allodynia and hyperalgesia.10–12
The symptoms of endometriosis, including pain, are considered significant sources of both physiological and psychological stress occurring throughout the reproductive life of these patients.13–15 We have previously demonstrated that physical stress (using the Morris water maze) can exacerbate the size and severity of the lesions in an animal model of endometriosis.16,17 However, we did not examine the impact on pain. Interestingly, the pain and symptoms of endometriosis do not always correlate with the severity of the condition (such as the size of the lesions).18 It is known that stress can cause a hyperalgesic effect,19,20 but it can also activate endogenous pain inhibitory systems.21 Under physical and/or mental stress, the pituitary releases β-lipoprotein like hormones that are posttranscriptionally processed into smaller molecules such as endorphins, enkephalins, and dynorphins also known as endogenous opioids,22 a phenomenon known as stress-induced analgesia/antinociception.23 The different endogenous opioids can activate their receptors, such as mu opioid receptor (MOR), and produce analgesia by inhibiting the excitability of sensory nerves and/or inhibiting the release of pro-inflammatory neuropeptides (substance P [SP], calcitonin gene-related peptide).24 In previous experiments, a role for stress has been shown to be involved in the secretion of endogenous opioids since mice lacking β-endorphin have a decreased latency on the hot plate test compared to those that have the endogenous opioids suggesting that the opioids (in this case β-endorphin) mediate antinociception.21
One of the most relevant neuropeptides involved in pain signaling is SP22 that acts primarily on NK-1 receptors (NK-1R) and its effects extend to the surrounding undamaged tissues causing secondary hyperalgesia.25 Interestingly, endometriosis displays similar features to other visceral pain disorders such as irritable bowel syndrome (IBS).7 In the pathogenesis of IBS, the inflammatory cells have an important role. It is well known that mucosal mast cells are located near the enteric nerves and secrete histamines, cytokines, proteases, and eicosanoids that are known to sensitize visceral sensory nerve fibers.26–28 The SP in the intestinal tract is mainly produced by nerve terminals and endocrine cells such as mast cells. In previous studies, the increased expression of SP was closely related with the number of mast cells in the lamina propria of rats with IBS.26 The number of mast cells, the optical density, and positive expressing areas of SP and SP receptors were greater in rats with IBS than in normal rats.26 In our animal model of endometriosis, we have found an increase in mast cell expression,17 but it is not yet known whether there is a difference in SP expression.
In our previous studies, we have demonstrated that endometriosis alone is able to dysregulate the expression of MOR in the periaqueductal gray area in the brain when compared to sham animals suggesting that endometriosis has an impact on altering signaling at central pain processing areas.29 Also, we have previously shown in the rat model of endometriosis that a physical stressor (forced swim test) can have a beneficial effect by reducing the severity and number of developed endometriosis vesicles, counteracting the allodynic effect that endometriosis causes. It increases the expression of MOR, endomorphin-2, and decreases the expression of SP in the dorsal part of lumbar region of the spinal cord.30 However, in endometriosis, it is still unknown if exposure to a psychological stressor can elicit changes in nociception. Here, we studied the effect of a repeated psychological stress (7 days) on pain perception and spinal cord expression of various pain signaling mediators in a rat model of endometriosis.
Methods
Animal Model
Female Sprague Dawley rats weighing 200 to 250 g were singly housed at 23°C in a 12-hour light/dark cycle with food and water ad libitum. They were randomly assigned to 1 of the 3 groups—sham-no stress, endo-no stress, and endo-stress, with 12 animals per group. The Institutional Animal Care and Use Committee from Ponce Health Sciences University approved all procedures.
Animals were handled (5 min/d) for 7 days prior to beginning the experiments in order to reduce manipulation stress, and vaginal cytological smears were carried out daily to verify reproductive cycles.16 Experiments were carried out at the same time of day (9 am to 12 pm) to minimize the influence of circadian rhythms.
Induction of Endometriosis
Endometriosis was induced surgically under pentobarbital anesthesia by autotransplanting pieces of the right uterine horn to the intestinal mesentery as previously described in detail.16,31 In sham-operated groups, the right uterine horn was massaged for 2 minutes and sutures were placed in the intestinal mesenteric area with no uterine implants. Similar to our previous report,30 endometriosis was allowed to progress for 21 days before sacrificing.
Stress Protocol and Corticosterone Measurement
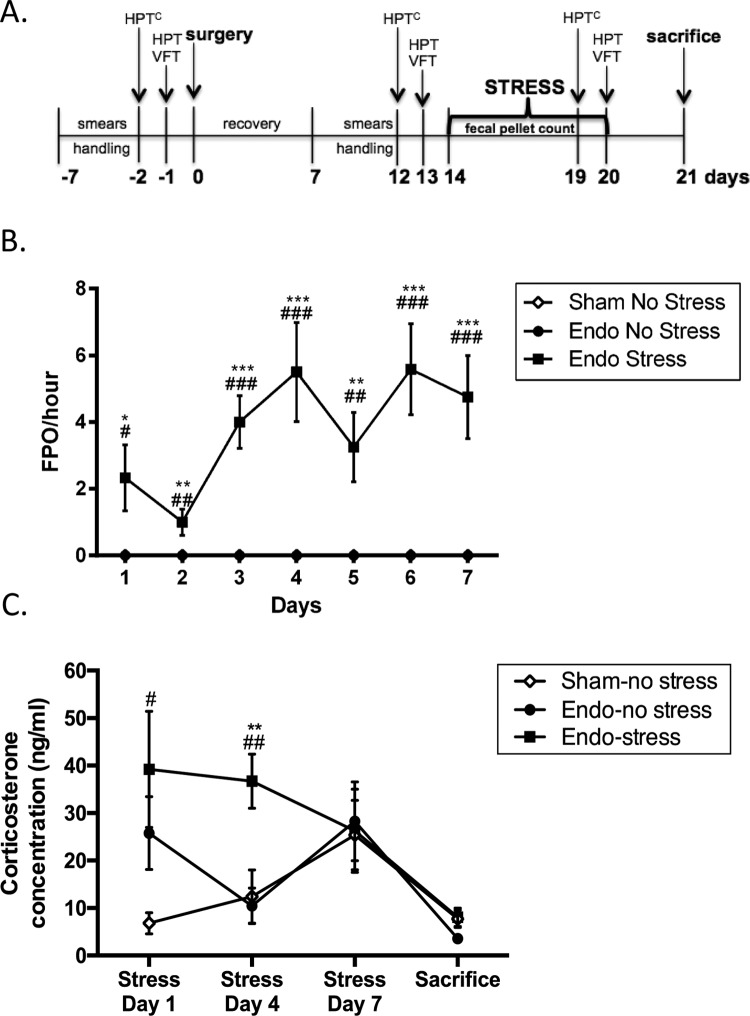
Animals were exposed to a psychological stressor (water avoidance) starting on day 14 after surgery (Figure 1A). Animals were brought to the room before the protocol for acclimatization (10 minutes). Rats were placed on a platform (11 cm × 10 cm) within a plastic enclosure (40.6 cm × 40.6 cm) with 25°C water up to 1 cm below the platform for 1 hour during 7 consecutive days at the same time each day (between 9:00 and 11:00 am). This protocol has been shown to cause a stress response in the animal since they are in an enclosed environment that they cannot escape from.32 Colonic propulsive activity (an index of anxiety) was assessed by counting the number of fecal pellets (FPO) expelled during each stress trial.16,33 The no-stress animals were kept in the home cage and handled for 5 min/d for 7 consecutive days, quantifying the FPO during the handling. Blood samples from the tail vein of the rats were obtained on days 1, 4, and 7 of the stress protocol, immediately after exposure to stress, and at the time of sacrifice under brief restraint with a plastic cone. Serum corticosterone levels were determined by enzyme-linked immunosorbent assay (IBL-America).
Figure 1.
Stress increases anxiety levels. A, All animals were subjected to psychological stress for 7 consecutive days after endometriosis induction. HPT indicates hot plate test; HPTC, hot plate test cold; VFT, Von Frey filaments. B, Endo-stress animals had increased fecal pellet counts (**P < .01, ***P < .001 vs endo-no stress animals, ## P < .01, ### P < .001 vs sham-no stress animals). C, Endo-stress animals had increased levels of corticosterone during the first (# P < .05 vs sham-no stress animals) and day 4 of stress (**P < .01 vs endo-no stress animals, ## P < .01 vs sham-no stress animals), which decreased over time (n = 12 ± standard error of the mean [SEM]).
Nociception Test
We used 2 well-characterized nociceptive tests. The hot plate is a commonly used method to measure nociception in rodents by testing the response threshold to thermal stimuli. This type of test has been used to measure analgesia and hyperalgesia in various animal models including endometriosis.34–36 Hyperalgesia is defined as increased sensitivity to pain or enhanced intensity of pain sensation. Rats were allowed to acclimatize in the testing room for 10 minutes, then placed inside the cylinder of a PanLab/Harvard (Barcelona, Spain) Hot Plate Apparatus (Model LE7406) heated to 52°C. The animals were exposed to habituation periods inside the apparatus the day before they were tested in the hot plate (days −2, 12, and 19). For habituation, rats were placed inside the cylinder for 40 seconds but the plate was at room temperature. On the days the animals were tested on the hot plate (days −1, 13, and 20; at 52°C), they were left in the cylinder for 40 seconds as described by Parikh et al21 and were video recorded for the whole time. Their latency was calculated as the amount of time it took them to first react. Each rat was tested only once per session.
Tactile sensitivity was assessed by measuring the threshold for withdrawal of the abdomen and hind paws to Von Frey filaments (Stoelting Co, Wood Dale, Illinois) as previously described by Hernandez et al.30 The Von Frey filament test is used to measure tactile allodynia in animal models and humans, where allodynia is defined as pain resulting from a stimulus, which would not normally provoke pain.30,37,38 The animals were tested in individual plastic glass enclosures with a wire mesh bottom (rats were left to acclimatize for ∼10 minutes prior to testing).39 The withdrawal response was assessed by the application of Von Frey filaments to the lower abdomen and plantar region of the hind paws (Stoelting Co). Each filament was applied in an up-down method until the hair bent slightly (∼1 second with interstimulus interval of 2-5 seconds), and the hairs were tested in ascending order of force.37 Three types of behaviors were considered as positive responses to filament stimulation—(1) sharp retraction of the abdomen, (2) immediate licking or scratching of the area of filament stimulation, or (3) jumping.37,39,40
Severity of Endometriosis and Collection of Tissue
At the time of sacrifice, all animals had a cytological smear taken to verify stage of the estrous cycle. After performing a laparotomy, the peritoneal cavity was examined for the presence of implants and the original sutures. The longest length and width of the implants were measured with a digital caliper. Classification of vesicles was carried out as previously described.16,41 Vesicles were assigned the following grades: <2 mm in length = grade 2; >2 mm but <4.5 mm = grade 3; >4.5 mm but <6 mm = grade 4; >6 mm = grade 5. If a vesicle did not develop, grade 1 was assigned. Tissue segments from colon, uterus, and vesicles were fixed in 10% formalin.
Colonic Damage and Mast Cells Expression
The whole colon was examined for macroscopic damages using an established, previously well-defined scoring system.42 Colon tissue slices (4 μm) were stained with hematoxylin and eosin to determine the extent of inflammatory infiltration and the appearance of the underlying muscles layers. Histological assessment of damage was performed using previously published criteria.16,43 Toluidine blue was used to stain the mast cells in the different tissues (colon, uterus, and vesicles).
Spinal Cord
The spinal cord is the most vital signaling link between the brain and the body and vice versa. Specifically, the lumbar dorsal area of the spinal cord connects with the pelvic organs, hindlimbs, and colon.44 The internal structure of the spinal cord exhibits a pattern of lamination where each lamina indicates different functions. The dorsal horn is linked to sensory information and the ventral horn contains mainly motor neurons. Therefore, preserving anatomical structure when verifying changes in receptors and peptides of interest is important to provide a clearer idea of their function and subsequent effects in the animals. At the time of sacrifice after collecting tissue samples, rat spinal cord was fixed by aortic arch perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.6).45 The lumbar (L1-L6) spinal cord was removed and postfixed for 1 hour in 4% paraformaldehyde in 0.1 M PB then stored at −20°C in cryoprotectant solution (30% sucrose and 30% ethylene glycol in 0.1 M PB) before sectioning on a Leica Cryostat (30 μm thick) and mounting on charged slides.
Mu Opioid Receptor and NK-1R Quantification
Sections of the lumbar spinal cord were single labeled for immunofluorescent analysis of the NK-1R (rabbit polyclonal 1:250, sc-15323; Santa Cruz [Dallas, TX]) or MOR (rabbit polyclonal 1:100, sc-15310; Santa Cruz).46 Immunofluorescent staining and analysis were performed as previously described in detail in Hernandez et al30. The secondary antisera was goat anti-rabbit Dylight 549 (1:400; Jackson ImmunoResearch Labs, Inc, West Groove, PA). All images from the dorsal area were taken at the same time under the same illumination parameters. To prevent bias, cells were manually counted by a blinded observer using the program Image J 1.47m (Wayne Rasband, NIH, USA) and verified by a second blinded observer.
Substance P, Endomorphin, and Enkephalin Analysis
Sections of the lumbar spinal cord were single labeled for immunohistochemical analysis of SP (mouse monoclonal 1:200, MAB4375; R&D Systems [Minneapolis, MD]), endomorphin-2 (rabbit polyclonal, ab10289; Abcam, Cambridge, MA), and leu-enkephalin (rabbit polyclonal, AB5024; Abcam). The specificity of this antibody has been previously tested in rat dorsal root ganglion by immunofluorescence.45,47 In the case of SP, sections were stained and analyzed as previously describe in detail in Hernandez et al30.
Sections stained with endomorphin-2 or leu-enkephalin were heated in citrate solution (10 mM, pH 8.3) followed by an incubation with 3% hydrogen peroxide to block endogenous peroxidase. They were washed with PB and incubated with 5% bovine serum albumin (BSA) in Tris (TS, pH 7.6). Tissues were rinsed with PB and TS for 24 hours at room temperature and then for 24 hours at 4°C with primary antibodies: endomorphin-2 (1:300) or leu-enkephalin (1:200) in 0.1% of BSA in TS with 0.06% of Triton X-100. Sections were rinsed and incubated with secondary antibody (1:500, Biotin-SP-conjugated Donkey anti-rabbit, Jackson ImmunoResearch Laboratories, Inc, 711-065-152). They were then incubated with avidin DH and biotinylated horseradish peroxidase for 30 minutes (Vectastain ABC kit; Vector Laboratories [Burlingame, CA], cat # pk-6100). Control slides lacked primary antisera. Sections were dehydrated and cover-slipped using Cytoseal XYL (Richard Allan Scientific, Kalamazoo, MI, cat # 8312-4). All pictures from the dorsal area were taken using a CCD Camera on a Nikon 200 microscope at the same illumination level for all images. Analyses were the same as for SP staining.
Statistical Analysis
Graphs were prepared using GraphPad Prism 6.0 (GraphPad Software) and presented as mean difference ± SEM. A P value <.05 was considered statistically significant. A 1-way analysis of variance (ANOVA) was used for normally distributed variables (assessed by Shapiro-Wilk normality test), followed by the Tukey post hoc test. Student t test for normally distributed variables on the mast cell expression in the vesicles. In the case of Von Frey filaments data were transformed logarithmically and analyzed using ANOVA followed by Tukey post hoc test.
Results
Water Avoidance Causes Stress and Anxiety
The animals exposed to stress had significantly higher numbers of FPO expelled per minute during the stress protocol when compared to the animals that received no stress indicating that these animals were indeed anxious and that the water avoidance protocol was in fact an aversive environment (Figure 1B). On day 1, increased corticosterone was found in both endo groups but this only reached significance in the group exposed to stress (endo-stress group). On day 4 of stress, the endo-stress group had significantly higher corticosterone levels compared to sham-no stress and endo-no stress groups (Figure 1C), which decreased over time. This indicated that the animals exposed to the water avoidance were indeed stressed the first days of exposure but then the animals became accustomed to their environment resulting in decreased corticosterone levels. In contrast, the endo-no stress and sham-no stress animals had no statistical significant difference in their corticosterone levels during the protocol. On day 7, the animals were exposed to a different environment, such as the hot plate test, Von Frey filaments, and restraint in order to take blood samples, all on the same day. The stressed animals were already accustomed to an aversive environment since they were exposed to water avoidance stress (WAS) for 7 days but for the nonstressed animals, this is a completely new environment, which might account for increased corticosterone levels at this time point. By the time of sacrifice, the corticosterone levels for all groups decreased.
Stress Counteracts the Allodynic Effect Caused by Endometriosis But Does Not Reverse the Hyperalgesia
In the hot plate test, we found that the sham-no stress group had a significant decrease in latency following surgery but on their last exposure (day 20) to the hot plate their latency increased, indicating some recovery toward their normal pain threshold. Both endo groups developed hyperalgesia, showing decreased latency following surgery and also following stress but this only reached significance in the endo-stress animals (Figure 2A). The surgery by itself caused a deregulation in the pain threshold causing the animals to become more sensitive to pain. In these experiments, the WAS did not reverse this effect. This corroborates recent studies in mice exposed to immobilization stress before and after the induction of endometriosis, whereby the stress significantly decreases latency on the hot plate.36
Figure 2.
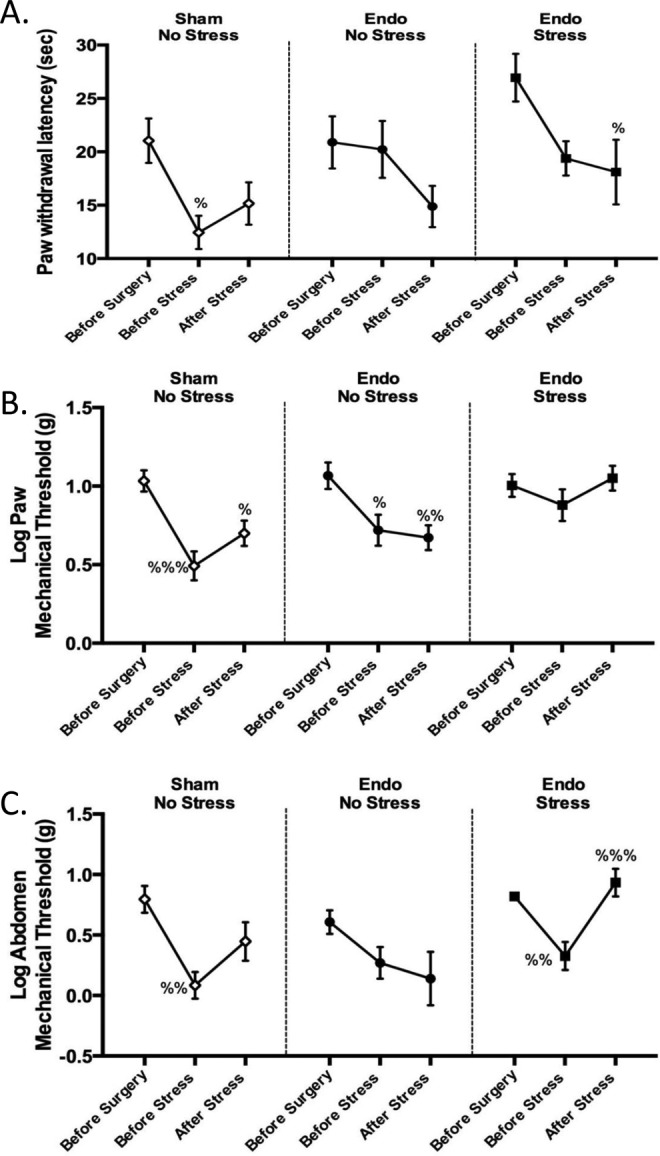
Effect of stress on nociception. A, There was a decrease in latency of paw withdrawal from before surgery to after surgery/before stress in the sham-no stress group and from before surgery to after stress in the endo-stress group (% P < .05 vs before surgery). B, Endo-no stress and sham-no stress groups decreased their tolerance to mechanical testing from their first exposure (% P < .05, %% P < .01, %%% P < .001 vs day 1). C, Sham-no stress and endo-stress groups demonstrated a decrease in sensitivity after surgery (%% P < .01 vs day 1), but only the endo-stress group recovered from the allodynic effect after the stress protocol (%%% P < .001 vs day 1; n = 12 ± standard error of the mean [SEM]).
With the Von Frey filaments, we observed that in the paw, both nonstressed groups (sham and endo) showed a decrease in the amount of force they can bear when tested before stress and after stress compared to the first baseline test (Figure 2B). This indicated that the animals developed an allodynic effect after surgery. In contrast, in the endo-stress group the force remained constant and increased on the third exposure (after stress).
In the abdomen, we found again that endo-no stress animals kept decreasing the amount of force they could bear through the different exposures (Figure 2C). Endo-stress and sham-no stress groups developed allodynia effect after surgery, but only the endo-stress group recovered from the allodynic effect. This indicated that the WAS helped the endo-stress group overcome the effect of the surgery on their tactile sensitivity in both areas (paw and abdomen).
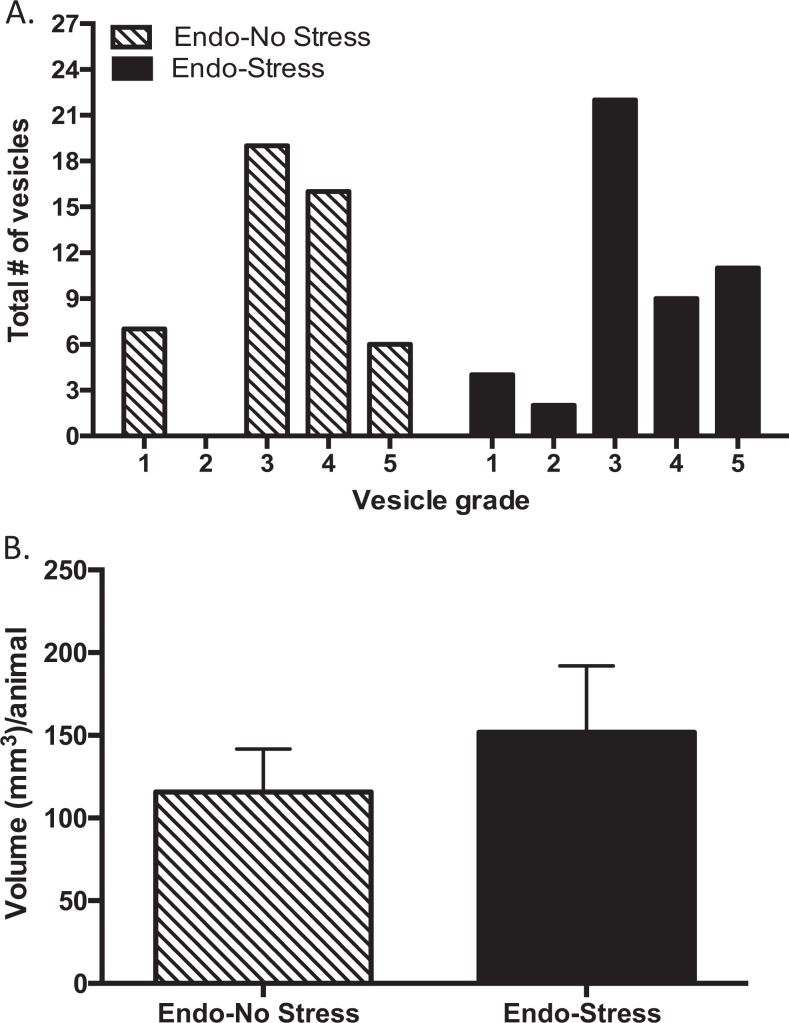
Higher Grade of Severity and More Implants Developed in the Endo-Stress Group
Although we found no significant difference in the volume (Figure 3B) or area (data not shown) of the vesicles on exposure to stress, there were more vesicles in the endo-stress group with a higher grade of severity. Specifically, there were more vesicles with grade 5 (where the vesicle was >6.0 mm) in the endo-stress group (22.92%) compared to the endo-no stress group (12.50%; Figure 3A). Also more implants in the endo-stress group developed into vesicles (91.7% vs 85.4%). These data replicate our previous observations showing that stress exacerbates endometriosis vesicle development.16,17
Figure 3.
Stress increased the size of the vesicles. A, Endometriosis animals exposed to stress developed vesicles with a higher grade of severity. The endo-stress group developed more vesicles with grade 5 (where the vesicle was >6.0 mm; 22.92%) when compared to the endo-no stress group (12.50%). B, There was no significant difference in overall average volume of the vesicles per animal although this tended to be higher in those receiving stress (n = 12 ± standard error of the mean [SEM]).
More Colonic Damage and Mast Cell Infiltration in the Endo-Stress Group
Macroscopic colonic damage was significantly higher in the endo groups when compared to the sham-no stress group. This difference appeared to be primarily due to the fact that the endo animals developed adhesions that were not present in the sham animals. Further, the endo-stress group had significantly higher damage when compared to the endo-no stress group (Figure 4A). This difference in damage can be accounted for by the presence of more adhesions of a greater severity in the endo-stress animals (accounting for 51.30% of the total macroscopic score vs 44.43% in endo-no stress animals) and a higher incidence of diarrhea (8.30% of animals vs 0.00%). When we examined the colon at the microscopic level, we found no differences between groups (data not shown).
Figure 4.
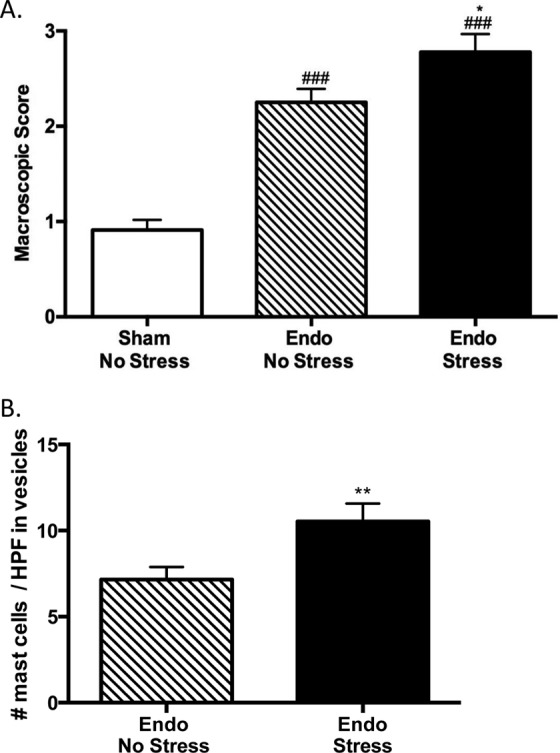
Stress worsens colonic damage and increases mast cell infiltration in the vesicles. A, Endo groups have more colonic damage when compared to sham-no stress group (### P < .001 vs sham-no stress group). The endo-stress group has more damage than the endo-no stress group (n = 12 ± SEM, *P < .05 vs endo-no stress group). B, There was a significant increase in mast cell numbers in the vesicles of endo-stress animals when compared to the endo-no stress animals (n = 30-34 vesicles ± standard error of the mean [SEM], **P < .01 vs endo-no stress animals).
We examined the presence of mast cells in the colon and vesicles by staining with toluidine blue. We found that there were no differences in mast cell numbers between groups in the colon and uterus (data not shown), although there was a tendency for more mast cells in the endo-stress group. In the vesicles, we found that there were significantly more mast cells in the endo-stress group when compared to the endo-no stress group (Figure 4B). This indicated that the stress caused an increase in inflammation as seen before with the macroscopic damage. This also correlates with previous experiments in our laboratory in which the Morris water maze stress caused more mast cell infiltration when compared to the nonstressed groups.16,17
Mu Opioid Receptor and NK-1R Spinal Cord Expression
Induction of endometriosis resulted in significantly less labeled cells immunoreactive for MORs in the dorsal part of the lumbar region of the spinal cord (Figure 5A) when compared to the sham-no stress animals. Regardless of exposing the animals to stress, the endometriosis by itself decreased MOR cell labeling in the endo animals compared to the sham-no stress animals reaching statistical significance (Figure 5A). Exposure of the endometriosis animals to stress did not significantly increase the number of MOR labeled cells. We found no significant differences in NK-1R expression in the dorsal part of the lumbar region of the spinal cord between groups (Figure 5B and C). All the labeled cells were mostly observed in the dorsal area with similar patterns between groups.
Figure 5.
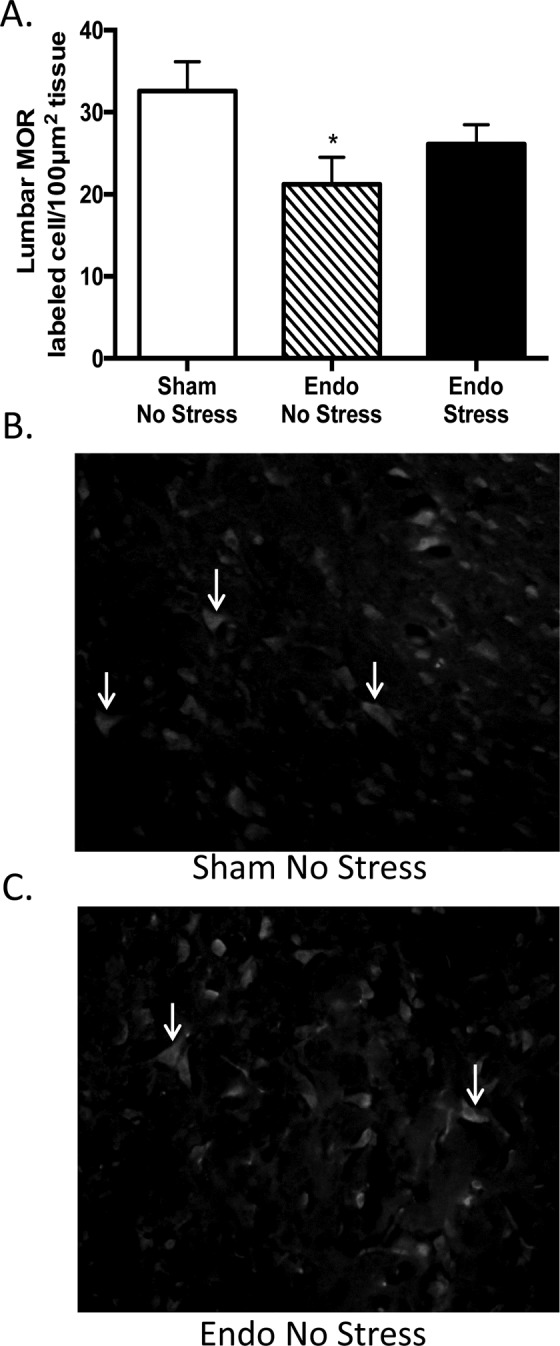
Endometriosis decreases Mu opioid receptor (MOR) expression in the spinal cord. A, Regardless of exposing the animals to stress, the endometriosis by itself decreased MORs cells labeling in the endo animals compared to the sham-no stress animals reaching statistical significance (*P < .05 vs sham-no stress animals). Representative MOR immunofluorescent staining of the lumbar region in (B) sham-no stress animals and (C) endo-no stress animals (1:100 at 400×; n = 11-12 ± standard error of the mean [SEM]).
Expression of Endomorphin, Enkephalin, and SP in Spinal Cord
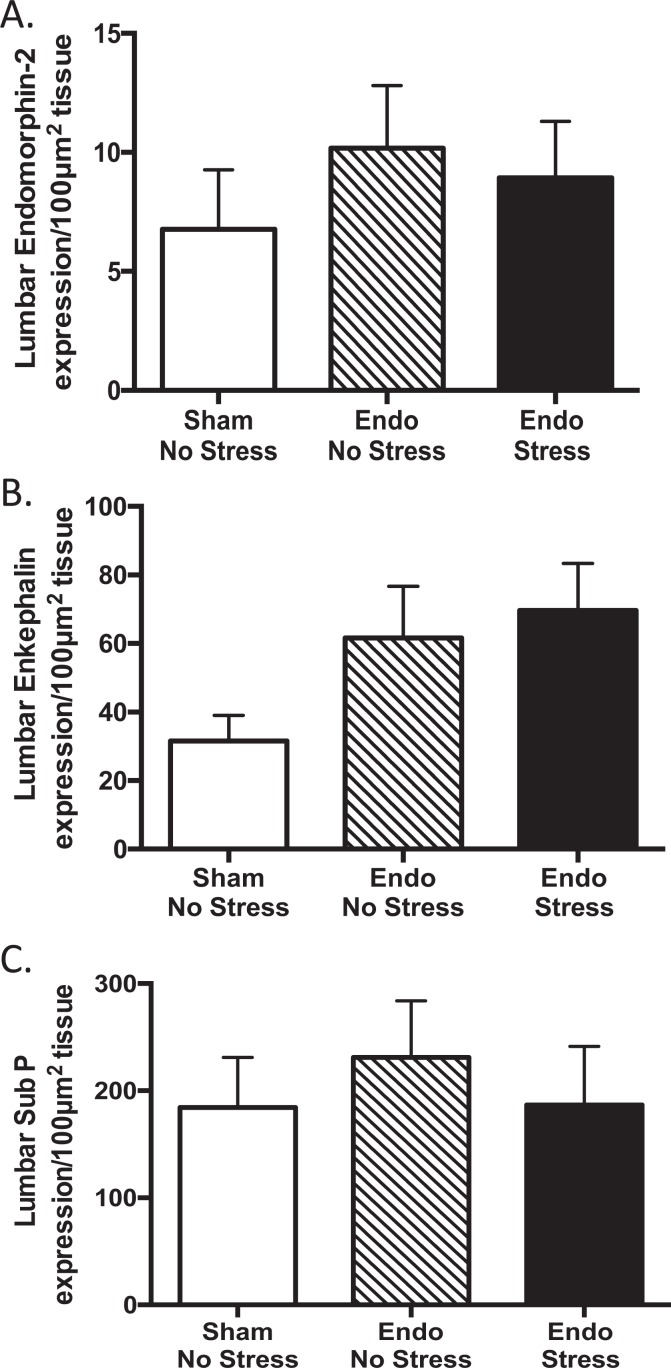
We found no significant differences in endomorphin-2 expression in the dorsal part of the lumbar region of the spinal cord between groups, although there was a trend toward increased expression in the endometriosis animals (Figure 6A). Similarly, the expression of enkephalin in the dorsal part of the lumbar region of the spinal cord showed that regardless of stress, endometriosis by itself increased enkephalin expression in the endo animals compared to the sham-no stress animals (Figure 6B). The pattern of expression was similar between groups.
Figure 6.
Endogenous opioid and substance P (SP) expression in the spinal cord of rats exposed to stress. A, There were no significant differences in endomorphin-2 expression between groups (n = 10-12 ± standard error of the mean [SEM]). B, Regardless of stress endometriosis by itself tended to increase enkephalin expression in the endo animals compared to the sham-no stress animals (n = 10-12 ± SEM). C, There were no significant differences between groups (n = 10-12 ± SEM).
Using the same procedure and analysis as for the endogenous opioids, we evaluated SP expression in the dorsal part of the lumbar region of the spinal cord and found no significant differences between groups (Figure 6C). We found the same pattern of immunolabeled SP fibers between groups.
Discussion
In the present study, we demonstrated how psychological stress in a rat model can impact the development of endometriosis and pain perception. Prior studies in our laboratory found that a psychological/physical stress, such as the Morris water maze, can exacerbate endometriosis symptoms in this model.16,17 It has been shown that psychological stress plays a substantial role in the exacerbation of many chronic inflammatory and painful conditions.48 Stress negatively impacts the regulatory process of inflammatory responses and neuroendocrine pathways, which in turn could lead to disease.49,50 Here, we subjected our animals to a psychological stressor (water avoidance) that did not involve any kind of physical exercise, contrary to the Morris water maze where the animals are also subjected to a physical component, since in recent previous studies we have found that physical activity could be beneficial in decreasing the pathophysiology of endometriosis and counteracting allodynic effects.30 In the current studies, the higher corticosterone levels found in the animals subjected to the water avoidance confirmed that they were stressed, and this was substantiated by the increased FPO output during the protocol.
In endometriosis animal models and in patients with endometriosis, the pain is mediated through peripheral and central sensitization, which is also commonly found in other pain syndromes such as painful bladder or IBS and as a consequence develop hyperalgesia and/or allodynia.10,51 Prior studies have found that after the induction of endometriosis, the animals experience hypersensitivity to a thermal stimulus.6 In the current study, we measured nociception at 3 different time points since it is known that stress can elicit pain relief (a phenomenon referred to as “stress-induced analgesia”) via an opioid and nonopioid pathway.21 We found that the endometriosis by itself decreased the pain threshold correlating with previous work by Zhao et al, Lu et al, and Long et al.6,35,36 Surprisingly, we also found that before surgery, although all the groups were under the same conditions, there were different baseline nociceptive reactions. However, this difference in baseline nociception was not statistically significant between groups and was not a confounding factor in our study since the subjects were compared to themselves for analysis. After exposing our animals to stress, there was an increased threshold in tactile sensitivity (allodynia) but not in thermal sensitivity, implying that the stressed animals develop hyperalgesia. In previous studies, it was observed that normal adult rats exposed to repeated water avoidance develop visceral hyperalgesia immediately after the last stress session, which was found to be long lasting, taking up to 40 days to return to baseline values.52 This could explain why our animals did not develop stress-induced analgesia. Another important aspect to consider is that rodents need surgery to induce endometriosis that is not akin to the natural process in women who develop the condition. It is worth noting that the sham group in our experiment acts as a control for the surgical procedure undergoing surgery but without implantation of tissue in the mesenteric area. This type of surgery is still invasive and can cause changes in nociception, which might explain the latency change but this effect does not continue throughout the protocol, contrary to our findings in the endo animals that were not exposed to stress. Even with its limitations, and those of the nociceptive tests utilized,34 this animal model is still one of the most appropriate to try to understand nociceptive changes since rodents share many similarities to patients including the presence of glands, stroma, and cysts. Further, published studies investigating the effects of the lesions on pain behavior in the rodent model have found that vaginal nociception (vaginal hyperalgesia) is increased in rats with endometrial lesions in a manner similar to women with endometriosis.53,54 Future studies could examine how long it takes the pain thresholds to return to normal baseline values or if the pain actually gets more severe over time.
We examined the expression of endogenous opioids, SP, and their respective receptors in the dorsal part of the lumbar region of the spinal cord. When we examined the endogenous opioids and pain pathways in the spinal cord, we found that the differences in pain perception cannot be explained by the differences in the expression of the receptors, neuropeptides, or opioid peptides. We only found a decrease in the expression of the MOR in the endo-no stress group compared to the sham-no stress group and a trend toward an increase expression of enkephalin in the Endo groups. It is important to remember that expression of the receptor does not necessarily correlate with protein ligand expression. Receptor expression is usually dynamic depending on whether there is chronic activation that can result in a decreased number of receptors due to internalization or degradation or might result in increased expression to improve chances for ligand binding.55 Downregulation of the receptor might occur if there is more ligand available, whereas upregulation might compensate for low expression of the ligand, which could be our case with the MOR and enkephalin. Being aware of this limitation, we decided to measure both the receptor and ligand in both pathways for the opioids (receptor: MOR and ligands: enkephalins and endomorphin) and pain (receptor: NK-1R and ligand: SP). In this way, we could look at how each was expressed and distributed in the tissue and observe if this expression correlates with the changes found in the behavioral analysis of nociception. Since we did not find any significant difference, we suggest that the changes in pain perception might be caused via alternate signaling pathways in the spinal cord. Follow-up studies should examine the delta opioid receptor since they preferentially inhibit mechanical pain, whereas the activation of spinal MOR preferentially inhibits thermal pain.56 Further, previous experiments have found that endomorphin prefers to bind to MOR and enkephalin prefers delta opioid receptors.57 However, it is important to note that the delta opioid receptor pathway is also important for the management of pain and should also be taken into consideration to help explain the differences in pain perception between our groups.
In our other studies using a physical stressor, we found similar nociceptive changes where animals exposed to stress maintain the hyperalgesia but provide beneficial effects by reducing perceived pain. Physical stress also increased the MOR-labeled cells back to normal levels and decreased SP in the spinal cord lumbar dorsal area, contrary to what we found with animals exposed to the psychological stressor. In those studies, we also observed the confounding effects of physical activity resulting in a significant reduction in vesicle size number, which suggest the potential benefits of exercise in this condition.30 Therefore, endometriosis manifestation could be exacerbated or not depending on the nature and duration of the stressor.
To our knowledge, no prior studies have identified the effect of repeated psychological stress, and to what extent psychological stress can affect the perception of pain in women with endometriosis. Ours is the first study to examine how psychological stress might affect pain perception in an animal model of endometriosis and if those effects can be correlated to what happens physiologically at the level of pain signaling in the spinal cord. In summary, our results suggest that psychological stress exacerbates endometriosis symptoms with increased visceral inflammation and as a consequence, maintains the hyperalgesia that is caused by the endometriosis but could also provide beneficial effects in the abdomen by reducing perceived pain. It is still unclear how the psychological stressor alleviates the effect of allodynia but based on our experiments these changes are not related to MOR, enkephalin, or endomorphin-2. Our data give us an insight as to how the pain receptors change and may help in the future for more targeted treatment options in patients; however, further studies are still needed to understand physiologically how pain perception is altered in this disease and which pathways are involved.
Acknowledgments
The authors would like to acknowledge the technical support of Abigail Ruiz and Mariano Colon during surgeries; Alcira Benítez for histological preparation; Anixa del C. Hernández, María Del C. Colon, Yadmarie Rivera, Mayra I. Gonzalez, and Abdon Lopez for assistance during the stress protocol and immunohistochemistry; Luis S. Hernandez for construction of water avoidance boxes and Von Frey filament table; Raymond A. Isidro for graph analysis; and helpful feedback from discussions with Drs Alan Gintzler, Dinah L. Ramos, and Idhaliz Flores.
Author Notes: Institution where work was done: Ponce Health Sciences University, Ponce Research Institute, Ponce, PR. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported in part by 1R15AT006373 (CBA, AT), R25GM082406 (SH), G12MD007579-27 (MAGIC Core and B.R.A.I.N. CORE) from the National Institutes of Health (NIH).
References
- 1. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 2. Schindler AE. Dienogest in long-term treatment of endometriosis. Int J Womens Health. 2011;3:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKinnon B, Bersinger N, Wotzkow C, Mueller MD. Endometriosis-associated nerve fibers, peritoneal fluid cytokine concentrations, and pain in endometriotic lesions from different locations. Fertil Steril. 2012;97(2):373–380. [DOI] [PubMed] [Google Scholar]
- 4. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598. [DOI] [PubMed] [Google Scholar]
- 5. Levy BS, Apgar BS, Surrey ES, Wysocki S. Endometriosis and chronic pain: a multispecialty roundtable discussion. J Fam Pract. 2007;56(3 suppl diagnosis):S15–S20. [PubMed] [Google Scholar]
- 6. Zhao T, Liu X, Zhen X, Guo SW. Levo-tetrahydropalmatine retards the growth of ectopic endometrial implants and alleviates generalized hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci. 2011;18(1):28–45. [DOI] [PubMed] [Google Scholar]
- 7. He W, Liu X, Zhang Y, Guo SW. Generalized hyperalgesia in women with endometriosis and its resolution following a successful surgery. Reprod Sci. 2010;17(12):1099–1111. [DOI] [PubMed] [Google Scholar]
- 8. Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain. 2003;4(7):372–380. [DOI] [PubMed] [Google Scholar]
- 9. As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122(5):1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339–356. [DOI] [PubMed] [Google Scholar]
- 13. Huntington A, Gilmour JA. A life shaped by pain: women and endometriosis. J Clin Nurs. 2005;14(9):1124–1132. [DOI] [PubMed] [Google Scholar]
- 14. Tariverdian N, Rücke M, Szekeres-Bartho J, et al. Neuroendocrine circuitry and endometriosis: progesterone derivative dampens corticotropin-releasing hormone-induced inflammation by peritoneal cells in vitro. J Mol Med (Berl). 2010;88(3):267–278. [DOI] [PubMed] [Google Scholar]
- 15. Toth B. Stress, inflammation and endometriosis: are patients stuck between a rock and a hard place? J Mol Med (Berl). 2010;88(3):223–225. [DOI] [PubMed] [Google Scholar]
- 16. Appleyard CB, Cruz ML, Hernández S, Thompson KJ, Bayona M, Flores I. Stress management affects outcomes in the pathophysiology of an endometriosis model. Reprod Sci. 2015;22(4):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cuevas M, Flores I, Thompson KJ, Ramos-Ortolaza DL, Torres-Reveron A, Appleyard CB. Stress exacerbates endometriosis manifestations and inflammatory parameters in an animal model. Reprod Sci. 2012;19(8):851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagabukuro H, Berkley KJ. Influence of endometriosis on visceromotor and cardiovascular responses induced by vaginal distention in the rat. Pain. 2008;132(suppl 1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myers B, Greenwood-Van Meerveld B. Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am J Physiol Gastrointest Liver Physiol. 2012;302(2):G260–G266. [DOI] [PubMed] [Google Scholar]
- 20. Moloney RD, O’Mahony SM, Dinan TG, Cryan JF. Stress-induced visceral pain: toward animal models of irritable-bowel syndrome and associated comorbidities. Front psychiatry. 2015;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parikh D, Hamid A, Friedman TC, et al. Stress-induced analgesia and endogenous opioid peptides: the importance of stress duration. Eur J Pharmacol. 2012;650(2-3):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosch PJ. The stress-pain connection: some historical considerations and new directions. Compr Ther. 1986;12(7):3–8. [PubMed] [Google Scholar]
- 23. Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88(3):184–202. [DOI] [PubMed] [Google Scholar]
- 24. Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14(3):249–258. [PubMed] [Google Scholar]
- 25. Harrison S, Geppetti P. Substance P. Int J Biochem Cell Biol. 2001;33(6):555–576. [DOI] [PubMed] [Google Scholar]
- 26. Ma XP, Tan LY, Yang Y, et al. Effect of electro-acupuncture on substance P, its receptor and corticotropin-releasing hormone in rats with irritable bowel syndrome. World J Gastroenterol. 2009;15(41):5211–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang GD, Wang XY, Liu S, et al. Innervation of enteric mast cells by primary spinal afferents in guinea pig and human small intestine. Am J Physiol Gastrointest Liver Physiol. 2014;307(7):G719–G731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shea-Donohue T, Stiltz J, Zhao A, Notari L. Mast cells. Curr Gastroenterol Rep. 2010;12(5):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torres-Reveron A, Palermo K, Hernández-López A, et al. Stress and endometriosis affects Mu opioid and NMDA receptors in areas relevant to pain processing. Reprod Sci. 2016;23(9):1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hernández S, Cruz ML, Torres-Reveron A, Appleyard CB. Impact of physical activity on pain perception in an animal model of endometriosis. J Endometriosis Pelvin Pain Disord. 2016;7(3):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44(5):684–694. [PubMed] [Google Scholar]
- 32. Bhatia N, Maiti PP, Choudhary A, et al. Animal models in the study of stress: a review. J Pharm Healthc Manag. 2011;2:42–50. [Google Scholar]
- 33. Maillot C, Million M, Wei JY, Gauthier A, Tache Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119(6):1569–1579. [DOI] [PubMed] [Google Scholar]
- 34. Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652. [PubMed] [Google Scholar]
- 35. Lu Y, Nie J, Liu X, Zheng Y, Guo SW. Trichostatin A, a histone deacetylase inhibitor, reduces lesion growth and hyperalgesia in experimentally induced endometriosis in mice. Hum Reprod. 2010;25(4):1014–1025. [DOI] [PubMed] [Google Scholar]
- 36. Long Q, Liu X, Qi Q, Guo SW. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor B2 . Hum Reprod. 2016;24(31):2506–2519. [DOI] [PubMed] [Google Scholar]
- 37. May V, Vizzard MA. urinary bladder dysfunction and altered somatic sensitivity in pituitary adenylate cyclase activating polypeptide knockout (PACAP−/−) mice. J Urol. 2011;183(2):772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tena B, Escobar B, Arguis MJ, Cantero C, Rios J, Gomar C. Reproducibility of electronic Von Frey and Von Frey monofilaments testing. Clin J Pain. 2012;28(4):318–323 [DOI] [PubMed] [Google Scholar]
- 39. Rudick CN, Schaeffer AJ, Klumpp DJ. Pharmacologic attenuation of pelvic pain in a murine model of interstitial cystitis. BMC Urol. 2009;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stagg NJ, Mata HP, Ibrahim MM, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ingelmo JM, Quereda F, Acién P. Intraperitoneal and subcutaneous treatment of experimental endometriosis with recombinant human interferon-alpha-2b in a murine model. Fertil Steril. 1999;71(5):907–911. [DOI] [PubMed] [Google Scholar]
- 42. Appleyard CB, Cruz ML, Rivera E, Hernández GA, Flores I. Experimental endometriosis in the rat is correlated with colonic motor function alterations but not with bacterial load. Reprod Sci. 2007;14(8):815–824. [DOI] [PubMed] [Google Scholar]
- 43. Hernández GA, Appleyard CB. Bacterial load in animal models of acute and chronic ‘reactivated’ colitis. Digestion. 2003;67(3):161–169. [DOI] [PubMed] [Google Scholar]
- 44. Anderson CR, Keast JR, McLachlan EM. Spinal autonomic preganglionic neurons: the visceral efferent system of the spinal cord. Spinal Cord. 2009;115–129.18542085 [Google Scholar]
- 45. Milner TA, Drake CT. Ultrastructural evidence for presynaptic Mu opioid receptor modulation of synaptic plasticity in NMDA-receptor-containing dendrites in the dentate gyrus. Brain Res Bull. 2001;54(2):131–140. [DOI] [PubMed] [Google Scholar]
- 46. Luo DS, Huang J, Dong YL, et al. Connections between EM2- and SP-containing terminals and GABAergic neurons in the mouse spinal dorsal horn. Neurol Sci. 2014;35(9):1421–1427. [DOI] [PubMed] [Google Scholar]
- 47. Xu JT, Zhao X, Yaster M, Tao YX. Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res. 2010;1336:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62(6):591–599. [PubMed] [Google Scholar]
- 49. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. [DOI] [PubMed] [Google Scholar]
- 50. Rivat C, Becker C, Blugeot A, et al. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150(2):358–368. [DOI] [PubMed] [Google Scholar]
- 51. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G42–G53. [DOI] [PubMed] [Google Scholar]
- 53. Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett. 2001;306(3):185–188. [DOI] [PubMed] [Google Scholar]
- 54. McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PLoS One. 2012;7(2):e31758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lauffenburger DA, Linderman J. Receptors: Models for Binding, Trafficking, and Signaling. 1st ed New York, NY: Oxford University Press; 1993. [Google Scholar]
- 56. Normandin A, Luccarini P, Molat JL, Gendron L, Dallel R. Spinal μ and δ opioids inhibit both thermal and mechanical pain in rats. J Neurosci. 2013;33(28):11703–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Koneru A, Satyanarayana S, Rizman R. Endogenous opioids: their physiological role and receptors. Glob J Pharmacol. 2009;3(3):149–153. [Google Scholar]