Abstract
Uterine leiomyoma are a common benign pelvic tumors composed of modified smooth muscle cells and a large amount of extracellular matrix (ECM). The proteoglycan composition of the leiomyoma ECM is thought to affect pathophysiology of the disease. To test this hypothesis, we examined the abundance (by immunoblotting) and expression (by quantitative real-time polymerase chain reaction) of the proteoglycans biglycan, decorin, and versican in leiomyoma and normal myometrium and determined whether expression is affected by steroid hormones and menstrual phase. Leiomyoma and normal myometrium were collected from women (n = 17) undergoing hysterectomy or myomectomy. In vitro studies were performed on immortalized leiomyoma (UtLM) and normal myometrial (hTERT-HM) cells with and without exposure to estradiol and progesterone. In leiomyoma tissue, abundance of decorin messenger RNA (mRNA) and protein were 2.6-fold and 1.4-fold lower, respectively, compared with normal myometrium. Abundance of versican mRNA was not different between matched samples, whereas versican protein was increased 1.8-fold in leiomyoma compared with myometrium. Decorin mRNA was 2.4-fold lower in secretory phase leiomyoma compared with proliferative phase tissue. In UtLM cells, progesterone decreased the abundance of decorin mRNA by 1.3-fold. Lower decorin expression in leiomyoma compared with myometrium may contribute to disease growth and progression. As decorin inhibits the activity of specific growth factors, its reduced level in the leiomyoma cell microenvironment may promote cell proliferation and ECM deposition. Our data suggest that decorin expression in leiomyoma is inhibited by progesterone, which may be a mechanism by which the ovarian steroids affect leiomyoma growth and disease progression.
Keywords: leiomyoma, proteoglycans, steroid hormones
Introduction
Uterine leiomyomas are the most common benign solid pelvic tumors in reproductive-age women and are associated with menorrhagia, pelvic pain, and infertility. Leiomyoma disease is the primary reason for hysterectomy in the United States and is associated with significant morbidity and health care costs of up to 5 billion dollars annually.1,2 The development of effective therapies that prevent and/or inhibit leiomyoma development and growth requires a clear understanding of the pathophysiology of leiomyoma disease.
Uterine leiomyomas are composed of modified smooth muscle cells and a large amount of extracellular matrix (ECM). Growth of leiomyomas involves proliferation of leiomyoma cells and increased deposition of ECM. Uterine leiomyomas have 50% more ECM compared with normal adjacent myometrial tissue.3–6 The ECM is thought to play a key role in leiomyoma growth and structural architecture, and the influence of the ECM on leiomyomas is generally considered to be affected by the composition, deposition, and function of specific ECM components, especially the proteoglycans biglycan, decorin, and versican.7
Biglycan, decorin, and versican are thought to affect leiomyoma growth by modulating growth factor activity in the leiomyoma cell microenvironment. Biglycan is a small leucine-rich proteoglycan found in the ECM of tissues such as bone and cartilage. It affects tissue-specific angiogenesis and the activity of ECM-associated growth factors, and it can inhibit the activity of transforming growth factor β (TGF-β), a major regulator of cell proliferation, differentiation, and ECM production. Decorin is also a small leucine-rich proteoglycan, structurally similar to biglycan. Decorin binds collagen and inhibits the formation of collagen fibrils.8 Like biglycan, decorin also affects tissue angiogenesis and decreases TGF-β-induced cell proliferation and ECM deposition.9–12 This concept is especially relevant for leiomyoma growth because TGF-β, and specifically the TGF-β3 isoform, is elevated in leiomyomas and, thus, may enhance leiomyoma cell proliferation and ECM deposition.3,13–15 Versican is a chondroitin sulfate proteoglycan found in soft tissues, and it functions to affect cell adhesion and migration in part through increasing TGF-β signaling. Versican is increased in leiomyomas and is modulated by estradiol in mouse uterine tissue.5,16
The underlying cause of leiomyomas is unknown. Clinical studies suggest that leiomyoma development and growth are affected by the steroid hormone milieu, especially estradiol and progesterone.17,18 The effects of estradiol and progesterone on proteoglycan gene expression in human leiomyoma compared with normal myometrium have not been explored. This study tested the hypothesis that the relative abundance of biglycan, decorin, and versican in leiomyoma differs compared to normal matched myometrium and that abundance of the proteoglycans varies by menstrual phase, suggesting hormonal modulation of expression. To test this hypothesis, levels of biglycan, decorin, and versican gene expression in leiomyoma and adjacent normal myometrium from women in the proliferative (estrogen dominant) and secretory (progesterone-dominant) phases of the menstrual cycle were measured. In addition, the effects of estradiol and progesterone on biglycan, decorin, and versican expression in immortalized leiomyoma and myometrial cell lines were examined.
Materials and Methods
Tissue
Women with symptomatic leiomyoma disease undergoing hysterectomy or myomectomy were included in the study. Approval to collect leiomyoma and normal myometrial tissue from medically indicated gynecologic procedures was obtained by the University Hospitals Case Medical Center institutional review board (IRB # 06-07-29). All tissue samples were classified as discarded tissue. Leiomyoma (intramural or subserosal and >2 cm in diameter) and normal myometrium (from a site at least 3 cm away from any tumors) were collected from all patient samples. All specimens were reviewed by a pathologist, and final pathology was confirmed as benign leiomyoma and normal myometrium. Data regarding age, race, current medications, and self-reported last menstrual period were obtained for all patients. Phase of menstrual cycle was determined in patient undergoing hysterectomy by histologic diagnosis of the endometrium according to the Noyes criteria.19 Patients on hormone therapy, who were postmenopausal, and those undergoing surgery for known gynecologic cancer were excluded from the study.
For RNA analysis, tissue was snap frozen in liquid nitrogen and then stored at −80°C. For proteoglycan isolation, tissue was rinsed in normal saline, weighed, frozen in dry ice ethanol, and stored at −80°C.
Immortalized Cell Lines
Immortalized cell lines of human leiomyoma (UtLM: provided by Dr Darlene Dixon, National Institute of Environmental Health Sciences20) and myometrium (hTERT-HM: provided by Dr William Rainey, Medical College of Georgia, Georgia21) were used to examine the effect of ovarian steroids on proteoglycan gene expression. Cells were cultured as described previously.20,21 Both of the immortalized cell lines at 80% confluency were exposed to 100 nmol/L estradiol, 10 nmol/L R5020 (progesterone agonist; Perkin Elmer, Waltham, Massachusetts), or vehicle (dimethyl sulfoxide ) for 24 hours and then processed for RNA extraction.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
Leiomyoma and normal myometrial tissue (each ∼500 mg) were homogenized (Ultra-Turrax Tissumizer; Tekmar Co, Cincinnati, Ohio) in 1 to 2 mL of TRIzol reagent (Life Technologies; Waltham, MA, USA) for 1 minute. Total RNA from tissue was then isolated according to the manufacturer’s protocol, and contaminating genomic DNA was degraded by DNase treatment (Applied Biosystems; Waltham, MA, USA). RNA was ethanol precipitated, resuspended in water, and quantified by absorbance at 260 nm. The same TRIzol protocol (lacking the homogenization step) was used to isolate RNA from UtLM and hTERT-HM cells.
For quantitative real-time polymerase chain reaction (RT-PCR), total RNA (300-600 ng) was reverse transcribed with random primers using superscript II reverse transcriptase (Life Technologies). Primers for specific target messenger RNAs (mRNAs) were designed using the Primer Express software (Applied Biosystems) based on published sequences (Table 1). The decorin and versican primers were designed to detect all known splice variants. Assays were optimized and validated for all primer sets by confirming that single amplicons of appropriate size and sequence were generated and that the priming and amplification efficiencies of all primer pairs were identical.
Table 1.
Primers Used to Measure Biglycan, Decorin, and Versican mRNA levels.
| Gene | Primers Forward (F) and Reverse (R) | Amplicon Size in Base Pairs (bp) | Genbank Number |
|---|---|---|---|
| Biglycan | F: 5′-TACAACGGCATCAGCCTCTTC-3′ | 78 bp | AB20949.1 |
| R: 5′-GTCAGTGACGCAGCGGAAA-3′ | |||
| Decorin | F: 5′-GCCAGAAAAAATGCCCAAAA-3′ | 67 bp | BC005322.1 |
| R: 5′-TCGCACTTTGGTGATCTCATTC-3′ | |||
| Versican | F: 5′-GCCTTTCCTATCACCTCGAGAA-3′ | 110 bp | BC004244.1 |
| R: 5′-CACGGCAACCCAAAATGACG-3′ |
The PCR was performed in the presence of SYBR Green (Applied Biosystems) in an ABI PRISM 7500 Sequence Detector (Applied Biosystems). The cycling conditions were 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. The cycle at which the fluorescence reached a preset threshold (cycle threshold = CT) was used for quantitative analyses. The threshold in each assay was set at a level where the rate of exponential increase in amplicon abundance was approximately parallel between all samples. Messenger RNA abundance data were expressed relative to the abundance of the constitutively expressed 18S ribosomal RNA (rRNA) using the ΔCT method, that is, relative mRNA abundance = 2−(CT gene of interest − CT 18S rRNA).23
Proteoglycan Isolation and Quantification
Biglycan, decorin, and versican were isolated as described previously.22 Briefly, tissue was minced and extracted with 4 mol/L guanidinium chloride containing protease inhibitors, and proteoglycans were isolated by anion exchange chromatography. The amount of glycosaminoglycans was determined for each sample with a Safranin O assay. For all gel electrophoreses, proteoglycans were loaded at equal amounts for each sample. Initial analyses were done by standard sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE), and the gels were stained with toluidine blue. For proteoglycan quantification, samples were electrophoresed in nondenaturing composite gels of agarose and polyacrylamide, which resolve based on size and charge. This was essential for resolving versican, which is too large to migrate on regular polyacrylamide gels and too large to transfer efficiently out of polyacrylamide gels. Composite gels have larger pores, which allow versican to migrate some distance into the gels and also to transfer efficiently out of the gels for immunoblotting. The gels were electrotransferred to immobilon-P membrane (EMD Millipore, Billerica, Massachusetts), and the blots were then probed with antibodies to specific proteoglycan core proteins (LF-121, a polyclonal antibody for human biglycan; 6B6, a monoclonal antibody for human decorin; and 3 antibodies for versican 12C5, 2B1, and anti-DPEAAE peptide (single letter amino acid code) accounting for the full length verscian core protein).22 The immunoblots were digitally scanned, and the bands were quantified by densitometry with ImageJ.
Statistical Analysis
Statistics
The RT-PCR and densitometry data are represented as means ± standard error of the mean. Nonparametric statistical analyses were applied after determining that the data were not normally distributed using the Kolmogorov-Smirnov test. Paired and unpaired data were compared using the Wilcoxon signed rank or Mann-Whitney U tests, respectively. A P value of less than .05 was used as the limit for significance.
Results
Leiomyoma and normal matched myometrium were obtained from 17 women, the majority of whom (14 of 17) were African American and the others (3 of 17) caucasian. The average patient age was 41 ± 6 years old. Menstrual stage was determined by endometrial dating in 11 of the 17 cases (5 proliferative and 6 secretory), all of which were hysterectomy specimens.
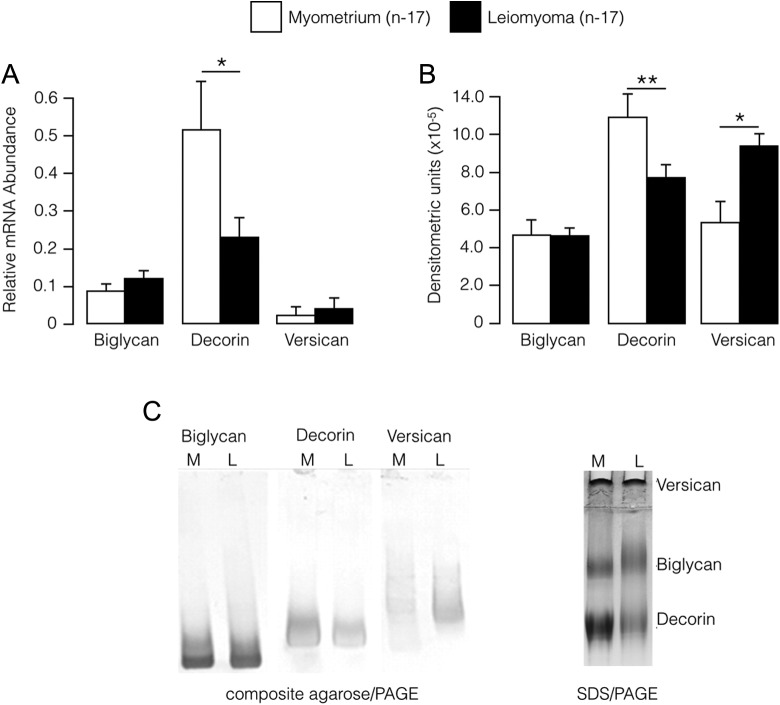
The relative abundance of decorin mRNA and protein was significantly lower in leiomyoma compared with matched adjacent myometrium. Abundance of decorin mRNA in leiomyoma was 2.6-fold less than in adjacent myometrium (P < .02), and abundance of decorin protein in leiomyoma was 1.4-fold less than in adjacent myometrium (P < .01; Figure 1). No differences were detected in biglycan protein and mRNA levels between the 2 tissues (Figure 1). The level of versican protein was increased 1.8-fold (P = .01) in leiomyoma compared with normal myometrium, but this was not matched by an increase in versican mRNA.
Figure 1.
A, Abundance (relative to 18S rRNA; mean ± SE) of mRNAs encoding proteoglycans in myometrium and leiomyoma. B, Proteoglycan protein abundance (mean ± SE) assessed by densitometry of immunoblot bands in myometrium and leiomyoma. C, Representative immunoblot analyses of the proteoglycans decorin, biglycan, and versican in myometrium (M) and leiomyoma (L) assessed by composite agarose/polyacrylamide gel electrophoresis (composite agarose/PAGE; left). Also shown is a typical result for SDS polyacrylamide gel electrophoreses (SDS/PAGE; right). *P < .05, **P < .01; Wilcoxon signed rank test. SE indicates standard error; mRNA, messenger RNA; PAGE, polyacrylamide gel electrophoresis ; SDS, sodium dodecyl sulfate.
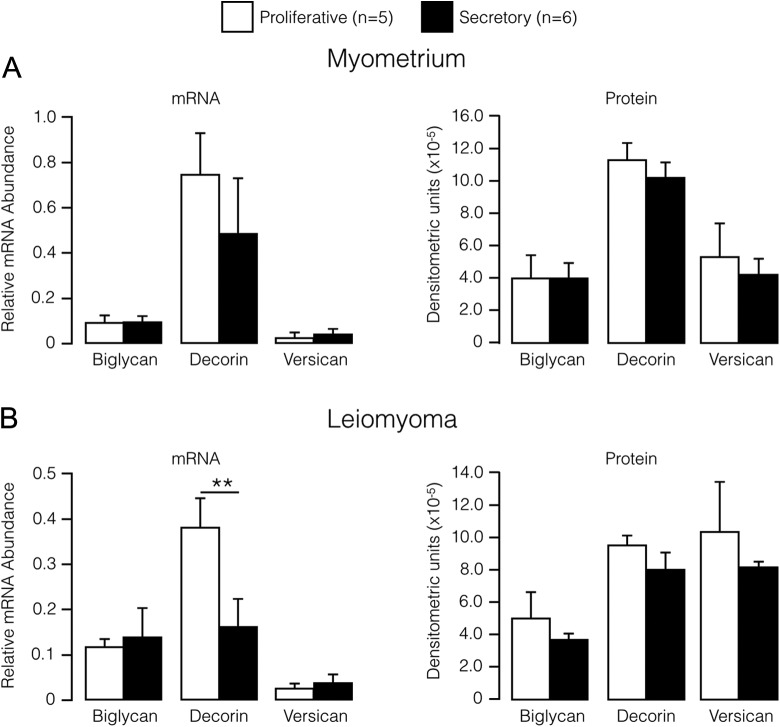
For myometrium tissue, no difference was detected at either the mRNA level or the protein level for any of the proteoglycans at the secretory phase compared to the proliferative phase (Figure 2). In contrast, a 2.4-fold decrease (P < .01) in decorin mRNA was detected in leiomyoma collected during the secretory phase compared with leiomyoma obtained during the proliferative phase (Figure 2). However, there was no corresponding phase-associated difference in leiomyoma decorin protein nor was a phase-associated difference observed for the other proteoglycans in leiomyoma tissue at either the mRNA or protein level (Figure 2).
Figure 2.
Relative abundance (mean ± SE) of proteoglycan mRNAs (left) and proteins (right) in myometrium (A) and leiomyoma (B) stratified by menstrual cycle phase. **P < .01; Wilcoxon signed rank test. SE indicates standard error; mRNA, messenger RNA.
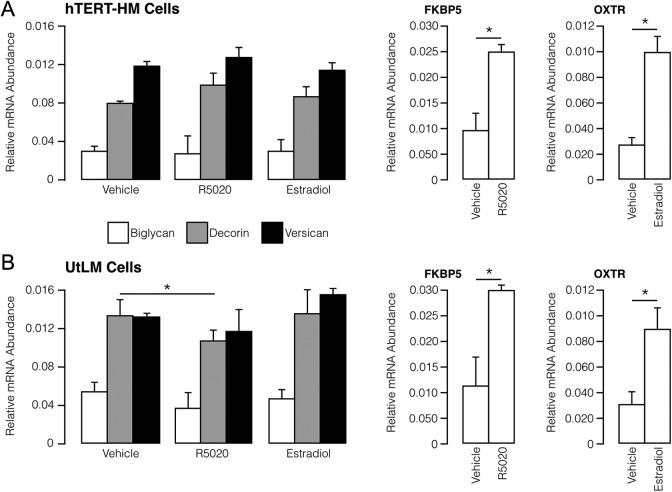
The effect of steroid hormones, estradiol and progesterone, on decorin, versican, and biglycan gene expression (measured by mRNA levels) was assessed in myometrial (hTERT-HM) and leiomyoma (UtLM) cell lines (Figure 3). Progesterone (in the form of the agonist R5020) and estradiol did not affect the relative abundance of proteoglycan mRNAs in the hTERT-HM cells. In the UtLM cells, R5020 decreased decorin mRNA by 1.3-fold (P = .01). No other hormone-related effects were observed on proteoglycan mRNA abundance in UtLM cells. As expected, R5020 increased expression of FK506 Binding Protein 5 (FK506) binding protein 5, a known progesterone responsive gene, and estradiol increased expression of the oxytocin receptor (OXTR), which is known to be increased by estrogen in myometrial cells.
Figure 3.
Relative abundance (mean ± SE) of mRNA encoding proteoglycan in myometrial hTERT-HM (A) and leiomyoma UtLM (B) cells under basal conditions (vehicle) and after exposure to estradiol (100 nmol/L) and R5020 (10 nmol/L). Open histograms are positive controls for responsiveness of progestin (FK506 Binding Protein 5 [FKBP5]; a progesterone-responsive gene) and estrogen (oxytocin receptor [OXTR]; an estrogen-responsive gene). *P < .05 using Mann-Whitney test.SE indicates standard error; mRNA, messenger RNA.
Discussion
The goal of this study was to determine whether the relative abundance of the proteoglycans biglycan, decorin, and versican differs in leiomyoma and myometrium, and whether expression of the genes encoding biglycan, decorin, and versican is modulated by the ovarian steroid hormones, estradiol and progesterone, in leiomyoma and myometrial cells. Our data show that in leiomyoma the relative level of decorin is decreased, and the relative level of veriscan is increased compared with matched normal myometrium. There was no difference in the relative level of biglycan in the 2 tissues. Decreased abundance of mRNA encoding decorin in leiomyoma obtained during the secretory phase compared with tissue obtained from uteri in the proliferative phase suggests that decorin expression in leiomyoma is decreased by progesterone, which is highest during the secretory phase. That conclusion is supported by data showing that R5020 (a progesterone agonist) significantly decreased decorin mRNA levels in leiomyoma cells (UtLM cells) but did not effect decorin expression in normal myometrial cells (hTERT-HM cells). Taken together, our findings demonstrate a clear difference in the proteoglycan spectrum in leiomyoma compared with matched myometrium and that this is, at least in part, due to progesterone suppression of decorin expression.
Current literature regarding the level and role of decorin in human leiomyoma pathophysiology is conflicting. Catherino et al23 reported no difference in decorin mRNA levels in leiomyoma compared to normal myometrium in 11 patients. However, patients in that study were in various stages of the menstrual cycle as determined by recalled last menstrual period, and some patients were receiving hormonal therapy. The present study determined menstrual cycle dating by histologic diagnosis of the endometrium, which is a more reliable way to categorize menstrual cycle stage. As we show here, menstrual cycle phase may alter decorin expression. It is also notable that Catherino et al23 used immunohistochemical staining of tissue sections to measure decorin levels and did not quantify the data by image analysis. This likely explains the discrepancy between their results and our data, since measuring the relative levels of proteoglycans by densitometry of the band intensities on immunoblots of isolated proteoglycans is a more quantitative way to compare the amount of proteoglycans in leiomyoma and normal myometrium. Consistent with our findings, Roth et al,24 using microarray analyses, reported lower decorin mRNA levels (by ∼30%) in leiomyoma compared with normal myometrium (although these authors referred to decorin by an older name: PG40).
Our finding of increased versican in leiomyomas is consistent with other reports.22 However, we did not observe a coordinate increase of versican mRNA in leiomyomas, which contrasts with at least one study.23 Differences in the efficiency of versican mRNA translation in leiomyoma and normal myometrium may be the reason our data reflect a higher versican proteoglycan with no difference in mRNA levels. Discrepancy between versican mRNA versus protein in mouse myometrium has been reported, suggesting that versican steady state is influenced by posttranscriptional modifications.25
In leiomyoma, a significant decrease in decorin mRNA abundance was detected in tissue obtained during the secretory phase compared with the proliferative phase, but this was not associated with a decrease in decorin protein. The discrepancy between mRNA and protein may be related to the dynamics of ECM turnover, as ECM molecules in some nonuterine tissues have been reported to turnover slowly.26 Thus, the decrease in decorin mRNA may not be manifested in a cycle-associated change in ECM protein, whereas the net effect of decreased decorin expression over multiple cycles leads to an overall decrease in leiomyoma decorin compared with its level in normal myometrium. Taken together with our finding that progesterone inhibits decorin expression in UtLM but not hTERT-HM cells, the data suggest that decorin expression in leiomyoma is inhibited by progesterone. This is important in light of studies showing that progesterone increases leiomyoma proliferative activity, and that antiprogesterone agents such as asoprisnil and ulipristal acetate reduce leiomyoma burden and disease progression by inhibiting cell proliferation and angiogenesis, promoting apoptosis, and decreasing ECM deposition.27–40
Limitations to our study exist. Our sample size is small, particularly for the mRNA and protein data stratified to menstrual cycle phase. Also, we did not have a control of normal myometrium from a uterus that lacks leiomyomas. To address this issue, we instead compared normal and affected tissue from the same patient, but the presence of leiomyoma and/or pathologic symptoms from leiomyoma may affect the adjacent myometrium. Thus, we acknowledge that the presence of leiomyoma disease could affect the mRNA or protein data from adjacent presumably “normal” myometrium.
In summary, we propose that progesterone repression of leiomyoma decorin is somehow associated with adverse disease pathophysiology. Progesterone-mediated inhibition of decorin expression by leiomyoma cells may be the mechanistic link between progesterone and leiomyoma disease progression via effects on the functional relationship between ECM and growth factor activity. Morbidities caused by uterine leiomyoma disease are directly related to tumor size, and tumor size is primarily associated with the amount and composition of tumor ECM,6,7 which affects the activity of growth factors in the tumor microenvironment. TGF-β expression and abundance are increased in leiomyomas, and this growth factor stimulates cell proliferation and ECM production and deposition.3,7,13,14,23 Decorin inhibits TGF-β activity and as such a reduction in decorin levels in the leiomyoma ECM could establish a microenvironment that enhances TGF-β-induced cell proliferation and aberrant ECM deposition.9 Thus, the reduced decorin, likely promoted by progesterone, may establish a local microenvironment that promotes a positive TGF-β-induced feedback loop leading to increased cell proliferation and the deposition of an ECM that further promotes TGF-β signaling. In this context, decorin may be a key proteoglycan in leiomyoma pathogenesis and targeted therapies to modulate its level in leiomyoma ECM, either directly or by affecting progesterone signaling, may be an effective strategy to treat leiomyoma disease.
Acknowledgments
We thank Lora Jennifer Mesiano for assistance in preparing this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants from the National Institutes of Health to (1R01CA163562-01 to AIC), the Lerner Fund (to JHL), and the L. David and E. Virginia Baldwin Fund (to AIC).
References
- 1. Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006;195(4):955–964. [DOI] [PubMed] [Google Scholar]
- 2. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73(5):1006–1011. [DOI] [PubMed] [Google Scholar]
- 4. Arslan AA, Gold LI, Mittal K, et al. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20(4):852–863. [DOI] [PubMed] [Google Scholar]
- 5. Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28(3):169–179. [DOI] [PubMed] [Google Scholar]
- 7. Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78(1):1–12. [DOI] [PubMed] [Google Scholar]
- 8. Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136(3):729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346(6281):281–284. [DOI] [PubMed] [Google Scholar]
- 10. Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54(11):1205–1216. [DOI] [PubMed] [Google Scholar]
- 11. Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;280(6): L1327–1334. [DOI] [PubMed] [Google Scholar]
- 12. Jarvelainen H, Puolakkainen P, Pakkanen S, et al. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14(4):443–452. [DOI] [PubMed] [Google Scholar]
- 13. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. [DOI] [PubMed] [Google Scholar]
- 14. Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86(2):913–920. [DOI] [PubMed] [Google Scholar]
- 15. Norian JM, Malik M, Parker CY, et al. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16(12):1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salgado RM, Favaro RR, Martin SS, Zorn TM. The estrous cycle modulates small leucine-rich proteoglycans expression in mouse uterine tissues. Anat Rec (Hoboken). 2009;292(1):138–153. [DOI] [PubMed] [Google Scholar]
- 17. Cook JD, Walker CL. Treatment strategies for uterine leiomyoma: the role of hormonal modulation. Semin Reprod Med. 2004;22(2):105–111. [DOI] [PubMed] [Google Scholar]
- 18. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. [DOI] [PubMed] [Google Scholar]
- 19. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263. [DOI] [PubMed] [Google Scholar]
- 20. Carney SA, Tahara H, Swartz CD, et al. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82(6):719–728. [DOI] [PubMed] [Google Scholar]
- 21. Condon J, Yin S, Mayhew B, et al. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67(2):506–514. [DOI] [PubMed] [Google Scholar]
- 22. Carrino DA, Mesiano S, Barker NM, Hurd WW, Caplan AI. Proteoglycans of uterine fibroids and keloid scars: similarity in their proteoglycan composition. Biochem J. 2012;443(2):361–368. [DOI] [PubMed] [Google Scholar]
- 23. Catherino WH, Leppert PC, Stenmark MH, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40(3):204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roth TM, Klett C, Cowan BD. Expression profile of several genes in human myometrium and uterine leiomyoma. Fertil Steril. 2007;87(3):635–641. [DOI] [PubMed] [Google Scholar]
- 25. Salgado RM, Covarrubias AC, Favaro RR, Serrano-Nascimento C, Nunes MT, Zorn TM. Estradiol induces transcriptional and posttranscriptional modifications in versican expression in the mouse uterus. J Mol Histol. 2013;44(2):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–39031. [DOI] [PubMed] [Google Scholar]
- 27. Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79(3):900–906. [DOI] [PubMed] [Google Scholar]
- 28. DeManno D, Elger W, Garg R, et al. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 2003;68(10-13):1019–1032. [DOI] [PubMed] [Google Scholar]
- 29. Chen W, Ohara N, Wang J, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2006;91(4):1296–1304. [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Ohara N, Wang Z, et al. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21(7):1869–1877. [DOI] [PubMed] [Google Scholar]
- 31. Maruo T. Progesterone and progesterone receptor modulator in uterine leiomyoma growth. Gynecol Endocrinol. 2007;23(4):186–187. [DOI] [PubMed] [Google Scholar]
- 32. Sasaki H, Ohara N, Xu Q, et al. A novel selective progesterone receptor modulator asoprisnil activates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated signaling pathway in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2007;92(2):616–623. [DOI] [PubMed] [Google Scholar]
- 33. Levens ED, Potlog-Nahari C, Armstrong AY, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morikawa A, Ohara N, Xu Q, et al. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Hum Reprod. 2008;23(4):944–951. [DOI] [PubMed] [Google Scholar]
- 35. Yoshida S, Ohara N, Xu Q, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28(3):260–273. [DOI] [PubMed] [Google Scholar]
- 36. Nieman LK, Blocker W, Nansel T, et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril. 2011;95(2):767–772.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. [DOI] [PubMed] [Google Scholar]
- 38. Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Adv Ther. 2012;29(8):655–663. [DOI] [PubMed] [Google Scholar]
- 39. Biglia N, Carinelli S, Maiorana A, D’Alonzo M, Lo Monte G, Marci R. Ulipristal acetate: a novel pharmacological approach for the treatment of uterine fibroids. Drug Des Devel Ther. 2014;8:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Donnez J, Vazquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101(6):1565–1573.e1-8. [DOI] [PubMed] [Google Scholar]