Abstract
Estrogen and progesterone regulate proliferation and differentiation of epithelial cells in the female genital tract. We investigated the effects of these hormones on reconstructed human organotypic vaginal epithelial tissue models (EpiVaginal). We ascertained that epithelial cells in the tissue models express estrogen and progesterone receptors. Treatment with estradiol-17β (E2) significantly increased epithelium thickness and transepithelial electrical resistance (TEER), whereas progesterone (P) treatment resulted in thinning of the epithelium and decreased TEER when compared with untreated controls. Exposure to E2 increased (1) the expression of the progesterone receptor B (PR-B), (2) accumulation of glycogen in suprabasal cells, (3) epithelial differentiation, and (4) the expression of a number of gene pathways associated with innate immunity, epithelial differentiation, wound healing, and antiviral responses. These findings indicate that EpiVaginal tissues are hormone responsive and can be used to study the role of female reproductive hormones in innate immune responses, microbial infection, and drug delivery in the vaginal mucosa.
Keywords: vagina, epithelium, hormones, estrogen, immunity, in vitro model
Introduction
The lower female genital tract provides a physical and immunological barrier to the entry of sexually transmitted pathogens.1,2 The vaginal and ectocervical mucosa, which are contiguous and histologically indistinguishable from each other, are both nonkeratinizing, stratified squamous epithelia. The epithelial cells are interconnected by numerous adherens junctions that provide an effective structural barrier to prevent entry of pathogens across the vaginal/ectocervical mucosa.3 The epithelium also provides innate immune protection against bacterial or viral infection and transmits molecular signals to underlying immune cells following pathogenic challenge.4
The physical and immunological properties of the vaginal microenvironment are regulated by reproductive hormones that are important in the development and maintenance of tissue structure and function during the menstrual cycle, contraceptive use, and pregnancy.2,5–9 For instance, estrogen (estradiol-17β [E2]) stimulates proliferation of epithelial cells and increases epithelial thickness during the proliferative stage of the menstrual cycle whereas progesterone (P) dominates the secretory phase of the menstrual cycle and promotes maturation of epithelial cells.10–12 Administration of high doses of exogenous P to female rhesus monkeys suppressed ovarian production of E2 and resulted in: (1) increased acquisition of simian immunodeficiency virus (SIV) across the vaginal mucosa, (2) genetic alteration of the virus, (3) increased peripheral viral load, and (4) enhanced progression to disease.13 Conversely, treatment of macaques with topical or systemic E2 protected the animals against vaginal SIV transmission.14 Hence, understanding the effects of reproductive hormones on tissue structure and innate immune responses in the vaginal microenvironment could enhance our knowledge of the pathophysiology of sexually transmitted infections (STIs) and lead to their prevention.15–17
Clinical studies which examined the association between contraceptive use and risk of HIV-1 acquisition in women have provided conflicting results. Recent studies from Kenya and Thailand reported an elevated risk for HIV-1 infection among women using the injectable progestin contraceptive depot medroxyprogesterone acetate.18–24 Postmenopausal women who have low estrogen levels and a relatively thinned vaginal epithelium were found to be 4- to 8-fold more susceptible to HIV-1 transmission.25 Hormones may affect HIV infection of the vaginal epithelium through alterations in (1) intercellular junctions and barrier function, (2) innate and adaptive immune responses, (3) activation and migration of immune cells, (4) the pH of the microenvironment, (5) inflammatory responses of epithelial, immune, or stromal cells, and (6) expression levels of cellular receptors for pathogenic organisms.25,26 Alterations in vaginal epithelium physiology, structure, and immunological responsiveness by P have been implicated in susceptibility to genital tract infections such as HIV-1.27–29
To date, most studies on the effects of reproductive hormones on vaginal tissue structure and susceptibility to STIs have used animal models, explant tissues, and cells in monolayer culture. Caveats in the use of animal models to mimic human vaginal tissue responses include anatomical differences of the reproductive tract, species variations in hormonal responsiveness, and limited susceptibility to human pathogens.30–32 For instance, the vaginal mucosa of rodents and macaques contain little glycogen and have a neutral pH,33,34 whereas the human vaginal epithelium produces high levels of glycogen which support robust lactobacillus colonization; importantly, the lactobacilli produce lactic acid that creates an acidic vaginal pH.35 Vaginal explant tissues are unsuitable for studies of hormonal effects on the vaginal epithelium because of their short life span, rapid deterioration in culture, and high degree of intersubject variation.36 Vaginal epithelial cell monolayers are also unsuitable because they lack normal tissue stratification and differentiation that characterize the in vivo microenvironment.37
Here we demonstrate the use of human vaginal tissue models, reconstructed from primary human vaginal epithelial cells and fibroblasts, to study the effect of reproductive hormones on the vaginal mucosa. The tissue models mimic their in vivo counterparts in that they express hormone receptors and respond to hormonal stimulation. The hormone-treated in vitro 3-dimensional (3D) vaginal tissue models are anticipated to be valuable tools to address basic biological questions regarding the role of female reproductive hormones in tissue differentiation/stratification, barrier function, microbial invasion, and innate immunity in the vaginal mucosal microenvironment.
Materials and Methods
Source of Cells for EpiVaginal Tissue Models
Human vaginal and ectocervical tissues were obtained from healthy women of reproductive age (age 37-44) undergoing hysterectomies for benign indications at the Boston Medical Center (Boston, Massachusetts); the tissue procurement protocol was approved by the Institutional Review Board of Boston University School of Medicine. Pieces of tissue were placed in Dulbecco’s Modified Eagle’s Medium (DMEM; Cambrex, Maryland) containing penicillin streptomycin and gentamycin (10 mg/mL, Cambrex) and processed within 24 hours of surgical removal. The underlying connective tissue was dissected away from epithelial layers and cultured in DMEM plus 10% fetal bovine serum (FBS) at 37°C and 5% CO2 for isolation of fibroblasts. Epithelial and fibroblast cell isolation and cryopreservation were performed as described previously.38
EpiVaginal tissue Reconstruction
Partial thickness vaginal/ectocervical tissue
Cryopreserved epithelial cells from a single donor were thawed and plated into 150-mm culture dishes. When the cell density reached 60% to 70% confluence, the cells were trypsinized, counted, and seeded onto polycarbonate tissue culture treated microporous membrane cell culture inserts (Millipore Corporation, Bedford, Massachusetts). Inserts were cultured at 37°C, 5% CO2, 98% rH for 4 days submerged and 7 days at the air–liquid interface using a serum-free differentiation medium (VEC-100-MM, MatTek Corporation, Ashland, Massachusetts) in the presence or absence of hormones to produce the epithelium-only EpiVaginal tissue model hereafter called partial thickness vaginal/ectocervical tissue (VEC-PT).
Full-thickness vaginal/ectocervical tissue
To mimic the in vivo situation, a tissue model that contains epithelial cells and fibroblasts, referred to as a full-thickness EpiVaginal tissue (VEC-FT) model, was also developed. Briefly, normal fibroblasts were obtained from the human ectocervical tissues by standard collagenase treatment and expanded in DMEM medium containing 10% FBS (DMEM-10). For the preparation of lamina propria equivalents, a collagen solution was prepared by mixing 1.6 mL of 0.1% acetic acid and chilled 10× Hank’s buffer supplemented with phenol red to obtain a final collagen concentration of 3.2 mg/mL. The pH of the collagen solution was adjusted to 7.2 by adding drops of 1 N NaOH. Fibroblasts (in DMEM-10) were added to the collagen solution to obtain a final concentration of 1 × 106 cells/mL. Finally, the collagen–fibroblast mixture was poured into tissue culture inserts and allowed to gel by incubating the inserts at 37°C for 1 hour. The gel was equilibrated with 2.0 mL of DMEM-10 and cultured for 24 hours. Thereafter, autologous vaginal-ectocervical epithelial cells were seeded onto the matrix and the epithelial cell/fibroblast matrix was cultured in a manner similar to that used for differentiation of the VEC-PT tissues described previously.38 The epithelial cell/fibroblast matrices were cultured for 4 days under submerged conditions using proprietary medium formulated at MatTek and then for an additional 7 days at air–liquid interface in medium with or without specific reproductive hormones and are available for purchase. To avoid potential artifacts caused by phenol red and steroid hormones in the culture medium, serum-free, hydrocortisone-free, phenol red-free medium was used in all hormone experiments.
Tissue Model Quality Control: Tissue Standardization and Structural Features
The VEC-FT and VEC-PT tissues used in this study were qualified using a standardized quality control assay. Tissues were exposed to a positive control (1% Triton X-100) and negative control (ultrapure H2O) and the resulting tissue viability (as determined using the MTT assay) versus exposure time data were determined. These data were used to calculate the exposure time required to reduce tissue viability to 50% (ET-50).38,39 Tissues were used in the present studies only if: (a) the ET-50 for the positive control was >0.7 hour, (b) tissues exposed to the negative control gave an optical density reading >1.0, and (c) the difference in MTT viability between duplicate tissues was <15%. The resemblance of the in vitro reconstructed tissue to the native vaginal-ectocervical tissue has been confirmed histologically using hematoxylin and eosin (H&E) staining as well as ultrastructurally by transmission electron microscopy.38
Hormones
17β-Estradiol and progesterone were purchased from Sigma-Aldrich (St Louis, Missouri). Stock solutions were prepared by solubilizing the hormones in absolute ethanol. Aliquots of the diluted hormones were stored at −70oC. For most experiments, E2 concentrations of 10 and 100 nmol/L and P concentrations of 100 and 550 nmol/L were used to represent peak menstrual cycle and pregnancy levels, respectively.40,41 The high estradiol dose also approximates that used clinically for postmenopausal women.42
Histology
The reconstructed VEC-PT and VEC-FT tissues were fixed with 10% formalin overnight. The tissues were embedded in paraffin and sections were cut (5-7 μm thick), stained with H&E, and photographed using a Nikon Diaphot microscope.
Transepithelial Electrical Resistance
Changes in barrier function were quantified using transepithelial electrical resistance (TEER) measurements. TEER monitors the presence of functional intercellular junctions, which are responsible for barrier function and which regulate paracellular permeation of water and solutes. The TEER measurements were made using an EVOM volt-ohmmeter equipped with an Endohm electrode chamber (World Precision Instruments, Sarasota, Florida). The TEER values (reported in ohm × cm2) were calculated by multiplying raw resistance measurements by the area of the tissue (0.6 cm2).
Immunohistochemistry
To detect expression of estrogen receptor (ER) and progesterone receptor (PR) in the tissues, 5-μm-thick paraffin sections were mounted on glass slides, deparaffinized, and rehydrated using a graded series of ethanols from 100% to 95%, to 70% EtOH made up with molecular grade deionized distilled water. An antigen retrieval protocol was used to unmask reactive epitopes. Sections were immersed for 30 seconds in a citrate buffer (pH 6.0) in a pressure cooker heated to 125 C. The slides were allowed to cool to room temperature after which they were washed extensively with distilled water and placed in Tris buffered saline containing 0.1% Tween (TBST) for 5 minutes. The sections were incubated for 30 minutes with a serum free protein blocking solution (0.25% casein in PBS, containing stabilizing protein and 0.015 mol/L sodium azide; Dako, Carpintaria, California) which was drained from the sections prior to the application of the primary antibodies. Tissue sections were incubated with a specific antibody directed against the full length α form of ER (ER-α) or against the N-terminal PR (1:20 dilution; Zymed Laboratories, San Francisco, California, now Invitrogen) for 60 minutes at room temperature. Primary antibodies were detected using an alkaline phosphatase detection system (EnVision, Dako: an immunohistochemical visualization system employing goat antirabbit and goat antimouse immunoglobulins conjugated to an alkaline phosphatase labeled polymer) and visualized by incubating the sections with a substrate for alkaline phosphatase (Fast Red, Dako, dissolved in napthol phosphate in Tris-HCL buffer) which stains positive elements in cells red. Visualization of positive cells in the sections was improved by counterstaining in aqueous hematoxylin and mounting in a glycerin-based mounting medium.
Periodic Acid–Schiff’s Staining
The Periodic Acid–Schiff’s (PAS; Sigma, St. Louis, Missouri) stain was used to localize glycogen within the VEC tissues. Briefly, tissues were fixed with 10% formalin, paraffin embedded, cut into 4-5 μm sections, and mounted on microscopic slides. The sections were deparaffinized, hydrated, oxidized in 0.5% periodic acid solution, and processed as described previously.38
RNA Isolation and Quantification
RNA from the tissues was isolated using an RNAqueous kit (Ambion, Inc, Austin, Texas) following the manufacturer’s recommendations. The quantity of RNA from each sample was determined spectrophotometrically and the integrity of isolated RNA was checked by agarose gel electrophoresis. This procedure yielded between 10 and 15 μg of high-quality total RNA per VEC-FT or VEC-PT tissue; RNA was stored at −70°C to 80°C.
Reverse Transcription Polymerase Chain Reaction for ER-α, ER-β and PR
The primer sequences for ER-α (exons 1-3 and 3-6), ER-β, and PR have been described previously.43 Total RNA of 130 ng were reverse transcribed using the SuperScript One-step Reverse Transcription Polymerase Chain Reaction (RT-PCR) system with platinum Taq DNA polymerase (Invitrogen, Carlsbad, California) following the manufacturer’s recommendations. The thermal cycler was programmed so that complementary DNA (cDNA) synthesis was followed immediately by PCR amplification. The cDNA synthesis was performed by incubating samples with RT-PCR master mix and reverse transcriptase enzyme at 48°C for 30 minutes. Next, the reaction mixtures were heated to 95°C for 6 minutes for the initial PCR activation. This protocol was chosen to minimize nonspecific product amplification. The PCR program utilized 35 cycles of 1 minute duration at 94°C (denaturation), 1 minute (annealing temperature) at 55°C for ER-α, 58°C for ER-β, and 55°C for PR, and 1 minute at 72°C (extension), followed by a final extension at 72°C for 10 minutes, and a hold temperature of 4°C. GAPDH was used as the amplification control. After PCR, the products were resolved on a 2% agarose gel and stained with ethidium bromide. The images were captured using an Ultraviolet transilluminator.
Microarray
Affimetrix Genechip Human Gene 1.0 ST arrays were used to analyze RNA isolated from hormone treated and untreated VEC-PT and VEC-FT tissues. For each treatment group, 4 separate tissues were pooled and homogenized in Trizol. The RNA samples were sent to the Boston University Microarray Core for messenger RNA (mRNA) extraction and microarray analysis. Data analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics resource.44 Changes of >2-fold in mRNA expression were considered significant.
Quantitative PCR
The cDNA was generated using 1 μg RNA per 20 μL reaction with the High-Capacity cDNA Reverse Transcription Kit (Life Technologies) according to the manufacturer’s instructions. TaqMan Gene Expression Assays (Life Technologies inventoried assays catalogue# 4331182; Life Technologies, Grand Island, New York) were used in quantitative PCR (qPCR): KRT20 (Hs00300643_m1), TFF1 (Hs00907239_m1), MUC1 (Hs00159357_m1), GYS2 (Hs00608677_m1), MUCL1(Hs00536495_m1), and run in an Applied Biosystems 7300 Real Time PCR System according to the manufacturer’s instructions. Quadruplicates of 1 μL cDNA from each sample were normalized to 18S expression (Life Technologies) and relative quantification (versus untreated control tissue) was determined by the comparative CT method.
Results
Expression of ER and PR in the Vaginal Tissue Models
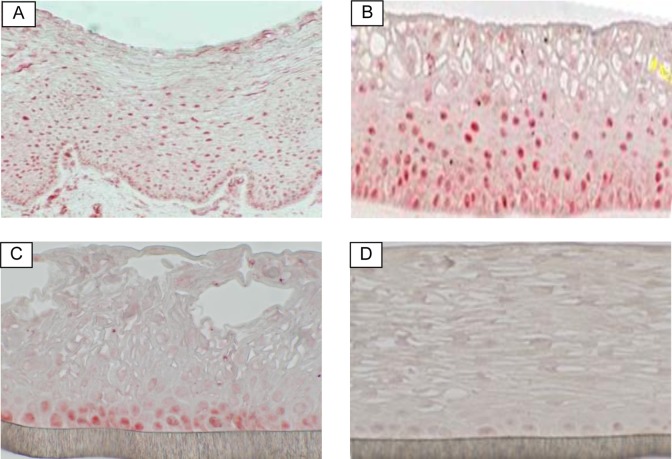
Since the actions of E2 and P are mediated through specific receptors that serve as ligand-inducible transcriptional activators, we examined the expression of ERs and PRs in the VEC-FT and VEC-PT tissue models. Tissues were analyzed by immunohistochemistry (IHC) using specific ER or PR antibodies. Immunohistochemistry demonstrated that ER-α was expressed by epithelial cells in ectocervical explant tissues (positive control; Figure 1A). Similarly, ER-α was expressed by epithelial cells in both the VEC-PT and VEC-FT tissue models. Estrogen receptor was expressed in both the basal and suprabasal layers in the VEC-FT tissue model (Figure 1B) in a similar pattern to that seen in the ex vivo vaginal tissue (Figure 1A), however, ER was confined to the basal cell layer in the VEC-PT model (Figure 1C). Expression of PR was rarely observed in the epithelial tissue layer and only a few fibroblasts were positive for PR staining (data not shown).
Figure 1.
Immunohistological staining of estradiol receptor (red) in: (A) human vaginal-ectocervical tissue explant (positive control), (B) full-thickness vaginal-ectocervical (VEC-FT) tissue model, (C) partial thickness vaginal-ectocervical tissue model (VEC-PT), and (D) VEC-PT tissue negative control (without primary antibody). (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
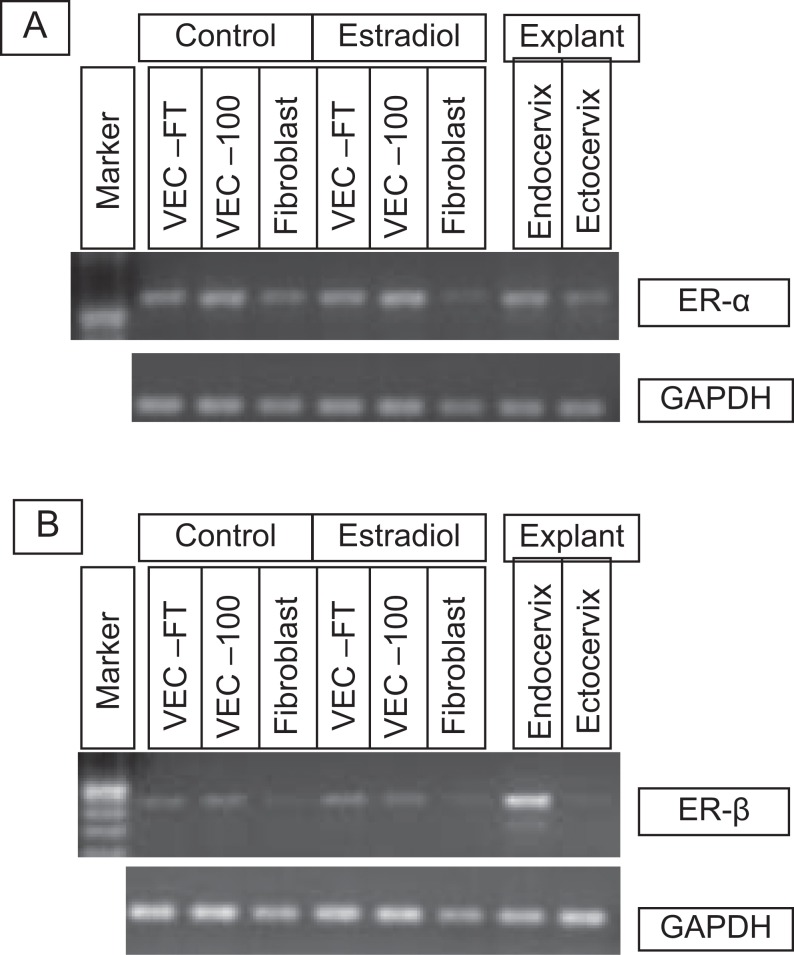
To determine the contribution of fibroblasts to expression of ER-α and ER-β in the vaginal mucosa, tissue reconstructs were made using: (1) fibroblasts only, (2) vaginal-ectocervical epithelial cells (partial thickness VEC-PT), and (3) vaginal-ectocervical epithelial cells and fibroblasts (full-thickness VEC-FT). RNA isolated from explant ectocervical and endocervical tissues were used as controls. RNA was isolated from these tissues and gene expression levels were determined using RT-PCR. The results showed that: (1) ER-α is expressed by fibroblasts and epithelial cells in the VEC-PT and VEC-FT tissues (Figure 2A) and (2) ER-β is not expressed by fibroblasts but is expressed by epithelial cells in the VEC-PT and VEC-FT tissues (Figure 2B). Treatment of the tissues with E2 did not significantly alter the expression of ER-α or ER-β.
Figure 2.
Reverse transcription polymerase chain reaction (RT-PCR) results showing expression of estrogen receptors, ER-α and ER-β, in the VEC tissue models following E2 treatment. VEC-FT or VEC-PT (VEC-100) tissues were reconstructed for 11 days and then cultured in the presence of estradiol (10 nmol/L) or medium alone for an additional 7 days. Primary fibroblasts were also cultured for 7 days with and without E2 treatment. RNA was isolated and RT-PCR was performed using specific primer pairs for ER-α and ER-β. Explant endocervical and ectocervical tissues were included as controls. VEC-FT indicates full-thickness vaginal-ectocervical; VEC-PT, partial thickness vaginal-ectocervical.
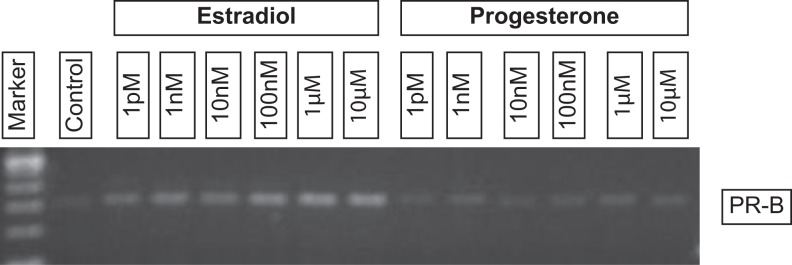
To determine whether estrogen up-regulates expression of PR as has been shown in endometrial cells and uterine tissue,45,46 RNA was isolated from hormone-treated and untreated VEC-FT tissues and analyzed by RT-PCR. The results showed a concentration dependent up-regulation of PR by estradiol at concentrations >1 nmol/L (Figure 3).
Figure 3.
Reverse transcription polymerase chain reaction (RT-PCR) results showing expression of progesterone receptor (PR-B) in hormone-treated tissues. Mature VEC-FT tissues (day 11) were exposed to estradiol or progesterone for 7 days. On day 18, RNA was isolated and RT-PCR was performed using specific primers for PR-B. As shown, PR-B expression is upregulated by estradiol but not by progesterone. VEC-FT indicates full-thickness vaginal-ectocervical.
Microarray Analysis
To identify genes and pathways affected by hormonal treatment, microarray analysis was performed on hormone-treated VEC-PT and VEC-FT tissues using the Affimetrix Human Gene array 1.0 chip. Microarray experiments confirmed the expression of ER and PR in the vaginal tissue models (Table 1); the ER-α, the membrane bound ER, and the PR membrane complexes 1 and 2 were highly expressed in the tissues, whereas the ER-β, the classical PR, and the androgen receptor were only weakly expressed (Table 1). An overview of the number of genes altered by hormonal treatments in VEC-FT and VEC-PT models is presented in Table 2. The VEC-PT model was more affected by hormone treatment than the full thickness model (eg, 345 vs 113 genes were upregulated by E2 treatment). Several immune genes were among those affected by E2 treatment in VEC-PT tissues (Table 3).
Table 1.
Expression of Hormone Receptors in the Partial Thickness (VEC-PT) and Full Thickness (VEC-FT) Vaginal-Ectocervical Tissue Models.a
| Entrez Gene ID | Hormone Receptor | Partial Thickness | Full Thickness |
|---|---|---|---|
| ESR1 | Estrogen receptor 1 | 9.6 | 8.0 |
| ESR2 | Estrogen receptor 2 (ER-beta | 4.3 | 4.3 |
| GPER | Membrane bound ER receptor | 7.0 | 6.6 |
| PGR | Progesterone receptor | 4.1 | 3.9 |
| PGRMC1 | Progesterone Receptor Membrane Complex 1 | 11.1 | 11.1 |
| PGRMC2 | Progesterone Receptor Membrane Complex 2 | 9.2 | 9.4 |
| AR | Androgen Receptor | 5.3 | 5.6 |
aMicroarray data are presented as log transformed relative intensity units (log2). 7.0 is the median, and 5.3 is the first quartile (1/4) for the entire gene database. Values under 5.3 are considered not significant; genes between 5.3 and 7.0 are expressed at low level, and genes over 7.0 are expressed at high level.
Table 2.
Description of Global Gene Expression in Response to 100 nmol/L Estradiol or 10 nmol/L Estradiol + 10 nmol/L Progesterone (Versus Untreated Control Tissues Grown and Harvested at the Same Time).
| VEC Thickness | Genes upregulated (fold increase >2 vs control) | Genes downregulated (fold decrease >2 vs control) | |
|---|---|---|---|
| Partial (PT) | E2 | 345 | 141 |
| E2 + P | 210 | 63 | |
| Full (FT) | E2 | 113 | 14 |
| E2 + P | 14 | 0 |
Abbreviations: VEC, vaginal/ectocervical tissue.
Table 3.
Change in Expression Levels of Selected Immune-Related Genes in VEC-PT Grown in the Presence of 100 nmol/L Estradiol.
| Symbol | Immune Genes Regulated by E2 in VEC-PT | Fold Change |
|---|---|---|
| Upregulated genes | ||
| IL1F6 | Interleukin 1 family, member 6 (epsilon) | 12.1 |
| TFF1 | Trefoil Factor 1 | 10.2 |
| IL13RA2 | Interleukin 13 receptor, alpha 2 | 8.98 |
| IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 | 4.58 |
| IFIT3 | Interferon-induced protein with tetratricopeptide repeats 3 | 3.41 |
| IL1RL1 | Interleukin 1 receptor-like 1 | 3.41 |
| IL2RG | Interleukin 2 receptor, gamma | 3.35 |
| CISH | Cytokine inducible SH2-containing protein | 3.22 |
| CD22 | CD22 molecule | 2.97 |
| IL23A | Interleukin 23, alpha subunit p19 | 2.68 |
| IL1A | Interleukin 1, alpha | 2.63 |
| CD14 | CD14 molecule | 2.61 |
| IL1R2 | Interleukin 1 receptor, type II | 2.59 |
| GYS2 | Glycogen synthase 2 (liver) | 2.49 |
| IFI44 | Interferon-induced protein 44 | 2.48 |
| ESAM | Endothelial cell adhesion molecule | 2.39 |
| IL1F5 | Interleukin 1 family, member 5 (delta) | 2.37 |
| TNFSF11 | Tumor necrosis factor ligand superfamily, member 11 | 2.35 |
| CCL20 | Chemokine (C-C motif) ligand 20 | 2.33 |
| HSPB8 | Heat shock 22 kDa protein 8 | 2.29 |
| MUCL1 | Mucin-like 1 | 2.28 |
| Downregulated genes | ||
| GALC | Galactosylceramidase | −2 |
| APOBEC3G | Apolipoprotein B mRNA editing enzyme 3G | −2.08 |
| CXCR2 | Chemokine (C-X-C motif) receptor 2 | −2.13 |
| CCL28 | Chemokine (C-C motif) ligand 28 | −2.21 |
| CASP1 | Caspase 1, apoptosis-related cysteine peptidase | −2.35 |
| IL33 | Interleukin 33 | −2.53 |
| CD36 | CD36 molecule (thrombospondin receptor) | −2.88 |
| TLR3 | Toll-like receptor 3 | −5.62 |
| CASP14 | Caspase 14, apoptosis-related cysteine peptidase | −6.22 |
| KRT20 | Keratin 20 | −13.65 |
Abbreviations: VEC-PT, partial thickness vaginal/ectocervical tissue.
The DAVID44 analysis was performed to understand the many biological processes involving epithelial differentiation and estrogen responses. In response to treatment with 100 nm E2, many processes, molecular functions, and pathways in the VEC-PT tissue were affected including immune response (P = .00013), wound healing (P = .001), regulation of I-κB kinase/nuclear factor (NF-κB) cascade (P = .005), defense response (P = .01), regulation of cell division (P = .01), antiviral response (P = .02), antiapoptosis (P = .02), and immune response-activating cell surface receptor signaling pathway (P = .04). Molecular functions identified included IL1 R receptor binding (P = .003), cytokine activity (P = .01): TGFβ2, IL36RA, CCL20, IL23A, IL1α, SECTM1, TNFRSF11B, IL36A, TNFSF11, and growth factor activity (P = .03). Kegg pathways identified included 6 genes representing Fc-gamma receptor (FcγR)-mediated phagocytosis (P = .05). Specific ontology (or vocabulary describing gene products in a species independent manner) of genes downregulated in 100 nm E2 VEC-PT included positive regulation of cell adhesion (P = .01), negative regulation of cell proliferation (P = .03), cell projection organization (P = .04), protein kinase cascade (P = .04), and other nonoverlapping cytokine activity (P = .04): FAM3B, TNFSF15, IL33, BMP3, BMP7, and CCL28. Kegg pathways identified decreased leukocyte transendothelial migration (P = .03) and O-glycan biosynthesis (P = .04). Therefore, estrogen appears to affect many pathways in these models relevant to innate immunity and cell migration.
We confirmed the regulated expression of the following selected genes by quantitative polymerase chain reaction: (1) genes upregulated by hormones in the microarray experiment—Glycogen Synthase 2, Mucin Like 1, Trefoil Factor 1; (2) genes downregulated in the microarray study—Keratin 20; and genes unaffected in the microarray study—Mucin1. Figure 4 shows relative quantification of these genes across treatment groups; 85% of the qPCR data agrees in direction and magnitude with the array data, as would be expected for array validation by qPCR (where one may expect 80% concordance between Array and qPCR data47).
Figure 4.
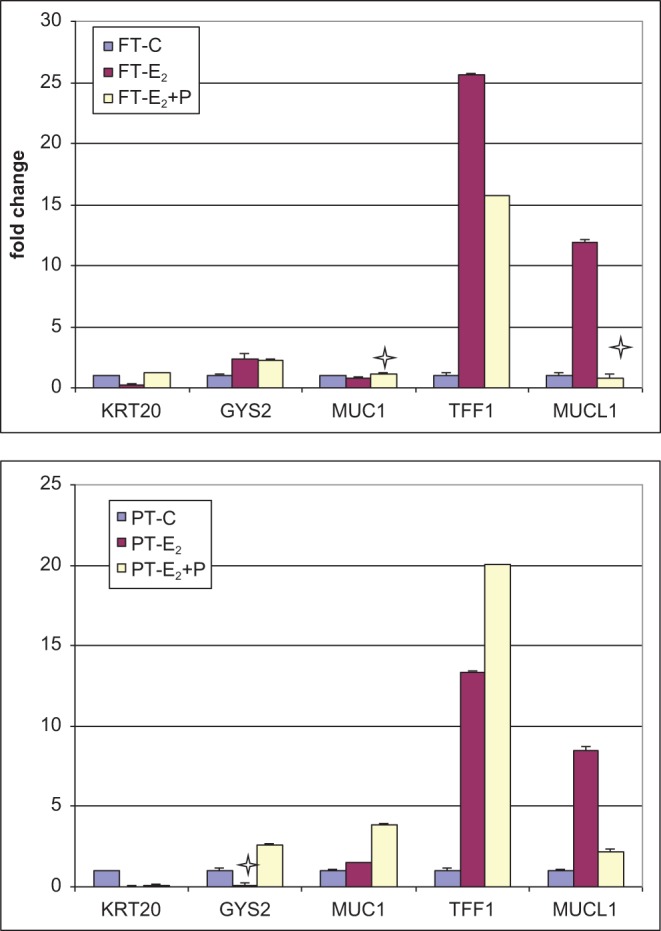
Quantitative PCR results showing fold change in gene expression relative to the untreated control and normalized to 18 S in full thickness (VEC-FT) and partial thickness (VEC-PT) tissues following exposure to estradiol-17β (E2) and progesterone (P). The x-axis denotes official gene symbols of genes verified by qRT-PCR. Stars show data points where there is a lack of correlation between the gene product concentration and the relative quantification by microarray (15%). FT-C: untreated control VEC-FT tissue; FT-E: VEC-FT tissue treated with 100 nmol/L E2; FT-E + P: VEC-FT tissue treated with 10 nmol/L E2+ 10 nmol/L P; PT-C: untreated control VEC-PT tissue; PT-E: VEC-PT tissue treated with 100 nmol/L E2; PT-E + P: VEC-PT tissue treated with 10 nmol/L E2+ 10 nmol/L P. PCR indicates polymerase chain reaction; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
Effect of Hormones on Tissue Thickness and Barrier Properties
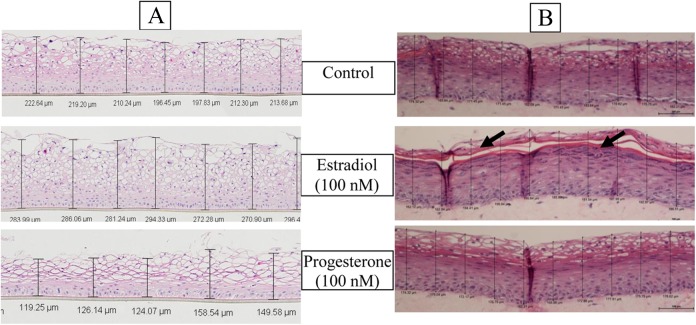
To investigate the effect of hormones on tissue architecture and barrier properties, tissues were reconstructed in culture medium supplemented with E2 or P from the time of seeding (day 0) of the vaginal epithelial cells. After 11 days of culture, the tissues were fixed, cryosectioned, and H&E stained. The thickness of the tissue cross-sections was measured at 10 different locations using a Nikon microscope together with image analysis software (NIS Elements, Melville, New York). Representative tissue histology is shown in Figure 5. Quantitative tissue thickness and barrier function (TEER) data from 3 individual experiments (N = 3 lots or 3 different patient samples) are summarized in Table 4. As shown in Table 4, the average epithelial thickness of the control partial thickness (VEC-PT) tissues was 197 ± 12.8 μm versus 276 ± 28.7 μm (P value < .001) for the E2-treated tissues and 112 ± 24.5 μm (P value < .005) for the P-treated tissues. The VEC-PT tissues treated with a combination of estradiol and P (1:1) remained relatively unaffected. As shown, TEER values increased with tissue thickness (eg, E2-treated tissues), decreased with decreased tissue thickness (eg, P-treated tissues), and remained unchanged for E2- and P-treated tissues (similar to the thickness of the control tissue). The VEC-FT tissue showed similar results: tissue thickness and TEER increased due to treatment with E2, decreased due to treatment with P, and were not significantly affected by treatment with E2 + P (Table 4). In short, estradiol increased epithelial thickness, barrier integrity, and differentiation whereas progesterone alone decreased vaginal barrier thickness and barrier integrity.
Figure 5.
Effect of hormones on tissue thickness. H&E stained cross-sections of (A) VEC PT or (B) VEC-FT tissues. Tissue thickness was measured after 11 days of culture in medium supplemented with hormones. Thickness was measured at 10 different locations and an average thickness was calculated. Estradiol treatment resulted in increased tissue thickness in the partial thickness model and enhanced cornification (arrow) in the full thickness tissue. Progesterone decreased tissue thickness in partial thickness model, but did not significantly alter tissue thickness in the full thickness tissue model.
Table 4.
Tissue Thickness Versus TEER With Hormone Treatment.
| Hormone Treatment | VEC-FT | VEC-PT | ||||
|---|---|---|---|---|---|---|
| Reproductive Hormone | Concentration (nm) | Mean Tissue Thickness (μm) ± SD | Mean TEER (Ω × cm2) ± SD | Mean Tissue Thickness (μm) ± SD | Mean TEER (Ω × cm2) ± SD | |
| Untreated Control | 0 | 107 ± 26.8 | 109 ± 13.7 | 197 ± 12.8 | 91 ± 9.8 | |
| Estradiol | 100 | 188 ± 8.8** | 134 ± 7.8** | 276 ± 28.7* | 120 ± 11.7** | |
| Progesterone | 100 | 71 ± 36.5 | 93 ± 7.8** | 112 ± 24.5** | 61 ± 7.3** | |
| Estradiol+ progesterone | 10:10 | 103 ± 24.0 | 112 ± 11.3 | 167 ± 57.6 | 92 ± 14.1 | |
| * = P < .01 | ||||||
| ** = P < .005 | ||||||
Abbreviations: VEC-PT, partial thickness vaginal/ectocervical tissue; VEC-FT, full thickness vaginal/ectocervical tissue; SD, standard deviation; TEER, transepithelial electrical resistance.
a Effect of endocrine hormones on tissue thickness and TEER in 3 separate experiments (3 lots). Vaginal-ectocervical tissues EpiVaginal tissues full (FT) and partial (PT) thickness were cultured for 11 days in medium supplemented with hormones. At the end of the culture period of each lot, tissue thickness was measured at 10 different locations and an average thickness for the 3 lots was calculated. Estradiol treatment resulted in increased tissue thickness and TEER in the VEC-FT and VEC-PT tissue models.
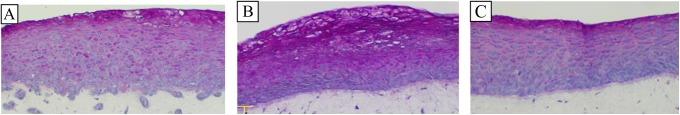
To model the highest reproductive hormone levels that are physiologically relevant in vivo, we next investigated the effect of pregnancy levels of E2 and P48 and the estradiol level found in topical vaginal cream. The VEC-FT tissues were reconstructed and cultured for 72 hours in medium supplemented with E2 (80 nmol/L), P (550 nmol/L), and a combination of E2 and P (E2 = 80 nmol/L, P = 550 nmol/L). Tissues treated with E2 (80 nmol/L) were significantly thicker and more cornified than tissues grown in medium without E2. In contrast, P-treated tissues developed a less differentiated, thinner epithelium. Treatment of tissues with a combination of P and E2 resulted in tissue structure that was similar to untreated controls with slight cornification between the glycogen filled and suprabasal cell layers (Figure 6).
Figure 6.
Effect of hormones on glycogen production in the VEC-FT tissues. PAS stained cross-sections of tissues treated with: (A) no hormone (control), (B) E2 (100 nmol/L), and (C) progesterone (100 nmol/L) are shown. Periodic Acid–Schiff (PAS) stains glycogen reddish-pink. Glycogen was observed in all tissues, increasing in amount as the apical surface is approached. Estradiol treated tissues had the highest glycogen content as evidenced by more intense staining in the suprabasal cell layers. Basal cells showed little or no staining. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Hormones and Glycogen Distribution in the Epithelium
One of the hallmarks of the vaginal epithelium is the accumulation of glycogen in the suprabasal layers. Hence, we investigated the role of female reproductive hormones in the deposition of glycogen in the organotypic VEC-FT model by PAS staining. Results showed the presence of glycogen in the suprabasal and apical but not in the basal cell layers of the vaginal epithelium (Figure 6A). As the epithelial cells differentiate and ascend toward the tissue surface, the level of accumulated glycogen increases resulting in intense PAS staining of the apical layers of the epithelium.35 The E2 treatment of the tissue model resulted in intense staining for glycogen in the suprabasal cell layers (Figure 6B), whereas P-treated tissue had reduced glycogen staining compared to either control or the E2-treated tissue (Figure 6C). A similar pattern of glycogen distribution also occurred in the VEC-PT tissue model (data not shown).
Discussion
The vaginal/ectocervical epithelium provides a robust physical barrier that prevents entry of microorganisms through the mucosa of the lower female genital tract. Disruption of this barrier can result in increased permeability which ultimately can result in inflammatory conditions and increased susceptibility to infection.49 Here, we have shown that E2 increases the thickness of the vaginal epithelium resulting in enhanced barrier function. Also, E2-treated tissues showed large increases in the accumulation of glycogen in the suprabasal and apical epithelial cells, similar to effects of estradiol reported for women.35 Such alterations in the vaginal epithelial morphology and physiology may affect populations of endogenous lactobacilli that depend on vaginal glycogen stores and prevent infection by pathogens by maintaining an acidic pH.50,51
Under physiological conditions, different cell types coexist in a 3D format in which they exchange biochemical and mechanical signals (cellular “cross-talk”) that help determine tissue properties such as differentiation and barrier function. Hence, a full-thickness (VEC-FT) tissue model consisting of a fibroblast-containing lamina propria with epithelial layers was reconstructed to mimic the vaginal tissue microenvironment where cross-talk between epithelial and stromal (fibroblast) cells can be simulated. In order to examine the effect of female reproductive hormones on stromal–epithelial interactions, the VEC-FT tissue was cultured in medium supplemented with reproductive hormones. Morphological and histological analysis showed that E2 induced tissue thickening and differentiation. Since E2 does not usually induce as much differentiation in the VEC-PT epithelial tissue model, increased tissue differentiation in the full-thickness model suggests a potential hormone induced cross-talk between epithelial cells and stromal cells. Such features mimic the in vivo situation52 and make the tissue model more physiologically relevant than monolayer culture systems. In mice, it has been hypothesized that E2-mediated changes on uterine epithelial cells were facilitated indirectly via the underlying stroma, which is ER-α positive.52 As has been described previously, it is also possible that interactions between E2 and growth factors–growth factor receptors (epidermal growth factor), insulin-like growth factor-I, hepatocyte growth factor, and keratinocyte growth factor might be involved in stromal fibroblast–epithelial cell interactions which can lead to epithelial differentiation, growth, and function.8 Thus, conditions such as exposure to estradiol that increase tissue thickness and promote cornification are likely to provide a formidable barrier to pathogen entry into the vaginal mucosa when compared to those conditions (eg, P) which decrease the number of epithelial layers or compromise membrane integrity of the tissue.
Although ERs were expressed predominantly by the epithelium, the classical cytoplasmic PR was poorly expressed in the tissue model; however, gene array data showed expression of membrane bound receptors, PR membrane complexes 1 and 2, by the epithelial tissues which may serve as alternative PR in the epithelium. The expression of PR by stromal cells has been reported in endometrial biopsies.53 Others have also shown the presence of ER-α and ER-β in skin fibroblasts and the coexpression of ER-α with ER-β may indicate an important regulatory function of estrogen in skin.54 These findings demonstrate that the reconstructed tissue model recapitulates the in vivo type responses and highlight the relevance of the tissue model to study hormone-induced biological changes in the vaginal mucosa. Since hormone signaling is important in a number of disease processes, reconstructed vaginal-ectocervical tissue could provide useful models to study therapeutic agents that target the ER-α, ER-β, or PR. In addition, these findings indicate that the vaginal tissue model could be used to screen: (1) vaginal formulations and other feminine care products with estrogenic effects, and (2) the safety (toxicity and irritation) and efficacy of therapeutic agents and microbicides against viral, bacterial, and fungal infections that can colonize the female genital tract55–57 under various hormonal conditions.
Microarray, RT-PCR, and IHC experiments confirmed the expression of ERs and PRs in the VEC models and defined estrogen effects on a number of pathways relevant to innate immunity and cell migration. Noteworthy, is our observation that the VEC-FT was less responsive to hormonal stimulation than VEC-PT. Given that the VEC-FT is comprised of a greater proportion of terminally differentiated epithelial cells making up the physiological stratum corneum (enucleated, glycogen filled cells) as well as basal fibroblasts that promote this cornification,37 this observation is not unexpected. Cornification slows active growth of cells; the VEC-PT model, after 11 days growth, was thicker but with lower average TEER than the VEC-FT model. In VEC-PT, more genes were altered as observed by microarray, increasing the ability of DAVID analysis to classify them into pathways: several immune genes were upregulated by E2 treatment of partial thickness tissues such as interferon-induced genes, interleukin genes, cytokines, and cell adhesion molecule endothelial cell adhesion molecule. Upregulated biological processes involving epithelial differentiation and estrogen responses as well as immune response, wound healing, regulation of NF-κB cascade, defense response, regulation of cell division, antiviral response, antiapoptosis, and immune response-activating cell surface receptor signaling pathways according to DAVID analysis. Kegg pathways identified 6 upregulated genes representing FcγR-mediated phagocytosis. Genes downregulated in E2 VEC-PT included positive regulation of cell adhesion, negative regulation of cell proliferation, cell projection organization, protein kinase cascade, and other nonoverlapping cytokine activity. Kegg pathways classified decreased leukocyte transendothelial migration and O-glycan biosynthesis. These data support enhanced immune defense and impaired leucocyte migration through epithelial tissue following estrogen treatment.
In conclusion, we have developed a vaginal-ectocervical tissue model which expresses ERs and PRs similar to in vivo tissues. The model is hormone responsive as exemplified by the gene array data which show alteration in many biological processes including immunological responses as a result of estradiol treatment. The reconstructed tissue model will have applications in the study of hormonal effects on: (1) tissue stratification and architecture, (2) microbial entry, translocation and transmission, (3) modulation of innate immune responses, (4) tissue barrier function, and (5) drug delivery via the vaginal mucosa. The model will also be useful in preclinical screening of topically applied chemicals and microbicides intended for therapeutic and prophylactic uses. In short, we describe in this manuscript the first hormone responsive human-primary cell based organotypic vaginal/ectocervical tissue model that can be used to enhance our understanding of the mechanisms by which reproductive hormones affect vaginal morphology and physiological function.
Footnotes
Author’s Note: Summary Sentence: This report characterizes effects of reproductive hormones on epithelial differentiation, structure, function, and gene expression in an organotypic human vaginal tissue model; these changes may influence innate immune responses, microbial infection mechanisms, and drug delivery in the vaginal mucosa.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by NIH grants 5U01AI070914, U19AI084048, U19AI096398, and R44ES015641, UL1-TR000157.
References
- 1. Anderson DJ. Genitourinary Immune Defense In: Holmes K, Sparling P, Stamm W, et al., eds. Sexually Transmitted Diseases, ed. New York: McGraw-Hill; 2007. [Google Scholar]
- 2. Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57 (1-2):61–79. [DOI] [PubMed] [Google Scholar]
- 3. Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85 (1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53 (2):65–76. [DOI] [PubMed] [Google Scholar]
- 5. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73 (6):1253–1263. [DOI] [PubMed] [Google Scholar]
- 6. Hafner LM, Cunningham K, Beagley KW. Ovarian steroid hormones: effects on immune responses and Chlamydia trachomatis infections of the female genital tract. Mucosal Immunol. 2013;6 (5):859–875. [DOI] [PubMed] [Google Scholar]
- 7. Kaul R, Hirbod T. Genital epithelial cells: foot soldiers or fashion leaders? J Leukoc Biol. 2010;88 (3):427–429. [DOI] [PubMed] [Google Scholar]
- 8. Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR. Innate immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr Women Health Rev. 2008;4 (2):102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seavey MM, Mosmann TR. Estradiol-induced vaginal mucus inhibits antigen penetration and CD8(+) T cell priming in response to intravaginal immunization. Vaccine. 2009;27 (17):2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cotreau MM, Chennathukuzhi VM, Harris HA, et al. A study of 17beta-estradiol-regulated genes in the vagina of postmenopausal women with vaginal atrophy. Maturitas. 2007;58 (4):366–376. [DOI] [PubMed] [Google Scholar]
- 11. Molander U, Milsom I, Ekelund P, Mellstrom D, Eriksson O. Effect of oral oestriol on vaginal flora and cytology and urogenital symptoms in the post-menopause. Maturitas. 1990;12 (2):113–120. [DOI] [PubMed] [Google Scholar]
- 12. Sjoberg I, Cajander S, Rylander E. Morphometric characteristics of the vaginal epithelium during the menstrual cycle. Gynecol Obstetr Invest. 1988;26 (2):136–144. [DOI] [PubMed] [Google Scholar]
- 13. Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2 (10):1084–1089. [DOI] [PubMed] [Google Scholar]
- 14. Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000;182 (3):708–715. [DOI] [PubMed] [Google Scholar]
- 15. Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J Virol. 2005;79 (5):3107–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fidel PL, Jr, Cutright J, Steele C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun. 2000;68 (2):651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77 (8):4558–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin HL, Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178 (4):1053–1059. [DOI] [PubMed] [Google Scholar]
- 19. Ungchusak K, Rehle T, Thammapornpilap P, Spiegelman D, Brinkmann U, Siraprapasiri T. Determinants of HIV infection among female commercial sex workers in northeastern Thailand: results from a longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12 (5):500–507. [DOI] [PubMed] [Google Scholar]
- 20. Kiddugavu M, Makumbi F, Wawer MJ, et al. Hormonal contraceptive use and HIV-1 infection in a population-based cohort in Rakai, Uganda. AIDS. 2003;17 (2):233–240. [DOI] [PubMed] [Google Scholar]
- 21. Polis CB, Wawer MJ, Kiwanuka N, et al. Effect of hormonal contraceptive use on HIV progression in female HIV seroconverters in Rakai, Uganda. AIDS. 2010;24 (12):1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13 (9):797–808. [DOI] [PubMed] [Google Scholar]
- 23. Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24 (11):1778–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12 (1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31 (1):79–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88 (2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22 (15):1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25 (15):1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheffield JS, Wendel GD, Jr, McIntire DD, Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci. 2009;16 (1):20–31. [DOI] [PubMed] [Google Scholar]
- 30. Mingjia L, Short R. How oestrogen or progesterone might change a woman’s susceptibility to HIV-1 infection. Aust N Z J Obstet Gynaecol. 2002;42 (5):472–475. [DOI] [PubMed] [Google Scholar]
- 31. Van Esch E, Cline JM, Buse E, Wood CE, de Rijk E, Weinbauer GF. Summary comparison of female reproductive system in human and the Cynomolgus Monkey (Macaca fascicularis). Toxicol Pathol. 2008(36):171S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rakasz E, Lynch RG. Female sex hormones as regulatory factors in the vaginal immune compartment. Int Rev Immunol. 2002;21 (6):497–513. [DOI] [PubMed] [Google Scholar]
- 33. Gregoire AT, Parakkal PF. Glycogen content in the vaginal tissue of normally cycling and estrogen and progesterone-treated rhesus monkeys. Biol Reprod. 1972;7 (1):9–14. [DOI] [PubMed] [Google Scholar]
- 34. Larsen B, Markovetz AJ, Galask RP. Role of estrogen in controlling the genital microflora of female rats. Appl Environ Microbiol. 1977;34 (5):534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT. A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility. AIDS Res Human Retroviruses. 2012;28 (1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson DJ, Pudney J, Schust DJ. Caveats associated with the use of human cervical tissue for HIV and microbicide research. AIDS. 2010;24 (1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014;71 (6):618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ayehunie S, Cannon C, Lamore S, et al. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol In Vitro. 2006;20 (5):689–698. [DOI] [PubMed] [Google Scholar]
- 39. Ayehunie S, Cannon C, Larosa K, Pudney J, Anderson DJ, Klausner M. Development of an in vitro alternative assay method for vaginal irritation. Toxicology. 2011;279 (1-3):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44 (7):883–887. [DOI] [PubMed] [Google Scholar]
- 41. Levitz M, Young BK. Estrogens in pregnancy. Vitam Horm. 1977;35:109–147. [DOI] [PubMed] [Google Scholar]
- 42. Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause. 2009;16 (1):30–36. [DOI] [PubMed] [Google Scholar]
- 43. Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60 (12):3175–3182. [PubMed] [Google Scholar]
- 44. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4 (1):44–57. [DOI] [PubMed] [Google Scholar]
- 45. Prange-Kiel J, Rune GM, Zwirner M, Wallwiener D, Kiesel L. Regulation of estrogen receptor alpha and progesterone receptor (isoform A and B) expression in cultured human endometrial cells. Exp Clin Endocrinol Diabetes. 2001;109 (4):231–237. [DOI] [PubMed] [Google Scholar]
- 46. Ing NH, Tornesi MB. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod. 1997;56 (5):1205–1215. [DOI] [PubMed] [Google Scholar]
- 47. Morey JS, Ryan JC, Van Dolah FM. Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol Proced Online. 2006;8:175–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peck JD, Hulka BS, Poole C, Savitz DA, Baird D, Richardson BE. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11 (4):361–368. [PubMed] [Google Scholar]
- 49. Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6 (4):e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mirmonsef P, Gilbert D, Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65 (3):190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van de Wijgert JH, Verwijs MC, Turner AN, Morrison CS. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS. 2013;27 (13):2141–2153. [DOI] [PubMed] [Google Scholar]
- 52. Buchanan DL, Kurita T, Taylor JA, Lubahn DB, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification, and cornification. Endocrinology. 1998;139 (10):4345–4352. [DOI] [PubMed] [Google Scholar]
- 53. Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod. 1998;4 (4):407–412. [DOI] [PubMed] [Google Scholar]
- 54. Thornton MJ. The biological actions of estrogens on skin. Exp Dermatol. 2002;11 (6):487–502. [DOI] [PubMed] [Google Scholar]
- 55. Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunology Med Microbiol. 2003;38 (1):13–22. [DOI] [PubMed] [Google Scholar]
- 56. Fatakdawala H, Uhland SA. Hydrogen peroxide mediated transvaginal drug delivery. Int J Pharm. 2011;409 (1-2):121–127. [DOI] [PubMed] [Google Scholar]
- 57. Doncel GF, Clark MR. Preclinical evaluation of anti-HIV microbicide products: New models and biomarkers. Antiviral Res. 2010;88 (suppl 1):S10–S18. [DOI] [PubMed] [Google Scholar]