Abstract
Visfatin is both a systemic adipocytokine and the cytosolic enzyme, nicotinamide phosphoribosyl transferase (Nampt). This is a longevity protein, which extends the lifespan of human cells by activating sirtuin 1 (SIRT1). In this study, we sought a role for these proteins in obese pregnant women, who experience more postterm deliveries. Thus, 78 women (26 lean, 24 overweight, and 28 obese) were recruited and maternal blood and placental tissue collected prior to term labor. Plasma levels were measured by enzyme-linked immunosorbent assay and quantitative immunohistochemistry used for placenta. We confirmed maternal plasma interleukin 6 increased according to prepregnancy body mass index (BMI; P < .0001) and showed a linear relationship between BMI and syncytiotrophoblast visfatin/Nampt (P = .021) but not with its levels in maternal plasma. Both systemic and placental visfatin/Nampt were significantly associated with placental SIRT1 levels (P = .028 and .017). Thus, higher visfatin/Nampt may prevent a labor-associated decrease in SIRT1 leading to postterm delivery in obesity.
Keywords: visfatin, Nampt, SIRT1, pregnancy, obesity
Introduction
Obesity remains a growing public health problem both in the United States and globally. Obesity in pregnancy has been well documented to cause increased rates of both maternal and fetal complications, including gestational diabetes, preeclampsia, cesarean delivery, wound infection,1,2 stillbirth,3 congenital anomalies,4–6 and macrosomia.7 During pregnancy, the placenta has a significant role in the coordination of both maternal and fetal metabolism and is central to successful fetal development. It achieves this by production of numerous regulatory molecules, many of which act within the placenta and fetal membranes, while others are also secreted into the maternal or fetal systemic circulations. Some of these regulatory molecules are proinflammatory cytokines with adipose tissue as their primary site of production, and therefore called “adipocytokines“ or “adipokines.” Indeed, adipose tissue is the largest endocrine organ in the human and an increase in adipose tissue mass correlates with maternal body mass index (BMI). Many of these adipokines are secreted from adipose tissue into the maternal circulation, target the placenta, and thereby alter its homeostatic regulation. This can alter growth and development of the fetus downstream.8 Moreover, the complex cross talk between maternal adipose tissue and the fetal-placental unit is likely to influence the development of subsequent childhood obesity and the metabolic syndrome.9
Many adipokines are synthesized by and are likely to act locally within the placenta, one of these is visfatin, although produced primarily by visceral fat,10 it is also a product of the placenta and fetal membranes.11 The nomenclature for visfatin is confusing; originally called pre-B-cell colony enhancing factor (PBEF), due to its expression by lymphocytes and synergy with interleukin (IL) 7 and stem cell factors to promote B-cell precursor growth.12 Our laboratory identified the PBEF gene when stretching human amniotic epithelial cells.11 This was the first demonstration of its intrauterine production and we subsequently showed that as a cytokine it participates in the initiation of labor.13 In 2002, PBEF was identified as the cytosolic enzyme, nicotinamide phosphoribosyl transferase (Nampt), which had been sought for several decades and is an essential enzyme and rate-limiting step in nicotinamide adenine dinucleotide (NAD) biosynthesis.14 However, a few years later, it was shown to be an adipokine, produced primarily by visceral fat and was again renamed, visfatin.10 Thus, PBEF/visfatin/Nampt is both a secreted cytokine from adipose tissue and placenta, as well as an intracellular enzyme. Within the cells of production, it appears to reside in separate, independently regulated intracellular pools, depending upon whether its function is intra or extracellular.15 However, a specific receptor for this molecule has yet to be identified and its action on the insulin receptor is now highly controversial.10
The availability of a commercial enzyme-linked immunosorbent assay (ELISA) kit for PBEF/visfatin spawned numerous reports on its systemic levels in a variety of normal and pathological conditions. In one study, no difference was found in the plasma concentrations in men and nonpregnant women, but its levels significantly correlated with measures of obesity; BMI and body fat content.16 This study also showed that plasma visfatin concentrations positively correlated with visceral but negatively correlated with subcutaneous visfatin messenger RNA (mRNA) expression.16 Another study focused on nonpregnant obese women and showed higher serum visfatin concentrations in these women compared to normal-weight controls.17 In pregnant women of normal weight, visfatin levels in serum significantly increased between 19 and 26 weeks of gestation when compared to those between 11 and 14 weeks or 27 and 34 weeks of gestation. However, in overweight patients, the median maternal visfatin concentration showed no such change, leading to the suggestion that visfatin levels correspond to the rate of maternal weight gain.18 Most studies in pregnant women have determined differences in visfatin levels in the systemic circulation in gestational pathologies, especially in gestational diabetes and preeclampsia. Although the placenta also produces visfatin, most of these reports have focused only on the systemic levels. However, one study measured visfatin in the maternal circulation and in the placenta and showed that placental expression was significantly higher in women with gestational diabetes than in controls and that serum visfatin concentrations positively correlated with its expression in placenta, rather than in adipose tissue.19
Visfatin, as the intracellular enzyme Nampt, has the critical function of recycling NAD+ and regulating the enzymes promoting cell survival.20 Nampt also controls the activity of sirtuin 1 (SIRT1; a silent information regulator 2-related protein) and increases its activity in mammals.20 Although mammals have 7 sirtuins, SIRT1 and SIRT2 have NAD+ dependent histone deacetylase activity, which is associated with transcriptional repression and the resolution of inflammation.21 Indeed, Nampt is a longevity protein shown to extend the lifespan of human smooth muscle cells by activating SIRT1 and restraining the accumulation of p53.22 The production of human SIRT1 and SIRT2 has recently been demonstrated in the syncytiotrophoblast and cytotrophoblast of the placenta and in the amniotic epithelium and chorionic cytotrophoblast of the fetal membranes and shown to be anti-inflammatory.23 Most recently, the same group has shown that SIRT1 represses matrix metalloproteinase 9 (MMP-9) expression in the fetal membranes.24 Normal human labor is associated with both a systemic inflammatory state25 and a localized intrauterine inflammatory response.26,27 We hypothesized that the role of systemic and placental visfatin may be altered in obese pregnant women, thus affecting the onset of parturition since elevated prepregnancy BMI has been associated with postterm delivery.28–30 Thus, the aims of this study were to determine the effects of maternal obesity on systemic and placental PBEF/visfatin/Nampt and SIRT1 and their possible association with postterm delivery.
Materials and Methods
Patient Selection and Tissue Collection
Approval was obtained from the Western Institutional Review Board, and pregnant women at term (>37 weeks gestation) who were undergoing an elective prelabor cesarean section at Kapiolani Medical Center for Women and Children (Honolulu, Hawaii) were recruited between August and December 2012. Exclusion criteria included labor, premature rupture of membranes, multiple gestation, pregestational diabetes, acute infection at the time of delivery, maternal inflammatory/connective tissue/autoimmune disease, and maternal use of steroids. After meeting inclusion and exclusion criteria, a detailed consent form was signed by the patient prior to the performance of any study procedure or data collection. These samples have been previously used for a study on leptin and its receptor.31 However, because visfatin is part of the inflammatory response, all tissues were re-examined histologically by a placental pathologist (KT) for signs of inflammation/infection. Of the original samples, 6 showed mild inflammation and were excluded, leaving 78 for this study.
The recruited patients were divided according to their prepregnancy BMI: lean, N = 26 (BMI 16.0 to 24.9), overweight N = 24 (BMI 25.0 to 29.9), and obese N = 28 (BMI ≥ 30). Information regarding the demographics of the mother (age, pregravid weight, height, substance use history, gravity, parity, gestational age at delivery, temperature at admission, medical history, and medication use), pregnancy complications (gestational diabetes, preeclampsia, uterine growth restriction, shoulder dystocia, and macrosomia), and neonatal information (gender, birth weight, Apgar scores, presence of respiratory distress syndrome, admission to neonatal intensive care unit, hypoglycemia, hyperbilirubinemia, and neonatal malformation) were collected.
All patients were fasted for at least 10 hours prior to delivery, blood was collected into EDTA tubes, centrifuged and the supernatant plasma immediately stored at −80°C. All plasma samples were aliquoted and stored at −80°C until assayed, they were never thawed and refrozen before use. One full-thickness sample of the placenta, excluding basal plate, was taken at the site of cord insertion within 15 minutes of delivery. This was fixed in neutral buffered formaldehyde for 72 hours and stored in 70% ethanol until embedded in paraffin.
Maternal Plasma Analyses
Maternal plasma IL-6 was measured using the Quantikine ELISA kit from R&D Systems, Minneapolis, Minnesota, with an intra-assay coefficient of variation of 2.6%. Measurement values below the sensitivity of this assay (0.7 pg/mL) were expressed as 0.7 pg/mL. Visfatin was measured with an ELISA kit from Phoenix Pharmaceuticals Inc. Burlingame, California, with an intra-assay coefficient of variation of 10%. All samples were assayed in duplicate.
Immunohistochemistry and Quantitation
Paraffin-embedded tissue blocks were sectioned (5 µm) onto slides, deparaffinized in a graded series of xylenes and ethanols, then rinsed in distilled water. An antigen retrieval step was performed for both antibodies. For visfatin/Nampt, the slides were heated for 20 minutes at 95°C in 10-mmol/L sodium, citrate buffer, pH 6.0. For SIRT1 staining, slides were heated for 10 minutes at 95°C in 10 mmol/L Tris/1 mmol/L EDTA, pH 9.0. The Vectastain Elite ABC kit (Vector Labs, Burlingame, California) was used according to the manufacturer’s protocol. Following antigen retrieval, slides were treated with 0.3% hydrogen peroxide in methanol to remove endogenous peroxidase activity. Nonspecific binding was blocked with 2.5% normal goat serum for 30 minutes followed by incubation with predetermined concentrations of one of the antibodies as follows: rabbit monoclonal antibody to visfatin/Nampt (Abcam, Cambridge, Massachusetts, catalog number 109210, 0.25 µg/mL) or rabbit polyclonal antibody to SIRT1 (Sigma-Aldrich, St. Louis, Missouri, catalog number S5322, 0.25 µg/mL). A rabbit immunoglobulin G (IgG) monoclonal isotype control (Abcam, catalog number 125938) was used as a negative control for visfatin and a normal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, California, catalog number 2027) was used as the SIRT1 control at the same concentration as the primary antibodies. The 3,3-diaminobenzidine substrate solution was used to visualize the antigens. Slides were rinsed and counterstained with Gill’s hematoxylin, cleared and mounted in Pro-Texx mounting media (Lerner Laboratories, Pittsburgh, Pennsylvania).
A multispectral imaging system comprised an Olympus BX51 microscope (Olympus America Inc, Mellville, New York) and a CRI Nuance spectral analyzer (Caliper Life Sciences, Hopkinton, Massachusetts) was used to obtain bright-field image cubes between 420 and 700 nm wavelength at 20 nm intervals. Inform software (version 1.4.0; PerkinElmer, Waltham, Massachusetts) was used to quantitate the unmixed image data. The average signal intensity per pixel was obtained from 5 different fields of villous tissue from each patient. The signal intensity for visfatin/Nampt and SIRT1 in the syncytiotrophoblast was quantified at ×400.
Statistical Analysis
Data were analyzed using SAS statistical software (SAS Institute Inc, Cary, North Carolina, version 9.3). Continuous data were summarized as means ± standard deviations. Analysis of variance was used to examine the differences in baseline characteristics between the 3 BMI groups: lean, overweight, and obese. The relationships between the intensity of placental immunostaining for visfatin/Nampt and BMI and with the intensity of SIRT1 immunostaining were evaluated using linear regression models. Statistical significance was set at P < .05.
Results
Demographic Data
We recruited 84 patients, but 4 had histologic evidence of mild chorionitis and another 2 mild chorioamnionitis, these 6 patients were excluded, leaving 78 patients for final analysis. Patient demographics by BMI groups are shown in Table 1. There were no statistical differences in the mean age, average gestational age at delivery, gestational weight gain, in lean, overweight, and obese women. The average gestational age was 39 weeks. The mean birth weight and placental weight were significantly different by maternal BMI (P = .041 and P = .003, respectively). Obese women had infants with higher birth weight (3581 grams) compared to lean (3254 grams) and overweight women (3460 grams). Obese women had a higher mean placental weight (563 grams) compared to lean (473 grams) and overweight women (523 grams).
Table 1.
Patient Demographics.
| Lean (N = 26) | Overweight (N = 24) | Obese (N = 28) | P Value | |
|---|---|---|---|---|
| Mean age, years | 32.96 ± 5.77 | 30.75 ± 5.33 | 30.64 ± 4.90 | .215 |
| BMI, kg/m2 | 21.17 ± 2.17 | 27.65 ± 1.31 | 36.82 ± 6.46 | <.001 |
| Mean gestational age at delivery, weeks | 39.00 ± 0.66 | 39.04 ± 0.53 | 39.24 ± 0.46 | .233 |
| Mean gestational weight gain, kg | 13.47 ± 5.22 | 15.45 ± 6.60 | 13.06 ± 6.32 | .336 |
| Mean birth weight, g | 3254.31 ± 410.34 | 3459.54 ± 538.27 | 3581.29 ± 455.13 | .041 |
| Mean placental weight, g | 472.96 ± 92.39 | 523.00 ± 98.77 | 562.93 ± 100.87 | .003 |
Abbreviation: BMI, body mass index.
Maternal IL-6 and Visfatin
In order to validate our sample set for this study, we measured the levels of maternal plasma IL-6, previously shown by others to be elevated in obese pregnant women.32 Indeed there was significantly increased IL-6 (P < .0001) in our samples with increasing maternal BMI (Figure 1A). On the other hand, the levels of maternal plasma visfatin showed no relationship with maternal BMI (Figure 1B) or with birth weight (data not shown). However, visfatin/Nampt is also produced by the placental syncytiotrophoblast, and when its protein was quantitated, it was significantly increased with maternal BMI and the relationship was linear (P = .021; Figure 1C). There was no relationship between placental visfatin/Nampt and birth weight (data not shown). Since IL-6 is capable of stimulating the production of visfatin,33 we expected a relationship between maternal plasma IL-6 and placental visfatin/Nampt production, but this association was not significant (Figure 1D). Similarly, there was no correlation between maternal plasma visfatin and the placental visfatin/Nampt (data not shown). Examples of visfatin/Nampt immunolocalized in the syncytiotrophoblast of the placental villi are shown in Figure 1E. There was less staining in the syncytiotrophoblast of lean patients (Figure 1Ea) compared to the obese patients (Figure 1Eb). A negative control from an obese patient is shown in Figure 1Ec.
Figure 1.
Maternal plasma IL-6, visfatin, and placental visfatin/Nampt. A, Maternal plasma IL-6 increased linearly as maternal body mass index (BMI) increased (P < .0001). B, Maternal plasma visfatin showed no relationship with increasing maternal BMI (P = .314). C, Placental visfatin/Nampt in the syncytiotrophoblast was significantly and linearly increased with increasing maternal BMI (P = .021). D, There was no relationship between IL-6 in maternal plasma and placental visfatin/Nampt in the syncytiotrophoblast (P = .727). E, Examples of placental immunostaining for visfatin/Nampt in (a) lean (b) obese women, showing darker staining in the syncytiotrophoblast (SN) in the obese patient, (c) negative control. Original magnification 400× for all sections. IL indicates interleukin. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Placental SIRT1
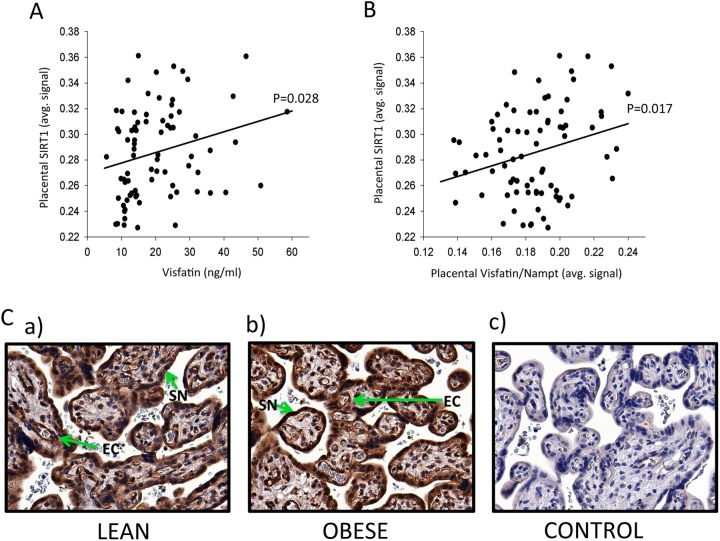
It is known that visfatin, acting as Nampt, is the rate-limiting enzyme for NAD+ biosynthesis in human cells and extends the life span of human smooth muscle cells by activating SIRT1 and restraining the accumulation of p53.22,34 Therefore, we immunolocalized and quantitated SIRT1, previously shown to be a product of the syncytiotrophoblast,23 in our samples. There was significantly increased SIRT1 in the syncytiotrophoblast with increasing maternal plasma visfatin (P = .028), Figure 2A. There was also increased syncytiotrophoblast SIRT1 with increasing syncytiotrophoblast visfatin/Nampt (P = .017; Figure 2B). Thus, both maternal systemic visfatin and placental visfatin were positively correlated with the levels of SIRT1 in the syncytiotrophoblast. However, the placental SIRT1 level did not differ by maternal BMI (data not shown). The immunolocalization of SIRT1 was performed with the same antibody as used by Lappas et al.23 Examples from a lean patient (Figure 2Ca) and an obese patient (Figure 2Cb) are shown, together with a negative control (Figure 2Cc). There was strong SIRT1 staining in the syncytiotrophoblast of all patients, with weak staining in the vascular endothelial cells, which could not be adequately quantified due the weak signal.
Figure 2.
Maternal plasma visfatin and placental visfatin/Nampt and SIRT1. A, Maternal plasma visfatin significantly increased SIRT1 in the placental syncytiotrophoblast (P = .028). B, Placental visfatin/Nampt significantly increased placental SIRT1 in placental syncytiotrophoblast (P = .017). C, Examples of immunostaining for SIRT1 in (a) lean and (b) obese women, there was strong SIRT1 staining in the syncytiotrophoblast (SN) of all patients and weak staining in the vascular endothelial cells (EC), (c) negative control. Original magnification 400× for all sections. SIRT1 indicates sirtuin 1.
Maternal Gestational Diabetes and its Effects on IL-6, Visfatin, and SIRT1
Of the 78 patients used in this study, 10 had maternal gestational diabetes (GDM). Of these, 8 were controlled with diet alone and 2 were controlled with medication. There were no significant differences in systemic levels of IL-6 or visfatin when women with and without GDM were compared. Similarly, placental visfatin/Nampt and SIRT1 levels were not statistically different between women with versus those without GDM.
Discussion
This study shows for the first time a relationship between maternal prepregnancy BMI and the levels of visfatin/Nampt in the syncytiotrophoblast, but not with its levels in maternal plasma. We also show relationships between placental levels of visfatin/Nampt and SIRT1 and maternal plasma visfatin and placental SIRT1. Thus, we suggest that visfatin/Nampt, acting within the syncytiotrophoblast as the rate-limiting component in the NAD biosynthesis pathway, is a potential biological mechanism by which the placenta is protected against the cellular stress of obesity.35 Indeed eukaryotes have evolved elaborate mechanisms to survive periods of adversity and visfatin/Nampt is one such stress-responsive gene that modulates cell survival.15 Its overexpression causes a 55% increase in total intracellular NAD+and one effect of this is a boost in SIRT1 activity.34 This in turn can influence DNA repair mechanisms, protects telomeres, and reduces inflammation.35 SIRT1 is a class III histone deacetylase and as a transcription factor inhibits NFkB and its target genes.35 Thus, it may affect a number of placental hormones involved in the regulation of an inflammatory reaction. However, these have anti-inflammatory properties and we have focused here on visfatin/Nampt that appears to be the driver for syncytiotrophoblast survival in a hostile environment and is likely to be involved in the increased risk of a postterm delivery when the prepregnancy maternal weight is elevated.28–30 Indeed, an unidentified biological mechanism to account for this phenomenon has been suggested by these authors, which is of clinical importance because of its association with both maternal and fetal complications.36 All patients in our study had an elective prelabor cesarean section between 38 and 40 weeks of gestation. Although this eliminated the effects of labor and delivery on the placenta and specifically on both visfatin and SIRT1, it was not possible to determine what their natural gestational age at spontaneous delivery would have been. Thus, a major limitation of our study was the requirement of having to eliminate patients with spontaneous delivery. However, studying spontaneous deliveries is a challenge in itself because it is not routine obstetric practice to allow patients to delay delivery beyond 41 to 42 weeks of gestation.
In this study, we first confirmed an increase in circulating IL-6, in obese pregnant women.32 Previously, we had shown that IL-6 added in vitro to amniotic epithelial cells induced a significant increase in visfatin expression,37 and that in synovial fibroblasts IL-6 trans-signaling regulates visfatin in a STAT-3-dependent manner.33 Since IL-6 was elevated in maternal plasma according to BMI and maternal blood bathes the placenta, we expected there to be a relationship between its levels and the visfatin/Nampt expressed in the syncytiotrophoblast. However, this was not the case. Therefore, other cytokines in the circulation of obese patients, either alone or in combination, may be affecting syncytiotrophoblast production of visfatin/Nampt. Alternatively, prepregnancy obesity, which causes an exaggerated inflammatory response in the placenta with associated accumulation of multiple types of inflammatory cells38 and increased expression of chemotactic cytokines,32 may increase placental production of visfatin/Nampt. These potential relationships are shown diagrammatically in Figure 3.
Figure 3.
Possible relationships between maternal and placental cytokines and syncytiotrophoblast levels of visfatin/Nampt and SIRT1. Interleukin 6 (IL-6) in maternal plasma was significantly related to prepregnancy BMI (P < .0001) but unrelated to levels of visfatin/Nampt in the syncytiotrophoblast (P = .727). However, visfatin/Nampt in the syncytiotrophoblast was linearly and significantly (P = .021) related to maternal BMI (not shown). Other proinflammatory cytokines in the maternal circulation or those produced within the placenta may be causing increased syncytiotrophoblast visfatin/Nampt in obesity (dashed lines). The visfatin in maternal plasma was unrelated to maternal BMI but maybe related to the amount of visceral fat present (dashed line). Both the plasma visfatin and placental visfatin/Nampt increased placental levels of SIRT1 (P = .028 and .017, respectively), and SIRT1 is anti-inflammatory and a negative regulator of the matrix metalloproteinases. However, there was no relationship between placental SIRT1 and maternal BMI. This suggests that the increased visfatin/Nampt in obesity may keep SIRT1 stable in the syncytiotrophoblast, delaying the proinflammatory events leading to spontaneous labor and causing a greater incidence of postterm deliveries in obese patients. BMI indicates body mass index; SIRT1, sirtuin 1.
The visfatin levels in the maternal circulation, unlike its levels in the syncytiotrophoblast, were unrelated to maternal BMI. Visfatin is thought to be produced by visceral but not subcutaneous adipose tissue,10,16 but in order to measure visceral adipose tissue mass, computed tomography is required, which is unacceptable in pregnant patients due to the potential teratogenicity of radiation exposure. Therefore, a relationship between visceral adipose tissue mass is a possibility as shown in Figure 3. Visfatin mRNA expression was shown to be 7 times greater in omental fat of pregnant women compared to nonpregnant lean control women.39 However, this increased mRNA did not correlate with levels of visfatin in serum. These data confirm other previous observations of no correlation between visfatin levels in the maternal circulation and maternal BMI18 and these authors suggested that circulating maternal visfatin levels may be related to the rate of maternal weight gain during gestation. However, our data on weight gained showed no correlation with circulating visfatin levels (data not shown). Thus, an association between circulating visfatin and obesity, BMI and visceral fat depot in pregnancy, continues to remain uncertain.18
It has been established that visfatin/Nampt increases the activation of SIRT115,20 and we show here that both systemic visfatin and placental visfatin/Nampt significantly increased syncytiotrophoblast SIRT1 levels (P = .028 and P = .017, respectively). This is interesting because in the circulation, visfatin is a secreted extracellular protein, while within the syncytiotrophoblast, it is an intracellular modulator. The circulating visfatin would be acting on its potential and specific, but unknown membrane receptor to cause this effect, while the placental visfatin/Nampt would be acting as the enzyme Nampt within the syncytiotrophoblast. Furthermore, there was no correlation between the levels of systemic and placental visfatin in this study, suggesting that placental visfatin was not secreted from the syncytiotrophoblast into maternal blood. Our use of immunohistochemistry to detect visfatin/Nampt within the syncytiotrophoblast did not allow us to distinguish between the extracellular and intracellular forms of the protein. To do this would be difficult, although we have previously attempted it using amniotic epithelial cells in vitro.40 Others have also concluded that visfatin is likely to be acting more as an autocrine/paracrine factor than as a classical blood borne endocrine agent.39 Indeed, we further suggest that it acts in an intracrine manner, as Nampt in the syncytiotrophoblast. Further studies are needed to confirm this, using measurements of syncytiotrophoblast NAD+ and nicotinamide.
Nampt as the rate-limiting enzyme for NAD+ biosynthesis from nicotinamide represents the important salvage pathway by which NAD+ is generated. NAD+ can also be produced de novo, but together these 2 pathways are the major contributors to NAD biosynthesis. NAD is a major coenzyme in numerous oxidation–reduction reactions. Its chemistry allows it to readily accept and donate electrons in reactions leading to the generation of ATP, universally required by cells for most energy-consuming processes.41 These intracellular activities are essential for certain cell survival reactions, including those linked to the sirtuin family of deacytelases.22 Indeed, visfatin/Nampt is important in the activation of SIRT1,15 and SIRT1 is a negative regulator of proinflammatory cytokines42,43 and MMPs.24,44 For this reason, we measured SIRT1 in the syncytiotrophoblast and although visfatin/Nampt levels in both this tissue and in the maternal circulation were significantly related to its levels, there was no direct relationship between syncytiotrophoblast SIRT1 and maternal BMI. We propose that an increase in SIRT1 is not required for the prolongation of pregnancy in obese patients, because the higher visfatin/Nampt continues to drive the production of SIRT1, whereas normally it would decrease in association with spontaneous labor,23 thereby allowing the inflammatory processes associated with labor to take place.
In Figure 3, we summarize a potential mechanism for the higher incidence of postterm pregnancy in obese women. Higher prepregnancy BMI increased IL-6 in maternal plasma and the levels of visfatin/Nampt in the placental syncytiotrophoblast. However, the IL-6 levels, at least in maternal plasma, did not drive the visfatin/Nampt in the syncytiotrophoblast. Other proinflammatory cytokines either from maternal adipose tissue or produced by the placenta may be causing the increased syncytiotrophoblast visfatin/Nampt in obesity. On the other hand, the visfatin levels in maternal plasma were unrelated to maternal BMI but may be related to the amount of visceral fat. Both plasma visfatin and the visfatin/Nampt in the syncytiotrophoblast increased the production of SIRT1 in the syncytiotrophoblast. It has been shown by Dr Lappas and colleagues23 that term spontaneous labor is associated with decreased SIRT1 levels in the syncytiotrophoblast and she proposed that this decrease would allow the normal inflammatory processes associated with spontaneous labor to take place. Therefore, we propose (Figure 3) that although SIRT1 was unrelated to maternal BMI, its levels would be prevented from declining by the increased visfatin/Nampt; this would in turn prevent the proinflammatory events leading to spontaneous labor from taking place in a timely manner. The effect of this SIRT1 directed placental anti-inflammatory milieu could therefore cause a delay in the onset of spontaneous labor. Therefore, visfatin/Nampt, in both the maternal circulation and as the enzyme Nampt in the syncytiotrophoblast, is likely to have important roles in the prolongation of syncytiotrophoblast survival in times of placental stress.
Acknowledgments
The authors acknowledge Ms Sandra Yamamoto for her assistance with the laboratory aspect of this study. We also acknowledge Ms Ann Marie Savage and the nurses of the Family Birth Center at Kapiolani Medical Center for Women and Children for their support in patient recruitment and sample collection. Finally, we acknowledge all the patients who donated their time and samples for this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by John A. Burns School of Medicine, University of Hawaii, Department of Obstetrics, Gynecology, and Women’s Health. Statistical analyses were supported in part by grants from the National Institute of Minority Health and Health Disparities (U54MD007584 and G12MD007601) at the National Institutes of Health.
References
- 1. Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190 (4):1091–1097. [DOI] [PubMed] [Google Scholar]
- 2. Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118 (2 Pt 1):305–312. [DOI] [PubMed] [Google Scholar]
- 3. Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112 (4):403–408. [DOI] [PubMed] [Google Scholar]
- 4. Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003;111 (5 Part 2):1152–1158. [PubMed] [Google Scholar]
- 5. Honein MA, Moore CA, Watkins ML. Subfertility and prepregnancy overweight/obesity: possible interaction between these risk factors in the etiology of congenital renal anomalies. Birth Defects Res A Clin Mol Teratol. 2003;67 (8):572–577. [DOI] [PubMed] [Google Scholar]
- 6. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301 (6):636–650. [DOI] [PubMed] [Google Scholar]
- 7. Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191 (3):964–968. [DOI] [PubMed] [Google Scholar]
- 8. Bohler H, Jr, Mokshagundam S, Winters SJ. Adipose tissue and reproduction in women. Fertil Steril. 2010;94 (3):795–825. [DOI] [PubMed] [Google Scholar]
- 9. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115 (3):e290–e296. [DOI] [PubMed] [Google Scholar]
- 10. Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307 (5708):426–430. [DOI] [PubMed] [Google Scholar]
- 11. Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187 (4):1051–1058. [DOI] [PubMed] [Google Scholar]
- 12. Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14 (2):1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of pre-B-cell colony-enhancing factor on the human fetal membranes by microarray analysis. Am J Obstet Gynecol. 2003;189 (4):1187–1195. [DOI] [PubMed] [Google Scholar]
- 14. Rongvaux A, Shea RJ, Mulks MH, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32 (11):3225–3234. [DOI] [PubMed] [Google Scholar]
- 15. Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp. Gerontol. 2006;41 (8):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berndt J, Kloting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54 (10):2911–2916. [DOI] [PubMed] [Google Scholar]
- 17. Zahorska-Markiewicz B, Olszanecka-Glinianowicz M, Janowska J, et al. Serum concentration of visfatin in obese women. Metabolism. 2007;56 (8):1131–1134. [DOI] [PubMed] [Google Scholar]
- 18. Mazaki-Tovi S, Romero R, Kusanovic JP, et al. Maternal visfatin concentration in normal pregnancy. J Perinat Med. 2009;37 (3):206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma Y, Cheng Y, Wang J, Cheng H, Zhou S, Li X. The changes of visfatin in serum and its expression in fat and placental tissue in pregnant women with gestational diabetes. Diabetes Res Clin Pract. 2010;90 (1):60–65. [DOI] [PubMed] [Google Scholar]
- 20. Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279 (49):50754–50763. [DOI] [PubMed] [Google Scholar]
- 21. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404 (1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van der Veer E, Ho C, O’Neil C, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282 (15):10841–10845. [DOI] [PubMed] [Google Scholar]
- 23. Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84 (1):167–178. [DOI] [PubMed] [Google Scholar]
- 24. Poljak M, Lim R, Barker G, Lappas M. Class I to III histone deacetylases differentially regulate inflammation-induced matrix metalloproteinase 9 expression in primary amnion cells. Reprod Sci. 2014;21 (6):804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180 (2 Pt 1):499–506. [DOI] [PubMed] [Google Scholar]
- 26. Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195 (2):394 e391–e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16 (2):206–215. [DOI] [PubMed] [Google Scholar]
- 28. Stotland NE, Washington AE, Caughey AB. Prepregnancy body mass index and the length of gestation at term. Am J Obstet Gynecol. 2007;197 (4):378 e371–e375. [DOI] [PubMed] [Google Scholar]
- 29. Caughey AB, Stotland NE, Washington AE, Escobar GJ. Who is at risk for prolonged and postterm pregnancy? Am J Obstet Gynecol. 2009;200 (6):683 e681–e685. [DOI] [PubMed] [Google Scholar]
- 30. Halloran DR, Cheng YW, Wall TC, Macones GA, Caughey AB. Effect of maternal weight on postterm delivery. J Perinatol. 2012;32 (2):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsai PJ, Davis J, Bryant-Greenwood G. Systemic and placental leptin and its receptors in pregnancies associated with obesity. Reprod Sci. 2015;22 (2):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roberts KA, Riley SC, Reynolds RM, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32 (3):247–254. [DOI] [PubMed] [Google Scholar]
- 33. Nowell MA, Richards PJ, Fielding CA, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthrit Rheum. 2006;54 (7):2084–2095. [DOI] [PubMed] [Google Scholar]
- 34. Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23 (2):164–170. [DOI] [PubMed] [Google Scholar]
- 35. Horio Y, Hayashi T, Kunimoto R. Cellular and molecular effects of sirtuins in health and disease. Clin Sci. 2011;121:191–203. [DOI] [PubMed] [Google Scholar]
- 36. Norwitz ER, Snegovskikh VV, Caughey AB. Prolonged pregnancy: when should we intervene? Clin Obstet Gynecol. 2007;50 (2):547–557. [DOI] [PubMed] [Google Scholar]
- 37. Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Bryant-Greenwood GD. Genomic organization of the gene coding for PBEF and expression in human fetal membranes. J Mol Endocrinol. 2001;26 (2):107–117. [DOI] [PubMed] [Google Scholar]
- 38. Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29 (3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morgan SA, Bringolf JB, Seidel ER. Visfatin expression is elevated in normal human pregnancy. Peptides. 2008;29 (8):1382–1389. [DOI] [PubMed] [Google Scholar]
- 40. Kendal-Wright CE, Hubbard D, Gowin-Brown J, Bryant-Greenwood GD. Stretch and inflammation-induced pre-B cell colony-enhancing factor (PBEF/visfatin) and interleukin-8 in amniotic epithelial cells. Placenta. 2010;31 (8):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rongvaux A, Andris F, Van-Gool F, et al. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25 (7):683–690. [DOI] [PubMed] [Google Scholar]
- 42. Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an anti-inflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 2008;177 (8):861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen Z, Ajmo JM, Rogers CQ, et al. Role of SIRT1 in regulation of LPS or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol. 2009;296 (5):G1047–G1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakamuru Y, Vuppusetty C, Wada H, et al. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23 (9):2810–2819. [DOI] [PubMed] [Google Scholar]