Abstract
Approximately 20% of pregnant women smoke despite intentions to quit. Smoking cessation drugs, such as nicotine replacement therapy (NRT) and bupropion, are recommended treatments. Adverse cardiovascular outcomes in offspring have raised concerns about NRT’s safety during pregnancy. However, the effect of bupropion is unknown. Using a rat model, we determined whether NRT and bupropion interventions during pregnancy are safer than continued smoking on offspring’s cardiovascular function. Male offspring of controls and dams exposed to cigarette smoke (1.6 packs/day, inhalation), nicotine (2 mg/kg/d subcutaneously), and bupropion (13 mg/kg twice daily orally) were assessed for fetoplacental weight, cardiac function, blood pressure, and vascular reactivity. Fetoplacental weights were decreased and spontaneous beating and intracellular calcium in neonatal cardiomyocytes were increased in smoking, nicotine, and bupropion offspring; however, these effects were more accentuated in smoking followed by nicotine and bupropion offspring. Increased heart rate and decreased cardiac output, stroke volume, and left ventricular percent posterior wall thickening were observed in smoking, nicotine, and bupropion offspring. The left ventricular mass was reduced in smoking and nicotine but not in bupropion offspring. Blood pressure was higher with decreased endothelium-dependent relaxation and exaggerated vascular contraction to angiotensin II in smoking and nicotine offspring, with more pronounced dysfunctions in smoking than nicotine offspring. Maternal bupropion did not impact offspring’s blood pressure, endothelium-dependent relaxation, and vascular contraction. In conclusion, maternal nicotine intervention adversely affects offspring’s cardiovascular outcomes, albeit less severely than continued smoking. However, bupropion causes cardiac derangement in offspring but does not adversely affect blood pressure and vascular function.
Keywords: nicotine, bupropion, offspring, cardiac function, vascular function
Introduction
Smoking during pregnancy is associated with a higher risk of poor pregnancy outcomes, including placental abruption, miscarriage, preterm birth, and fetal death.1,2 In addition, maternal smoking is related to short- and long-term health risks for the fetus, such as fetal growth restriction and increased risks of obesity, diabetes, asthma, behavioral disorders, and cancer.3–6 However, studies on offspring’s cardiovascular outcomes have presented mixed results. Some studies show that maternal smoking increases infant, childhood, and adolescence blood pressure by 0.9 to 5.4 mm Hg.7–12 Some studies found no effect,13–15 and one study found an interaction between prenatal smoking exposure and gestational length (ie, preterm and full term leading to decreased and increased offspring blood pressure, respectively).16 Pooled data in a meta-analysis estimated the effect to be 0.62 mm Hg.17 These associations of maternal smoking with blood pressure in the offspring are limited to observational studies, and they may be confounded by several factors, such as nutrition, dose and time of exposure, lack of compliance, and genetic factors. In addition, smoking mothers are more likely to drink alcohol and abuse other recreational drugs, which may also confound the outcomes.18 Thus, controlled animal studies utilizing pregnant animals subjected to cigarette smoking would help confirm a definitive link between maternal smoking and long-term cardiovascular function.
The broad detrimental effects of maternal smoking have led some public health scientists to believe that stopping smoking during pregnancy would lead to positive maternal and fetal outcomes. Accordingly, studies show that smoking cessation interventions during pregnancy have reduced the prevalence of low birthweight, preterm births, and infant morbidity and mortality.19 Although smoking cessation counseling is recommended as the first-line intervention for pregnant smokers, many women continue to smoke.20 For women who do not respond to counseling, clinical practice guidelines recommend that pharmacological interventions be considered for smoking cessation.21 Nicotine replacement therapy (NRT) and bupropion are commonly advocated smoking cessation agents. Nicotine replacement therapy is the only Food and Drug Administration–approved aid for smoking cessation during pregnancy and is the first-line pharmacotherapy for pregnant women who cannot quit smoking by other means.21 However, the use of NRT during pregnancy continues to be controversial since recent clinical studies with NRT in pregnant women have reported adverse pregnancy effects, including increased fetal heart rate and aortic pulsatility, decreased fetal movements, reduced umbilical artery blood velocity, intraventricular hemorrhage, neonatal convulsions, and congenital heart diseases.22–26 In addition, animal studies have clearly demonstrated that maternal nicotine has toxic effects on the fetus, leading to low birth weight and cardiovascular dysfunctions, such as adult-onset hypertension, reduced endothelial relaxation, enhanced vascular smooth muscle contractility, and increased heart susceptibility to ischemia and reperfusion injury.27–34 Although it is conceivable that substituting “clean” nicotine without the 4000 other toxins that are inhaled while smoking35,36 is safer than tobacco smoking, it is important to perform controlled animal studies to directly compare maternal NRT versus smoking and determine the health benefits and relative safety of NRT over smoking on fetal cardiovascular outcomes. These studies are particularly important because the prescriptions for NRT to pregnant women are steadily increasing.37
Bupropion is an approved and highly prescribed antidepressant for pregnant women and is also a highly effective smoking cessation agent compared to NRT.38–41 Bupropion does not deliver nicotine, like NRT, but it inhibits dopamine and norepinephrine reuptake and attenuates stimulant effects of nicotine on nicotinic acetylcholine receptors.42,43 To date, there is limited clinical evidence available regarding the safety of bupropion use during pregnancy.36,44,45 Despite this lack of data, studies have reported that 3% of pregnant smokers take bupropion during pregnancy.46,47 Preclinical data obtained from ex vivo and in vitro studies indicate that bupropion has a better short-term fetal safety profile than nicotine.48–50 However, the long-term effect of maternal bupropion treatment on offspring cardiovascular function is not known. In this study, using a pregnant rat model of cigarette smoke (via inhalation), NRT, and bupropion exposures at clinically relevant concentrations, we determined whether (1) maternal smoking during pregnancy affects fetal and adult cardiovascular function; (2) NRT intervention is relatively safer than continued smoking on fetal and adult life cardiovascular function; and (3) other therapies, such as bupropion, during gestation are safe for the developing fetus and its cardiovascular system.
Materials and Methods
Animals and Treatment
All experimental procedures were approved by the Animal Care and Use Committee at The University of Texas Medical Branch (UTMB) at Galveston and were in accordance with the guidelines published by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). Timed-pregnant Sprague Dawley rats (gestational day [GD] 3) were purchased from Harlan (Houston, Texas) and housed in temperature-controlled rooms (23°C) with a 12:12 hour light/dark cycle and with unlimited access to food and water. The animals were randomly divided into control, cigarette smoking, nicotine, and bupropion groups (n = 12 in each group) and exposed to these treatments from GD 4 to until delivery to focus on the consequences of in utero exposure. The cigarette smoke exposure was performed at UTMB’s inhalation core facility. Briefly, animals were exposed to mainstream cigarette smoke generated from 3R4F reference cigarettes (1.6 packs per day; Tobacco Health Research Institute, Lexington, Kentucky) using an automated cigarette generation system (Jaeger-Baumgartner cigarette smoking machine; CH Technologies, Westwood, New Jersey) for 6 hours a day. Chamber levels of total particulate matter (TPM) were monitored throughout the exposure using an Electro-Medical Measurement Systems (CH Technologies Westwood, New Jersey); mean TPM levels were determined gravimetrically from filters weighed before and after sampling. The control rats were put in an identical chamber but exposed to air for the same time period. This cigarette smoke exposure paradigm produced plasma cotinine levels of 198 ± 20 ng/mL in the dams, equivalent to that observed in moderate human smokers.51,52 Nicotine was administered to pregnant rats through a subcutaneous osmotic minipump at 2 mg/kg/d, as described previously.31,32 This dose of nicotine resulted in blood cotinine levels of 217 ± 38 ng/mL, which closely resembles humans using transdermal nicotine patch.53,54 Control rats received saline from the osmotic minipump. Bupropion was gavaged orally (13 mg/kg/d) twice daily. This dose increased plasma levels of bupropion and its metabolite hydroxybupropion to 765 ± 168 ng/mL and 850 ± 146 ng/mL, respectively, which mimic average steady-state Cmax levels in pregnant women.55,56 Cotinine, bupropion, and hydroxybupropion levels in rats’ plasma were analyzed by liquid chromatography–mass spectrometry (LC-MS), as described by us previously.57 A subset of animals (n = 6 in each group) were killed on GD 20 using carbon dioxide inhalation; their placentas were isolated and blotted to remove fluids and blood, and the junctional and labyrinth zones were separated and weighed. Fetal weights were also measured. Other dams in all groups were allowed to deliver at term. The number of pups in the control and treatment litters were adjusted to 8 pups per dam to ensure equal nutrient access for all offspring (pups with weights at each extreme were removed from the study). The ratio of male to female pups remained equivalent after culling, when possible. Heart/cardiomyocytes were isolated from 1-day-old pups to determine spontaneous beating frequency and intracellular calcium ([Ca2+]i) and for RNA analysis. The pups were weaned at 21 days of age, males and females were housed separately, and only males were used for the study. Animals were reared up to 6 months of age, and cardiac function and blood pressure were determined using ultrasound and telemetry, respectively. Then, the animals were killed and mesenteric arteries were isolated for vascular reactivity studies. Although each treatment group had its own control (ie, air inhalation, subcutaneous saline, and oral saline), the studied outcomes did not significantly differ between these control groups, hence combined control data are presented for easy comparison across and between treatment groups.
Isolation of Neonatal Cardiomyocytes and Spontaneous Beating Frequency
Neonatal ventricular myocytes were isolated and cultured as described previously.58,59 Briefly, 1-day-old pups were euthanized, and their hearts were immediately excised. The ventricles were separated, transferred into ice-cold phosphate-buffered saline (Sigma, St Louis, Missouri), and quickly digested with 0.04% collagenase II (Fisher, Newark, Delaware) and 0.05% pancreatin (Sigma) at 37°C. The dispersed cells were suspended in DMEM-M19 (4:1) with 10% fetal bovine serum (Gibco, Grand Island, New York) and plated on a dish for 90 minutes at 37°C to allow attachment of fibroblasts and endothelial cells. Then, the unattached cardiomyocytes in the supernatants were collected, cultured on glass cover slips for 4 to 7 days, placed on a stage-mounted microscope, and observed for several minutes to calculate the spontaneous cell beats per minute, as described previously.60
Measurement of [Ca2+]i Concentration
The measurements of [Ca2+]i were performed with Fura-2 methodology.61 Briefly, neonatal cardiomyocytes cultured on glass bottom dishes were loaded with 5-μM Fura-2 AM (Life Technology, Grand Island, New York) in HEPES-buffered salt solution (HBSS; 152 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH 7.4) for 1 hour in an incubator humidified with 95% air/5% CO2, washed 3 times with HBSS, and then incubated in that medium for 30 minutes for dye esterification. The cells that show beating property (which are characteristic of cardiomyocytes) were focused for [Ca2+]i measurement. Fluorescence quantitation was performed with the Nikon Eclipse TS100 inverted microscope equipped with a 20× S Fluor objective (Nikon, Japan), Fura-2 filter set (Chroma, Bellows Falls, Vermont), Basler scA640-74fm CCD camera (Basler AG, Germany), and shutter wheel changer (Lambda LS 10-B; Sutter Instruments, Novato, California) controlled by a computer using InCytIm2 imaging software (University of Cincinnati, Ohio). Changes in Fura-2 fluorescence intensities emitted at 2 excitation wavelengths (340 and 380 nm) were acquired, and [Ca2+]i was determined from Fura-2 ratio images using InCytIm2 imaging software (Version 5.0). During each scanning session, 6 individual cardiomyocytes were randomly selected and continuously imaged for 3 minutes to monitor basal [Ca2+]i levels.
Reverse Transcription and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from neonatal heart using TRIzol reagent (Invitrogen, Grand Island, New York) and purified by RNeasy cleanup kit (QIAGEN Inc., Valencia, California). Total RNA quality was determined using an ND-1000 model Nanodrop (Thermo Fisher Scientific, Newark, Delaware). One microgram of total RNA was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, California), and real-time reverse transcription PCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad) with gene-specific primers for L-type Ca2+ channels (Cacna1c, Cacna1d, Cacna1s, and Cacna1f), sarcoplasmic reticulum Ca2+ ATPases (Atp2a1, Atp2a2, and Atp2a3), Na+/Ca2+ exchangers (Slc8A1, Slc8A1, and Slc8A3), ryanodine receptors (Ryr1, 2, and 3), protein kinase C isoforms (Pkc α, δ, and ∊), and transient receptor potential channels (TRPC; Trpc1, Trpc2, Trpc3, Trpc4, Trpc5, Trpc6, and Trpc7). Primers were designed based on the Ensembl Rat genome version Rnor_6.0 using Primer3 and purchased from Integrated DNA Technologies (Coralville, Iowa; Table 1). Results were calculated using the 2−ΔΔCT method and expressed as fold changes in the treatment group versus control. All reactions were performed in duplicate, and β-actin was used as an internal control.
Table 1.
Primer Sequences for L-Type Ca2+ Channels, Sarcoplasmic Ca2+ ATPase, Na+/Ca2+ Exchanger, Ryanodine Receptors, Protein Kinase C Isoforms, and Transient Receptor Potential Channels.
| Gene Name | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Cacna1c | GATTCGATGTGAAGGCACTGAGAGC | ATGGCCTTGATGATGGAGTTCAGGA |
| Cacna1d | AATCAGGAGGCTTTGATGTCAAAGCC | AGGGGGACCATGGCTTTTATAATGGAG |
| Cacna1s | CCATCATCTTGCTCACCATCTTCGC | GAGAAGACGATGAGGAAGAAGTACTCCAG |
| Cacna1f | TGAGCTACCTTGGGTGTACTTTGTGAG | GCTTCTCCCGAAGCTTCTGAAAGTC |
| Ryr1 | CGAGGACGAGGTCCAGTTTTTGCGGACG | ACACAACTTGAGCTGCTCCTTGAGCA |
| Ryr2 | GATGTCAAATCAGCACGAATGGGATCC | TCGTGCTGCGATCTGGATAAGTTCA |
| Ryr3 | AATGCTTGCCAACACCGTTGAAAATGG | CTGAAGGAGTGCCGCAGGAGAATTGCA |
| Pkc α | GATTTACCTGAAGGCAGAGGTCACAGAT | GGGTCAGGAATAAGTTTCAGCTTCACGTA |
| Pkc δ | GCCTTTCTGTGCCGTGAAGATGA | ATAGATGTGGGCGTCGAATGTTGACT |
| Pkc ∊ | GCGGAAACACCCTTATCTAACCCAAC | GGAACATGAGGTCTCCACCGTTTACATA |
| Trpc1 | ATATACTGCAGCTGCTTTTGGACTACGG | GTGATGATCGTTTTGGCCGATGGTTAAG |
| Trpc2 | ATAGCCATGGAGAACCAGATTCCAGTC | ACTTTCCTCCTCTCCTTGTT |
| Trpc3 | GGCCAAAGTAAACCCTGCTTTTACCAC | GACAACAGAAGTCACTTCAGAGAGTCCAAATA |
| Trpc4 | GAGTGGATGATATTACCGTGGGTCCT | CACCAGTCGTGGATGTAATCCTGAAGT |
| Trpc5 | CCAAGCTGAAGGTGGCAATCAAATATCAC | AGTGTTTTCTTCGCCATCCAGGGAA |
| Trpc6 | TTTAGAACTCAGCAATGAGCTGGCAGT | TCCAGGAGACCCACAACAAAATCCTT |
| Trpc7 | GCATCAAACTTGCCATTAAGTACGAAGTCAA | GATAGACTGTTGCCGTAAGCCTGAGAG |
| Atp2a1 | ATGACCATGGCCTTGTCTGTGTTG | AGATGGAACCGAGAAGCCAGATGTT |
| Atp2a2 | TTGCTGGAACTTGTGATCGAGCAGT | GGCTGTAATCGTTTCTTCACCTTCTTCG |
| Atp2a3 | TGTACGTAGGCCTGGCTACAGTG | TGGATTGTCTTCAGAGCACTTCAGGAAG |
| Slc8A1 | CATTCTAGGCGAACACACCAAGCTG | GGCCAGGTTCGTCTTCTTAATGAGTTTG |
| Slc8A2 | GTTTTTAGAGGCAGTTACAGTGAGCGC | AAGTGCATCACGTAGTCAAAGCAGGAT |
| Slc8A3 | GGGAGCTGGAGTTTAAGAATGATGAAACG | CAAGGGCAATGAAGAAATTCTCTTGCCT |
Abbreviations: Atp, ATPases; Pkc, protein kinase C; Ryr, ryanodine receptors; Slc, Na+/Ca2+ exchangers; Trpc: transient receptor potential channels.
Heart Functional Analysis Using Cardiac Ultrasound
At 6 months of age, offspring underwent echocardiography evaluation as reported previously.62 Animals were anesthetized with inhaled continuous isoflurane via facemask. After using a chemical depilatory agent (Nair; Church & Dwight Co., Princeton, New Jersey) to remove chest wall hair, transthoracic M-mode, B-mode, and pulsed Doppler echocardiography were performed using an MS250 probe with a frequency of 13 to 24 MHz and 30-µm resolution to capture 240 frames per second (Vevo 2100; VisualSonics, Toronto, Ontario, Canada). Long-axis measurements at the minor chord dimension were taken in a standard long-axis view. Short-axis measurements were taken just below the level of the midpapillary muscle. Vevo 2100 software was used to calculate end-diastolic and end-systolic interventricular septal thickness, left ventricular percent posterior wall thickness, heart rate, cardiac output, stroke volume, left ventricular mass, and left ventricular ejection fraction and fractional shortening for each animal.
Blood Pressure Measurement by Radiotelemetry
Mean arterial pressure in conscious free-moving rats was determined using the telemetry system as previously described.63 Briefly, rats were anesthetized with 2.5% isoflurane, and a flexible catheter attached to a radio transmitter (TA11PA-C10; Data Sciences, Minneapolis, Minnesota) was inserted into the left femoral artery. After surgery, rats were housed in individual cages and allowed to recover for a week. Blood pressure measurements obtained with a 10-second sampling period were averaged and recorded every 10 minutes, 24 hours a day, using the software (Dataquest 4.0; Data Sciences, St. Paul, Minnesota) provided by the manufacturer. Averaged 24-hour blood pressure was calculated for each animal.
Vascular Reactivity Studies
Rats were killed, and mesenteric arcade was removed and placed directly into ice-cold Krebs buffer (in mM: NaCl, 119; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.17; NaHCO3, 25; KH2PO4, 1.18; EDTA, 0.026; and d-glucose, 5.5; pH 7.4). Under a dissecting microscope, the third-order mesenteric resistance arteries (100-200 µm diameter) were dissected. Mesenteric vessels were cut into 1.5-mm segments, and two 25-µm wires were threaded though the lumen of the vessel segment. One wire was attached to a stationary support driven by a micrometer, whereas the other was attached to an isometric force transducer (Multi Myograph, Model 610M; Danish Myo Technologies, Aarhus, Denmark). The myograph organ bath (6 mL) was filled with Krebs buffer maintained at 37°C and aerated with 95% O2/5% CO2. The vessels were then washed and incubated for 30 minutes under zero force. Then, the rings were normalized to an internal diameter of 0.9 of L13.3kPa using a normalization software package (Myodata; Danish Myotechnology, Aarhus, Denmark). The rings were then assessed for vascular function. The presence of intact endothelium in the vascular preparations was confirmed by observing the relaxation response to acetylcholine (10−6 M; Sigma) in rings precontracted with phenylephrine (10−6 M; Sigma), as described previously.64 Endothelium-dependent relaxation responses to cumulative concentrations of acetylcholine (10−9-10−5 M) in endothelium-intact arterial rings and endothelium-independent relaxation responses to sodium nitroprusside (10−9-10−6 M; Sigma) in rings precontracted with phenylephrine (10−6 M) were determined. In endothelium-denuded arterial rings, vascular contractile responses to cumulative additions of angiotensin II (10−13 to 3 × 10−8 M; Sigma) were determined. Since angiotensin II causes tachyphylaxis, only 1 dose response per arterial ring was measured.
Statistical Analysis
Contractile responses to angiotensin II were calculated as percentages of its maximal contraction. Relaxant responses to acetylcholine were calculated as inhibition percentages of the phenylephrine-induced contraction. Sigmoidal functions were modeled to individual dose–response curves (GraphPad Prism, San Diego, California). Pharmacological concentration–response curves were defined by the negative log concentration that produced half of the maximum effect (pD2) and by the maximum response (E max). Data from multiple offspring of the same dam were averaged and presented as the datum for 1 dam, with the “n” representing the number of dams. Statistical comparisons were made by analysis of variance followed by the Bonferroni post hoc test. All values are expressed as means ± standard error (SE). Differences are considered statistically significant at P < .05.
Results
Maternal Smoking and Smoking Cessation Agents Decrease Fetal and Placental Weights
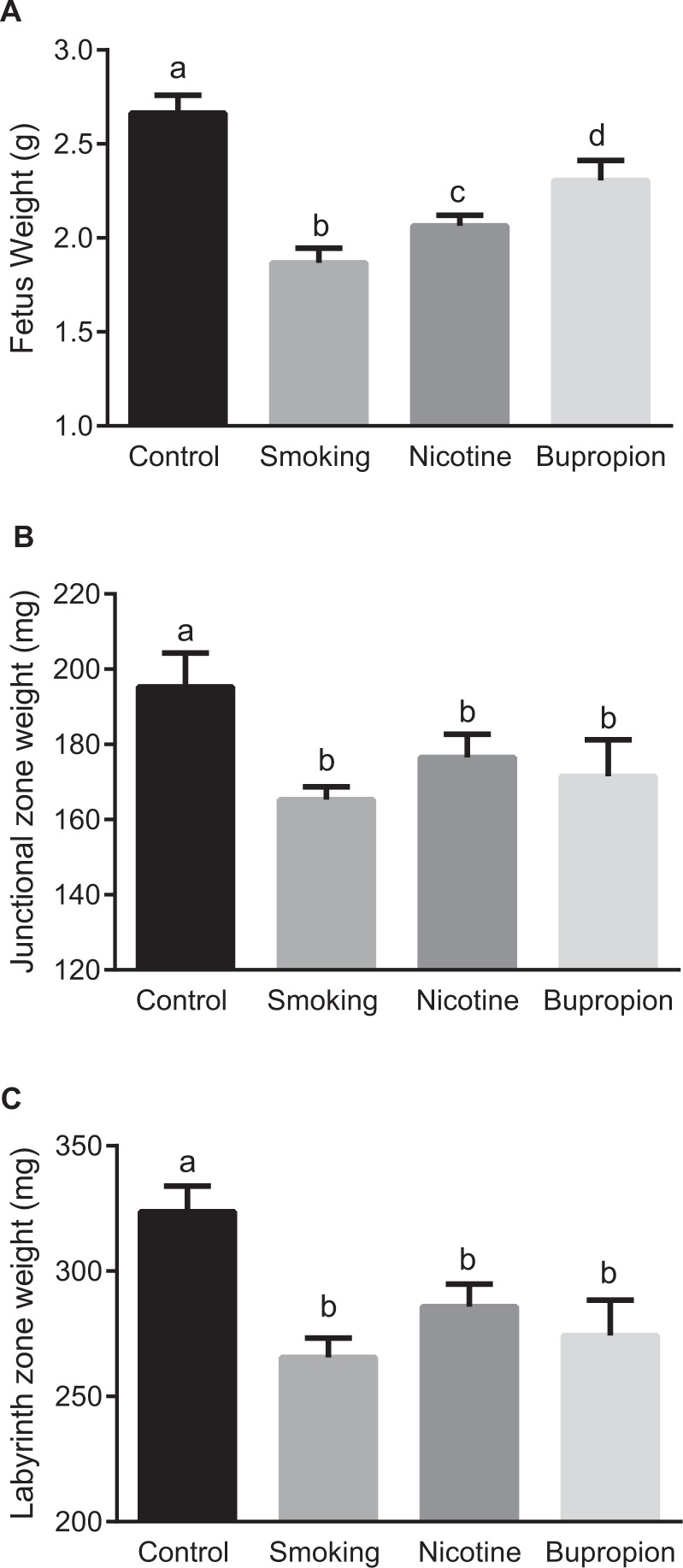
Maternal smoking, nicotine, and bupropion treatments caused significant decreases in body weight of near-term (20-day) fetuses compared with controls; however, the effect was more pronounced in smoking (−17%), followed by nicotine (−9%) and then bupropion (−5%; Figure 1A; P < .05; n = 6 in each group).
Figure 1.
Fetal and placental weights were reduced in pregnant rats exposed to cigarette smoke, nicotine, and bupropion. The animals were killed on gestational day (GD) 20, and (A) fetal, (B) placental junctional zone, and (C) placental labyrinth zone weights were measured. Data are expressed as mean ± standard error of the mean (SEM) of 6 rats in each group. Bars with different alphabet superscripts differ significantly (P < .05).
Placental weights (junctional and labyrinth zones) were significantly lower in smoking, nicotine, and bupropion groups compared with controls (Figure 1B and C; P < .05; n = 6 in each group). No significant difference in weights was found between smoking-, nicotine-, and bupropion-treated placentas (Figure 1B and C; n = 6 in each group).
Compared to controls, maternal body weight at term (GD 20) was significantly decreased by smoking (−16.34%) but not by nicotine (−6.41%) and bupropion (−6.08%) treatments (Supplementary Figure 1). There was no significant difference in the food intake between the control and smoking-, nicotine-, and bupropion-treated groups over the course of gestation (Supplementary Figure 2). No significant differences were noted in mean litter sizes between control (12.4 ± 2.7), smoking (11.7 ± 1.2), nicotine (13.1 ± 1.8), and bupropion (12.4 ± 2.3) dams (n = 6 in each group).
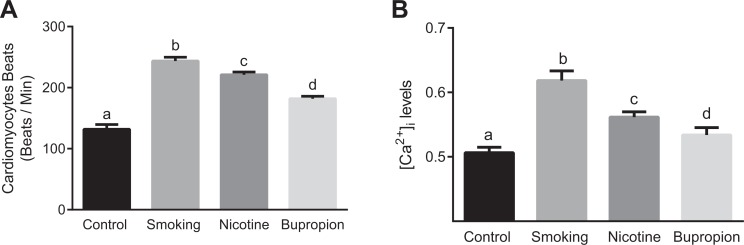
Figure 2.
Maternal cigarette smoke, nicotine, and bupropion exposure increased spontaneous beating and [Ca2+]i in neonatal cardiomyocytes. Cardiomyocytes isolated from day-old pups were examined for (A) spontaneous beating frequency and (B) [Ca2+]i levels. Cultured cardiomyocytes were observed for several minutes under microscope to calculate spontaneous beats per minute. Fura-2-loaded cardiomyocytes were examined for [Ca2+]i levels that is expressed as F340/380 ratio. All data are expressed as mean ± standard error of the mean (SEM); n = 6 in each group. Bars with different alphabet superscripts differ significantly (P < .05).
Increased Spontaneous Beating and [Ca2+]i in Cardiomyocytes
To address the impact of smoking and smoking cessation agents on neonatal cardiomyocyte function, single cells were observed over several minutes to quantify beating frequency and [Ca2+]i levels. Spontaneous beating frequency (Figure 2A) and basal [Ca2+]i levels (Figure 2B) were significantly increased in smoking, nicotine, and bupropion offspring compared to controls (P < .05; n = 6 in each group). The magnitude of increase in beating frequency and basal [Ca2+]i levels was more pronounced in cardiomyocytes of the smoking group than the nicotine group. The beating frequency and basal [Ca2+]i levels in the nicotine group were significantly higher compared to the bupropion group (Figure 2A and B; P < .05; n = 6 in each group).
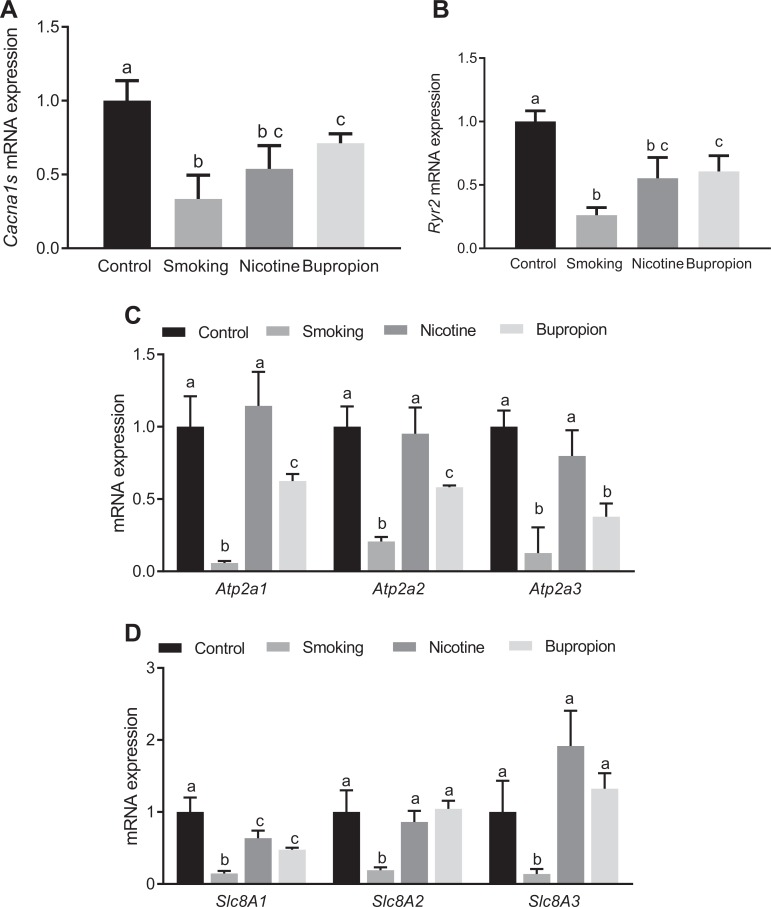
Impaired Expression of Cardiac Signaling Genes in Heart
To determine whether increased spontaneous beating of isolated fetal cardiomyocytes correlated with alteration of excitation–contraction signaling molecules, messenger RNA levels of L-type calcium channels, ryanodine receptors, protein kinase C, and transient receptor potential channels were determined using quantitative real-time PCR. The expression of L-type calcium channel Cacna1s (Figure 3A) and ryanodine receptor Ryr2 (Figure 3B) showed significant downregulation in the smoking-, nicotine-, and bupropion-treated groups compared to controls (P < .05; n = 6 in each group). The expression of L-type calcium channel Cacna1c was not different between the control and treatment groups (data not shown), and other L-type calcium channels (Cacna1d and Cacna1f) and ryanodine receptors (Ryr1 and Ryr3) were below detectable limits. Moreover, Ca2+ ATPases (Atp2a1, Atp2a2, and Atp2a3) were significantly downregulated in the smoking and bupropion groups (P < .05; n = 6 in each group) but not in the nicotine group compared to controls (Figure 3C). The Na+/Ca2+ exchangers (Slc8A1, Slc8A2, and Slc8A3) were downregulated in the smoking group compared with controls, and only Slc8A1 was downregulated in the nicotine and bupropion groups compared with controls (P < .05; n = 6 in each group; Figure 3D).
Figure 3.
Maternal cigarette smoking, nicotine, and bupropion exposure decreased L-type calcium channel and ryanodine receptor messenger RNA (mRNA) expression in neonatal hearts. Real-time reverse transcriptase polymerase chain reaction (PCR) was used to assess (A) Cacna1s (calcium channel, voltage-dependent, L-type, alpha-1S subunit), (B) Ryr2 (ryanodine receptor), (C) Ca2+ ATPases (Atp2a1, Atp2a2, and Atp2a3), and (D) Na+/Ca2+ exchangers (Slc8A1, Slc8A1, and Slc8A3) mRNA expression. Quantitation of fold change in Cacna1s, Ryr2, Atp2a1, Atp2a2 Slc8A1, Slc8A1, and Slc8A3 was normalized relative to β-actin levels. Values are given as means ± standard error of the mean (sem) of 6 rats in each group. Bars with different alphabet superscripts differ significantly (P < .05).
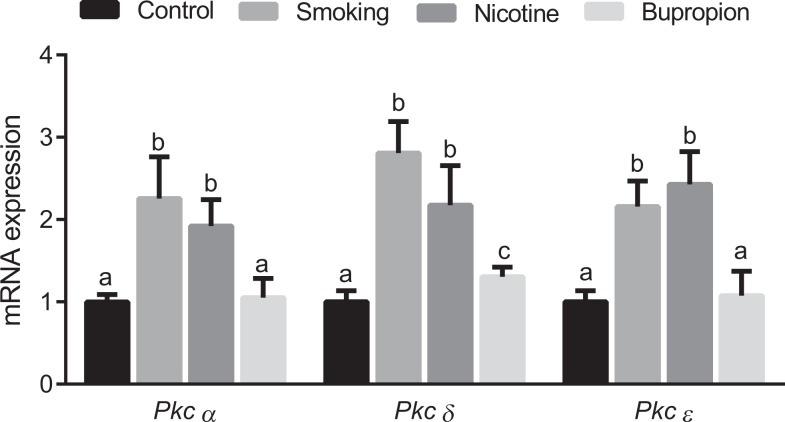
Expression of protein kinase C-α, -δ, and -∊ was significantly higher in the smoking- and nicotine-exposed groups compared to controls (Figure 4; P < .05; n = 6 in each group). Only protein kinase C-δ was significantly higher in the bupropion group compared to controls (Figure 4; P < .05; n = 6 in each group). Overall, the expression of protein kinase C was less affected by bupropion treatment compared to smoking and nicotine exposures.
Figure 4.
Maternal cigarette smoke, nicotine, and bupropion exposure increased protein kinases C isoforms messenger RNA (mRNA) expression in neonatal hearts. Real-time reverse transcriptase polymerase chain reaction (PCR) was used to assess protein kinase C-α, -δ, and -∊ mRNA expression. Quantitation of fold change in protein kinase C was normalized relative to β-actin levels. Values are given as means ± standard error of the mean (sem) of 6 rats in each group. Bars with different alphabet superscripts differ significantly (P < .05).
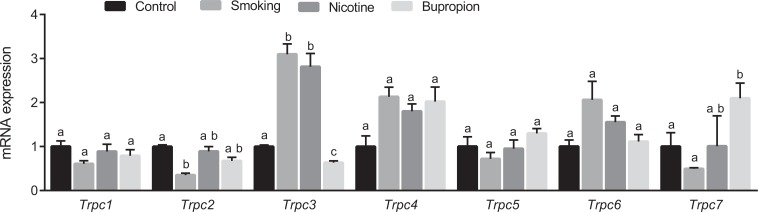
Transient receptor potential channels were differentially expressed depending on exposure. In smoking offspring, Trpc2 was downregulated, whereas Trpc3 was upregulated. In the nicotine offspring, Trpc3 was upregulated. In the bupropion offspring, Trpc3 was downregulated and Trpc7 was upregulated (Figure 5; P < .05; n = 6 in each group).
Figure 5.
Maternal cigarette smoke, nicotine, and bupropion exposure dysregulates messenger RNA (mRNA) of transient receptor potential channels (Trpc1, Trpc2, Trpc3, Trpc4, Trpc6, and Trpc7) in the neonatal heart. Quantitation of fold change in Trpc was normalized relative to β-actin levels. Values are given as means ± standard error of the mean (sem) of 6 rats in each group. Bars with different alphabet superscripts differ significantly (P < .05).
Cardiac Dysfunction in Adult Offspring
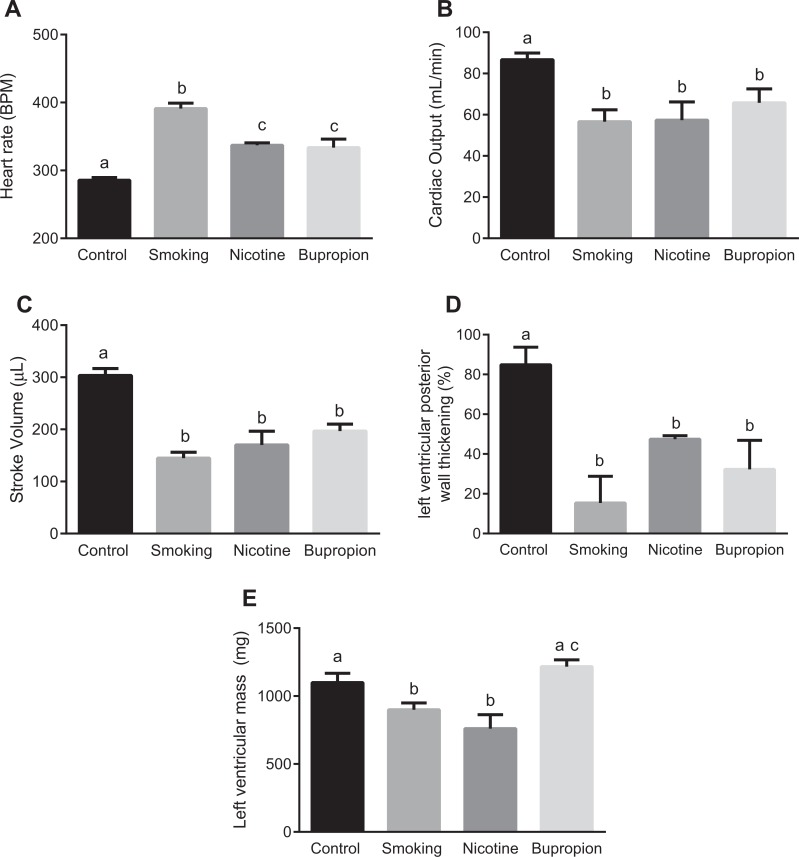
We next examined the cardiac function in 6-month-old adult offspring using ultrasound. At 6 months of age, the offspring’s body weights were not significantly different between the control and treatment groups (control: 506.5 ± 8.07, smoking: 495.5 ± 12.10, nicotine: 498.5 ± 4.15, and bupropion: 508.833 ± 5.98). Heart rate was significantly higher in smoking, nicotine, and bupropion offspring compared to controls; however, the magnitude of increase was greater in smoking than nicotine and bupropion offspring (Figure 6A; P < .05; n = 6 in each group). No significant difference was found in heart rate between nicotine and bupropion offspring (Figure 6A). Cardiac output, stroke volume, and left ventricular percent posterior wall thickening were significantly decreased in smoking, nicotine, and bupropion offspring compared to controls (Figure 6B-D; P < .05; n = 6 in each group). Compared to controls, the left ventricular mass was significantly reduced in smoking and nicotine but not in bupropion offspring (Figure 6E; P < .05; n = 6 in each group). There were no significant differences in interventricular septal thickness, ejection fraction, and fractional shortening between groups (data not shown).
Figure 6.
Cardiac morphology and functions were altered in offspring of dams exposed to cigarette smoke, nicotine, and bupropion. Cardiac morphology was analyzed in 6-month-old offspring by echocardiography to assess (A) heart rate, (B) cardiac output, (C) stroke volume, (D) left ventricular percent posterior wall thickening, and (E) left ventricular mass. Values are given as means ± standard error of the mean (sem) of 6 rats in each group. Bars with different alphabet superscripts differ significantly (P < .05).
Elevated Blood Pressure in the Adult Offspring
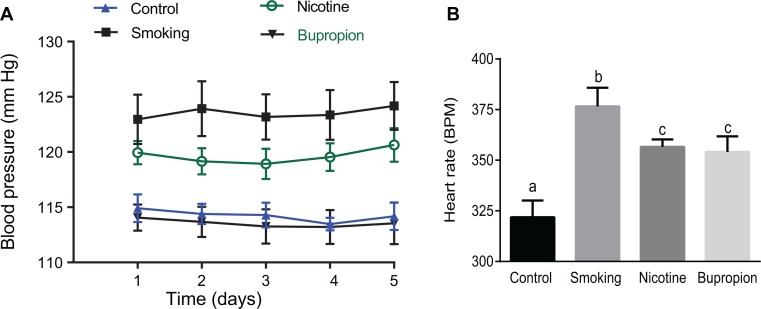
Blood pressure was significantly higher in the 6-month-old smoking (124 ± 2.22 mm Hg) and nicotine (120 ± 1.13 mm Hg) offspring compared to controls (115 ± 1.2 mm Hg); however, the magnitude of increase was significantly higher in the smoking (+9 mm Hg) compared to nicotine (+5 mm Hg) offspring (Figure 7A; P < .05; n = 6 in each group). The arterial pressure in the bupropion offspring (114 ± 2.1 mm Hg) was comparable to that in the controls (115 ± 1.2 mm Hg; Figure 7A; P < .05; n = 6 in each group). Heart rate recorded from conscious free-moving rats was significantly higher in smoking, nicotine, and bupropion offspring compared to controls; however, the magnitude of increase was greater in smoking than nicotine and bupropion offspring (Figure 7B; P < .05; n = 6 in each group). No significant difference was found in heart rate between nicotine and bupropion offspring (Figure 7B).
Figure 7.
Maternal smoke and nicotine but not bupropion exposure increased blood pressure in adult offspring. Heart rate was significantly higher in smoking, nicotine, and bupropion offspring compared to controls. (A) Blood pressure and (B) heart rate were measured 24 hours per day by telemetry at 6 months of age for 5 days. Values are given as means ± standard error of the mean (sem) of 6 rats in each group. Bars with different alphabet superscripts differ significantly (P < .05).
Decreased Endothelium-Dependent Relaxation
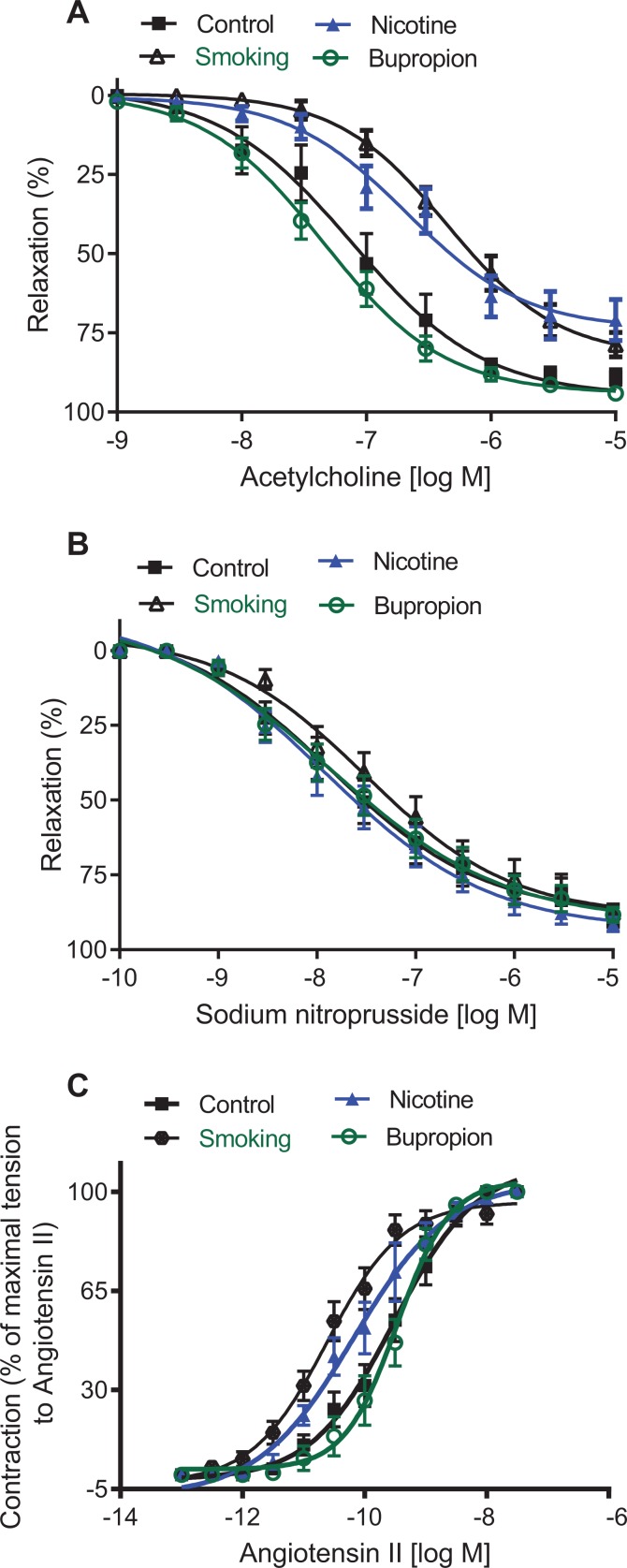
Endothelium-dependent acetylcholine-induced relaxation in mesenteric arterial rings was significantly reduced in smoking- (pD2 = 6.13 ± 0.09) and nicotine-treated groups (pD2 = 6.33 ± 0.12) compared to their controls (pD2 = 7.03 ± 0.12; Figure 8A; P < .05; n = 6 in each group). Besides, the maximal vascular relaxation to acetylcholine was significantly decreased in smoking (Emax: 78.71% ± 3.81%) and nicotine exposure (Emax: 71.35% ± 6.55%) compared to controls (Emax: 98.68% ± 0.73%; Figure 8A; P < .05; n = 6 in each group). On the other hand, acetylcholine-induced relaxation in the bupropion offspring was not significantly altered (pD2 = 7.27 ± 0.11; Emax: 94.28 ± 1.62%) compared to controls (Figure 8A; n = 6 in each group). Endothelium-independent vascular relaxation to sodium nitroprusside in mesenteric arteries was not significantly different between groups (Figure 8B; n = 6 in each group).
Figure 8.
Maternal smoke and nicotine but not bupropion exposure altered vascular function in adult offspring. Mesenteric arterial rings were isolated from 6-month-old rats to assess (A) endothelium-dependent relaxation and (B) endothelium-independent relaxation. Endothelium-intact mesenteric arterial rings were precontracted with phenylephrine (10−6 M) and examined for relaxation to cumulative additions of acetylcholine or sodium nitroprusside and (C) vascular contractile responses. Endothelium-denuded mesenteric arterial rings were contracted to cumulative additions of angiotensin II. Values are given as means ± standard error of the mean (Sem) of 6 rats in each group.
Exaggerated Contractile Response to Angiotensin II
Angiotensin II induced a dose-dependent increase in contractile responses in endothelium-denuded mesenteric arterial rings in all 4 groups. However, angiotensin II-mediated dose-dependent contractions were exaggerated, with a leftward shift in smoking (pD2 = 10.62 ± 0.12) and nicotine (pD2 = 10.12 ± 0.15) offspring compared to controls (pD2 = 9.51 ± 0.11; Figure 8C; P < .05; n = 6 in each group). The pD2 for angiotensin II was significantly higher in arteries of smoking than nicotine offspring. Angiotensin II-induced contractile responses were comparable between bupropion offspring (pD2 = 9.47 ± 0.10) and controls (Figure 8C; n = 6 in each group).
Discussion
For the first time, this study has demonstrated that cigarette smoking during pregnancy in rats leads to cardiac abnormalities and hypertension in the adult offspring, with an associated decrease in endothelium-dependent relaxation and exaggerated vascular contractile responses to angiotensin II. Maternal nicotine administration at concentrations used for NRT also induced similar cardiac and vascular abnormities in the offspring, albeit at a lesser magnitude than maternal smoking. However, bupropion administration caused minimal fetal and adult cardiac abnormalities but no adverse changes in vascular function and blood pressure. These results indicate that the use of NRT has significant cardiovascular risk, whereas bupropion has less risk for the development of adult-onset cardiovascular dysfunctions than nicotine; however, translation of these findings to human warrants further studies.
In our study, we observed that maternal smoking, nicotine, and bupropion exposure during pregnancy induced fetal growth restriction, which is in accordance with previous human and animal studies.28,33,65–68 In addition, the present study revealed the magnitude of fetal growth restriction is different between maternal exposures, with a more pronounced effect in the smoking group, followed by the nicotine and then bupropion groups. These adverse effects on fetal growth could be from indirect action of these maternal exposures on the placenta. This theory is supported by the fact that maternal smoking causes lower placental weights and changes essential features of placental function, such as progesterone production,69 estrogen metabolism,70 and amino acid transport.71 In addition, nicotine is shown to have a direct vasoconstrictive effect on pregnant uterine arteries33 and decrease uterine blood flow to the fetoplacental unit.72 These changes may conceivably have a detrimental effect contributing for fetal growth restriction in smoking and nicotine offspring. On the other hand, these agents can directly cause fetal damage. Indeed, studies have shown that nicotine readily crosses the placenta, and maternal cigarette smoking produces higher nicotine concentrations in fetal circulation than that experienced by the mother.73,74 Bupropion is also known to cross placenta.75 Thus, it is possible that fetal growth restriction may have an indirect effect on placental function and a direct effect on fetal organogenesis.
It is generally accepted that smoking and smoking cessation drugs mediate their therapeutic effects via specific interaction with a neurotransmitter signaling event in the central nervous system.42,43,76–78 However, most of these agents also interact with ion channels, which make these agents candidates for cardiotoxicity during embryonic development. In the present study, the spontaneous beating of isolated neonatal cardiomyocytes was increased following smoking, nicotine, and bupropion treatments, which is consistent with the increased fetal heart rate observed in smoking mothers,79 maternal nicotine treatments in rats, and chewing nicotine gums in humans.80,81 In addition, bupropion is known to induce fetal arrhythmias.82 The increased beating observed ex vivo in cardiomyocytes indicates that this effect is independent of endogenous factors and possibly involves some inherent alterations in cardiomyocyte function. An increase in intracellular Ca2+ via the L-type Ca2+ channels, sarcoplasmic reticulum Ca2+ ATPase, and the ryanodine receptors is an important cellular process for excitation–contraction coupling.83 Intriguingly, the expressions of Cacna1s and Ryr2 in neonatal hearts were lower following exposure to smoking, nicotine, and bupropion, yet the spontaneous beating of the cardiomyocytes and [Ca2+]i levels were higher. It is unclear whether the decreases in Cacna1s and Ryr2 expressions are an attempt to compensate for increased beating frequency and [Ca2+]i levels. Thus, other mechanisms, possibly the increased expression of protein kinases C and TRPCs and decreased expression of Ca2+ ATPases and Na+/Ca2+ exchangers observed in the smoking-, nicotine-, and bupropion-exposed fetuses, may play a role in contributing to increased spontaneous beating and [Ca2+]i in cardiomyocytes.84–87 The increased expression of TRPCs (which are known to increase Ca2+ influx through activation of receptor-activated and store-operated mechanisms)86,87 may play a role in increasing [Ca2+]i in cardiomyocytes of smoking, nicotine, and bupropion offspring. Indeed, studies have shown that nicotine increases [Ca2+]i through upregulation and activation of Trpc3.88 It is interesting to note that the minimal upregulation of protein kinase C in bupropion-exposed neonatal hearts correlates well with the marginal increase in beating frequency. Also, the decreased expression of Ca2+ ATPase and Na2+/Ca2+ exchanger may contribute to the decrease in reuptake of Ca2+ into the sarcoplasmic reticulum or removal by the sarcolemma leading to increase in [Ca2+]i. Further studies that delineate the exact signaling mechanism, including measurement of intracellular Ca2+ transients with selective inhibitors,89 may help us understand the underlying changes that contribute to increased beating in cardiomyocytes and [Ca2+]i of smoking, nicotine, and bupropion groups.
It is now well established that a variety of insults, when experienced in the prenatal period, can have long-term influences on the health of the individual. In the present study, the increased beating frequency of neonatal cardiomyocytes was maintained through adult life as evidenced by the increased heart rates in smoking, nicotine, and bupropion offspring. Whether altered control of cardiac sympathetic and parasympathetic activity or other mechanisms contribute to increased heart rate is not clear.80 Prenatal nicotine is shown to alter the tone of cholinergic neurons through a programmed disruption, suggesting a possible deficiency in cholinergic activity in smoking/nicotine offspring.90 The decreased cardiac output in the smoking, nicotine, and bupropion offspring may presumably be due to decreased stroke volume. In addition, this study identified changes in heart morphology, including decreased left ventricular percent posterior wall thickness in smoking, nicotine, and bupropion offspring. This decrease in ventricular wall thickness, together with the finding of decreased left ventricular mass, suggests that maternal smoking and nicotine may compromise myocardial development and function. Consistent with this notion, previous studies show that maternal smoking and nicotine decreased cardiomyocyte numbers in the fetal heart,91 reduced the size of embryonic organs—including heart,91,92 and increased risks for congenital heart defects.93 Also studies have shown that maternal nicotine exposure leads to elevated left ventricle myocardial infarct size and decreased post-ischemic recovery following ischemia in the offspring.34 It is also possible that the impaired cardiac function may be secondary to altered respiratory, metabolic, and hemodynamic functions.30 The exact causes of impaired cardiac parameters are unknown, and further investigation at an ultrastructural, biochemical, and genetic basis may prove to be revealing.
The adult offspring of both smoke- and nicotine-exposed dams exhibited increased blood pressure. This result is consistent with the reports in children of smoking mothers7–12,94 and offspring of nicotine-exposed pregnant rats.27,95–97 However, this study provides new data that the magnitude of hypertension is different between smoking and nicotine offspring, with smoking offspring having a more pronounced effect (mean increase of 9 mm Hg in smoking vs 5 mm Hg in nicotine offspring). This outcome suggests that the smoking offspring are more susceptible and hypertension is more severe than in nicotine offspring. Interestingly, bupropion offspring had no blood pressure alterations, despite the fact that bupropion treatment has been reported to cause increased blood pressure in nonpregnant humans.98
To dissect the contribution of the vasculature to the altered blood pressure changes, we examined alterations in vascular function in the offspring. Responses to acetylcholine, an endothelium-dependent vasodilator, to sodium nitroprusside, an endothelium-independent vasodilator, and to angiotensin II, a vasoconstrictor, were investigated. Mesenteric vasorelaxation to acetylcholine was reduced in smoking and nicotine offspring compared to controls, but no differences could be detected to sodium nitroprusside. These observations suggest that it is not the smooth muscle vasodilating capability that is reduced in smoking and nicotine offspring but some function related to the endothelium. This result is in accordance with previous reports of altered endothelial function in adult offspring of maternal nicotine-exposed dams.28,33 In the present study, angiotensin II-induced contractile responses were significantly increased in smoking and nicotine offspring compared to controls. Because smoke and nicotine exposure caused increases in angiotensin II-induced contractions in the absence of functional endothelium, we suggest the enhanced arterial sensitivity to angiotensin II primarily occurred in the vascular smooth muscle cells. It is possible that the vascular effects could be primary effects or secondary effects related to differences in blood pressure. Other studies in prenatally nicotine-exposed rats have also reported exaggerated vasomotor responses to angiotensin II in other vascular beds, such as the aorta.28 The magnitude of vascular dysfunction is different between smoking and nicotine offspring, with smoking offspring having more pronounced impairment in endothelium-dependent relaxation and greater exaggeration in vasoconstriction to angiotensin II, which relates to a proportional increase in blood pressure. Although smoke and nicotine exposure in pregnant rats achieved comparable increases in plasma cotinine levels similar to those in moderate human smokers51,52 and in humans who use transdermal nicotine patch,54 the exaggerated adverse cardiovascular effect in the smoking offspring may be attributed to additional toxic components of cigarette smoking.35,36 The finding that endothelium-dependent relaxation response and angiotensin II-induced contractile response are preserved in the bupropion offspring suggests that the vascular function is not affected by developmental exposure to bupropion.
By controlling for external confounders present in human epidemiological studies, this study provides strong evidence of adverse consequences of maternal smoking on the cardiovascular health of the offspring, implicating the need to develop strategies that facilitate smoking cessation for pregnant women. The magnitude of outcomes in the fetus (fetal weight and beating frequency of cardiomyocytes) and adults (cardiac dysfunction, blood pressure, and vascular relaxation and contraction) is more severe in the smoking than in the nicotine offspring, yet the consequences are significant enough to raise potential safety concerns for the use of NRT during pregnancy. There was fetal growth restriction and cardiac derangement; however, the lack of adverse effects of developmental exposure to bupropion on adult life vascular function and blood pressure control is novel and intriguing. Similar findings that maternal bupropion does not cause significant metabolic and reproductive dysfunction in the offspring have been reported.68 Although animal studies are reassuring, further clinical studies are required to evaluate the safety and efficacy of bupropion for smoking cessation during pregnancy.
Supplementary Material
Acknowledgments
The authors thank Drs. Bill Ameredes and Lance Hallberg of the Sealy Center for Environmental Health and Medicine and the Department of Preventive Medicine and Community Health for helping with cigarette smoking exposure at the inhalation facility.
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support from the National Institute of Health (NIH) through grants HL119869 (S.K.), and DA030998 and HD047891 (T.N.N.) is greatly appreciated.
Supplemental Material: The online supplemental figures are available at http://journals.sagepub.com/doi/suppl/10.1177/1933719116673199.
References
- 1. Einarson A, Riordan S. Smoking in pregnancy and lactation: a review of risks and cessation strategies. Eur J Clin Pharmacol. 2009;65(4):325–330. [DOI] [PubMed] [Google Scholar]
- 2. Rogers J. Tobacco and pregnancy. Reprod Toxicol. 2009;28(2):152–160. [DOI] [PubMed] [Google Scholar]
- 3. Horta BL, Victora CG, Menezes AM, Halpern R, Barros FC. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol. 1997;11(2):140–151. [DOI] [PubMed] [Google Scholar]
- 4. Antonopoulos CN, Sergentanis TN, Papadopoulou C, et al. Maternal smoking during pregnancy and childhood lymphoma: a meta-analysis. Int J Cancer. 2011;129(11):2694–2703. [DOI] [PubMed] [Google Scholar]
- 5. Durmus B, Kruithof CJ, Gillman MH, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R Study. Am J Clin Nutr. 2011;94(1):164–171. [DOI] [PubMed] [Google Scholar]
- 6. Haynes A, Cooper MN, Bower C, Jones TW, Davis EA. Maternal smoking during pregnancy and the risk of childhood type 1 diabetes in Western Australia. Diabetologia. 2014;57(3):469–472. [DOI] [PubMed] [Google Scholar]
- 7. Blake KV, Gurrin LC, Evans SF, et al. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev. 2000;57(2):137–147. [DOI] [PubMed] [Google Scholar]
- 8. Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Davey SG. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater-University study of pregnancy and its outcomes. Circulation. 2004;110(16):2417–2423. [DOI] [PubMed] [Google Scholar]
- 9. Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13(11):2021–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawlor DA, Smith GD. Early life determinants of adult blood pressure. Curr Opin Nephrol Hypertens. 2005;14(3):259–264. [DOI] [PubMed] [Google Scholar]
- 11. Geerts CC, Grobbee DE, van der Ent CK, et al. Tobacco smoke exposure of pregnant mothers and blood pressure in their newborns: results from the wheezing illnesses study Leidsche Rijn Birth Cohort. Hypertension. 2007;50(3):572–578. [DOI] [PubMed] [Google Scholar]
- 12. Hogberg L, Cnattingius S, Lundholm C, D’Onofrio BM, Langstrom N, Iliadou AN. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. J Hypertens. 2012;30(4):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brion MJ, Leary SD, Smith GD, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49(6):1422–1428. [DOI] [PubMed] [Google Scholar]
- 14. Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14(8):935–941. [PubMed] [Google Scholar]
- 15. Whincup PH, Cook DG, Shaper AG. Early influences on blood pressure: a study of children aged 5-7 years. BMJ. 1989;299(6699):587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morley R, Leeson PC, Lister G, Lucas A. Maternal smoking and blood pressure in 7.5 to 8 year old offspring. Arch Dis Child. 1995;72(2):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brion MJ, Leary SD, Lawlor DA, Smith GD, Ness AR. Modifiable maternal exposures and offspring blood pressure: a review of epidemiological studies of maternal age, diet, and smoking. Pediatr Res. 2008;63(6):593–598. [DOI] [PubMed] [Google Scholar]
- 18. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25(2):95–101. [DOI] [PubMed] [Google Scholar]
- 19. Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009;(3):CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filion KB, Abenhaim HA, Mottillo S, et al. The effect of smoking cessation counselling in pregnant women: a meta-analysis of randomised controlled trials. BJOG. 2011;118(12):1422–1428. [DOI] [PubMed] [Google Scholar]
- 21. Wong S, Ordean A, Kahan M. Substance use in pregnancy. J Obstet Gynaecol Can. 2011;33(4):367–384. [DOI] [PubMed] [Google Scholar]
- 22. Cooper S, Lewis S, Thornton JG, et al. The SNAP trial: a randomised placebo-controlled trial of nicotine replacement therapy in pregnancy–clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technol Assess. 2014;18(54):1–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oncken CA, Hardardottir H, Hatsukami DK, Lupo VR, Rodis JF, Smeltzer JS. Effects of transdermal nicotine or smoking on nicotine concentrations and maternal-fetal hemodynamics. Obstet Gynecol. 1997;90(4 pt 1):569–574. [DOI] [PubMed] [Google Scholar]
- 24. Lindblad A, Marsal K, Andersson KE. Effect of nicotine on human fetal blood flow. Obstet Gynecol. 1988;72(3 pt 1):371–382. [PubMed] [Google Scholar]
- 25. Windsor R, Oncken C, Henningfield J, Hartmann K, Edwards N. Behavioral and pharmacological treatment methods for pregnant smokers: issues for clinical practice. J Am Med Womens Assoc. 2000;55(5):304–310. [PubMed] [Google Scholar]
- 26. Swamy GK, Roelands JJ, Peterson BL, et al. Predictors of adverse events among pregnant smokers exposed in a nicotine replacement therapy trial. Am J Obstet Gynecol. 2009;201(4):354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51(4):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao D, Huang X, Lawrence J, Yang S, Zhang L. Fetal and neonatal nicotine exposure differentially regulates vascular contractility in adult male and female offspring. J Pharmacol Exp Ther. 2007;320(2):654–661. [DOI] [PubMed] [Google Scholar]
- 29. Xiao D, Dasgupta C, Li Y, Huang X, Zhang L. Perinatal nicotine exposure increases angiotensin ii receptor-mediated vascular contractility in adult offspring. PLoS One. 2014;9(9):e108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116(2):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiao D, Wang L, Huang X, Li Y, Dasgupta C, Zhang L. Protective effect of antenatal antioxidant on nicotine-induced heart ischemia-sensitive phenotype in rat offspring. PLoS One. 2016;11(2):e0150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiao D, Huang X, Li Y, Dasgupta C, Wang L, Zhang L. Antenatal antioxidant prevents nicotine-mediated hypertensive response in rat adult offspring. Biol Reprod. 2015;93(3):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao D, Huang X, Yang S, Zhang L. Direct effects of nicotine on contractility of the uterine artery in pregnancy. J Pharmacol Exp Ther. 2007;322(1):180–185. [DOI] [PubMed] [Google Scholar]
- 34. Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther. 2008;324(1):331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stedman RL. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68(2):153–207. [DOI] [PubMed] [Google Scholar]
- 36. Swauger JE, Steichen TJ, Murphy PA, Kinsler S. An analysis of the mainstream smoke chemistry of samples of the U.S. cigarette market acquired between 1995 and 2000. Regul Toxicol Pharmacol. 2002;35(2 pt 1):142–156. [DOI] [PubMed] [Google Scholar]
- 37. Brose LS, McEwen A, West R. Association between nicotine replacement therapy use in pregnancy and smoking cessation. Drug Alcohol Depend. 2013;132(3):660–664. [DOI] [PubMed] [Google Scholar]
- 38. Alwan S, Reefhuis J, Rasmussen SA, Friedman JM; National Birth Defects Prevention Study. Patterns of antidepressant medication use among pregnant women in a United States population. J Clin Pharmacol. 2011;51(2):264–270. [DOI] [PubMed] [Google Scholar]
- 39. Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;(1):CD000031. [DOI] [PubMed] [Google Scholar]
- 40. Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340(9):685–691. [DOI] [PubMed] [Google Scholar]
- 41. Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. J Addict Dis. 2005;24(2):19–23. [DOI] [PubMed] [Google Scholar]
- 42. Paterson NE. Behavioural and pharmacological mechanisms of bupropion’s anti-smoking effects: recent preclinical and clinical insights. Eur J Pharmacol. 2009;603(1-3):1–11. [DOI] [PubMed] [Google Scholar]
- 43. Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295(1):321–327. [PubMed] [Google Scholar]
- 44. Cressman AM, Pupco A, Kim E, Koren G, Bozzo P. Smoking cessation therapy during pregnancy. Can Fam Physician. 2012;58(5):525–527. [PMC free article] [PubMed] [Google Scholar]
- 45. Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078. [DOI] [PubMed] [Google Scholar]
- 46. Rigotti NA, Park ER, Chang Y, Regan S. Smoking cessation medication use among pregnant and postpartum smokers. Obstet Gynecol. 2008;111(2 pt 1):348–355. [DOI] [PubMed] [Google Scholar]
- 47. Anderka M, Romitti PA, Sun L, Druschel C, Carmichael S, Shaw G; National Birth Defects Prevention Study. Patterns of tobacco exposure before and during pregnancy. Acta Obstet Gynecol Scand. 2010;89(4):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nekhayeva IA, Nanovskaya TN, Pentel PR, Keyler DE, Hankins GD, Ahmed MS. Effects of nicotine-specific antibodies, Nic311 and Nic-IgG, on the transfer of nicotine across the human placenta. Biochem Pharmacol. 2005;70(11):1664–1672. [DOI] [PubMed] [Google Scholar]
- 49. Earhart AD, Patrikeeva S, Wang X, et al. Transplacental transfer and metabolism of bupropion. J Matern Fetal Neonatal Med. 2010;23(5):409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X, Abdelrahman DR, Zharikova OL, et al. Bupropion metabolism by human placenta. Biochem Pharmacol. 2010;79(11):1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. [DOI] [PubMed] [Google Scholar]
- 52. Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285(3):931–945. [PubMed] [Google Scholar]
- 53. Benowitz NL, Zevin S, Jacob P., III Sources of variability in nicotine and cotinine levels with use of nicotine nasal spray, transdermal nicotine, and cigarette smoking. Br J Clin Pharmacol. 1997;43(3):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gariti P, Alterman AI, Barber W, Bedi N, Luck G, Cnaan A. Cotinine replacement levels for a 21 mg/day transdermal nicotine patch in an outpatient treatment setting. Drug Alcohol Depend. 1999;54(2):111–116. [DOI] [PubMed] [Google Scholar]
- 55. Zhu AZ, Cox LS, Nollen N, et al. CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther. 2012;92(6):771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suckow RF, Smith TM, Perumal AS, Cooper TB. Pharmacokinetics of bupropion and metabolites in plasma and brain of rats, mice, and guinea pigs. Drug Metab Dispos. 1986;14(6):692–697. [PubMed] [Google Scholar]
- 57. Wang X, Vernikovskaya DI, Abdelrahman DR, Hankins GD, Ahmed MS, Nanovskaya TN. Simultaneous quantitative determination of bupropion and its three major metabolites in human umbilical cord plasma and placental tissue using high-performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2012;70:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peng M, Huang L, Xie Z, Huang WH, Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J Biol Chem. 1996;271(17):10372–10378. [DOI] [PubMed] [Google Scholar]
- 59. Gopalakrishnan K, Morgan EE, Yerga-Woolwine S, et al. Augmented rififylin is a risk factor linked to aberrant cardiomyocyte function, short-QT interval and hypertension. Hypertension. 2011;57(4):764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Webster DR, Patrick DL. Beating rate of isolated neonatal cardiomyocytes is regulated by the stable microtubule subset. Am J Physiol Heart Circ Physiol. 2000;278(5):H1653–H1661. [DOI] [PubMed] [Google Scholar]
- 61. Sathishkumar K, Yallampalli U, Elkins R, Yallampalli C. Raf-1 kinase regulates smooth muscle contraction in the rat mesenteric arteries. J Vasc Res. 2010;47(5):384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Poole AT, Vincent KL, Olson GL, et al. Effect of lactation on maternal postpartum cardiac function and adiposity: a murine model. Am J Obstet Gynecol. 2014;211(4):424.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chinnathambi V, Yallampalli C, Sathishkumar K. Prenatal testosterone induces sex-specific dysfunction in endothelium-dependent relaxation pathways in adult male and female rats. Biol Reprod. 2013;89(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chinnathambi V, Selvanesan BC, Vincent KL, et al. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. 2014;64(2):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5(3):231–241. [DOI] [PubMed] [Google Scholar]
- 66. Abel EL. Smoking during pregnancy: a review of effects on growth and development of offspring. Hum Biol. 1980;52(4):593–625. [PubMed] [Google Scholar]
- 67. Perkins SL, Belcher JM, Livesey JF. A Canadian tertiary care centre study of maternal and umbilical cord cotinine levels as markers of smoking during pregnancy: relationship to neonatal effects. Can J Public Health. 1997;88(4):232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. De LN, Hyslop JR, Nicholson CJ, Morrison KM, Gerstein HC, Holloway AC. Postnatal metabolic and reproductive consequences of fetal and neonatal exposure to the smoking cessation drug bupropion. Reprod Sci. 2013;20(10):1156–1161. [DOI] [PubMed] [Google Scholar]
- 69. Piasek M, Blanusa M, Kostial K, Laskey JW. Placental cadmium and progesterone concentrations in cigarette smokers. Reprod Toxicol. 2001;15(6):673–681. [DOI] [PubMed] [Google Scholar]
- 70. Zhu BT, Cai MX, Spink DC, et al. Stimulatory effect of cigarette smoking on the 15 alpha-hydroxylation of estradiol by human term placenta. Clin Pharmacol Ther. 2002;71(5):311–324. [DOI] [PubMed] [Google Scholar]
- 71. Pastrakuljic A, Derewlany LO, Koren G. Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta. 1999;20(7):499–512. [DOI] [PubMed] [Google Scholar]
- 72. Resnik R, Brink GW, Wilkes M. Catecholamine-mediated reduction in uterine blood flow after nicotine infusion in the pregnant ewe. J Clin Invest. 1979;63(6):1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20(2):115–126. [DOI] [PubMed] [Google Scholar]
- 74. Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8(6):384–395. [DOI] [PubMed] [Google Scholar]
- 75. Fokina VM, West H, Oncken C, et al. Bupropion therapy during pregnancy: the drug and its major metabolites in umbilical cord plasma and amniotic fluid. Am J Obstet Gynecol. 2016;215(4):497.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99–110. [PubMed] [Google Scholar]
- 77. Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97(10):1331–1337. [DOI] [PubMed] [Google Scholar]
- 78. Janes AC, Jensen JE, Farmer SL, Frederick BD, Pizzagalli DA, Lukas SE. GABA levels in the dorsal anterior cingulate cortex associated with difficulty ignoring smoking-related cues in tobacco-dependent volunteers. Neuropsychopharmacology. 2013;38(6):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oncken C, Kranzler H, O’Malley P, Gendreau P, Campbell WA. The effect of cigarette smoking on fetal heart rate characteristics. Obstet Gynecol. 2002;99(5 pt 1):751–755. [DOI] [PubMed] [Google Scholar]
- 80. Feng Y, Caiping M, Li C, et al. Fetal and offspring arrhythmia following exposure to nicotine during pregnancy. J Appl Toxicol. 2010;30(1):53–58. [DOI] [PubMed] [Google Scholar]
- 81. Jolma CD, Samson RA, Klewer SE, Donnerstein RL, Goldberg SJ. Acute cardiac effects of nicotine in healthy young adults. Echocardiography. 2002;19(6):443–448. [DOI] [PubMed] [Google Scholar]
- 82. Leventhal K, Byatt N, Lundquist R. Fetal cardiac arrhythmia during bupropion use. Acta Obstet Gynecol Scand. 2010;89(7):980–981. [DOI] [PubMed] [Google Scholar]
- 83. Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87(4):275–281. [DOI] [PubMed] [Google Scholar]
- 84. Ventura C, Zinellu E, Maninchedda E, Fadda M, Maioli M. Protein kinase C signaling transduces endorphin-primed cardiogenesis in GTR1 embryonic stem cells. Circ Res. 2003;92(6):617–622. [DOI] [PubMed] [Google Scholar]
- 85. Steinberg SF, Goldberg M, Rybin VO. Protein kinase C isoform diversity in the heart. J Mol Cell Cardiol. 1995;27(1):141–153. [DOI] [PubMed] [Google Scholar]
- 86. Kuwahara K, Wang Y, McAnally J, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116(12):3114–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci U S A. 2010;107(145):7000–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li N, Si B, Ju JF, et al. Nicotine induces cardiomyocyte hypertrophy through TRPC3-mediated Ca/NFAT signalling pathway. Can J Cardiol. 2016;32(10):1260.e1–1260.e10. [DOI] [PubMed] [Google Scholar]
- 89. Brain KL, Trout SJ, Jackson VM, Dass N, Cunnane TC. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106(2):395–403. [DOI] [PubMed] [Google Scholar]
- 90. Abreu-Villaca Y, Seidler FJ, Slotkin TA. Does prenatal nicotine exposure sensitize the brain to nicotine-induced neurotoxicity in adolescence? Neuropsychopharmacology. 2004;29(8):1440–1450. [DOI] [PubMed] [Google Scholar]
- 91. Chou HC, Chen CM. Maternal nicotine exposure during gestation and lactation induces cardiac remodeling in rat offspring. Reprod Toxicol. 2014;50:4–10. [DOI] [PubMed] [Google Scholar]
- 92. Anblagan D, Jones NW, Costigan C, et al. Maternal smoking during pregnancy and fetal organ growth: a magnetic resonance imaging study. PLoS One. 2013;8(7):e67223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sullivan PM, Dervan LA, Reiger S, Buddhe S, Schwartz SM. Risk of congenital heart defects in the offspring of smoking mothers: a population-based study. J Pediatr. 2015;166(4):978–984. [DOI] [PubMed] [Google Scholar]
- 94. Williams S, Poulton R. Twins and maternal smoking: ordeals for the fetal origins hypothesis? A cohort study. BMJ. 1999;318(7188):897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee RM. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol. 2008;590(1-3):264–268. [DOI] [PubMed] [Google Scholar]
- 96. Pausova Z, Paus T, Sedova L, Berube J. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene-environment interaction. Kidney Int. 2003;64(3):829–835. [DOI] [PubMed] [Google Scholar]
- 97. Fox KA, Longo M, Tamayo E, et al. Sex-specific effects of nicotine exposure on developmental programming of blood pressure and vascular reactivity in the C57Bl/6J mouse. Am J Obstet Gynecol. 2012;207(3):208.e1–9. [DOI] [PubMed] [Google Scholar]
- 98. Hays JT, Ebbert JO. Bupropion for the treatment of tobacco dependence: guidelines for balancing risks and benefits. CNS Drugs. 2003;17(2):71–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.