Abstract
Fetal growth restriction (FGR) is commonly associated with perinatal morbidity and mortality. Nitric oxide (NO) deficiency increases endothelin-1 (ET-1) production, and this increased ET-1 may contribute to the pathophysiology of NO deficiency-induced FGR. Using an endothelial NO synthase knockout mouse model of FGR, we sought to determine (a) the relative importance of maternal versus conceptus (fetal and placental) NO deficiency and (b) the contribution of ET-1 to the pathogenesis of FGR in this model. Fetal growth restriction occurred both with NO-deficient conceptuses in the setting of maternal NO production and with maternal NO deficiency in the setting of NO-producing conceptuses. Placental ET-1 expression was increased in NO-deficient dams, ET receptor A (ETA) production increased in endothelial nitric oxide synthase+/− placentas, and antagonism of ETA prevented FGR. These results demonstrate that both maternal and conceptus NO deficiency can contribute to FGR and suggest a role for ETA antagonists as therapeutic agents in FGR.
Keywords: mouse, fetal growth restriction, endothelin antagonism, nitric oxide synthase gene knockout, pregnancy
Introduction
Fetal growth restriction (FGR) affects approximately 5% of human pregnancies in developed countries.1 Fetal growth restriction is associated with an increased risk of fetal morbidity and mortality as well as short-term and lifelong morbidity.2-4 Growth-restricted neonates exhibit an increased risk of developing respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis.5-7 Lifelong consequences of FGR include neurologic morbidity along with cognitive deficits as well as a propensity to develop metabolic syndrome, diabetes, cardiovascular disease, infertility, and cancer.8-11
Fetal growth restriction may be caused by abnormalities of the fetal (eg, anomalies and aneuploidy), maternal (eg, nutritional and life-style choices), or placental (eg, ischemic insult) compartments. While there are many potential etiologies of FGR, the single most commonly recognized cause is uteroplacental insufficiency. In the majority of cases, the pathogenesis of FGR is not understood in spite of intense investigation.
Vasoactive agents, including nitric oxide (NO) and endothelin-1 (ET-1), have been implicated in the pathophysiology of human FGR.12,13 There is close interaction between the signals that regulate each of these vasoactive molecules. Endothelin-1 is one of the most potent endogenous vasoconstrictors known. Nitric oxide, a vasodilator, inhibits the production of ET-1.14 Consequently, reduced NO (eg, NO synthase [NOS] inhibition) encourages the production of ET-1. Endothelin-1 produces vasoconstriction via the ET receptor A (ETA). The binding affinity of ETA is increased as NO is reduced.15 Mechanistically, NO can bind the ETA cysteine cluster and modify receptor affinity,16 therefore decreased NO can lead to increased ETA receptor affinity.
The endothelial NOS (eNOS) gene knockout mouse (eNOS−/−) does not produce any eNOS protein, therefore it is NO deficient compared with its normal parent strains. Homozygous eNOS−/− mice are hypertensive, but heterozygous (eNOS+/−) mice have been shown to have normal blood pressure,17-20 indicating that the eNOS activity of the heterozygous mice is adequate for maintaining normal vascular physiologic requirements. It has been shown that eNOS−/− mating pairs produce growth-restricted pups.21-23 By mating eNOS+/− females with eNOS−/− males, we were able to investigate the impact on fetal growth of NO-deficient conceptuses in NO-producing and NO-deficient dams.
Endothelin-1 has been implicated in the pathophysiology of several animal models of FGR, including the eNOS gene knockout mouse.24-27 Increased ET-1 expression in eNOS−/− mice has been reported in nonreproductive tissues, particularly in the mesenteric arteries.28 The role of ET-1 in this model of FGR is unknown.
Our objective was to determine whether fetal or maternal eNOS deficiency is more significant in the development of FGR. Secondarily, we sought to determine the significance of increased endothelin expression on the FGR observed with eNOS deficiency.
Methods
Animals
Mice (12-16 weeks old) were purchased from The Jackson Laboratory, Bar Harbor, Maine, housed in 12-hour light–dark cycles and allowed free access to a standard laboratory rodent diet and water. Animal care and the conduct of all experiments were approved by the NorthShore University HealthSystem Research Institute Animal Care and Use Committee.
Experimental Groups and Treatments
Seven groups of maternal mice were used for these experiments. All treatments were conducted concurrently. Virgin homozygous (eNOS−/−) female mice were mated with eNOS−/− males (B6.129P2-Nos3tm1Unc) to produce eNOS−/− conceptuses (both fetus and placenta having the same genotype) carried by eNOS−/− dams (group 1; n = 21 dams). Additional virgin eNOS−/− female mice were mated with wild type C57BL/6J male mice (eNOS+/+) to produced eNOS+/− conceptuses carried by eNOS−/− dams (group 2; n = 16 dams). Some of the female eNOS+/− pups from these matings were allowed to reach maturity and were mated with eNOS−/− males to produce both eNOS−/− and eNOS+/− conceptuses carried by eNOS+/− dams (group 3; n = 16 dams). Homozygous wild-type matings were also used as controls (group 4; n = 15 dams). These mice were treated with vehicle (20% ethyl alcohol, 40% propylene glycol, in 0.04 mol/L NaOH) for the ETA antagonist used in the groups identified in the next paragraph.
In separate groups of eNOS+/+ and eNOS−/− mice, mated to produce homozygous eNOS+/+ conceptuses (group 5; n = 8 dams) or eNOS−/− conceptuses (group 6; n = 6 dams) as well as heterozygous eNOS+/− conceptuses (group 7; n = 5 dams), on gestation days 13 to 19 an ETA antagonist (ABT-546, 20 mg/kg/d, Abbott Laboratories, Abbott Park, Illinois) was administered subcutaneously to dams via an osmotic pump (implanted under 2%-3% isoflurane anesthesia). This dose has been shown to restore normal fetal growth in other rodent models of FGR (data not shown). Vehicle-treated mice described above served as treatment controls. The ETA antagonist is a nonpeptide compound with a high affinity for ETA receptors (Ki = 0.46 nmol/L) and a low affinity for ETB receptors (Ki = 13 000 nmol/L), thus an ETA selectivity of approximately 28 000-fold.29
Genotyping
All fetuses from female eNOS+/− mice mated with male eNOS−/− mice were genotyped. Tail clippings (3 mm) from fetuses were incubated overnight at 55°C in lysis buffer (100 mmol/L NaCl, 10 mmol/L Tris [pH 8.0], 25 mmol/L EDTA, 0.5% sodium dodecyl sulfate) containing 0.1 mg/mL proteinase K. DNA was extracted in phenol/chloroform and then precipitated from the aqueous phase with 100% ethanol at 0°C for 1 hour followed by centrifugation at 14 000 rpm for 5 minutes. The pellet was washed with 75% ethanol at 14 000 rpm for 10 minutes and then dissolved in Tris-EDTA buffer (10 mmol/L Tris [pH 8.0] and 1 mmol/L EDTA). The polymerase chain reaction (PCR) primers used were designed by Jackson Laboratory for eNOS mouse genotyping and were synthesized by Sigma (St Louis, Missouri). The wild-type primer (oIMR1824) sequence was 5'-GGC CAG TCT CAG AGC CAT AC-3'; the mutant primer (oIMR0094) sequence was 5'-TGG CTA CCC GTG ATA TTG CT-3'; and the common primer (oIMR1823) sequence was 5'-ATT TCC TGT CCC CTG CCT TC-3'. Polymerase chain reaction was carried out on a GeneAmp PCR System 9700 (Applied Biosystems) thermal cycler in a total volume of 25 µL of Tris-EDTA buffer with 2.5 mmol/L MgCl2, 0.2 mmol/L deoxynucleotide, 0.5 µmol/L of each primer, 0.0125 U/µL Taq DNA polymerase, and 2 µL DNA in 3.5 µL bromophenol blue DNA loading buffer. The reaction mixture was heated at 94°C for 3 minutes followed immediately by 30 cycles of PCR consisting of 35 seconds of denaturation at 94°C, 1 minute of annealing at 65°C, and 1 minute of extension at 72°C. Reactions were then held at 72°C for another 2 minutes before cooling to 4°C. Electrophoresis was carried out on a 1.5% agarose gel. Bands were observed under ultraviolet light. Heterozygous eNOS+/− mice exhibited bands of 442 and 500 base pairs (bp), homozygous eNOS+/+ mice were represented by a single 442-bp band, and homozygous eNOS−/− mice by a single 500-bp band.
Fetal and Placental Weights
The mice were euthanized on gestation day 19, and a hysterotomy was performed. Fetal and placental weights were documented and fetuses were genotyped. Fetal and placental weights were compared among the experimental groups. Fetal–placental weight ratios, which some investigators use as a surrogate indicator of placental efficiency, were determined and compared among the experimental groups.
Placental PreproET-1 messenger RNA Expression
Placentas were homogenized with a PowerGen 125 homogenizer (Fisher Scientific, Waltham, Massachusetts) at 0°C in RLT buffer (Qiagen, Valencia, California), and total RNA was extracted using the RNeasy Mini kit (#74104, Qiagen) following the manufacturer’s instructions. The concentration and purity (260/280 nm ratio greater than 1.8) of RNA were determined on the Nanodrop 2000 UV Spectrophotometer (Thermo Scientific, Wilmington, Delaware). It was then stored at −80°C for reverse transcription-PCR (RT-PCR).
Reagents for RT-PCR were from Life Technologies (Foster City, California). For each sample, 1 µg total RNA was reverse transcribed at 37°C for 1 hour in a total of 10 µL reaction mixture using Taqman Reverse Transcription Reagents (N808234). The resulting complementary DNA (cDNA) was amplified by PCR using 1.5 µL cDNA and the Taqman assay for ET-1 (Mm00438656_m1), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_g1), and Taqman Gene Expression Master Mix (4369016) in a total volume of 25 µL on a Stratagene MX3000P real-time PCR instrument, per the Life Technologies time and temperature specifications. After heating at 50°C for 2 minutes and 95°C for 10 minutes, 40 cycles of PCR were run with 15 seconds of denaturation at 95°C and 60 seconds of annealing at 60°C. The internal reference GAPDH was used for normalization of the data. The ΔΔCt method was used to calculate gene expression, and each reaction was done in triplicate.
Protein Assay by Western Immunoblot
Cytosolic proteins
Placentas were homogenized and sonicated in radioimmunoprecipitation assay lysis buffer containing protease inhibitors (Santa Cruz Biotechnology [SCBT], Dallas, Texas) at a concentration of 100 µL/mg of tissue and then centrifuged at 10 000 g for 10 minutes at 4°C. The supernatant was recentrifuged at 10, 000 g and then collected for protein analysis.
Membrane proteins
Placentas were homogenized on ice in 10 volumes (w/v) of 10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4) containing 0.25 mol/L sucrose, 3 mmol/L EDTA, and protease inhibitors (5 µg/mL pepstatin A and 0.1 mmol/L phenylmethylsulfonyl fluoride [PMSF]) using a Tekmar mark II Tissumizer at 13 500 rpm. The homogenate was centrifuged at 1000 × g for 15 minutes at 4°C. The supernatant was collected, and the pellet was resuspended in the same buffer, rehomogenized, and recentrifuged. The combined supernatant was centrifuged at 16 000 × g for 25 minutes at 4°C, and then the pellet was sonicated in 50 mmol/L Tris (pH 7.4) containing 50 mmol/L EDTA, 1% TritonX-100, 150 mmol/L NaCl and protease inhibitors and recentrifuged at 16 000 × g as before. The supernatant was collected for membrane protein analysis.
Immunoblots
Equal amounts of protein were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis on 15% (for ET-1) or 10% (for endothelin receptors) SDS–polyacrylamide gels and transferred to 0.2-micron nitrocellulose membranes. Immunoblots were performed using primary antibodies specific for ET-1 (1:1000; ab190741, Abcam, Cambridge, Massachusetts), ETA (1:250, AER-001, Alomone Labs, Jerusalem, Israel), ETB (1:200, AER-002, Alomone), or Na+/K+ ATPase (1:5000, NB300-146, Novus Biologicals, Littleton, Colorado). Background was blocked in 5% nonfat dry milk and then the blots were incubated overnight at 4°C in anti-ET-1, anti-ETA, anti-ETB, or anti-Na+/K+ ATPase in 5% bovine serum albumin (Cell Signaling Technology [CST], Beverly, Massachusetts). After washing in Tris-buffered saline/Tween-20 (TBST), the nitrocellulose membranes were incubated as appropriate in antimouse or antirabbit immunoglobulin G–horseradish peroxidase (HRP) conjugate (1:1000, CST) or antiactin–HRP (1:500, SCBT) in 5% nonfat dry milk for 1 hour at 25°C. After washing in TBST, the labeled proteins were detected by chemiluminescence (SCBT) according to instructions by the manufacturer. Protein bands for ET-1 (24 kDa), ETA (48 kDa), and ETB (49 kDa) were identified using molecular weight markers, quantified using densitometry software (UVP LabWorks, Upland, California), and normalized using actin (43 kDa) as a loading control for cytosol or Na+/K+ ATPase (112 kDa) for cell membranes. The relative intensity of each band was determined as the ratio of the intensity of the band divided by the corresponding loading control band intensity.
Statistical Analyses
Results are presented as mean ± standard error and were compared statistically with P < .05 considered significant. Multiple group comparisons were made using an analysis of variance (ANOVA) with post hoc Newman-Keuls, Kruskal-Wallis nonparametric ANOVA with post hoc Dunn’s, or 2-way ANOVA, as appropriate.
Results
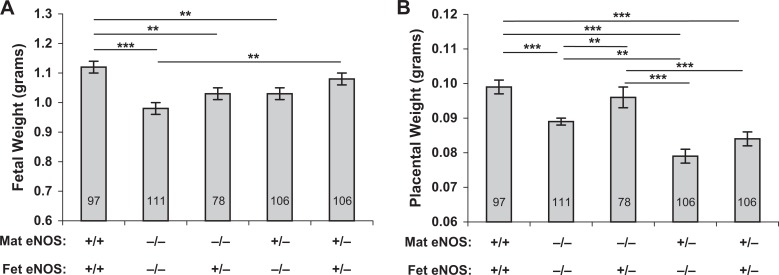
Fetal weights as affected by maternal and fetal eNOS genotype are presented in Figure 1. Fetal weights from vehicle-treated eNOS−/− mice were significantly reduced compared with those from normal (eNOS+/+) controls (0.98 ± 0.02 vs 1.12 ± 0.02, P < .001). Weights of eNOS+/− fetuses carried by eNOS−/− dams, as well as eNOS−/− fetuses carried by NO-producing (eNOS+/−) dams, were both significantly lower than those of eNOS+/+ fetuses carried by eNOS+/+ dams (1.03 ± 0.02 vs 1.12 ± 0.02, P < .01). Further, no significant difference was observed in the weights of eNOS−/− or eNOS+/− fetuses carried by eNOS+/-− dams (1.03 ± 0.02 vs 1.08 ± 0.02). However, when both fetus and dam were eNOS+/−, fetal weights were not significantly different from normal controls.
Figure 1.
Fetal (A) and placental (B) growth in mice of varying fetal and maternal eNOS genotype. Fetal weights were significantly lower, compared with eNOS+/+ mice, in all eNOS−/− dams and in eNOS+/− dams carrying eNOS−/− conceptuses. Fetal weights in eNOS+/− dams carrying eNOS+/− conceptuses were not different from eNOS+/+ controls. Placental weights were lower in eNOS−/− dams carrying eNOS−/− conceptuses and in all eNOS+/− dams regardless of conceptus genotype, compared with eNOS+/+ mice. Data are presented as mean ± SE; *P < .05, **P < .01, ***P < .001 for comparisons indicated by the lines above the bars, by ANOVA. Number of fetuses (n) in each group is indicated by the number in each bar. eNOS indicates, endothelial nitric oxide synthase; SE, standard error; ANOVA, analysis of variance.
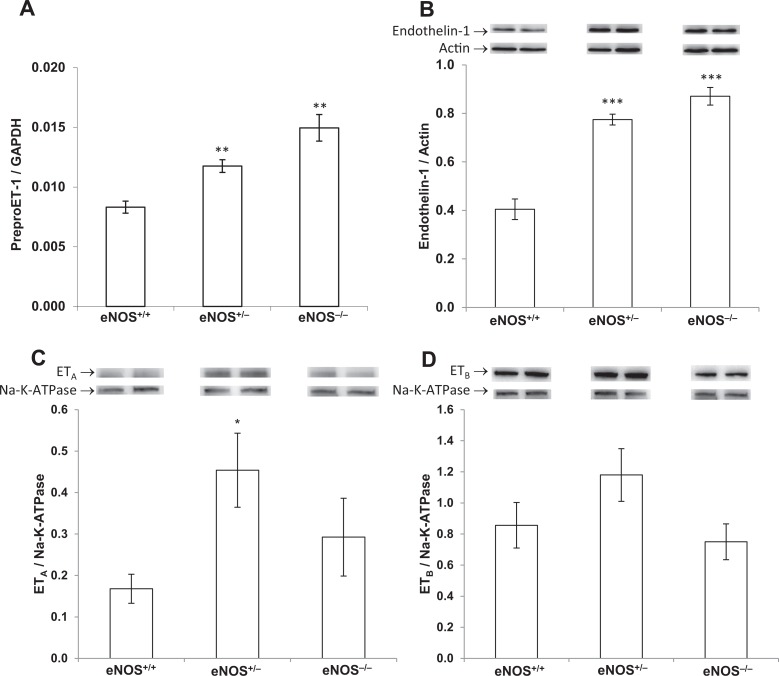
Placental preproET-1 messenger RNA (mRNA) expression was 2-fold higher in eNOS−/− mice than in eNOS+/+ and significantly increased but intermediate between the 2 in eNOS+/− placentas (P < .01, Figure 2A). This corresponded with a 2-fold increase in placental ET-1 production in eNOS−/− mice and 1.9-fold in eNOS+/− compared to eNOS+/+ controls (P < .001, Figure 2B). The ETA receptor levels were variable and not significantly increased in eNOS−/− but were increased 2.6-fold in eNOS+/− compared with eNOS+/+ placentas (P < .05, Figure 2C). Placental ETB receptor expression was not significantly altered by the eNOS genotype of the placentas (Figure 2D).
Figure 2.
PreproET-1 mRNA expression (A) and ET-1 (B), ETA receptor (C), and ETB receptor (D) protein production in the placentas from conceptuses of the indicated genotypes. Comparative mRNA expression was determined by real-time quantitative reverse transcription–polymerase chain reaction, and results were normalized to the internal control gene, GAPDH. Protein expression was determined by Western blot and quantified by densitometry normalized to actin. Relative molecular masses are 24 kDa (ET-1), 43 kDa (actin), 48 kDa (ETA), 49 kDa (ETB), and 112 kDa (Na-K-ATPase). Images of representative bands are exhibited. PreproET-1 mRNA and protein expression were significantly increased in eNOS+/− and eNOS−/− placentas compared with eNOS+/+ controls, while ETA was increased in eNOS+/− placentas, and ETB was not significantly affected by eNOS genotype. Data are presented as mean ± SE; *P < .05, **P < .01, ***P < .001; by ANOVA. eNOS indicates, endothelial nitric oxide synthase; SE, standard error; ANOVA, analysis of variance; mRNA, messenger RNA; ET-1, endothelin-1; ETA, endothelin receptor A; ETB, endothelin receptor B; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
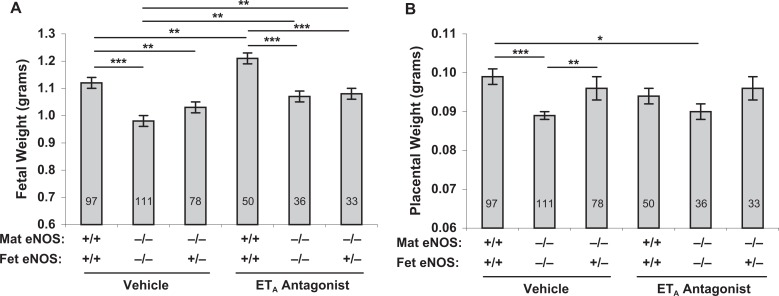
The impact of an ETA antagonist on fetal weight is presented in Figure 3. Fetal weights from eNOS−/− females treated with an ETA antagonist were significantly improved in comparison with those from vehicle-treated eNOS−/− controls (1.07 ± 0.02 vs 0.98 ± 0.02, P < .05) and were not significantly different from normal (eNOS+/+) controls (1.07 ± 0.02 vs 1.12 ± 0.02). Fetal weights were significantly increased in eNOS+/+ mice treated with an ETA antagonist compared with vehicle-treated eNOS+/+ mice (P < .01). Two-way ANOVA revealed significant effects of genotype and treatment (P < .0001 for each), but not the interaction of the 2, on fetal weights.
Figure 3.
Fetal (A) and placental (B) growth in control and ETA antagonist-treated pregnant mice. Lower fetal weights in eNOS−/− dams were significantly improved by ETA antagonism. Lower placental weights in eNOS−/− dams carrying eNOS−/− conceptuses remained significantly lower than control eNOS+/+ placental weights even with ETA antagonism. Data are presented as mean ± SE; *P < .05, **P < .01, ***P < .001 for comparisons indicated by lines above the bars; by ANOVA. Two-way ANOVA revealed significant effects for treatment and genotype (P < .0001 for each), but not for interaction, on fetal weight only. Number of fetuses (n) in each group is indicated by the number in each bar. eNOS indicates, endothelial nitric oxide synthase; SE, standard error; ANOVA, analysis of variance; ETA, endothelin receptor A.
Placental weights were significantly less in vehicle-treated eNOS−/− mice than in eNOS+/+ mice (P < .001, Figures 1 and 3). Placental weights remained lower in ETA antagonist-treated eNOS−/− mice compared with eNOS+/+ mice (P < .05). Placental weights were significantly lower in all eNOS−/− and eNOS+/− mice compared with eNOS+/+ mice (P < .001) with the exception of eNOS−/− dams carrying eNOS+/− fetuses, which were also significantly improved compared with eNOS−/− dams carrying eNOS−/− fetuses (P < .05).
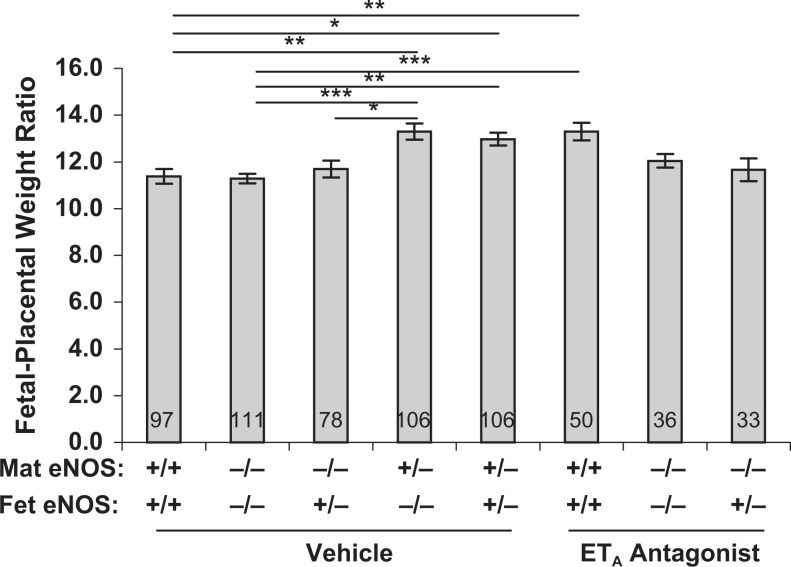
Fetal/placental weight ratios (Figure 4) were significantly increased in eNOS+/− dams regardless of fetal eNOS genotype. This ratio was also increased in eNOS+/+ mice by the presence of the ETA antagonist. However, it was not significantly changed by an ETA antagonist in eNOS−/− dams regardless of fetal eNOS genotype.
Figure 4.
Fetal/placental weight ratios as a surrogate indicator of placental efficiency in the experimental groups used in this study. This ratio was maintained at normal or higher levels regardless of genotype or treatment. Data are presented as mean ± SE; *P < .05, **P < .01, ***P < .001 for comparisons indicated by lines above the bars; by Kruskal-Wallis nonparametric ANOVA. Number of conceptuses (n) in each group is indicated by the number in each bar. SE, standard error; ANOVA, analysis of variance.
There were no statistically significant differences in litter sizes among the groups (Table 1). Lower litter size did not appear to increase fetal weight (or vice versa) as indicated by the fact that lower litter size absolute values (vehicle-treated eNOS−/− dams) were associated with lower fetal weights. Further, the groups with the highest litter size absolute values (ETA antagonist-treated eNOS−/− dams carrying eNOS+/− conceptuses and vehicle-treated eNOS+/− dams) had similar fetal weights as the group with the lowest litter size absolute value (vehicle-treated eNOS−/− dams carrying eNOS+/− conceptuses).
Table 1.
Litter Sizes.a
| Genotype | |||||||
| Maternal | eNOS+/+ | eNOS−/−- | eNOS−/− | eNOS+/+ | eNOS−/−- | eNOS−/− | eNOS+/− |
| Fetal | eNOS+/+ | eNOS−/− | eNOS+/− | eNOS+/+ | eNOS−/− | eNOS+/− | eNOS+/− & −/− |
| Treatment | Vehicle | Vehicle | Vehicle | ABT-546 | ABT-546 | ABT-546 | Vehicle |
| Fetuses/litter | 6.5 ± 0.6 | 5.3 ± 0.5 | 4.9 ± 0.5 | 6.3 ± 0.9 | 6.0 ± 0.7 | 6.6 ± 0.7 | 6.6 ± 0.5 |
Abbreviations: eNOS, endothelial nitric oxide synthase; SE, standard error.
aMean ± SE.
Discussion
Our data demonstrate that either maternal or conceptus NO production deficiency can contribute to the pathophysiology of FGR. Fetal growth restriction has been described previously in eNOS−/− mice21,30 and our results correspond with these other investigators’ results. Our results also show that, compared to fetal growth when neither conceptus nor dam is NO deficient (both eNOS+/+), fetal growth is significantly compromised when NO-producing heterozygous (eNOS+/−) conceptuses are carried by NO-deficient (eNOS−/−) maternal mice, demonstrating the significance of maternal NO production. Kulandavelu et al,31 in a study of this same issue, also reported reduced fetal weights in both eNOS−/− and eNOS+/− fetuses carried by eNOS−/− dams. However, they concluded that fetal growth was determined by conceptus genotype because eNOS+/− fetal weights and umbilical vein blood flow were both intermediate between those of eNOS+/+ and eNOS−/− in same-genotype dams and significantly different from both. Our data show that eNOS+/− fetal weights are significantly greater than those of eNOS−/−, both carried by eNOS+/− dams, but are not different from weights of eNOS+/+ fetuses. Pallares et al,32 in a magnetic resonance imaging-based study at gestation day 13.5, measured 8 fetal size parameters and placental thickness in eNOS+/+, eNOS+/−, and eNOS−/− fetuses all carried by eNOS+/− dams. Corresponding with our results, they showed that fetal size was reduced only in eNOS−/−, not eNOS+/−, fetuses in eNOS+/− dams. Placental thickness was reduced in eNOS−/−, but not eNOS+/−, placentas in eNOS+/− dams, corresponding with our eNOS−/− placental weight results. Although they showed no reduction in placental thickness in eNOS+/− placentas in eNOS+/− dams, we did show placental weight reduction in these placentas. Their study, besides being conducted at an earlier time in gestation, did not include measurements of placental diameters. Additionally, in our study, the same fetal weights resulted whether an eNOS+/− conceptus was carried by an eNOS−/− dam or an eNOS−/− conceptus was carried by an eNOS+/− dam. The fact that, when compared to their NO-producing cohorts, homozygous NO-deficient (eNOS−/−) fetuses are significantly growth restricted even when carried by NO-producing (eNOS+/−) dams, demonstrates the importance of conceptus NO production. These results are supported by a previous report showing that uterine blood flow in eNOS−/− dams was reduced to approximately half the level in eNOS+/+ mice and was reduced about one-third in eNOS+/− dams.30 Corresponding with our results, these investigators also reported that both maternal and conceptus eNOS contribute to uterine blood flow and thus to fetal growth.
Fetal growth restriction can be caused by various factors, both fetal and maternal, and can be asymmetrical (reduction of selected organs or at specific times during pregnancy; often related to nutritional delivery) or symmetrical (uniform reduction throughout pregnancy; often related to intrinsic factors). Our study focused on pregnancy outcome at term in these mice. Others have shown that eNOS deficiency in mice produces an asymmetrical FGR characterized by reduced body weight and crown-rump length that are not always detectable early in pregnancy but that worsen throughout pregnancy.21,23,31 This suggests that FGR in these mice is related less to the genotype per se of the mice and more to the effects of the eNOS genotypes on blood flow and resultant nutrient and gas exchange.
Placental ET-1 expression was increased in both eNOS+/− and eNOS−/− dams. This result corroborates what has been shown by others in tissues from nonpregnant eNOS−/− mice.28 This result also corresponds with reported increased placental ET-1 expression accompanying human FGR33 and elevated circulating ET-1 levels in hypertensive human pregnancies diagnosed with preeclampsia, a condition that is often accompanied by FGR.34 There is an increasing body of evidence which suggests that the vascular response to reduced NOS activity involves not only the lack of NO production but also an increase in ET-1 production. In hypertensive rats, vascular ET-1 mRNA expression and plasma ET-1 protein increase in response to NOS inhibition35 while no such changes occur in nonpregnant normotensive rats.36 However, with NOS inhibited during pregnancy, not only do NO levels decrease, but we37 and others38 have observed significant increases in plasma ET-1 levels. Since ET-1 is primarily locally active, uteroplacental tissue levels may be more significant than plasma levels as indicators of its importance in pregnancy and FGR. Our studies showing prevention of NOS inhibition-induced FGR by an ETA antagonist25 suggest that ET-1 is of primary importance in the pathophysiology of FGR in the rat model and correlate with the findings reported herein for mice. Increased production of the ETA receptor, particularly in eNOS+/− placentas, may increase the sensitivity of the placental vasculature to the vasoconstrictive effects of ET-1. Our results suggest not only increased vasoactivity from increased ET-1 but also increased tissue sensitivity from increased ETA.
Mechanistically, blockade of NO production in vitro has been shown to increase the production of ET-1.39 Conversely, increased NO reduces the production of ET-1 by activation of soluble guanylate cyclase40 and suppression of nuclear factor κB.41 Additionally, NO has a role in terminating the ET-1 signal at the ETA receptor by displacing bound ET-1 and interfering with the postreceptor pathway for calcium mobilization.16,42,43 Nitric oxide can reversibly bind the C-terminal cluster of cyteine residues of ETA and modify its affinity to its ligand.16 With reduced NO in the eNOS−/− mouse, ETA affinity for ET-1 would be expected to rise or at least to be maintained high. The absence of eNOS, then, would both directly attenuate vasodilation by NO and indirectly potentiate vasoconstriction by ET-1.
Endothelin, acting via the ETA receptor, has a prominent role in the pathophysiology of FGR in pregnant eNOS−/− mice. Maternally administered ETA antagonism prevents FGR in this model even with both maternal and fetal NO deficiency. The role of endothelin is less prominent in the placenta than in the fetus. Placental growth in eNOS−/− mice was not improved by maternal treatment with an ETA antagonist. While our results do not rule out possible effects of ET-1 on placental vascularity or amino acid transporters, these parameters are beyond the scope of this work and there have been no reports to indicate such effects by ET-1 in the placenta. We have reported reduced placental blood flow in response to ET-1, in the pregnant rat, resulting in reduced placental mass that is not fully restored with ETA antagonism.44 We have suggested that this decreased placental perfusion is a primary physiologic mechanism producing the reduced placental mass. However, placental function, in spite of reduced placental size, is apparently sufficient to support normal fetal growth in ETA antagonist-treated eNOS−/− mice carrying eNOS−/− conceptuses. This result corresponds with that of Hefler et al21 showing no placental abnormalities in pregnant eNOS−/− mice. The significantly improved fetal weights in eNOS−/− mice treated with the ETA antagonist were accompanied by fetal/placental weight ratios that were maintained at a normal level. This ratio did not fall significantly below normal in any treatment group, regardless of changes in placental mass. We have also observed the same phenomenon of ETA antagonism not increasing placental size in a rat model of NOS inhibition-induced FGR.45 The preserved placental mass in eNOS−/− mice carrying eNOS+/− conceptuses suggests fetal/placental involvement in the determination of placental growth. It is conceivable that molecular signals from the eNOS+/− fetuses in the context of an eNOS−/− dam stimulate increased placental growth, which the eNOS−/− fetuses cannot do. Conversely, it is possible that eNOS−/− fetuses can generate such signals but that the eNOS−/− placentas cannot respond to growth signals even if they are being produced.
Endothelin has a major impact on fetal growth. We have published extensively on the role of endothelin in various rat models of FGR and on the amelioration of FGR in these models by ETA antagonism. Endothelin induced by hypoxia or NOS inhibition37 reduces uteroplacental blood flow and fetal growth which are both completely restored by ETA antagonism.44,46 Endothelin antagonists are known to cross the placenta. We have shown that maternally administered ETA antagonists enter the fetal compartment but plasma levels reach only 2% of maternal levels.47 Further, rat pups born to dams that were treated with ETA antagonists during the last one-third of pregnancy exhibited normal weight, survival, postnatal growth, and oxygen saturation as well as normal gestational age at birth and normal litter sizes.47 Endothelin antagonists are teratogenic when administered to the dam during fetal organogenesis. For this reason, all of our experiments limit administration of ET antagonists to late pregnancy, after the completion of organogenesis. Under these conditions, no teratogenic effects from ET antagonists have ever been observed in any of our animal models.
Whether blood pressure significantly affects fetal growth in eNOS−/− mice is uncertain. Hefler et al48 showed increased maternal blood pressure across all 3 trimesters of eNOS−/− mouse pregnancy, whereas others have shown that while eNOS−/− mice are normally hypertensive,18 blood pressure is not significantly elevated during pregnancy49,50 compared to eNOS+/+ mice. It should be noted, however, that stroke volume and cardiac output have been shown to be decreased in pregnant eNOS−/− mice compared with wild type,50 possibly contributing physiologically to the decreased fetal weights in the eNOS−/− mice.
In summary, maternal as well as conceptus NO deficiency both contribute to the development of FGR. Endothelin-1, acting through the ETA receptor, contributes to the pathophysiology of FGR induced by reduced maternal and conceptus eNOS expression. In the setting of NO deficiency, ETA-selective antagonism improves fetal growth. Endothelin-1 and NO are important vasoactive mediators that both appear to have an important role in the regulation of fetal growth, most likely through regulation of uteroplacental perfusion.51,52 Our limited understanding of the regulation of uteroplacental perfusion and the paucity of therapeutic agents to improve it effectively limits our ability to treat FGR. The results of the current investigation provide further evidence of a prominent role for ET-1 in the pathophysiology of FGR and further suggest a role for ETA antagonists as therapeutic agents in FGR.
Acknowledgements
The authors are grateful to Jane Turbov, MSLIS, and Rebecca Rosales, BS, for their expert technical assistance with the reverse transcription-polymerase chain reaction and Western blotting.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant number HD046968.
References
- 1. Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6(suppl 3):332–336. [PubMed] [Google Scholar]
- 2. Barker DJP. The long-term outcome of retarded fetal growth. Clin Obstet Gynecol. 1997;40(4):853–863. [DOI] [PubMed] [Google Scholar]
- 3. Mcintire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. New Engl J Med. 1999;340(16):1234–1238. [DOI] [PubMed] [Google Scholar]
- 4. Lackman F, Capewell V, Richardson B, Dasilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001;184(5):946–953. [DOI] [PubMed] [Google Scholar]
- 5. Fanaroff AA, Wright LL, Stevenson DK, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991 through December 1992. Am J Obstet Gynecol. 1995;173(5):1423–1431. [DOI] [PubMed] [Google Scholar]
- 6. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. Am J Obstet Gynecol. 2000;182(1 pt 1):198–206. [DOI] [PubMed] [Google Scholar]
- 7. Damodaram M, Story L, Kulinskaya E, Rutherford M, Kumar S. Early adverse perinatal complications in preterm growth-restricted fetuses. Aust N Z J Obstet Gynaecol. 2011;51(3):204–209. [DOI] [PubMed] [Google Scholar]
- 8. Low JA, Handley-Derry MH, Burke SO, et al. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am J Obstet Gynecol. 1992;167(6):1499–1505. [DOI] [PubMed] [Google Scholar]
- 9. Sallout B, Walker M. The fetal origin of adult diseases. J Obstet Gynaecol. 2003;23(5):555–560. [DOI] [PubMed] [Google Scholar]
- 10. Chernausek SD. Update: consequences of abnormal fetal growth. J Clin Endocrinol Metab. 2012;97(3):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Juul A, Almstrup K, Andersson AM, et al. Possible fetal determinants of male infertility. Nat Rev Endocrinol. 2014;10(9):553–562. [DOI] [PubMed] [Google Scholar]
- 12. Morris NH, Sooranna SR, Learmont JG, et al. Nitric oxide synthase activities in placental tissue from normotensive, pre-eclamptic and growth retarded pregnancies. Br J Obstet Gynaecol. 1995;102(9):711–714. [DOI] [PubMed] [Google Scholar]
- 13. Harvey-Wilkes KB, Nielsen HC, D’alton ME. Elevated endothelin levels are associated with increased placental resistance. Am J Obstet Gynecol. 1996;174(5):1599–1604. [DOI] [PubMed] [Google Scholar]
- 14. Myatt L, Brewer AS, Langdon G, Brockman DE. Attenuation of the vasoconstrictor effects of thromboxane and endothelin by nitric oxide in the human fetal-placental circulation. Am J Obstet Gynecol. 1992;166(1 pt 1):224–230. [DOI] [PubMed] [Google Scholar]
- 15. Neerhof MG, Jilling T, Synowiec S, Khan S, Thaete LG. Altered endothelin receptor binding in response to nitric oxide synthase inhibition in the pregnant rat. Reprod Sci. 2008;15(4):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goligorsky MS, Tsukahara H, Magazine H, Andersen TT, Malik AB, Bahou WF. Termination of endothelin signaling: Role of nitric oxide. J Cell Physiol. 1994;158(3):485–494. [DOI] [PubMed] [Google Scholar]
- 17. Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377(6546):239–242. [DOI] [PubMed] [Google Scholar]
- 18. Shesely EG, Maeda N, Kim H-S, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93(23):13176–13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stauss HM, Nafz B, Mrowka R, Persson PB. Blood pressure control in eNOS knock-out mice: comparison with other species under NO blockade. Acta Physiol Scand. 2000;168(1):155–160. [DOI] [PubMed] [Google Scholar]
- 20. Quaschning T, Voss F, Relle K, et al. Lack of endothelial nitric oxide synthase promotes endothelin-induced hypertension: lessons from endothelin-1 transgenic/endothelial nitric oxide synthase knockout mice. J Am Soc Nephrol. 2007;18(3):730–740. [DOI] [PubMed] [Google Scholar]
- 21. Hefler LA, Reyes CA, O’brien WE, Gregg AR. Perinatal development of endothelial nitric oxide synthase-deficient mice. Biol Reprod. 2001;64(2):666–673. [DOI] [PubMed] [Google Scholar]
- 22. Van Der Heijden OWH, Essers YPG, Fazzi G, Peeters LLH, De Mey JGR, Van Eys GJJM. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod. 2005;72(5):1161–1168. [DOI] [PubMed] [Google Scholar]
- 23. Pallares P, Gonzalez-Bulnes A. Intrauterine growth retardation in endothelial nitric oxide synthase-deficient mice is established from early stages of pregnancy. Biol Reprod. 2008;78(6):1002–1006. [DOI] [PubMed] [Google Scholar]
- 24. Thaete LG, Neerhof MG, Caplan MS. Endothelin receptor A antagonism prevents hypoxia-induced intrauterine growth restriction in the rat. Am J Obstet Gynecol. 1997;176(1 pt 1):73–76. [DOI] [PubMed] [Google Scholar]
- 25. Thaete LG, Neerhof MG, Silver RK. Differential effects of endothelin A and B receptor antagonism on fetal growth in normal and nitric oxide-deficient rats. J Soc Gynecol Investig. 2001;8(1):18–23. [PubMed] [Google Scholar]
- 26. Thaete LG, Neerhof MG. Endothelin and platelet-activating factor: significance in the pathophysiology of ischemia/reperfusion-induced fetal growth restriction in the rat. Am J Obstet Gynecol. 2006;194(5):1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neerhof MG, Khan S, Synowiec S, Qu X-W, Thaete LG. The significance of endothelin in platelet activating factor-induced fetal growth restriction. Reprod Sci. 2012;19(11):1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Labonté J, Brochu I, Simard E, D’orleans-Juste P. Distinct modulation of the endothelin-1 pathway in iNOS-/- and eNOS-/- mice. Can J Physiol Pharmacol. 2008;86(8):516–525. [DOI] [PubMed] [Google Scholar]
- 29. Wu-Wong JR, Dixon DB, Chiou WJ, et al. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: in vitro studies. Clin Sci. 2002;103(suppl 48):107s–111s. [DOI] [PubMed] [Google Scholar]
- 30. Kulandavelu S, Whiteley KJ, Qu D, Mu J, Bainbridge SA, Adamson SL. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension. 2012;60(1):231–238. [DOI] [PubMed] [Google Scholar]
- 31. Kulandavelu S, Whiteley KJ, Bainbridge SA, Qu D, Adamson SL. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension. 2013;61(1):259–266. [DOI] [PubMed] [Google Scholar]
- 32. Pallares P, Perez-Solana ML, Torres-Rovira L, Gonzalez-Bulnes A. Phenotypic characterization by high-resolution three-dimensional magnetic resonance imaging evidences differential effects of embryo genotype on intrauterine growth retardation in NOS3-deficient mice. Biol Reprod. 2011;84(5):866–871. [DOI] [PubMed] [Google Scholar]
- 33. Faxén M, Nasiell J, Lunell N-O, Blanck A. Differences in mRNA expression of endothelin-1, c-fos and c-jun in placentas from normal pregnancies and pregnancies complicated with preeclampsia and/or intrauterine growth retardation. Gynecol Obstet Invest. 1997;44(2):93–96. [DOI] [PubMed] [Google Scholar]
- 34. Schiff E, Ben-Baruch G, Peleg E, et al. Immunoreactive circulating endothelin-1 in normal and hypertensive pregnancies. Am J Obstet Gynecol. 1992;166(2):624–628. [DOI] [PubMed] [Google Scholar]
- 35. Sventek P, Li J-S, Grove K, Deschepper CF, Schiffrin EL. Vascular structure and expression of endothelin-1 gene in L-NAME-treated spontaneously hypertensive rats. Hypertension. 1996;27(1):49–55. [DOI] [PubMed] [Google Scholar]
- 36. Fujita K, Matsumura Y, Miyazaki Y, Takaoka M, Morimoto S. Role of endothelin-1 in hypertension induced by long-term inhibition of nitric oxide synthase. Eur J Pharmacol. 1995;280(3):311–316. [DOI] [PubMed] [Google Scholar]
- 37. Neerhof MG, Thaete LG. Chronic NOS-inhibition-induced IUGR is associated with increased circulating endothelin-1 levels in the rat. Hypertens Pregnancy. 1997;16(2):319. [Google Scholar]
- 38. Edwards DL, Arora CP, Bui DT, Castro LC. Long-term nitric oxide blockade in the pregnant rat: effects on blood pressure and plasma levels of endothelin-1. Am J Obstet Gynecol. 1996;175(2):484–488. [DOI] [PubMed] [Google Scholar]
- 39. Kourembanas S, Mcquillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993;92(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):l984–l991. [DOI] [PubMed] [Google Scholar]
- 41. Ohkita M, Takaoka M, Sugii M, Shiota Y, Nojiri R, Matsumura Y. The role of nuclear factor-kappa B in the regulation of endothelin-1 production by nitric oxide. Eur J Pharmacol. 2003;472(3):159–164. [DOI] [PubMed] [Google Scholar]
- 42. Thompson A, Valeri CR, Lieberthal W. Endothelin receptor A blockade alters hemodynamic response to nitric oxide inhibition in rats. Am J Physiol. 1995;269(2 pt 2):h743–h748. [DOI] [PubMed] [Google Scholar]
- 43. Maric C, Aldred GP, Antoine AM, et al. Actions of endothelin-1 on cultured rat renomedullary interstitial cells are modulated by nitric oxide. Clin Exp Pharmacol Physiol. 1999;26(5-6):392–398. [DOI] [PubMed] [Google Scholar]
- 44. Thaete LG, Kushner DM, Dewey ER, Neerhof MG. Endothelin and the regulation of uteroplacental perfusion in nitric oxide synthase inhibition-induced fetal growth restriction. Placenta. 2005;26(2-3):242–250. [DOI] [PubMed] [Google Scholar]
- 45. Neerhof MG, Synowiec S, Khan S, Thaete LG. Pathophysiology of chronic nitric oxide synthase inhibition-induced fetal growth restriction in the rat. Hypertens Pregnancy. 2011;30(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thaete LG, Dewey ER, Neerhof MG. Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J Soc Gynecol Investig. 2004;11(1):16–21. [DOI] [PubMed] [Google Scholar]
- 47. Thaete LG, Khan S, Synowiec S, Dayton BD, Bauch J, Neerhof MG. Endothelin receptor antagonist has limited access to the fetal compartment during chronic maternal administration late in pregnancy. Life Sci. 2012;91(13-14):583–586. [DOI] [PubMed] [Google Scholar]
- 48. Hefler LA, Tempfer CB, Moreno RM, O’brien WE, Gregg AR. Endothelial-derived nitric oxide and angiotensinogen: blood pressure and metabolism during mouse pregnancy. Am J Physiol Regul Integ Comp Physiol. 2001;280(1):r174–r182. [DOI] [PubMed] [Google Scholar]
- 49. Shesely EG, Gilbert C, Granderson G, Carretero CD, Carretero OA, Beierwaltes WH. Nitric oxide synthase gene knockout mice do not become hypertensive during pegnancy. Am J Obstet Gynecol. 2001;185(5):1198–1203. [DOI] [PubMed] [Google Scholar]
- 50. Kulandavelu S, Qu D, Adamson SL. Cardiovascular function in mice during normal pregnancy and in the absence of endothelial NO synthase. Hypertension. 2006;47(6):1175–1182. [DOI] [PubMed] [Google Scholar]
- 51. Boulanger C, Lüscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85(2):587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richard V, Hogie M, Clozel M, Löffler B-M, Thuillez C. In vivo evidence of an endothelin-induced vasopressor tone after inhibition of nitric oxide synthesis in rats. Circulation. 1995;91(3):771–775. [DOI] [PubMed] [Google Scholar]