Abstract
The nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway is one of the primary pathways responsible for the cellular defense system against oxidative stress. Oxidative stress-induced apoptosis is a causal event in diabetic embryopathy. Thus, the Nrf2 pathway may play an important role in the induction of diabetic embryopathy. In the present study, we investigated the potentially protective effect of the Nrf2 activator, vinylsulfone, on high glucose-induced cellular stress, apoptosis, and neural tube defects (NTDs). Embryonic day 8.5 (E8.5) whole mouse embryos were cultured in normal (5 mmol/L) or high (16.7 mmol/L) glucose conditions, with or without vinylsulfone. At a concentration of 10 μmol/L, vinylsulfone had an inhibitory effect on high glucose-induced NTD formation, but it was not significant. At a concentration of 20 μmol/L, vinylsulfone significantly reduced high glucose-induced NTDs. In addition, 20 μmol/L vinylsulfone abrogated the high glucose-induced oxidative stress markers lipid hydroperoxide (LPO), 4-hydroxynonenal (4-HNE), and nitrotyrosine-modified proteins. The high glucose-induced endoplasmic reticulum (ER) stress biomarkers were also suppressed by 20 μmol/L vinylsulfone through the inhibition of phosphorylated protein kinase RNA-like ER kinase (PERK), inositol requiring protein 1α (IRE1a), eukaryotic initiation factor 2α (eIF2a), upregulated C/EBP-homologous protein (CHOP), binding immunoglobulin protein (BiP), and x-box binding protein 1 (XBP1) messenger RNA splicing. Furthermore, 20 μmol/L vinylsulfone abolished caspase 3 and caspase 8 cleavage, markers of apoptosis, in embryos cultured under high glucose conditions. The Nrf2 activator, vinylsulfone, is protective against high glucose-induced cellular stress, caspase activation, and subsequent NTD formation. Our data suggest that vinylsulfone supplementation is a potential therapy for diabetes-associated neurodevelopmental defects.
Keywords: Nrf2 activator, high glucose, oxidative stress, apoptosis, diabetic embryopathy
Introduction
Preexisting maternal diabetes adversely impacts embryonic development by significantly increasing the risk of neural tube defects (NTDs).1–3 Evidence from clinical and experimental studies4–12 reveals that hyperglycemia enhances reactive oxygen species (ROS) generation and concomitantly inhibits endogenous antioxidant expression, leading to oxidative stress. Our studies and those of others have shown that hyperglycemia-induced oxidative stress causes endoplasmic reticulum (ER) stress, which triggers cell apoptosis in the developing neuroepithelium, ultimately leading to NTD formation.13–26
Animal studies have shown that a variety of antioxidants can suppress hyperglycemia-induced NTDs both in vivo and in vitro.17,21,27–33 For example, multivitamins ameliorate maternal diabetes-induced NTD formation in rodent models.29 In addition, using rodent models, we have revealed the protective effects of several natural compounds against maternal diabetes-induced NTDs.21,28,31 However, multivitamins fail to reduce maternal diabetes-induced birth defects in humans,32,34,35 and the protective effects of other naturally occurring compounds in human pregnancy have yet to be confirmed. Therefore, new therapeutics that inhibit oxidative stress and prevent NTDs in human pregnancy need to be developed.
The nuclear factor erythroid 2-related factor 2 (Nrf2) and its negative regulator, Kelch-like ECH-associated protein 1 (Keap1), are important cellular defense mechanisms for combating oxidative stress.36 The Nrf2 regulates environmental stress response by inducing expression of antioxidant enzyme genes such as heme oxygenase 1 (HO-1), NAD(P)H quinine oxidoreductase 1 (NGC1), and glutamatecysteine ligase (GCL).37–39 A previous study has demonstrated that overexpression of Nrf2 dramatically inhibits hypertrophic factor-induced ROS production in both cardiomyocytes and cardiac fibroblasts, while knockdown of Nrf2 exerts opposite effects in both cells.40 Another study showed that Nrf2 signaling is involved in the induction of an antioxidant response in ethanol-exposed embryos.10 Additionally, others have observed that diabetic downregulation of Nrf2 activity contributes to oxidative stress-induced insulin resistance in cardiac cells in vivo and in vitro.41
More recently, attention has focused on identifying small molecular activators of the Nrf2/Keap1 pathway, including sulforaphane in cruciferous vegetables, caffeic acid phenethylester in bee propolis,42 cinnamic aldehyde in cinnamon bark,43 and bardoxolone methyl which is currently being explored to treat type 2 diabetes.44 Many of these pathway activators have shown promising results relevant to diabetic complications.31,45 Taken together, these observations indicate that Nrf2 activators may be ideal therapeutic candidates for preventing diabetic embryopathy.
We and others have demonstrated that oxidative stress, subsequent ER stress, and aberrant apoptosis in developing embryos lead to NTDs.1–3 In the present study, we utilized a cell-permeable Nrf2 activator, vinylsulfone,46 which can effectively induce overall Nrf2 cellular accumulation and nuclear translocation to assess its effect on NTD formation in mouse embryos cultured under high glucose conditions. We further revealed the impact of vinylsulfone on high glucose-induced cellular stress and apoptosis.
Materials and Methods
Animals and Whole-Embryo Culture
Wild-type C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). The procedures for animal use were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
The procedure of whole-embryo culture has been described previously.21 Briefly, C57BL/6J mice were paired overnight. The next morning was designated embryonic day 0.5 (E0.5) if a vaginal plug was present. Mouse embryos at E8.5 were dissected out of the uteri in phosphate-buffered saline (Invitrogen, La Jolla, California). The parietal yolk sac was removed using a pair of fine forceps, and the visceral yolk sac was left intact. Embryos (4 per bottle) were cultured in 25% Tyrode salt solution (Sigma, St Louis, Missouri) and 75% rat serum freshly prepared from male rats. The embryos were cultured at 37°C with 30 rpm rotation in a roller bottle system. The culture bottles were gassed 5% O2/5% CO2/90% N2 for the first 24 hours and at 20% O2/5% CO2/75% N2 for the last 12 hours. Embryos were cultured for 24 or 36 hours with 5 mmol/L of glucose, a value close to the blood glucose level of nondiabetic mice, or 16.7 mmol/L of glucose, which is equivalent to the blood glucose level of diabetic mice, in the presence or absence of Nrf2 activator vinylsulfone (Millipore, Bedford, Massachusetts).
We used 0, 10, and 20 μmol/L of vinylsulfone in the whole embryo culture experiments. Embryos dissected from the visceral yolk sac after 24 hours were used for biochemical and molecular analyses. Embryos dissected from the visceral yolk sac after 36 hours were examined under a Leica MZ16F stereomicroscope (Leica Microsystems, Bannockburn, Illinois) to identify embryonic malformations. Images of the embryos were captured by a DFC420 5 MPix digital camera (Leica Microsystems). Normal embryos were classified as possessing a completely closed neural tube and no evidence of other malformations. Malformed embryos were classified as showing evidence of failed neural tube closure or an NTD. The NTDs were verified by histological sections.
Hematoxylin–Eosin Staining
Embryos were fixed in methacarn (methanol, 60%; chloroform, 30%; glacial acetic acid, 10%), embedded in paraffin and cut into 8-μm sections. After deparaffinization and rehydration, all specimens then underwent hematoxylin and eosin staining in a standard procedure.
Lipid Hydroperoxide Quantification
The degree of lipid peroxidation was quantitatively assessed by the lipid hydroperoxide (LPO) assay, using the Calbiochem LPO assay kit (Millipore) and following the manufacturer’s instructions as described previously.21 Briefly, embryos cultured for 24 hours under normal and high glucose conditions, with and without increasing concentrations of vinylsulfone, were homogenized in high-performance liquid chromatography-grade water. The LPO of the embryonic tissue was extracted by deoxygenated chloroform, and then measured by the absorbance of 500 nm after reacting with chromogen. The results were expressed as mmol/L LPO per mg protein. Protein concentrations were determined by the BioRad DC protein assay kit (Hercules, California).
Detection of x-Box Binding Protein 1 Messenger RNA Splicing
The messenger RNA (mRNA) of x-box binding protein 1 (XBP1) was extracted from 24-hour cultured embryos and reverse transcribed using the QuantiTect reverse transcription kit (Qiagen, Frederick, Maryland). The polymerase chain reaction (PCR) primers for XBP1 were as follows: forward, 5’ GAACCAGGAGTTAAGAACACG- 3’ and reverse, 5’-AGGCAACAGTGTCAGAGTCC-3’. The mRNA splicing was detected by running a sucrose gel. If no XBP1 mRNA splicing occurred, a 205-base pair (bp) band was produced. When XBP1 splicing occurred, a 205-bp band and a 179-bp main band were produced.
Immunoblotting
Equal amounts of protein from cells were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto Immunobilon-P membranes (Millipore). Membranes were incubated in 5% nonfat milk for 1 hour, followed by incubation for 18 hours at 4°C with the following primary antibodies: phosphorylated protein kinase RNA-like ER kinase (p-PERK), PERK, phosphorylated eukaryotic initiation factor 2α (p-eIF2α), eIF2α, inositol requiring protein 1α (IRE1α; Cell Signaling Technologies, Danvers, Massachusetts), phosphorylated IRE1α (p-IRE1α; Abcam, Cambridge, Massachusetts), caspase 3, caspase 8, nitrotyrosine, and 4-hydroxynonenal (4-HNE; Millipore). Membranes were then exposed to horseradish peroxidase-conjugated goat antirabbit or antimouse secondary antibodies. To confirm that equivalent amounts of protein were loaded among the samples, membranes were stripped and probed with a mouse antibody against β-actin (Abcam). Signals were detected using the SuperSignal West Femto Maximum Sensitivity Substrate kit (Thermo Scientific, Waltham, Massachusetts). All experiments were repeated 3 times with the use of independently prepared cell lysates.
Statistical Analyses
Data are presented as means ± standard error of the mean. Embryonic samples from each replicate were from different dams. Statistical differences were determined by 1-way analysis of variance (ANOVA) using SigmaStat 3.5 software (Systat Software Inc, San Jose, California). In 1-way ANOVA, Tukey test was used to estimate the significance of the results (P < .05). The χ2 test was used to estimate the significance of the NTD rates.
Results
The Nrf2 Activator, Vinylsulfone, Ameliorates High Glucose-Induced NTDs
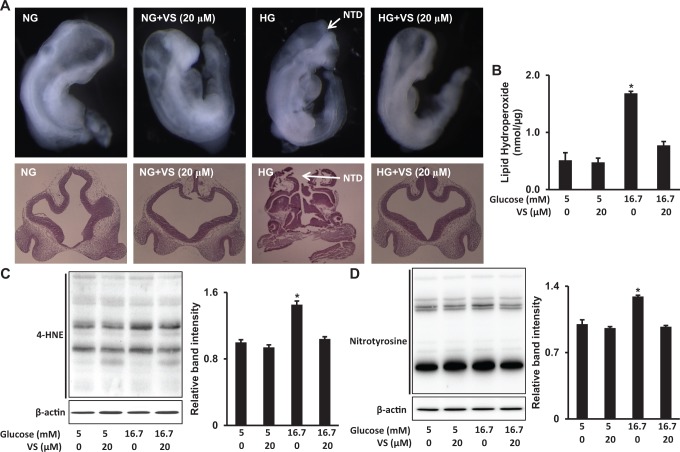
Mouse embryonic neurulation occurs from E8.5 through E10.5. Therefore, we harvested E8.5 mouse embryos and cultured them under normal (5 mmol/L) or high (16.7 mmol/L) glucose conditions, with or without 10 or 20 µmol/L of vinylsulfone. The NTD incidence of embryos cultured under high glucose conditions was significantly higher than that of embryos cultured under normal glucose conditions (Table 1, Figure 1A). Treatment with 10 µmol/L vinylsulfone slightly reduced NTD formation in embryos cultured under high glucose conditions but not significantly (Table 1, Figure 1A). However, the NTD incidence of embryos exposed to high glucose was significantly reduced by treatment with 20 µmol/L vinylsulfone, compared to that of embryos without vinylsulfone treatment (Table 1, Figure 1A). Therefore, we used 20 µmol/L vinylsulfone in our subsequent experiments. We also treated the cultured embryos with 20 µmol/L vinylsulfone under normal glucose (5 mmol/L) conditions but did not observe a significant change in NTD incidence (Table 1, Figure 1A).
Table 1.
Vinylsulfone Ameliorates High Glucose-Induced Neural Tube Defects.
| Group | Total Embryos | Embryos With NTDs | NTD Rate, % |
|---|---|---|---|
| NG | 15 | 1 | 6.67 |
| NG + VS (20 μmol/L) | 15 | 2 | 13.33 |
| HGa | 15 | 10 | 66.67 |
| HG + VS (10 μmol/L) | 15 | 5 | 33.33 |
| HG + VS (20 μmol/L) | 15 | 3 | 20.00 |
Abbreviations: VS, vinylsulfone; HG, high glucose (16.7 mmol/L); NG, normal glucose (5 mmol/L); NTDs, neural tube defects.
aHG group is statistically significant from the NG, NG + VS (20 μmol/L), HG + VS (10 μmol/L), and HG + VS (20 μmol/L) groups. Statistical significance was analyzed by χ2 test.
Figure 1.
Vinylsulfone (VS) inhibits high glucose-induced oxidative stress and consequent neural tube defects (NTD) formation. A, Closed and open neural tube structures of 36-hour cultured embryos under high glucose (HG, 16.7 mmol/L) or normal glucose (NG, 5 mmol/L) conditions, with or without 20 µmol/L VS. B and C, Protein levels of 4-hydroxynonenal (4-HNE) and nitrotyrosine-modified proteins in 24-hour cultured embryos. Experiments were repeated 3 times using 3 embryos from 3 different dams. *indicates statistical significance (P < .05).
Vinylsulfone Inhibits High Glucose-Induced Oxidative Stress and Nitrosative Stress
In the developing embryo, maternal diabetes induces ROS production, which results in oxidative stress and induces lipid peroxidation.1–3,27 Additionally, ROS can react with iNOS-induced nitric oxide to generate reactive nitrogen species (RNS), which cause nitrosative stress.1–3,47 To explore whether vinylsulfone treatment could ameliorate high glucose-induced oxidative and nitrosative stress in cultured embryos, we measured LPO levels, the lipid peroxidation marker 4-HNE, and nitrotyrosine-modified proteins.
High glucose significantly increased the levels of LPO, 4-HNE, and nitrotyrosine-modified proteins in cultured embryos (Figure 1B, C, and D). Treatment with 20 µmol/L vinylsulfone blocked high glucose-increased LPO, 4-HNE, and nitrotyrosine-modified protein levels (Figure 1B, C and D). These findings suggest that vinylsulfone suppresses high glucose-induced oxidative and nitrosative stress.
Vinylsulfone Abrogates High Glucose-Induced ER Stress
Maternal diabetes has been shown to cause ER stress in the embryo, specifically in the neuroepithelium, which is associated with hyperglycemia-induced NTDs.18,20,23,24 To determine whether vinylsulfone reduces high glucose-induced ER stress in cultured embryos, we measured the following ER stress markers: p-PERK, p-IRE1α, p-eIF2α, C/EBP-homologous protein (CHOP), and binding immunoglobulin protein (BiP; Figure 2). In whole embryo culture, vinylsulfone treatment abrogated high glucose-induced ER stress markers (Figure 2).
Figure 2.
Vinylsulfone (VS) abrogates high glucose-induced endoplasmic reticulum (ER) stress. Protein levels of phosphorylated protein kinase RNA-like ER kinase (p-PERK) and PERK (A), inositol requiring protein-1α (p-IRE1α) and IRE1α (B), eukaryotic initiation factor 2α (p-eIF2α) and eIF2α (C), C/EBP-homologous protein (CHOP; D), and binding immunoglobulin protein (BiP; E) were determined in 24-hour cultured embryos under normal glucose (5 mmol/L) or high glucose (16.7 mmol/L) conditions, with or without 20 µmol/L vinylsulfone. Experiments were repeated 3 times using 3 embryos from 3 different dams. * indicates significant difference compared to other groups (P < .05).
XBP1 mRNA splicing is a robust ER stress marker. To test whether vinylsulfone could prevent high glucose-induced XBP1 mRNA splicing, we analyzed XBP1 splicing through reverse transcription PCR. Cultured embryos exposed to high glucose exhibited robust splicing of XBP1 mRNA, resulting in 2 bands at 205 and 179 bp from the PCR products, whereas the normal glucose group showed no XBP1 splicing, with 1 band of 205 bp (Figure 3). Vinylsulfone treatment diminished XBP1 splicing (Figure 3). These results demonstrate that vinylsulfone significantly reduces high glucose-induced ER stress in cultured embryos.
Figure 3.
Vinylsulfone (VS) blocks high glucose-induced x-box binding protein 1 (XBP1) messenger RNA (mRNA) splicing. XBP1 mRNA splicing in 24-hour cultured embryos under normal glucose (NG, 5 mmol/L) or high glucose (HG, 16.7 mmol/L), with or without 20 µmol/L VS, as determined by polymerase chain reaction (PCR). Because the XBP1 cleavage can be determined from the absence or the presence of the 179-bp band, 2 samples (n = 2; two embryos from 2 different dams) were used. Arrows indicate actual sizes of the bands.
Vinylsulfone Blocks High Glucose-Induced Caspase Activation
Maternal diabetes induces severe ER stress in the developing embryo, and ER stress causes apoptosis in the developing neuroepithelium, which leads to NTD formation.1–3 To ascertain whether vinylsulfone suppresses high glucose-induced apoptosis in cultured embryos, we assessed caspase 3 and caspase 8 cleavage, markers of apoptosis. High glucose increased the abundance of both cleaved caspase 3 and caspase 8, whereas vinylsulfone blocked high glucose-induced caspase cleavage (Figure 4).
Figure 4.
Vinylsulfone (VS) reduces high glucose-induced caspase activation. Protein levels of caspase 3 (A) and caspase 8 (B) in 24-hour cultured embryos under normal glucose (NG, 5 mmol/L) or high glucose (HG, 16.7 mmol/L), with or without 20 µmol/L VS. Experiments were repeated three times using three embryos from three different dams. *indicates statistical significance (P < 0.05).
Discussion
In the present study, we demonstrated that the Nrf2 activator vinylsulfone can reduce high glucose-induced NTD formation, oxidative stress, nitrosative stress, ER stress, and apoptosis in cultured embryos. We showed that vinylsulfone inhibits oxidative and nitrosative stress by reducing levels of LPO, 4-HNE, and nitrotyrosine-modified proteins in embryos cultured under high glucose. Vinylsulfone treatment also blocked high glucose-induced ER stress markers p-PERK, p-eIF2α, p-IRE1α, CHOP, and BiP. Consequently, high glucose-induced apoptosis and NTD formation were ameliorated by vinylsulfone.
Nrf2 is a nuclear transcription factor that induces gene expression of detoxifying enzymes and antioxidant proteins. Nrf2 signaling is a key mechanism for cellular protection and cell survival.37,48,49 Under normal cellular conditions, Nrf2 is bound to its inhibitor, Keap1. Keap1 is an adapter for the Cullin3/Ring Box1 (Cul3/Rbx1) E3 ubiquitin ligase complex, which ubiquitinates and degrades Nrf2.50–52 Under cellular stress conditions, Nrf2 dissociates from the Keap1 complex, stabilizes, translocates into the nucleus, and activates antioxidant response element (ARE)-mediated gene expression.53–56
Experimental evidence supports the hypothesis that oxidative stress is involved in the etiology of diabetic complications, including diabetic embryopathy.1–3 Maternal diabetes induces oxidative and nitrosative stress in the developing embryo by increasing ROS production and inhibiting endogenous antioxidant activity.1–3 Because a previous study showed that diabetes contributes to oxidative stress in adult cardiomyocytes by downregulating Nrf2 activity,41 we hypothesize that activating Nrf2 can inhibit oxidative stress in our diabetic embryopathy model. We propose that Nrf2 signaling is suppressed under high glucose conditions and that by adding an exogenous Nrf2 activator, such as vinylsulfone, Nrf2 signaling is restored and its downstream antioxidant genes are expressed and ameliorate hyperglycemia-induced ROS, RNS, and NTDs.
High glucose-induced oxidative stress and ER stress lead to apoptosis in neuroepithelial cells and consequent NTD formation.18,24 We have demonstrated that maternal diabetes-induced embryonic neuroepithelial cell apoptosis is caspase 3 and caspase 8 dependent.24 Here we showed that vinylsulfone blocks high glucose-induced apoptosis in embryos. This may be due to upregulation of the ER-resident antioxidant enzymes, Prx4, GPx7, and GPx8, by Nrf2 but more studies are needed to determine the exact mechanism of action.49
Nrf2 appears to play a cytoprotective role against oxidative stress in a wide array of degenerative and immunological illnesses.48,49,56 Therefore, the development of agents that activate the Nrf2/Keap1/ARE signaling pathway has been of great interest.57 The most well-known Nrf2 activators used in complementary medicine include sulforaphane, resveratrol, curcumin, cinnamic aldehyde, and pterostilbene.58
The therapeutic potential of Nrf2 activators in diabetic complications has been indicated in many experiments in animal models and in human trials.58 For example, one group found that sulforaphane improved the metabolic profile and reduced renal injury in mice with streptozotocin-induced diabetes.45 However, sulforaphane is not an ideal therapeutic for people because it can also target other proteins that could potentially cause damage.58 In a clinical study, curcumin effectively reduced the number of prediabetic individuals who progressed toward type 2 diabetes mellitus.59 However, dietary curcumin is poorly absorbed by the digestive system and undergoes glucuronidation and excretion before it is released into the systemic circulation, thereby exhibiting a limited protective effect.60 Other Nrf2 activators, such as bardoxolone methyl and tert-butylhydroquinone, have similar adverse effects in clinical trials, although they had promising outcomes in preclinical studies.43,61–63
Vinylsulfone is a chalcone derivative, in which a vinylsulfone group is introduced to the α, β-unsaturated carbonyl entity of chalcone.46 Chalcones are an important group of natural compounds that are abundantly present in fruits (citruses and apples), vegetables (tomatoes, shallots, bean sprouts, and potatoes), and spices such as licorice.64 Chalcone derivatives are being investigated for the development of efficient drugs for the treatment of several dreadful diseases such as cancer, diabetes, HIV, tuberculosis, and malaria.65 It has been demonstrated that vinylsulfone protects neurons of the brain in an animal models of Parkinson disease.46 Therefore, vinylsulfone is able to cross the blood–brain barrier. Although there is no direct evidence that chalcone derivates can across the placenta barrier and reach the fetus, chalcone derivatives inhibit adenosine triphosphate-binding cassette transporters, which are expressed in the placenta barrier.66–68 Therefore, it is likely that chalcone derivatives including vinylsulfone may be able to across the placenta and protect the fetus.
Here, we tested a new cell-permeable Nrf2 activator, vinylsulfone, which was developed in the laboratory, specifically targeting Nrf2 through its 2’-methoxy group and has demonstrated minimal toxicity in animals.46 Our findings are among the first to demonstrate that vinylsulfone possesses therapeutic potential for maternal diabetes-induced birth defects, including NTDs. However, the therapeutic potential of this Nrf2 activator needs to be further confirmed in animal studies before we can consider clinical trials for prospective therapeutic candidates.
Acknowledgements
We are grateful to Dr Julie Wu, Offices of the Dean and Public Affairs & Communications at the University of Maryland School of Medicine, and Dr Noah Fu, for critical reading and editing.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is supported by NIH R01DK083243, R01DK101972 (to Yang P), R01DK103024 (to Yang P and Reece EA) and a Basic Science Award (1-13-BS-220), American Diabetes Association (to Yang P).
References
- 1. Yang P, Reece EA, Wang F, Gabbay-Benziv R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am J Obstetr Gynecol. 2015;212(5):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang F, Reece EA, Yang P. Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. Am J Obstet Gynecol. 2015;213(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gabbay-Benziv R, Reece EA, Wang F, Yang P. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World J Diabetes. 2015;6(3):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dincer Y, Akcay T, Alademir Z, Ilkova H. Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutat Res. 2002;505(1-2):75–81. [DOI] [PubMed] [Google Scholar]
- 5. Sakamaki H, Akazawa S, Ishibashi M, et al. Significance of glutathione-dependent antioxidant system in diabetes-induced embryonic malformations. Diabetes. 1999;48(5):1138–1144. [DOI] [PubMed] [Google Scholar]
- 6. Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993;49(3):642–652. [DOI] [PubMed] [Google Scholar]
- 7. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–412. [DOI] [PubMed] [Google Scholar]
- 8. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. [DOI] [PubMed] [Google Scholar]
- 9. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23(5):599–622. [DOI] [PubMed] [Google Scholar]
- 10. Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008;10(12):2023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide Dismutase 1 in vivo Ameliorates Maternal Diabetes-Induced Apoptosis and Heart Defects through Restoration of Impaired Wnt Signaling. Circ Cardiovasc Genet. 2015;8(5):665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dong D, Reece EA, Lin X, Wu Y, AriasVillela N, Yang P. New development of the yolk sac theory in diabetic embryopathy: molecular mechanism and link to structural birth defects[published online September 30, 2015]. Am J Obstet Gynecol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pinter E, Reece EA, Leranth CZ, et al. Yolk sac failure in embryopathy due to hyperglycemia: ultrastructural analysis of yolk sac differentiation associated with embryopathy in rat conceptuses under hyperglycemic conditions. Teratology. 1986;33(1):73–84. [DOI] [PubMed] [Google Scholar]
- 14. Reece EA, Pinter E, Leranth CZ, et al. Ultrastructural analysis of malformations of the embryonic neural axis induced by in vitro hyperglycemic conditions. Teratology. 1985;32(3):363–373. [DOI] [PubMed] [Google Scholar]
- 15. Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583(9):1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013;62(3):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. Am J Obstet Gynecol. 2013;209(4):345 e1-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Xu C, Yang P. c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes. 2013;62(2):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang F, Wu Y, Quon MJ, Li X, Yang P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. Am J Physiol Endocrinol Metab. 2015;309(5):E487–E499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular stress, excessive apoptosis, and the effect of metformin in a mouse model of type 2 diabetic embryopathy. Diabetes. 2015;64(7):2526–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu Y, Wang F, Reece EA, Yang P. Curcumin ameliorates high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Am J Obstet Gynecol. 2015;212(6):802 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu H, Yu J, Dong D, Zhou Q, Wang JY, Yang P. The miR-322-TRAF3 circuit mediates the pro-apoptotic effect of high glucose on neural stem cells. Toxicol Sci. 2015;144(1):186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang F, Wu Y, Gu H, et al. Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes. 2015;64(3):973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang P, Li X, Xu C, Reece EA, Zielke HR, Wang F. Maternal hyperglycemia activates an ASK1-FoxO3a-caspase 8 pathway that leads to embryonic neural tube defects. Sci Signal. 2013;6(290):ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang F, Weng H, Quon MJ, et al. Dominant negative FADD dissipates the proapoptotic signalosome of the unfolded protein response in diabetic embryopathy. Am J Physiol Endocrinol Metab. 2015;309(10):E861–E873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu H, Yu J, Dong D, et al. High glucose-repressed CITED2 expression through miR-200b triggers the unfolded protein response and endoplasmic reticulum stress. Diabetes. 2016;65(1):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. Am J Obstet Gynecol. 2011;205(1):84 e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang P, Li H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. Am J Obstet Gynecol. 2010;203(1):75 e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reece EA, Wu YK, Zhao Z, Dhanasekaran D. Dietary vitamin and lipid therapy rescues aberrant signaling and apoptosis and prevents hyperglycemia-induced diabetic embryopathy in rats. Am J Obstet Gynecol. 2006;194(2):580–585. [DOI] [PubMed] [Google Scholar]
- 30. Reece EA, Wu YK, Wiznitzer A, et al. Dietary polyunsaturated fatty acid prevents malformations in offspring of diabetic rats. Am J Obstet Gynecol. 1996;175(4 pt 1):818–823. [DOI] [PubMed] [Google Scholar]
- 31. Xu C, Li X, Wang F, Weng H, Yang P. Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. Am J Physiol Endocrinol Metab. 2013;305(5):E667–E678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Correa A, Botto L, Liu Y, Mulinare J, Erickson JD. Do multivitamin supplements attenuate the risk for diabetes-associated birth defects? Pediatrics. 2003;111(5 pt 2):1146–1151. [PubMed] [Google Scholar]
- 33. Zhong J, Reece EA, Yang P. Punicalagin exerts protective effect against high glucose-induced cellular stress and neural tube defects. Biochem Biophys Res Commun. 2015;467(2):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159(12):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guallar E, Stranges S, Mulrow C, Appel LJ, Miller ER., III Enough is enough: Stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013;159(12):850–851. [DOI] [PubMed] [Google Scholar]
- 36. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. [DOI] [PubMed] [Google Scholar]
- 37. Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101(7):2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Vries HE, Witte M, Hondius D, et al. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45(10):1375–1383. [DOI] [PubMed] [Google Scholar]
- 39. Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li J, Ichikawa T, Villacorta L, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29(11):1843–1850. [DOI] [PubMed] [Google Scholar]
- 41. Tan Y, Ichikawa T, Li J, et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60(2):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee Y, Shin DH, Kim JH, et al. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: structural analysis for NFkappaB inhibition. Eur J Pharmacol. 2010;643(1):21–28. [DOI] [PubMed] [Google Scholar]
- 43. Dinkova-Kostova AT, Liby KT, Stephenson KK, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102(12):4584–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yates MS, Tauchi M, Katsuoka F, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6(1):154–162. [DOI] [PubMed] [Google Scholar]
- 45. Zheng H, Whitman SA, Wu W, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60(11):3055–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woo SY, Kim JH, Moon MK, et al. Discovery of vinyl sulfones as a novel class of neuroprotective agents toward Parkinson’s disease therapy. J Med Chem. 2014;57(4):1473–1487. [DOI] [PubMed] [Google Scholar]
- 47. Weng H, Li X, Reece EA, Yang P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. Am J Obstet Gynecol. 2012;206(5):448 e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ma Q. Role of nrf2 in oxidative stress and toxicity. Ann Rev Pharmacol Toxicol. 2013;53:401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaspar JW, Niture SK, Jaiswal AK. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24(19):8477–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dhakshinamoorthy S, Long DJ, 2nd, Jaiswal AK. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul. 2000;36:201–216. [DOI] [PubMed] [Google Scholar]
- 53. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769–789. [DOI] [PubMed] [Google Scholar]
- 54. Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. [DOI] [PubMed] [Google Scholar]
- 55. Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology 2008;246(1):24–33. [DOI] [PubMed] [Google Scholar]
- 56. Hybertson BM, Gao B. Role of the Nrf2 signaling system in health and disease. Clin Genet. 2014;86(5):447–452. [DOI] [PubMed] [Google Scholar]
- 57. Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol Res. 2015;91:104–114. [DOI] [PubMed] [Google Scholar]
- 58. Garber K. Biochemistry: a radical treatment. Nature. 2012;489(7417):S4–S6. [DOI] [PubMed] [Google Scholar]
- 59. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. [DOI] [PubMed] [Google Scholar]
- 61. Li H, Zhang L, Wang F, et al. Attenuation of glomerular injury in diabetic mice with tert-butylhydroquinone through nuclear factor erythroid 2-related factor 2-dependent antioxidant gene activation. Am J Nephrol. 2011;33(4):289–297. [DOI] [PubMed] [Google Scholar]
- 62. Hirose M, Yada H, Hakoi K, Takahashi S, Ito N. Modification of carcinogenesis by alpha-tocopherol, t-butylhydroquinone, propyl gallate and butylated hydroxytoluene in a rat multi-organ carcinogenesis model. Carcinogenesis. 1993;14(4):2359–2364. [DOI] [PubMed] [Google Scholar]
- 63. Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. [DOI] [PubMed] [Google Scholar]
- 64. Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337–353. [DOI] [PubMed] [Google Scholar]
- 65. Singh P, Anand A, Kumar V. Recent developments in biological activities of chalcones: a mini review. Eur J Med Chem. 2014;85:758–777. [DOI] [PubMed] [Google Scholar]
- 66. Vahakangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol. 2009;158(3):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rangel LP, Winter E, Gauthier C, et al. New structure-activity relationships of chalcone inhibitors of breast cancer resistance protein: polyspecificity toward inhibition and critical substitutions against cytotoxicity. Drug Des Devel Ther. 2013;7:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parveen Z, Brunhofer G, Jabeen I, Erker T, Chiba P, Ecker GF. Synthesis, biological evaluation and 3D-QSAR studies of new chalcone derivatives as inhibitors of human P-glycoprotein. Bioorg Med Chem. 2014;22(7):2311–2319. [DOI] [PubMed] [Google Scholar]