Abstract
The four jointed box 1 (FJX1) is a regulator of angiogenesis, and the levels of FJX1 are increased in several types of cancer. Angiogenesis plays a critical role in endometrial growth as well as in several gynecologic disorders including endometriosis. However, the function of FJX1 has not been studied in endometriosis. Therefore, we examined the levels of FJX1 in eutopic endometrium from women with or without endometriosis. The levels of FJX1 protein did not change in endometrial cells during the menstrual cycle in endometrium from women without endometriosis. However, its levels were significantly higher in the secretory phase of the eutopic endometrium from women with endometriosis when compared to women without endometriosis. Hypoxia-inducible factor-1α (HIF1α) is known as a key mediator of endometriosis by regulating genes essential to estrogen production, angiogenesis, proliferation, inflammation, and extracellular invasion. It has been reported that FJX1 induces an increase in HIF1α through posttranslational stabilization. The results of our Western blot analysis reveal a significant positive correlation between FJX1 and HIF1α proteins in endometrium of women with and without endometriosis. This overexpression of FJX1 was confirmed by sequential analysis of the eutopic endometrium during endometriosis progression, using an induced model of endometriosis in the baboon. Therefore, our results suggest that high levels of FJX1 proteins may play an important role in the pathogenesis of endometriosis.
Keywords: endometriosis, FJX1, endometrium
Introduction
Endometriosis affects 10% of women of reproductive age and presents clinically with chronic pelvic pain, dysmenorrhea, and infertility.1 It is defined as the presence of endometrial tissue with functional glands and stroma outside the uterus.2 Although the pathogenesis of endometriosis remains unclear, it has been accepted that Sampson’s theory of retrograde menstruation is the first point to develop endometriosis.3 Furthermore, coelomic metaplasia, lymphatic, and hematogenic spread of endometrial cells and recruitment of progenitor cells may lead to the endometriotic implant. Regardless of the exact mechanisms, endometrial implants require neovascularization to invade the ectopic site, proliferate, and establish an endometriotic lesion.4
Angiogenesis is under the control of dynamic balance between proangiogenic factors, such as the vascular endothelial growth factor (VEGF) family and inhibitors such as thrombospondin-1, angiostatin, and so on.5 Similar to other systems of hypoxia and tumor angiogenesis, vascular endothelial growth factor A (VEGF-A) plays the role of the principal positive angiogenic regulator in endometriotic lesions.6 The endometrium is a cyclic regenerating system, and hypoxia may be a key factor in regeneration of endometrial tissues during menstruation.7 Neovascularization is principally induced by hypoxia, while other triggers include tissue injury, inflammation, and altered hormonal milieu in patients with endometriosis.8 Hypoxia-inducible factor-1 α (HIF1α) promotes VEGF-A secretion by upregulation of VEGF-A gene.9
Hypoxia-inducible factor-1 α expression increases over the course of the secretory phase of the menstrual cycle and reaches a maximum in the functional layer of human endometrium during menstruation.10 Interestingly, the expression of HIF1α is higher in the eutopic endometrium from endometriosis patients compared to disease-free women.11,12 Our previous results also showed that aberrant activation of STAT3 led to increased HIF1α expression in the eutopic endometrial epithelial cells of women with endometriosis.13 In addition, inhibiting HIF1α expression suppresses the growth of endometriosis, partially through its downregulating angiogenic potential of endometrial stromal cells.14 Hypoxia-inducible factor-1 α regulates its downstream target genes involved in processes such as angiogenesis,15 glycolysis,16 cell fate-related,17 oncogenesis,18 and tumor suppression.19 Therefore, understanding the molecular mechanisms underlying HIF1α in the development and progression of endometriosis is critical.
The four jointed box 1, FJX1, is a notch-inducible secreted ligand that is homologous to the Drosophila four-jointed (fj) gene.20 Although the precise function of FJX1 in humans remains unclear, studies of different species suggest that FJX1 is involved in carcinogenesis. FJX1 gene amplification and increased mRNA expression of FJX1 have been demonstrated in several human cancers, and it is suggested as a candidate for regulation of angiogenesis.21–23 Although endometriosis is considered a benign disease, it also shows tumor-like behavior such as the metastasis of endometriotic tissue outside the uterus and infiltrating to the surrounding organ.
Angiogenesis plays an essential role in the growth and survival of endometriotic lesions. Four jointed box 1 is known to regulate the HIF1α protein and promotes angiogenesis in colorectal carcinoma.22 Four jointed box 1 is necessary for miR-720 upregulation, which is dependent upon HIF1α.24 However, FJX1 has not been studied in the endometrium or endometriosis. In the present study, we investigated the expression patterns of the FJX1 protein in eutopic endometrium from women with and without endometriosis, and its correlation with HIF1α, which was found to be crucially associated with endometriosis as a proangiogenic mediator.
Materials and Methods
Human Endometrial Samples
The study has been reviewed and approved by the institutional review board of Michigan State University (Grand Rapids, Michigan), Greenville Health System (Greenville, South Carolina), and the University of North Carolina (Chapel Hill, North Carolina). Informed consent was obtained from all participants. Human endometrial samples were obtained through the Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository, the Greenville Hospital System, and the University of North Carolina.
For Western blot analysis of eutopic endometrium between women with and without endometriosis, 27 total protein samples (9 per proliferative and 18 per secretory phase) were used from the eutopic endometrium of women with endometriosis, and 12 total protein samples (6 per proliferative and 6 per secretory phase) from women without endometriosis were used. For immunohistochemical analysis, 19 samples (7 per proliferative and 12 per secretory phase) from women with endometriosis were compared with 16 samples from the control group (4 per proliferative and 12 per secretory phase). Endometrium was obtained at the time of laparoscopic surgery or hysterectomy for benign gynecologic disease (ie, fibroids and prolapse). The presence or absence of endometriosis was confirmed by visualization and pathologic confirmation of endometriotic lesions during surgery. Women who were laparoscopically positive for this disease were placed in the endometriosis group, while control women without signs or symptoms of disease were recruited for the control group. Use of an intrauterine device (IUD) or hormonal therapies in the 3 months preceding surgery was exclusionary. Histologic dating of endometrial samples was performed by board-certified pathologist and subsequently confirmed by an experienced fertility specialist (B.A.L.).
Baboon Endometrium Samples
The use of this animal model was reviewed and approved by the Institutional Animal Care and Use Committees of both the University of Illinois at Chicago and Michigan State University. Endometriosis was induced by intraperitoneal inoculation of menstrual endometrium on 2 consecutive menstrual cycles as previously described.25 Endometriosis was confirmed by laparoscopic presence of endometriotic lesions, and endometrial tissues from baboons with endometriosis were collected by laparotomy during the mid-secretory phase. Eutopic endometrial tissues were collected from early secretory phase baboons at preinoculation and postinoculation.
Western Blot Analysis
Endometrial tissue was lysed using a lysis buffer (10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2.5 mM EDTA, and 0.125% (vol/vol) Nonidet P-40 supplemented with both a protease inhibitor cocktail (Roche, Indianapolis, Indiana) and a phosphatase inhibitor cocktail (Sigma Aldrich, St Louis, Missouri). Twenty micrograms of total protein was separated on 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred into a polyvinylidene difluoride membrane (Millipore Corp, Bedford, Massachusetts). After blocking with 0.5% (wt/vol) casein for 2 hours in phosphate-buffered saline (PBS) with 0.1% (vol/vol) Tween 20 (Sigma Aldrich), membranes were incubated with either anti-FJX1 (ab80264, Abcam) or anti- HIF1α (610958, BD Bioscience, San Jose, California) antibodies. Total α-tubulin (ab7291, Abcam, Cambridge, Massachusetts) levels were examined for loading controls. Following incubation with the primary antibody, membranes were exposed to a horseradish peroxidase–linked (HRP-) secondary antibody and positive immunostained observed using an enhanced chemiluminescence HRP substrate. The band density was determined by relative densitometry using Image J (National Institute of Health, USA) and normalized against the bands obtained for α-tubulin.
Immunohistochemistry
Uterine sections were blocked with 10% normal goat serum in PBS (pH 7.5) for immunohistochemistry (IHC). Sections were exposed to appropriate primary antibody (anti-FJX1, ab80264, Abcam or anti- HIF1α, 610958, BD Bioscience) in 10% normal goat serum in PBS (pH 7.5) overnight at 4°C. Sections were incubated with the appropriate secondary antibody (Vector Laboratories, Burlingame, California). Following exposure to the horseradish peroxidase–conjugated streptavidin substrate, positive immunoreactivity (brown precipitate) was detected using the Vectastain Elite DAB kit (Vector Laboratories). A semiquantitative grading system (H-score) was used to compare the immunohistochemical staining intensities.26 The H-score was calculated using the following equation: H-score = ∑ Pi (i), where i = intensity of staining with a value of 1, 2, or 3 (weak, moderate, or strong, respectively) and Pi is the percentage of stained cells for each intensity, varying from 0% to 100%.
Statistical Analysis
For Western blot analysis, differences in protein expression between the control group and women with endometriosis were compared following normalization against α-tubulin. For data containing more than 2 groups, a parametric Tukey-Kramer 1-way analysis of variance was used to test the null hypothesis of group differences, followed by a Wilcoxon test. For data from the baboon, a paired t test was used. The Spearman correlation coefficient was used to assess correlations between the levels of FJX1 and HIF1α in the control and endometriosis groups. All data are presented as mean ± standard error of the mean (SEM). P < .05 was considered statistically significant. All statistical analyses were performed using Instat package from GraphPad (San Diego, California).
Results
Overexpression of FJX1 in Secretory Endometrium From Women With Endometriosis
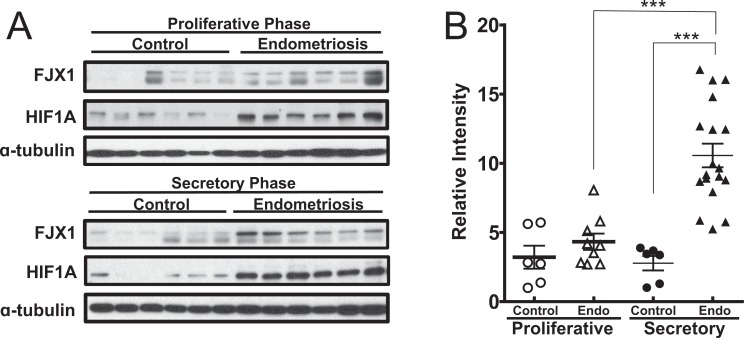
To determine whether FJX1 is dysregulated in endometriosis, we examined the levels of FJX1 proteins in eutopic endometrium from women with or without endometriosis using Western blot (Figure 1A). Western blot analysis was conducted on total protein lysates extracted from the endometrial tissue of women with and without endometriosis. The protein expression levels of FJX1 were significantly increased in the endometriosis group during the secretory phase (the mean of relative band density ± SEM: 10.57 ± 0.85) when compared with the control group (2.78 ± 0.52; P < .0001, Figure 1B). However, during the proliferative phase, it did not differ between the 2 groups (control vs endometriosis; 3.21 ± 0.83 vs 4.33 ± 0.59). In addition, the FJX1 protein was significantly increased in the secretory phase compared to the proliferative phase in women with endometriosis. These results suggest that the expression of FJX1 is associated with the menstrual cycle dependency in endometriosis.
Figure 1.
Enhanced expression of four jointed box 1 (FJX1) in secretory eutopic endometrium from women with endometriosis. A, Western blot analysis of FJX1 and hypoxia-inducible factor-1 α (HIF1α) proteins in eutopic endometrial lysate. B, Relative densitometric analysis showed the expression of FJX1 in endometriosis group during menstrual cycle. The results represent the mean ± standard error of the mean (SEM). Analysis of variance: ***P < .001.
Correlation Between FJX1 and HIF1α in Endometriosis
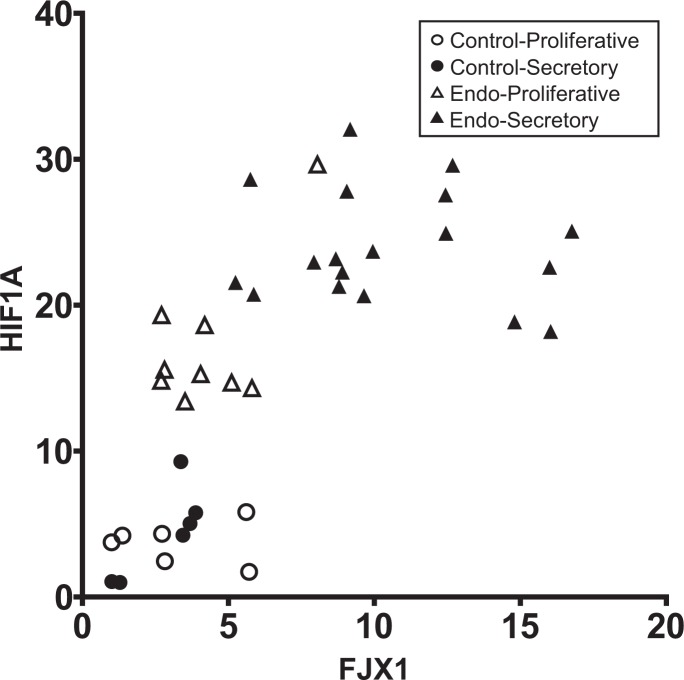
Hypoxia-inducible factor-1 α is known as a key mediator of endometriosis by regulating the genes that were essential to estrogen production, angiogenesis, proliferation, inflammation, and extracellular invasion.27 FJX1 regulates HIF1α protein levels via increasing protein stability rather than upregulation of translation, which contributed to increased endothelial tube formation in vitro.22 To determine whether the protein expression of FJX1 correlates with HIF1α expression with respect to endometriosis, we examined correlation between FJX1 and HIF1α using the results of Western blot analysis. Figure 2 showed the significant positive correlation between FJX1 and HIF1α proteins in the eutopic endometrium from women without (n = 12) and with (n = 27) endometriosis (Spearman correlation coefficient r = 0.6783, P < .0001). It suggests that positive correlation of the overexpression of FJX1 and HIF1α in the eutopic endometrium may play an important role in the development and progression of endometriosis.
Figure 2.
Correlation analysis of four jointed box 1 (FJX1) and hypoxia-inducible factor-1 α (HIF1α) in eutopic endometrium from women with and without endometriosis. A significant positive correlation was showed between FJX1 and HIF1α protein expression in control (n = 12) and endometriosis (n = 27; P < .0001, r = .6783).
Enhanced Protein Expression of FJX1 in Endometrial Epithelial Cell From Women With Endometriosis
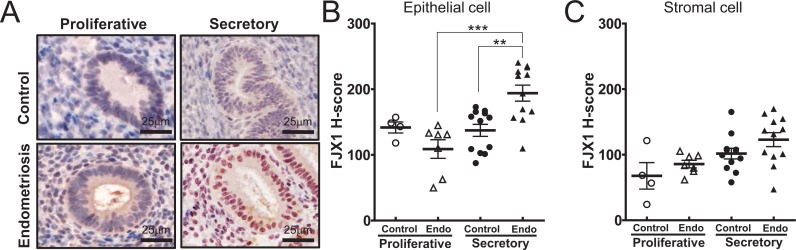
To gain insight into the cell-specific expression of FJX1, we examined the levels of FJX1 in eutopic endometrial tissues from spontaneously cycling women using immunohistochemical analysis (Figure 3A). Four jointed box 1 expression was detected in both the epithelial and stromal cells of endometrium from the proliferative and secretory phase in women without endometriosis. In the control group, the expression levels of FJX1 were not different throughout the stages of the menstrual cycle. However, significantly increased levels of FJX1 were detected in endometrial epithelial cells from women with endometriosis (mean H-score ± SEM; 194.03 ± 12.15) compared to the control group (137.36 ± 9.16) during the secretory phase (P < .01, Figure 3B). These findings are consistent with the pattern of expression seen in women with endometriosis by Western blot analyses. The stromal cells showed increasing trends in women with endometriosis; however, it did not reach statistical significance. These results suggest that increased FJX1 of epithelial cells may regulate the HIF1α protein, resulting in the upregulation of the proangiogenic factor in eutopic endometrium from women with endometriosis.
Figure 3.
Immunohistochemical staining of four jointed box 1 (FJX1) proteins in human endometrium of control and endometriosis. A, Representative light microscopic images of FJX1 immunostating in eutopic endometrium. B, Four jointed box 1 was highly detected in epithelial cells of secretory endometrium from women with endometriosis. C, Stromal cell showed increasing trends in women with endometriosis; however, they failed to achieve statistical significance. The results represent the H-score (mean ± standard error of the mean [SEM]). Analysis of variance: ***P <.001; **P < .005.
FJX1 Expression During Progression of Endometriosis in a Baboon Model
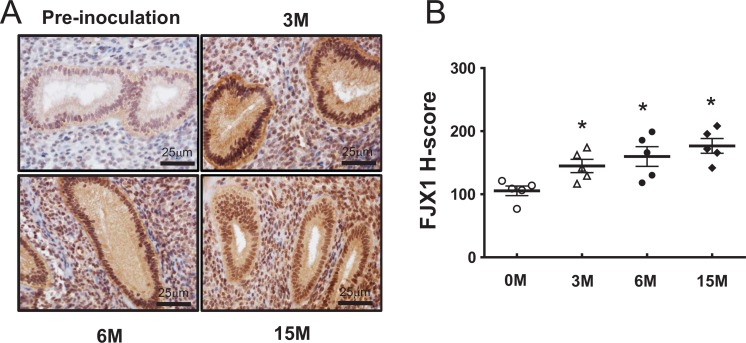
In the nonhuman primates, it is possible to induce endometriosis by inoculating menstrual products in the peritoneal cavity of baboons.28 These lesions are histologically and clinically similar to human ectopic endometriotic lesions.28–30 To determine whether FJX1 protein overexpression was also evident in this nonhuman primate model, we examined FJX1 expression in the eutopic endometrium of baboons following experimental induction of the disease. Four jointed box 1 was strongly detected in both the stroma and epithelium of baboon endometrium after 3 months from induction of endometriosis compared to preinoculation. Its expression was gradually increased with time-course pattern during endometriosis progression, but they did not reach significance (Figure 4). These results suggest that FJX1 may associate with the progression of endometriosis.
Figure 4.
Immunohistochemical staining of four jointed box 1 (FJX1) proteins in baboon endometrium of control and endometriosis. A, Representative light microscopic images of FJX1 immunostating in eutopic endometrium. B, Increased FJX1 expression in induced endometriosis of baboon. Four jointed box 1 was strongly detected in both stroma and epithelium of baboon endometrium after 3 months from the induction of endometriosis compared to preinoculation. The expression pattern was not changed during disease progression. Paired t test, *P < .05.
Discussion
In the present study, we demonstrate that FJX1 protein levels are significantly increased in eutopic endometrium from women with endometriosis compared to women without endometriosis during the secretory phase of menstruation. Four jointed box 1 is expressed not only in epithelial cells but also in stromal cells of endometrium. Epithelial cells show stronger expression of FJX1 than stromal cells. The human and mouse FJX1 genes have been described as the vertebrate homologs of the Drosophila fj gene,31 and the FJX1 protein is highly conserved in different species.32 Fj encodes a transmembrane type II glycoprotein that is partially secreted. The Drosophila protein is important for growth and differentiation of legs and wings and for proper development of the eyes.33,34 The murine FJX1 gene is also found in the mouse brain, peripheral nervous system, and epithelial cells of multiple organs during limb development.32 However, the exact function of FJX1 in humans is not known. Four jointed box 1 plays key roles in cancer-related pathways including Fat and Dachsous,35 Hippo-Yes-associated protein (YAP),36 Notch,37 and Janus kinase (JAK)/Signal Transducer and Activator of Transcription(STAT) signaling38 in other species. And several studies have shown that messenger RNA expression of FJX1 is increased in human cancers.21–23 FJX1 promotes angiogenesis, is associated with poor prognosis in colorectal carcinoma,22 and might serve as a tumor vascular marker in ovarian cancer.39
To gain insight into the cycle-dependent expression of FJX1, we compared the expression of FJX1 during the menstrual cycle. No significant difference could be observed regarding the FJX1 expression of healthy women during the menstrual cycle. In women with endometriosis, endometrial expression of FJX1 was significantly higher in the secretory phase than that of the proliferative phase. These menstrual cycle–specific changes of FJX1 suggest that ovarian hormones may affect FJX1 expression. It has been known that the Drosophila fj gene is modulated by Notch, Unpaired (JAK/STAT), and Wingless signals.40 Also, the murine FJX1 promoter has a conserved recombination signal binding protein-Jk (RBP-J) binding site that mediates canonical Notch signaling.32 Notch signaling regulates cell fate and critically influences cell proliferation, differentiation, and apoptosis in the primate endometrium. Notch1 is regulated by progesterone in endometrial stromal cells and modulates decidualization in primates.20 Consistently, Notch1 downregulation led to a significant decline of proliferation and migration in human endometrial stem cells from women with endometriosis, as well as the size of endometriotic lesions in murine models.41 Our results that FJX1 expression is increased during the secretory phase may suggest a possibility be related with Notch1 signaling activated by progesterone. It could be implicated in pathogenesis of endometriosis.
Hypoxia-inducible factor-1 α is a known key mediator of endometriosis by regulating genes essential to estrogen production, angiogenesis, proliferation, inflammation, and extracellular invasion.27 It has been reported that FJX1 induces an increase of HIF1α through posttranslational stabilization.22 The results of our Western blot analysis reveal a significant positive correlation between FJX1 and HIF1α proteins in women with and without endometriosis (Figure 2). This overexpression of FJX1 was confirmed by sequential analysis of the eutopic endometrium during disease progression, from baboons using this animal model of induced endometriosis. Four jointed box 1 was shown in colorectal carcinoma cells to promote endothelial cell capillary tube formation in an HIF1α-dependent manner.22 Four jointed box 1 induced an increase in HIF1α through posttranslational stabilization of HIF1α. It was associated with poor prognosis in colorectal cancer. And, FJX1 promotes angiogenesis through miR-720 upregulation in endothelial progenitor cells from patients with coronary artery disease.24 They revealed that knockdown of FJX1 with overexpression of HIF1α restored miRNA-720 levels, while knockdown of HIF1α with overexpressed FJX1 abolished miRNA-720, indicating FJX1 regulates miRNA-720 expression via HIF1α. These findings support our hypothesis that HIF1α is upregulated in eutopic endometrium from women with endometriosis and it may be related to FJX1. The underlying mechanism responsible for the expression of FJX1 and HIF1α in eutopic endometrial tissue remains to be clarified.
Aberrant leptin expression in endometriotic stromal cells was induced by elevated HIF1α.11 It was a contributor to stromal cell proliferation and the development of endometriosis. Decreased expression of dual specificity phosphatase-2 (DUSP2) is found in endometriotic stromal cells.42 Suppression of DUSP2 by HIF1α in eutopic stromal cells causes increased cyclooxygenase-2 (COX-2) gene sensitivity to pro-inflammatory cytokine stimulation.43 Since endometriosis is a cause of chronic pelvic pain, COX-2 regulation may be another target of FJX1. The COX-2 inhibitor (celecoxib) treatment was associated with decreased expression of FJX1 mRNA in paired rectal cancer samples, which came from 16 patient biopsies taken pre- and post-celocoxib treatment.44
In summary, we identified increased expression of novel protein FJX1 in the secretory endometrium from women with endometriosis. Although the underlying functional mechanism of FJX1 is not clear in humans, our findings provide the possible candidate of pathophysiologic mediation in endometriosis. The increased FJX1 and HIF1α proteins in eutopic endometrium may contributed to lesion establishment of refluxed endometrial tissues and disease progression. Determining the functional mechanism of FJX1 in endometriosis will be necessary and critical in focusing better therapeutic approaches.
Acknowledgments
We would like to thank the Human Female Reproductive Tract Biorepository and the Spectrum Health Medical Group, the Department of Obstetrics and Gynecology in Grand Rapids, Michigan (especially Elizabeth Leary, MD, Calvin Leazenby, MD, Diana Bitner, MD, and Christine Heisler, MD) and Meighan L. McAuliffe for their help in obtaining human samples.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NICHD R01HD084478 (to J.W.J) and R01HD083273 (A.T.F.).
References
- 1. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leone Roberti Maggiore U, Ferrero S, Mangili G, et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update. 2016;22(1):70–103. [DOI] [PubMed] [Google Scholar]
- 3. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110 143. [PMC free article] [PubMed] [Google Scholar]
- 4. Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update. 2007;13(4):331–342. [DOI] [PubMed] [Google Scholar]
- 5. McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 2000;6(1):45–55. [DOI] [PubMed] [Google Scholar]
- 6. Di Carlo C, Bonifacio M, Tommaselli GA, Bifulco G, Guerra G, Nappi C. Metalloproteinases, vascular endothelial growth factor, and angiopoietin 1 and 2 in eutopic and ectopic endometrium. Fertil Steril. 2009;91(6):2315–2323. [DOI] [PubMed] [Google Scholar]
- 7. Popovici RM, Irwin JC, Giaccia AJ, Giudice LC. Hypoxia and cAMP stimulate vascular endothelial growth factor (VEGF) in human endometrial stromal cells: potential relevance to menstruation and endometrial regeneration. J Clin Endocrinol Metab. 1999;84(6):2245–2248. [DOI] [PubMed] [Google Scholar]
- 8. Rocha AL, Reis FM, Taylor RN. Angiogenesis and endometriosis. Obstet Gynecol Int. 2013;2013:859619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. [DOI] [PubMed] [Google Scholar]
- 10. Critchley HO, Osei J, Henderson TA, et al. Hypoxia-inducible factor-1alpha expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2). Endocrinology. 2006;147(2):744–753. [DOI] [PubMed] [Google Scholar]
- 11. Wu MH, Chen KF, Lin SC, Lgu CW, Tsai SJ. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am J Pathol. 2007;170(2):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goteri G, Lucarini G, Montik N, et al. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis. Int J Gynecol Pathol. 2009;28(2):157–163. [DOI] [PubMed] [Google Scholar]
- 13. Kim BG, Yoo JY, Kim TH, et al. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30(5):1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang ZZ, Hu CP, Tang WW, et al. Wenshen Xiaozheng Tang suppresses the growth of endometriosis with an antiangiogenic effect. Climacteric. 2013;16(6):700–708. [DOI] [PubMed] [Google Scholar]
- 15. Tsai CH, Li CH, Liao PL, et al. NcoA2-dependent inhibition of HIF-1alpha activation is regulated via AhR. Toxicol Sci. 2015;148(2):517–530. [DOI] [PubMed] [Google Scholar]
- 16. Abu-Remaileh M, Aqeilan RI. Tumor suppressor WWOX regulates glucose metabolism via HIF1alpha modulation. Cell Death Differ. 2014;21(11):1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang Y, Wu GH, He GD, et al. The effect of silencing HIF-1alpha gene in BxPC-3 cell line on glycolysis-related gene expression, cell growth, invasion, and apoptosis. Nutr Cancer. 2015;67(8):1314–1323. [DOI] [PubMed] [Google Scholar]
- 18. Timani KA, Liu Y, Fan Y, Mohammad KS, He JJ. Tip110 regulates the cross talk between p53 and hypoxia-inducible factor 1alpha under hypoxia and promotes survival of cancer cells. Mol Cell Biol. 2015;35(13):2254–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365(6):537–547. [DOI] [PubMed] [Google Scholar]
- 20. Afshar Y, Miele L, Fazleabas AT. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012;153(6):2884–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarvinen AK, Autio R, Kilpinen S, et al. High-resolution copy number and gene expression microarray analyses of head and neck squamous cell carcinoma cell lines of tongue and larynx. Genes Chromosomes Cancer. 2008;47(6):500–509. [DOI] [PubMed] [Google Scholar]
- 22. Al-Greene NT, Means AL, Lu P, et al. Four jointed box 1 promotes angiogenesis and is associated with poor patient survival in colorectal carcinoma. PLoS One. 2013;8(7):e69660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chai SJ, Yap YY, Foo YC, et al. Identification of four-jointed box 1 (FJX1)-specific peptides for immunotherapy of nasopharyngeal carcinoma. PLoS One. 2015;10(11):e0130464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang HW, Huang TS, Lo HH, et al. Deficiency of the microRNA-31-microRNA-720 pathway in the plasma and endothelial progenitor cells from patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2014;34(4):857–869. [DOI] [PubMed] [Google Scholar]
- 25. Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis . Biol Reprod. 2013;88(2):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishibashi H, Suzuki T, Suzuki S, et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88(5):2309–2317. [DOI] [PubMed] [Google Scholar]
- 27. Zhan L, Wang W, Zhang Y, Song E, Fan Y, Wei B. Hypoxia-inducible factor-1alpha: a promising therapeutic target in endometriosis. Biochimie. 2016;123:130–137. [DOI] [PubMed] [Google Scholar]
- 28. Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann N Y Acad Sci. 2002;955:308–317; discussion 340-302, 396-406. [DOI] [PubMed] [Google Scholar]
- 29. Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod. 2009;15(10):577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D’Hooghe TM, Kyama CM, Chai D, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16(2):152–161. [DOI] [PubMed] [Google Scholar]
- 31. Ashery-Padan R, Alvarez-Bolado G, Klamt B, Gessler M, Gruss P. Fjx1, the murine homologue of the Drosophila four-jointed gene, codes for a putative secreted protein expressed in restricted domains of the developing and adult brain. Mech Dev. 1999;80(2):213–217. [DOI] [PubMed] [Google Scholar]
- 32. Rock R, Heinrich AC, Schumacher N, Gessler M. Fjx1: a notch-inducible secreted ligand with specific binding sites in developing mouse embryos and adult brain. Dev Dyn. 2005;234(3):602–612. [DOI] [PubMed] [Google Scholar]
- 33. Tokunaga C, Gerhart JC. The effect of growth and joint formation on bristle pattern in D. melanogaster . J Exp Zool. 1976;198(1):79–95. [DOI] [PubMed] [Google Scholar]
- 34. Yang CH, Axelrod JD, Simon MA. Regulation of frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108(5):675–688. [DOI] [PubMed] [Google Scholar]
- 35. Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321(5887):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao Y, Hata Y, Ikeda M, Withanage K. Mammalian Hippo pathway: from development to cancer and beyond. J Biochem. 2011;149(4):361–379. [DOI] [PubMed] [Google Scholar]
- 37. Buckles GR, Rauskolb C, Villano JL, Katz FN. Four-jointed interacts with dachs, abelson and enabled and feeds back onto the Notch pathway to affect growth and segmentation in the Drosophila leg. Development. 2001;128(18):3533–3542. [DOI] [PubMed] [Google Scholar]
- 38. Gutierrez-Avino FJ, Ferres-Marco D, Dominguez M. The position and function of the Notch-mediated eye growth organizer: the roles of JAK/STAT and four-jointed. EMBO Rep. 2009;10(9):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buckanovich RJ, Sasaroli D, O’Brien-Jenkins A, et al. Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol. 2007;25(7):852–861. [DOI] [PubMed] [Google Scholar]
- 40. Zeidler MP, Perrimon N, Strutt DI. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr Biol. 1999;9(23):1363–1372. [DOI] [PubMed] [Google Scholar]
- 41. He H, Liu R, Xiong W, et al. Lentiviral vector-mediated down-regulation of Notch1 in endometrial stem cells results in proliferation and migration in endometriosis. Mol Cell Endocrinol. 2016;434:210–218. [DOI] [PubMed] [Google Scholar]
- 42. Hsiao KY, Chang N, Lin SC, Li YH, Wu MH. Inhibition of dual specificity phosphatase-2 by hypoxia promotes interleukin-8-mediated angiogenesis in endometriosis. Hum Reprod. 2014;29(12):2747–2755. [DOI] [PubMed] [Google Scholar]
- 43. Wu MH, Lin SC, Hsiao KY, Tsai SJ. Hypoxia-inhibited dual-specificity phosphatase-2 expression in endometriotic cells regulates cyclooxygenase-2 expression. J Pathol. 2011;225(3):390–400. [DOI] [PubMed] [Google Scholar]
- 44. Johnson JC, Schmidt CR, Shrubsole MJ, et al. Urine PGE-M: a metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2006;4(11):1358–1365. [DOI] [PubMed] [Google Scholar]