Abstract
This study determined whether a progesterone (P) receptor (PR)-mediated mechanism regulates morphological characteristics associated with prepartum cervix remodeling at term and with preterm birth. With focus on the transition from a soft to ripe cervix, the cervix stroma of untreated controls had reduced cell nuclei density/area and less organized extracellular collagen, while the density of macrophages/area, but not neutrophils, increased just 2 days before birth (day 17 vs day 15 or 16.5 postbreeding). Preterm birth was induced within 24 hours of treatment on day 16 postbreeding with PR antagonist or ovariectomy (Ovx). Pure or mixed PR antagonists increased the density of macrophages in the cervix within 8 hours (day 16.5 postbreeding), in advance of preterm birth. However, neither PR antagonists nor P withdrawal after Ovx affected the densities of cell nuclei and neutrophils or extracellular collagen compared to the same day controls—an indication that the cervix was sufficiently remodeled for birth to occur. To block the effect of systemic P withdrawal, Ovx pregnant mice were given a PR agonist, either pure or mixed. These treatments forestalled preterm birth and prevented further morphological remodeling of the cervix. The resulting increase in macrophage density in cervix stroma following Ovx was only blocked by a pure PR agonist. These findings support the hypothesis that inflammatory processes in the prepartum cervix that include residency of macrophages, cellular hypertrophy, and extracellular collagen structure are regulated by genomic actions of PR in a final common mechanism both at term and with induced preterm birth.
Keywords: macrophage, neutrophil, collagen, onapristone, mifepristone, promegestone
Introduction
The cervix serves as a barrier to protect the womb from the vaginal ecosystem during pregnancy and as a gateway for birth. As pregnancy progresses, the morphological characteristics and biomechanical properties of the prepartum cervix reflect a dynamic remodeling process.1–3 Across species, before the onset of labor, the extracellular collagen matrix in the cervix restructures through a mechanism that resembles an inflammatory process.4–6 These changes occur in advance of the myometrial contractions during labor. Though available biopsy tissue is limited, evidence suggests that similar processes occur in the cervix of peripartum women.7,8
Progesterone (P) is recognized as essential to sustain pregnancy. As appreciated by Csapo and Wiest,9 circulating P declines before the onset of labor in some, but not in all, rodents, and in higher mammalian species there is no decline with the approach of term.10–12 However, in mice and rats, during the period when the cervix transitions from a soft to ripe structure, evidence clearly indicates that P in circulation remains near or at peak compared to that earlier in pregnancy or during the estrus cycle in nonpregnant controls.11 This period is characterized by extracellular collagen degradation, with respect to organization and decreased fibril length and diameter, a reduced cells density in the stroma, increased presence of macrophages, and enhanced innervation 3 to 5 days before birth depending upon the species.6,9,12–16 The consensus that cervix remodeling occurs while systemic P is comparatively high across mammals is consistent with the proposal by Mesiano et al17 that parturition may result from a local loss of the trophic effects of P.18–20 This functional withdrawal stems from the findings that preterm birth is induced in pregnant rodents by ovariectomy (Ovx) to remove the main source of P.21
Moreover, P receptors (PR) serve a critical role in cervix remodeling are demonstrated through studies of the mixed PR antagonist, mifepristone (RU486), that induces preterm birth in women and rodents.12,22–24 In women, RU486-induced termination of pregnancy increased collagenase and metalloproteinase activities, degraded extracellular collagen, and increased the density of immune cells in the cervix,19,25–28 which are characteristics of cervical ripening in rodents. In an early electron microscopic study of the guinea pig cervix, Hegele-Hartung et al observed an increased presence of morphologically identified macrophages and expansion of the extracellular matrix at the luminal–epithelial interface within 24 hours of treatment with the pure PR antagonist onapristone (Ona).29 Disorganization of collagen fibers surrounding both fibroblasts and macrophages was noted. However, effects were found only when guinea pigs were treated 7 days before term and, of critical importance, Ona did not induce preterm birth at any dose. In contrast, RU486 or Ona treatment of pregnant rats reduced the density of cell nuclei, degraded collagen structure, and enhanced residency by macrophages in the cervix within 8 hours, and preterm birth occurred 24 hours later.12 There is no comparable study of PR antagonist effects on cervix remodeling characteristics in mice, nor there has been focus on the transition from a soft to ripening cervix between 15 and 8 days of pregnancy in this species. Thus, to extend our current understanding of the importance of PR for remodeling and improving resolution of morphological changes early in the transition from a soft to ripe cervix, the first objective of this study was to use pure or mixed PR antagonists to determine whether PR in pregnant mice were similar to the characteristics associated with processes that occur at term.
The second objective of the present investigation was to further understand PR agonist effects on the remodeling process. Progestogens are well recognized to maintain pregnancy and block preterm delivery in a variety of species.9,30–33 In a nonpregnant mouse model for pregnancy, estradiol (E) and P treatments reduced cell nuclei density and degraded collagen organization in the cervix, similar to that found in untreated pregnant mice 3 to 5 days before birth.34 Within 24 hours of implant removal, P withdrawal increased the density of macrophages and neutrophils in the cervix stroma without a decline in cell nuclei density or evidence of further collagen structural degradation. Thus, PR-mediated actions may regulate aspects of structural and inflammatory processes for cervix remodeling. In fact, progestational treatments forestall parturition in women with a short cervix.28,35–38 In rats, the pure progestogen promegestone (R5020) blocks preterm birth and increases cervical resistance to stretch compared to the PR antagonist effects.39 Although the effects of pure PR agonists on characteristics associated with remodeling of the cervix are not known, in mice, medroxyprogesterone acetate (MPA) blocks inflammation-induced remodeling and prevents preterm birth.40 Thus, the second novel focus of the present report was to compare the effects of pure and mixed PR agonists to test the hypothesis that a PR-mediated mechanism regulates the morphological and structural characteristics in the prepartum cervix in a mouse model of preterm birth.
Materials and Methods
Primiparous CD-1 mice were obtained at 60 days of age from Harlan Laboratories (Livermore, California; n = 57). Mice were individually housed in the Loma Linda University (LLU) vivarium and maintained in a humidity-controlled room at 23°C, with a 12-hour light–dark cycle. Food and water were provided ad libitum. Animal care and experimental procedures followed the guidelines of the Office of Laboratory Animal Welfare (NIH) and American Association of Laboratory and Animal Science under a protocol approved by the LLU Institutional Animal Care and Use Committee. Unless otherwise specified, reagents were purchased from Sigma-Aldrich (St Louis, Missouri).
Experiment 1: PR-Antagonists-Induced Preterm Cervix Remodeling
On the morning of day 16 postbreeding (day 0 = first 24-hour mating period with male), pregnant mice were randomly allocated to 3 groups that were treated with a vehicle as a control (Veh, n = 10; 0.1 mL subcutaneous [sc] injection of a 1:7 ratio of ethanol to sesame oil), the pure PR receptor antagonist Ona (n = 8; 30 mg/kg body weight/0.1 mL vehicle sc, gift from Dr Robert Garfield), or RU486, a mixed PR antagonist (n = 10, 6 mg/kg 20 mg/L in vehicle, sc, M8046).41 Mice were asphyxiated with CO2, and the reproductive tract from the lower uterine horns through the upper portion of vagina that included the cervix was excised at 8 or 24 hours after treatment, that is, designated day 16.5 or postpartum on the morning the day pups were born (day 17 postbreeding), respectively, based on a time course from a previous PR agonist study.40 Reproductive tracts from an additional 6 pregnant mice on the morning of day 15 postbreeding served as pretreatment controls. The cervix was not weighed because gross morphology is not sufficiently accurate to define a boundary in the transition zone that distinguishes convergence of the uterine horns. Tissues were postfixed overnight in 4% paraformaldehyde, embedded in paraffin, and sectioned at 10 μm, as described previously.14
Experiment 2: PR Agonists Block Ovx-Induced Preterm Cervix Remodeling
Surgery was performed in all groups on the morning of day 16 postbreeding. Pregnant mice were anesthetized with 4% fluoroethane, then bilateral skin and abdominal wall incisions were made caudal to the diaphragm and parallel to the spinal cord to expose the fat body surrounding each ovary. These mice were randomly allocated to the following groups: (1) Sham controls (n = 29) remained ovary intact with the abdominal wall sutured, the flank skin incisions closed using wound clips, and 0.1 mL sesame oil injected into the nape of the neck. (2) Ovariectomized controls (Ovx, n = 9) had a portion of the fat pad that included the ovary ligated then removed and the abdominal wall incision closed with sutures. Silastic capsules filled with peanut oil (3 of 1 cm in length and 1 of 2 cm in length, outside diameter of 0.062 inch; #11-189-15G; Fisher Scientific, Asheville, NC) were inserted sc into the dorsal hindquarter before flank incisions were closed.34 (3) E ± P was used to assess the role of P in characteristics associated with remodeling the cervix, pregnant mice were Ovx, and silastic capsules inserted as already described (E + P Ovx; n = 9, one 2-cm capsule with10 μg E/mL peanut oil and 3 × 1 cm capsules with 1 g P/mL peanut oil, respectively) based on doses derived from treatments used in previous studies and that were empirically determined to sustain pregnancy.34,42 (4) On the morning of D18, the P capsules were removed from remaining mice (E-P Ovx, n = 4) to mimic the decline in P in circulation that occurs at term.43 For groups 5 and 6, pregnant mice were Ovx as described earlier and immediately injected with pure PR agonist promegestone (group 5: R5020 Ovx; n = 13, 8 mg/kg/0.1in sesame oil, intramuscularly; NLP004005MH Perkin-Elmer) or the mixed PR receptor agonist MPA (group 6: MPA Ovx; n = 13,10 mg/kg/0.1 mL sesame oil sc, M1620). Based on previous reports, dosing of progestogen treatments was based upon the efficacy to sustain pregnancy.39,44–48 At about 8 and 24 hours after treatment (days 16.5 and 17 postbreeding), 3 to 5 mice were killed, a segment of reproductive tract including the cervix was excised and processed as described previously. Reproductive tracts from 4 untreated pregnant mice on the morning of day 15 postbreeding served as additional pretreatment or sham-operated controls near the peak in systemic P and softening during pregnancy.
Processing and Analyses of Cervix Sections
Longitudinal sections of cervix that included vaginal and uterine tissues were stained with a Picrosirius red (PSR) stain kit (PK-4000; Polysciences, Warrington, Pennsylvania) to assess the extracellular collagen and cross-link organization.13,49,50 Optical density (OD) analysis of birefringence of circular polarized light is inversely proportional to collagen fibrillary organization. This staining procedure is the only method to assess extracellular collagen in the intact morphology of fixed tissues and has been used previously by several investigators in multiple reports about the cervix from rodents and human tissues.8,14,51 The inverse relationship of OD with hydroxyproline concentrations in the rodent cervix, as well as electron microscopic analysis of cervix stroma,16,19,52 further suggests this approach is predictive of disarray of collagen fibers and correlates well with changes in biomechanical properties of the cervix with the progress of pregnancy.53–55 Hydroxyproline content and other indirect measures of collagen content require dispersion of tissue and do not account for heterogeneity in cervix anatomy, that is, varying density of stromal fibroblasts in extracellular matrix from the cervical Os to the transition zone into the uterus, inclusion of basement and luminal membranes as well as remnants of vaginal and uterine tissues, or vascular changes that occur with the progress of pregnancy.54,56 The OD of circular polarized light from PSR stain birefringence may also reflect, to an extent, the collagen content in the cross-linked structures based upon the evidence that birefringence is directly correlated with hydroxyproline concentrations, collagen fiber length, and insoluble collagen during late gestation.52
Adjacent sections were processed by immunohistochemistry to identify F4/80-stained macrophages (1:800 dilution; product no. T-2006; Bachem, Torrance, California) or GR-1 neutrophils (1:100; MCA771G; Abd Serotec, Raleigh, North Carolina). The F4/80 monoclonal antibody has long been recognized as a marker for differentiated mature tissue-resident macrophage,57 while GR-1 has proven useful for studies of neutrophils in the cervix.58 Sections were counterstained to visualize cell nuclei with a 1% methyl green solution in acetate buffer (pH 7.2) or hematoxylin (# SH26; Fisher Scientific). Positive (previously run prepartum cervix) and negative control sections (10% goat serum with 3%Triton-X and antibody diluent substituted for primary antibody) were included in each immunohistochemistry procedure. Macrophages and neutrophils were clearly identified by dark brown stain against low background and in association with a methyl green counterstained cell nuclei. Immune cells and cell nuclei were counted in photomicrographs of the cervix stroma that extends from the lower Os up to the transition zone of striated fibers and smooth muscle leading into the uterine body. Lumen, epithelium, blood vessels, and other atypical structures were excluded. Analysis of 8 photomicrographs in 3 sections from each mouse/group (24 × 930,250 µm3/animal) via Image ProPlus (Rockville, MD) provides a replicable estimate of cell nuclei density and census of resident immune cells.12 Cell counts were independently replicated by multiple coauthors; coefficient of variation was <10%. Data for collagen and immune cell numbers/area were normalized to cell nuclei density for each animal to correct for variability in cellular hypertrophy within sections as well as among sections and individuals with respect to the progression of pregnancy and treatment.
Statistical Analyses
Data were normally distributed (Levene test), and group comparisons were made by 1-way analysis of variance (ANOVA) with either Tukey or Dunnett (all groups vs D15 Con or D16.5 Sham) for individual post hoc analysis (PRISM software version 3.03; GraphPad, La Jolla, CA). The Student t test was used to compare data between treatment groups on the same day postbreeding. P < .05 was considered significant for all tests.
Results
Progesterone Receptor Antagonists Induce Preterm Birth and Cervix Remodeling
Treatment with PR antagonists induced preterm birth compared to vehicle-treated controls. Mice given Ona or RU486 on the morning of day 16 postbreeding delivered pups by the next morning, within 24 hours, compared to mice given a vehicle in which birth occurred at term by the morning of day 19 postbreeding. An average of 11 pups/litter was found in each of the 3 groups based upon the head count and uterine implantation sites.
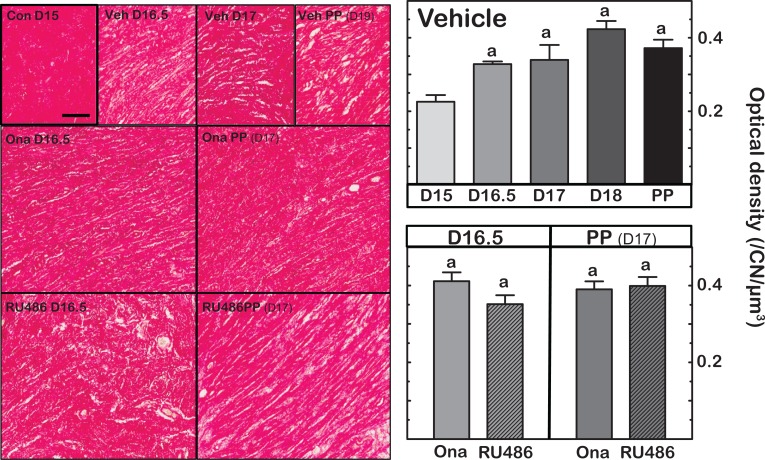
Optical density of birefringence from PSR-stained cervix sections from vehicle and PR-antagonist-treated mice was reduced between days 15 and 16.5 postbreeding (Figure 1—Left panels). This indicates deterioration in the extracellular collagen matrix in the cervix stroma by day 16.5 postbreeding. After day 16.5 postbreeding, the intensity of stain was comparable in sections of cervix from vehicle-treated and PR-antagonist-treated mice. Analysis of OD of PSR-stained sections support these observations (Figure 1—Right panels). Based upon light transmission (inverse of polarized light birefringence), the OD of cervix sections from mice on day 16.5 postbreeding, 8 hours after Ona or RU486 treatment, increased similar to that in vehicle controls (P < .05 vs OD of cervix sections from group on day 15 postbreeding). Optical density was not different among all groups on day 17 postbreeding, whether postpartum following PR antagonist treatment or prepartum in controls. In sections of cervix from controls, the present OD findings replicate the previous results in untreated mice.59 Thus, reduced collagen content and structure were similar in all groups.
Figure 1.
Left panels are representative photomicrographs of Picrosirius red-stained collagen in sections of cervix obtained on specified days postbreeding. PP indicates postpartum. Group designations are intact controls (Con), vehicle-injected (Veh), and mice given PR antagonist, onapristone (Ona), or mifepristone (RU486) on day 16.5 postbreeding, respectively, and prepartum 8 hours after treatment. Right panels are graphs of optical density (OD; mean ± standard error of the mean [SEM]; n = 3-10) of polarized light from birefringence of Picrosirius red–stained sections. Data were normalized to cell nuclei density/section to account for variability in the area of extracellular space, cell size, cell numbers, and morphology across sections, individuals, and groups. The term collagen degradation reflects disarray in collagen cross-linked fibers and possibly content/area as described in the Methods section. a P < .05 versus D15 Vehicle (analysis of variance [ANOVA] with Dunnett test).
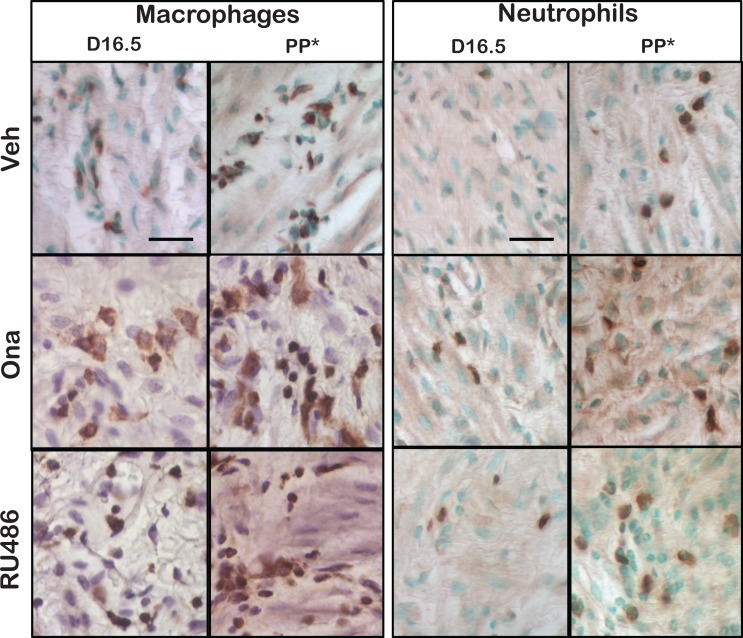
The gross morphology of the cervix in pregnant mice resembled macroscopic views previously presented for rat cervix.12 Immune cells stained dark brown with methyl green or hematoxylin counterstained cell nuclei (Figure 2). Photomicrographs were captured from regions of collagen-dense stroma that extended from the Os to a transition zone of striated fibers and cells that lacked smooth muscle and glands at the convergence of the uterine horns. With pregnancy, layers and thickness of columnar epithelium that lined the lumen, as well as size of stromal cells, appeared to increase. Later in pregnancy, more blood vessels with greater internal volume were observed in the subepithelium. Macrophages, but not neutrophils, were more abundant and morphologically distinct in the cervix stroma after PR antagonist treatment compared to Veh groups both in D16.5 and PP groups (day 17 vs day 19, respectively).
Figure 2.
Representative photomicrographs of macrophages (F4/80-stained) and neutrophils (GR-1-stained) with methyl green or hematoxylin counterstain in sections of cervix from mice on day 16.5 postbreeding or postpartum (PP*, indicates for Veh group postbreeding day 19 and for onapristone [Ona] and RU486 groups day 17 postbreeding). Mice were treated with vehicle (Veh) or PR antagonist (Ona or RU486).
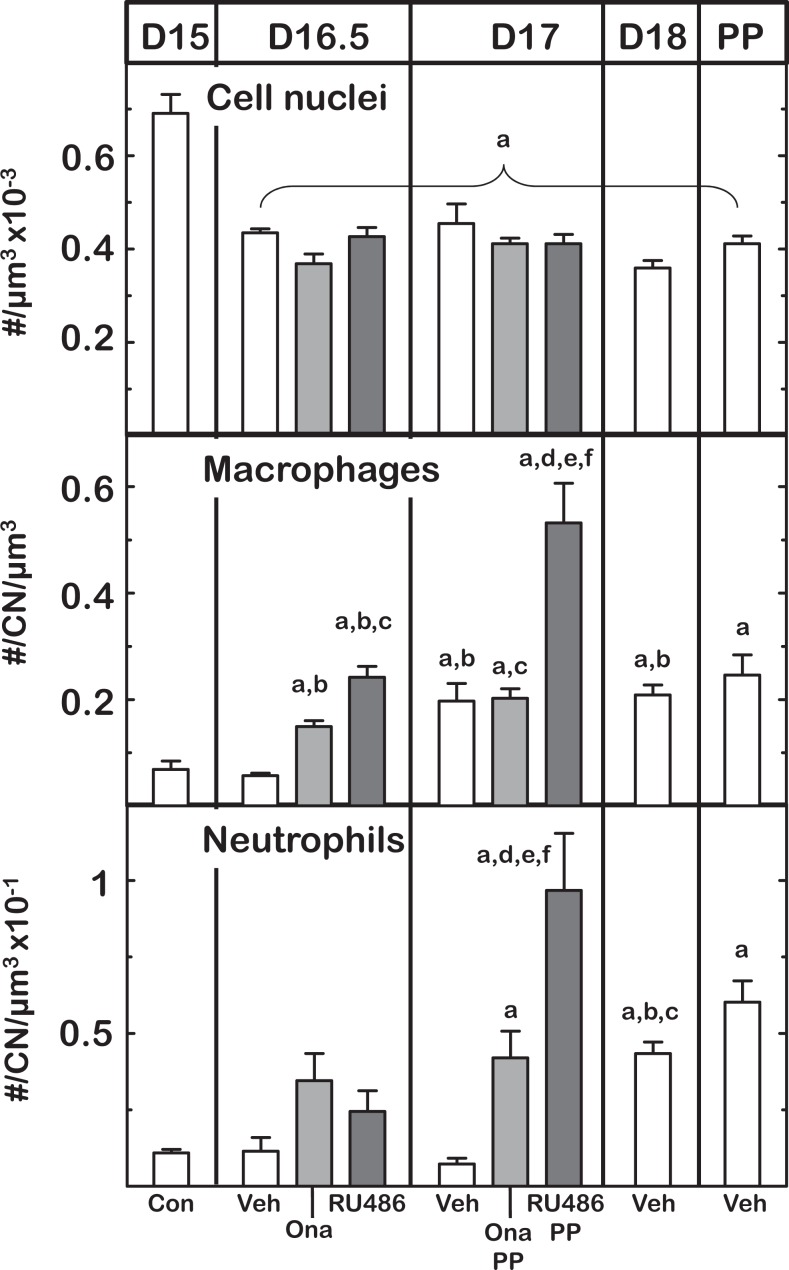
In vehicle-treated controls, characteristics associated with remodeling of the cervix paralleled previous findings in several other mouse strains and 2 strains of rats.12,14,51,58 Cell nuclei density in the stroma of cervix sections declined by day 16.5 postbreeding and plateaued thereafter (Figure 3). This decline in number of stromal cell nuclei/area of cervix occurred to the same extent in mice that were treated with either Ona or RU486 on day 16 postbreeding (P < .05). No significant differences in cell nuclei density were found in cervix sections among groups of mice whether in preterm or on the day of birth (P > .05 D17 Ona or Ru486 vs Veh D17 or Veh PP groups).
Figure 3.
Effects of PR antagonist treatment on cell nuclei (CN), density of macrophage, and neutrophils on days postbreeding and postpartum (PP). Groups and other designations are the same as in previous figures. Density of immune cells was normalized to CN to account for structural variability as detailed in the Methods section. Data are the mean ± standard error of the mean [SEM]. P < .05 vs aD15 controls (Con), bD16.5 vehicle-injected (Veh), cD16.5 onapristone (Ona), dD16.5 RU486, and fD17 Ona (analysis of variance [ANOVA]).
The density of resident macrophages increased in the prepartum cervix in controls and was advanced in PR-antagonist-treated mice (Figure 3 middle panel). In controls, macrophages per area of stroma in the cervix increased by postbreeding day 17 versus day 15 or 16.5. By comparison, macrophage density increased in the cervix from both groups of PR-antagonist-treated mice by day 16.5 postbreeding, within 8 hours of administration of Ona or RU486 (P < .05 Ona and RU486 vs Veh D15 and D16.5 groups; ANOVA with Tukey test days 15-18). The density of macrophages further increased in RU486-treated mice by the day of birth (P < .05 D17 vs D16.5 group). For neutrophils, an increased density was found in the cervix stroma on the day before and of birth in vehicle-treated controls as well as on the day of birth in PR antagonist-treated mice (24 hours posttreatment; P < .05 PP Ona and RU486 vs Veh D15). The findings in vehicle controls replicate previous results for the time course of residency by immune cells in another strain of untreated mice at term.14 Thus, PR antagonists increased the presence of macrophages earlier in the remodeling process and in advance of preterm birth.
Progesterone Receptor Agonists Delay Preterm Birth
Preterm birth occurred within 24 hours of ovary removal, while mice in the Sham and Ovx progestogen-treated groups gave birth at term. In Ovx progestogen-treated groups, births occurred on average on day 19 postbreeding, that is, 72 hours after Ovx and in Ovx + Veh controls 2 days later. The Ovx mice with E + P treatment on days 16 to 18 delivered on average on day 19 postbreeding, within 24 hours of removal of the P capsule on day 18. Similarly, Ovx mice given R5020 or MPA gave birth by the morning of day 19 postbreeding, similar to Sham controls. With the exception of Ovx + Veh controls in which preterm birth of pups was associated with high mortality (range 50%-100% stillbirth from prematurity or cannibalization), the number of pups/litter (average of 12-13 pups/dam based upon uterine implant sites or postpartum census) was not significantly different compared to Sham controls.
Progestational Treatments Forestall Preterm Remodeling of Cervix
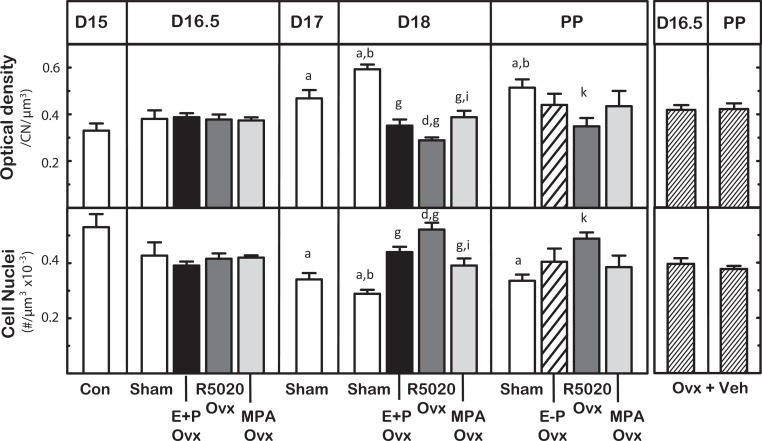
Structural characteristics associated with remodeling of the cervix at term were blocked by PR agonist treatments. In Sham controls, the OD of PSR-stained sections increased by day 17 postbreeding compared to day 15 in controls (Figure 4). The density of the cell nuclei in cervix stroma also decreased by day 17 postbreeding compared to D15. Cell nuclei per area further declined by the day before birth (P < .05 D18 vs D15 and D16.5 Sham groups). This result replicates the findings of controls in the antagonist for day 17 but not 16.5 postbreeding and indicates that the characteristics associated with remodeling of the cervix stroma had occurred at least 2 days before birth. Treatment with PR agonists blocked the increase in OD and decline in cell nuclei density by day 18 postbreeding relative to respective values in D18 controls. Thus, structural degradation of the prepartum cervix was similarly blocked by mixed and pure P agonist treatment. Of particular interest, R5020-treated mice on day 18 postbreeding had the least deterioration in extracellular collagen (lowest OD) and highest cell density compared to other groups on the day before birth. Moreover, neither OD nor cell nuclei density was affected about 8 hours after removal of the P implant (D18.5 E-P group data not shown) or the next day postpartum (P > .05 ANOVA D18 vs PP groups same treatment). Thus, the effect of progestogens on OD became evident by 24 hours after treatment.
Figure 4.
Characteristics associated with remodeling of cervix structure on postbreeding days before and on the day of birth (n = 3-7/group) from untreated controls (Con), Sham-operated (Sham), and mice ovariectomized (Ovx) on the morning of day 16 postbreeding that were treated with estradiol and progesterone (E + P Ovx) or a pure PR agonist (R5020 Ovx) or a mixed PR agonist (medroxyprogesterone acetate [MPA] Ovx) or with vehicle (Ovx + Veh). Mice in each group were killed 8, 24, or 48 hours later (D16.5, D17, or D18 postbreeding, respectively) or 8 hours after removal of the P capsule (E-P, D18.5 E-P data not shown because equivalent to D18 E + P group). Cervix were also taken from postpartum (PP) mice in the morning of the day pups delivered (PP average day 19 postbreeding except for 1 R5020-treated Ovx mouse that delivered on day 21 postbreeding). Ovx + veh-treated mice were prepartum on day 16.5, and all gave birth on day 17 postbreeding within 24 hours of removal of ovaries. Data are the mean ± standard error (SE) optical density (OD) of Picrosirius red-stain birefringence, an assessment of collagen content and structure normalized to cell nuclei density/section to account for variability in morphology and density of cell nuclei (CN) per area. P < .05 versus aD15 Con, bD16.5 Sham, dD16.5 R5020, gD18 Sham, iD18 R5020, or kPP Sham (analysis of variance [ANOVA] with Tukey test for individual comparisons for days 15 to 18 postbreeding groups or Dunnett test). See Methods section for further description of study details.
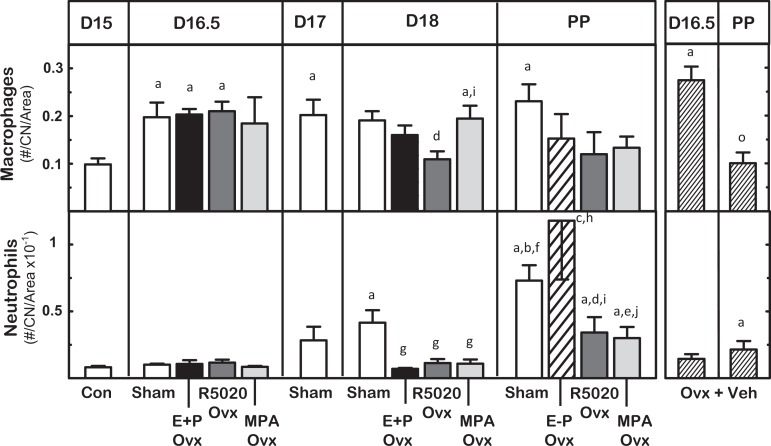
The presence of immune cells in cervix varied across subregions, with the progress of pregnancy and treatment. Macrophages and neutrophils in the cervix were found in subepithelium, near blood vessels, and in stroma. Within about 8 hours of treatments, more macrophages were present in the stroma of cervix sections obtained from D16.5 Sham, Ovx E+P, Ovx R5020, and OVX groups compared to that in pretreatment D15 controls (Figure 5; P < .05). Fewer macrophages were evident by day 18 postbreeding in the cervix from Ovx R5020-treated mice, equivalent to the density in D15 controls. On postbreeding day 19, postpartum Sham but not D17 Ovx vehicle-treated mice had sustained an increased density of macrophages in the stroma (P < .05 PP Sham vs D15 Con). Neutrophil density in stroma increased in D18 Shams and all postpartum groups, irrespective of treatment (P < .05 PP groups vs D15 Con and prepartum D18 same treatment).
Figure 5.
Effects of progestogen on density of resident macrophages and neutrophils in the cervix from groups of mice described in Figure 4. P < .05 versus aD15 controls (Con), bD16.5 Sham-operated (Sham), cD16.5 E + P ovariectomized (Ovx), dD16.5 R5020 Ovx, eD16.5 medroxyprogesterone acetate (MPA Ovx), fD17 Sham, gD18 Sham, hD18 mice ovariectomized with estradiol and progesterone (E + P Ovx) and D18.5 E-P Ovx (data not shown), iD18 R5020 Ovx, jD18 MPA Ovx, and oD16.5 mice ovariectomized with vehicle (Ovx + Veh).
Discussion
The present findings support the hypothesis that a PR-mediated mechanism regulates specific characteristics associated with remodeling of the prepartum cervix stroma in mice in a final common pathway in preterm and term birth. Withdrawal of the trophic effects of P using PR antagonists increased residency of macrophages in the prepartum cervix before preterm birth, an effect that supports previous findings in rats.12 Comparable remodeling changes before birth in controls and PR agonist-treated Ovx mice at term raise the possibility that a functional loss of P efficacy contributes to inflammatory processes that are associated with term birth. Although this macrophage-related inflammatory process was advanced by a blockade of the PR or delayed by PR agonists, no further change was evident in the extracellular collagen matrix or cell nuclei density of the stroma. Rather, these structural characteristics of the cervix appear to be adequately remodeled by sometime between the afternoon of day 16 and morning of day 17 postbreeding to allow for the transition into ripening and preterm or term birth. This observation was further supported in Ovx controls, where both optical and cell nuclei densities were not changed after removal of the ovaries. While further temporal resolution of the remodeling process is possible, the findings clearly focus on a time period of 2 or more days before term when the cervix ripens into a structure that is conducive for birth.
Complementing structural remodeling of the cervix by day 16.5 of pregnancy are the effects of PR modulators to regulate the presence of macrophages in the stroma. Within 8 hours, PR antagonists had increased the density of macrophages, prematurely compared to same day Veh controls and before preterm birth the next day. This enhanced presence of macrophages after PR antagonist treatment is analogous to the day 18 prepartum increase in the stroma of controls. The discovery that macrophage density increases in the cervix several days earlier in pregnancy than previously thought, given the novel study of additional groups on days 16 and 17 of pregnancy and effects of PR antagonists in this species, raises the possibility that a functional P withdrawal may also promote an increase in residency by macrophages at term.
By contrast, PR agonists did not necessarily reduce the presence of macrophages in the cervix (Figure 4; P > .05 on day 16.5 R5020 = MPA = Sham groups). Despite forestalled preterm birth, only R5020 blocked the increase in macrophage density that occurs in stroma by day 18 postbreeding in controls. Thus, the timing of effects of PR agonists, R5020 in particular, on macrophage residency coupled with the lack of change in structural characteristics of the cervix leads to the consideration that PR may regulate macrophage activities that contribute to the remodeling process. Support for this contention comes from increased presence of CD147 and CD169 macrophages,6 the so-called activation markers that indicate the potential for enhanced metalloproteinase and extracellular adhesion activities. Moreover, focus on macrophages, but not neutrophils, in the remodeling process seems justified by the lack of change in census of neutrophils until the last day of pregnancy (Figure 5) or in postpartum antagonist-treated mice. The conclusion that neutrophils may be involved in processes that restore the cervix to the nonremodeled state is consistent with the previous evidence that the presence of this immune cell increases late or after remodeling has occurred.14,58,60,61 The importance of macrophages is especially evident in the prepartum cervix from Ovx mice compared to PR agonist-treated groups or Sham controls where increased presence of macrophages occurred within 8 hours of Ovx, but CN, OD, and neutrophil numbers in the prepartum cervix remained unchanged. Thus, based upon responses to pure and mixed agonists in the day 18 postbreeding groups, the findings suggest that structural remodeling and reduced presence of macrophages in the cervix are mediated by actions of PR as part the mechanism that forestalled birth until term.
Pure PR modulators to induce or block preterm birth were just as or more effective as mixed PR modulators on characteristics associated with cervix remodeling. The effects of pure and mixed PR modulators differed mostly in magnitude, that is, more macrophages were present after RU486 versus Ona treatment, while R5020 appeared more potent than MPA to block morphological characteristics in the cervix associated with preterm birth. The few or limited effects of PR agonists on the density of macrophages may reflect an unresponsiveness to progestogens, that is, functional withdrawal, or cellular activities not assessed in the present study. With respect to cervix biomechanical properties, the pure PR agonist R5020 effectively reduces both pliability and collagenolysis.22 Genomic actions of PR antagonists and agonists used in the present study may inhibit pathways that involve STAT5A and HIF1A that regulate downstream activation of macrophage activating genes, including CCR7 and CCR2, and inhibition of angiogenesis-related genes such as Serpine1, tumor necrosis factor, and nuclear factor kappa B.56–60 Actions by PR modulators to affect the extracellular matrix in the cervix are likely to be indirect and necessitate a focus on the stroma because in rats, and more recently in mice, stromal fibroblasts stain for PR, but macrophages do not.12,62 Whether neutrophils in the cervix during pregnancy express steroid receptors are not known. In consideration of treatment effects on structural, inflammatory, and distensibility characteristics, the PR appears to have a predominant role in the final common mechanism that remodels and ripens the cervix in both rat and mouse models of preterm birth and at term.
In summary, structural and biomechanical changes in the prepartum cervix are necessary for parturition. Similarities in cervix remodeling characteristics from this study of PR antagonists and agonists in comparison to changes that occur at term indicate that a functional withdrawal of the P actions may mediate the shift from soft to ripening structure. In agreement with previous findings in other strains of mice and several strains of rats, characteristics of reduced collagen organization and possibly degradation, decreased cell nuclear density, and increased presence of macrophages are associated with remodeling of the cervix with the approach of term.6,12 Compared to these previous studies, additional groups during the transition from a soft to ripening cervix indicate that these remodeling characteristics occur earlier than previously found. The present study of pure and mixed PR antagonists and agonists is the first to indicate that genomic actions of PR regulate the census of macrophages in the cervix in association with advanced remodeling in PR antagonist-induced preterm birth and PR agonist-delayed preterm birth, respectively. These findings about rodents are consistent with the characteristics associated with peripartum remodeling of the cervix in other species and women at term and with preterm birth.8 ,22, 63 Thus, a common final mechanism for cervix remodeling may be conserved across a variety of mammalian species. Characteristics associated with remodeling may prove useful to assess the progress of prepartum changes in the cervix in preparation for birth whether preterm, as a morphometric correlate to a short cervix, or when labor fails to progress or at postterm. Understanding the mechanism through which PR regulates inflammatory processes in the cervix with respect to the census and phenotypes of macrophages in the cervix might improve current therapeutic uses of progestogens to forestall preterm birth64 or of PR antagonists to induce remodeling and facilitate birth.65
Acknowledgments
Imaging was performed in the LLUSM Advanced Imaging and Microscopy Core with support of NSF Grant MRI-DBI 0923559, as well as the efforts of Richard Chinnock, MD, Chair, Department of Pediatrics, Loma Linda University School of Medicine. We are grateful for the help of Donald R. Chase, MD, Professor of Pathology and Human Anatomy, California Tumor Tissue Registry, Loma Linda University. The technical assistance of Patricia Mazurek in manuscript proofing and referencing was appreciated.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by NIH HD054931 and the Department of Pediatrics.
References
- 1. Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38(2):267–279. [DOI] [PubMed] [Google Scholar]
- 2. Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25(1):69–79. [DOI] [PubMed] [Google Scholar]
- 3. Danforth DN, Veis A, Breen M, et al. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974;120(5):641–651. [DOI] [PubMed] [Google Scholar]
- 4. van Engelen E, de Groot MW, Breeveld-Dwarkasing VN, et al. Cervical ripening and parturition in cows are driven by a cascade of pro-inflammatory cytokines. Reprod Domest Anim. 2009;44(5):834–841. [DOI] [PubMed] [Google Scholar]
- 5. Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. [DOI] [PubMed] [Google Scholar]
- 6. Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod. 2012;87(5):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denison FC, Riley SC, Elliott CL, Kelly RW, Calder AA, Critchley HO. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6(6):541–548. [DOI] [PubMed] [Google Scholar]
- 8. Dubicke A, Ekman-Ordeberg G, Mazurek P, Miller L, Yellon SM. Density of stromal cells and macrophages associated with collagen remodeling in the human cervix in preterm and term birth. Reprod Sci. 2016;23(5):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Csapo AI, Wiest WG. An examination of the quantitative relationship between progesterone and the maintenance of pregnancy. Endocrinology. 1969;85(4):735–746. [DOI] [PubMed] [Google Scholar]
- 10. Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196(4):289–296. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R525–R545. [DOI] [PubMed] [Google Scholar]
- 12. Yellon SM, Dobyns AE, Beck HL, Kurtzman JT, Garfield RE, Kirby MA. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS One. 2013;8(12):e81340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodriguez HA, Kass L, Varayoud J, et al. Collagen remodelling in the guinea-pig uterine cervix at term is associated with a decrease in progesterone receptor expression. Mol Hum Reprod. 2003;9(12):807–813. [DOI] [PubMed] [Google Scholar]
- 14. Yellon SM, Oshiro BT, Chhaya TY, et al. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod. 2011;85(3):498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig. 2005;12(8):578–585. [DOI] [PubMed] [Google Scholar]
- 16. Feltovich H, Ji H, Janowski JW, Delance NC, Moran CC, Chien EK. Effects of selective and nonselective PGE2 receptor agonists on cervical tensile strength and collagen organization and microstructure in the pregnant rat at term. Am J Obstet Gynecol. 2005;192(3):753–760. [DOI] [PubMed] [Google Scholar]
- 17. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. [DOI] [PubMed] [Google Scholar]
- 18. Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. J Soc Gynecol Investig. 2002;9(3):125–136. [PubMed] [Google Scholar]
- 19. Clark K, Ji H, Feltovich H, Janowski J, Carroll C, Chien EK. Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events. Am J Obstet Gynecol. 2006;194(5):1391–1398. [DOI] [PubMed] [Google Scholar]
- 20. Kelly RW. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev. 1994;15(5):684–706. [DOI] [PubMed] [Google Scholar]
- 21. Milligan SR, Finn CA. Minimal progesterone support required for the maintenance of pregnancy in mice. Hum Reprod. 1997;12(3):602–607. [DOI] [PubMed] [Google Scholar]
- 22. Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery1. Hum Reprod. 1994;9(suppl 1):131–161. [DOI] [PubMed] [Google Scholar]
- 23. Fang X, Wong S, Mitchell BF. Effects of RU486 on estrogen, progesterone, oxytocin, and their receptors in the rat uterus during late gestation. Endocrinology. 1997;138(7):2763–2768. [DOI] [PubMed] [Google Scholar]
- 24. Dudley DJ, Dangerfield A, Edwin SS. Interleukin-10 (IL-10) prevents preterm birth in a mouse model of infection-mediated preterm labor. J Soc Gynecol Invest. 1996;3:67A. [Google Scholar]
- 25. Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone - A randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78(9):793–798. [PubMed] [Google Scholar]
- 26. Wing DA, Fassett MJ, Mishell DR. Mifepristone for preinduction cervical ripening beyond 41 weeks’ gestation: a randomized controlled trial. Obstet Gynecol. 2000;96(4):543–548. [DOI] [PubMed] [Google Scholar]
- 27. Leppert PC, Woessner JF., Jr The Extracellular Matrix of the Uterus, Cervix and Fetal Membranes: Synthesis, Degradation and Hormonal Regulation. Ithaca, NY: Ithaca Perinatology Press; 1991. [Google Scholar]
- 28. Romero R, Yeo L, Chaemsaithong P, Chaiworapongsa T, Hassan SS. Progesterone to prevent spontaneous preterm birth. Semin Fetal Neonatal Med. 2014;19(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hegele-Hartung C, Chwalisz K, Beier HM, Elger W. Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98.299): an electron microscopic study. Hum Reprod. 1989;4(4):369–377. [DOI] [PubMed] [Google Scholar]
- 30. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. [DOI] [PubMed] [Google Scholar]
- 31. Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293(14):675–680. [DOI] [PubMed] [Google Scholar]
- 32. Csapo A. Progesterone block. Am J Anat. 1956;98(2):273–291. [DOI] [PubMed] [Google Scholar]
- 33. Meis PJ. 17 hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol. 2005;105(5 pt 1):1128–1135. [DOI] [PubMed] [Google Scholar]
- 34. Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod. 2009;81(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453 e451–454; discussion 421. [DOI] [PubMed] [Google Scholar]
- 36. Durnwald CP, Lynch CD, Walker H, Iams JD. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. Am J Obstet Gynecol. 2009;201(4):410 e411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iams JD. Prevention of preterm parturition. N Engl J Med. 2014;370(19):1861. [DOI] [PubMed] [Google Scholar]
- 38. Byrns MC. Regulation of progesterone signaling during pregnancy: implications for the use of progestins for the prevention of preterm birth. J Steroid Biochem Mol Biol. 2014;139:173–181. [DOI] [PubMed] [Google Scholar]
- 39. Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Studies of cervical ripening in pregnant rats: effects of various treatments. Mol Hum Reprod. 2000;6(4):382–389. [DOI] [PubMed] [Google Scholar]
- 40. Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci. 2009;16(3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garfield RE, Gasc JM, Baulieu EE. Effects of the antiprogesterone RU 486 on preterm birth in the rat. Am J Obstet Gynecol. 1987;157(5):1281–1285. [DOI] [PubMed] [Google Scholar]
- 42. Milligan SR, Cohen PE. Silastic implants for delivering physiological concentrations of progesterone to mice1. Reprod Fertil Dev. 1994;6(2):235–239. [DOI] [PubMed] [Google Scholar]
- 43. Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20alpha-hydroxysteroid dehydrogenase. Mol Endocrinol. 2005;19(2):431–440. [DOI] [PubMed] [Google Scholar]
- 44. Philibert D, Bouchoux F, Degryse M, Lecaque D, Petit F, Gaillard M. The pharmacological profile of a novel norpregnance progestin (trimegestone). Gynecol Endocrinol. 1999;13(5):316–326. [DOI] [PubMed] [Google Scholar]
- 45. Alexander DP, Frazer JF, Lee J. The effect of steroids on the maintenance of pregnancy in the spayed rat. J Physiol. 1955;130(1):148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190(3):693–701. [DOI] [PubMed] [Google Scholar]
- 47. Elovitz MA, Mrinalini C. Can medroxyprogesterone acetate alter Toll-like receptor expression in a mouse model of intrauterine inflammation? Am J Obstet Gynecol. 2005;193(3 pt 2):1149–1155. [DOI] [PubMed] [Google Scholar]
- 48. Milligan SR, Cohen PE, Finn CA. The minimum requirements for oestradiol to induce uterine sensitivity for implantation and decidualization in mice. Hum Reprod. 1995;10(6):1502–1506. [DOI] [PubMed] [Google Scholar]
- 49. Luque EH, Munoz de Toro MM, Ramos JG, Rodriguez HA, Sherwood OD. Role of relaxin and estrogen in the control of eosinophilic invasion and collagen remodeling in rat cervical tissue at term. Biol Reprod. 1998;59(4):795–800. [DOI] [PubMed] [Google Scholar]
- 50. Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22(2):97–104. [Google Scholar]
- 51. Boyd JW, Lechuga TJ, Ebner CA, Kirby MA, Yellon SM. Cervix remodeling and parturition in the rat: lack of a role for hypogastric innervation. Reproduction. 2009;137(4):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu SY, Tozzi CA, Babiarz J, Leppert PC. Collagen changes in rat cervix in pregnancy--polarized light microscopic and electron microscopic studies. Proc Soc Exp Biol Med. 1995;209(4):360–368. [DOI] [PubMed] [Google Scholar]
- 53. Yoshida K, Jiang H, Kim M, et al. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS One. 2014;9(11):e112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Golichowski AM, King SR, Mascaro K. Pregnancy-related changes in rat cervical glycosaminoglycans. Biochem J. 1980;192(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoshida K, Reeves C, Vink J, et al. Cervical collagen network remodeling in normal pregnancy and disrupted parturition in Antxr2 deficient mice. J Biomech Eng. 2014;136(2):021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Collins JJ, Usip S, McCarson KE, Papka RE. Sensory nerves and neuropeptides in uterine cervical ripening. Peptides. 2002;23(1):167–183. [DOI] [PubMed] [Google Scholar]
- 57. Gordon S, Hamann J, Lin HH, Stacey M. F4/80 and the related adhesion-GPCRs. Eur J Immunol. 2011;41(9):2472–2476. [DOI] [PubMed] [Google Scholar]
- 58. Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74(2):236–245. [DOI] [PubMed] [Google Scholar]
- 59. Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod. 2008;78(3):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182(5):2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol. 2002;57(1-2):217–224. [DOI] [PubMed] [Google Scholar]
- 62. Heuerman AH, TT, Mesiano S, Yellon SM. The role of progesterone receptors in remodeling the cervix of prepartum mice. Biol Reprod. 94(4) Suppl, #23. [Google Scholar]
- 63. Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. [PubMed] [Google Scholar]
- 64. Cha J, Bartos A, Egashira M, et al. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest. 2013;123(9):4063–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stjernholm YM, Sahlin L, Eriksson HA, Bystrom BE, Stenlund PM, Ekman GE. Cervical ripening after treatment with prostaglandin E2 or antiprogestin (RU486). Possible mechanisms in relation to gonadal steroids. Eur J Obstet Gynecol Reprod Biol. 1999;84(1):83–88. [DOI] [PubMed] [Google Scholar]