Maize genotypes with more crown roots have more intensive topsoil foraging and superior phosphorus acquisition, growth, and yield in low-phosphorus soil.
Abstract
Suboptimal phosphorus (P) availability is a primary constraint to plant growth on Earth. We tested the hypothesis that maize (Zea mays) genotypes with large crown root number (CN) will have shallower rooting depth and improved P acquisition from low-P soils. Maize recombinant inbred lines with contrasting CN were evaluated under suboptimal P availability in greenhouse mesocosms and the field. Under P stress in mesocosms, the large-CN phenotype had 48% greater root respiration, 24% shallower rooting depth, 32% greater root length density in the topsoil, 37% greater leaf P concentration, 48% greater leaf photosynthesis, 33% greater stomatal conductance, and 44% greater shoot biomass than the small-CN phenotype. Under P stress in the field, the large-CN phenotype had 32% shallower rooting depth, 51% greater root length density in the topsoil, 44% greater leaf P concentration, 18% greater leaf photosynthesis, 21% greater stomatal conductance, 23% greater shoot biomass at anthesis, and 28% greater yield than the small-CN phenotype. These results support the hypothesis that large CN improves plant P acquisition from low-P soils by reducing rooting depth and increasing topsoil foraging. The large-CN phenotype merits consideration as a selection target to improve P capture in maize and possibly other cereal crops.
Maize (Zea mays) is a leading global crop, with importance for food security in developing nations (Grassini et al., 2013). Suboptimal phosphorus (P) availability is a primary limitation to plant growth in most agroecosystems (Lynch and Deikman, 1998; Vance et al., 2003; Lynch, 2007). In developing countries, most smallholder farmers cannot afford mineral fertilizer, and low soil P availability is a principal, pervasive constraint for crop production and, therefore, food security and economic development (Azeez et al., 2006; Worku et al., 2007). In developed countries, intensive P fertilization sustains high yields, but low utilization efficiency, limited reserves of high-grade phosphate ore deposits, high energy costs of producing fertilizer, as well as the environmental consequences of P effluents make intensive P fertilization unsustainable in the long term (Tilman et al., 2001; Zhang et al., 2008; Cordell et al., 2009). Therefore, developing cultivars with improved P acquisition is an important goal for global agriculture (Lynch, 2007).
The maize root system is composed of a primary root, a variable number of seminal roots, nodal roots arising from stem nodes, and lateral roots (Hochholdinger et al., 2004; Gao and Lynch, 2016). Crown roots are belowground nodal roots that are responsible primarily for soil resource acquisition during vegetative growth and remain important through reproductive development (Hoppe et al., 1986; Hochholdinger et al., 2004; Lynch, 2013; Yu et al., 2014). Crown root number (CN), consisting of the number of belowground nodal whorls and the number of roots per whorl, is a central feature of maize root architecture and varies from five to 62 among maize genotypes (Bayuelo-Jiménez et al., 2011; Gaudin et al., 2011; Trachsel et al., 2011; Burton et al., 2013; Saengwilai et al., 2014b; York and Lynch, 2015; Gao and Lynch, 2016). Results from mesocosms and field studies showed that maize lines with small CN had greater nitrogen (N) and water acquisition from deep soil strata under low-N or drought conditions (Saengwilai et al., 2014b; Gao and Lynch, 2016). However, the utility of CN for P acquisition from P-limiting soils has never been tested and is the focus of this study.
Phosphate is relatively immobile in soil, and P availability in surface soil strata is generally greater than that in subsoil strata because of the deposition of plant residues over time and the greater biological activity in surface strata (Lynch and Brown, 2001; Lynch, 2011, 2013). Therefore, root phenes associated with enhanced topsoil foraging are important for P acquisition (Lynch and Brown, 2001; Lynch, 2011; Richardson et al., 2011). Previous studies have shown that plants can improve P acquisition from low-P soils through increased topsoil foraging, enabled by increased root length density (Manske et al., 2000) and lateral root branching (Desnos, 2008; Lynch, 2011), through shallow root growth angles (Lynch and Brown, 2001; Ho et al., 2005; Zhu et al., 2005), and through reduced root metabolic costs, such as the formation of root cortical aerenchyma (Fan et al., 2003; Postma and Lynch, 2011a, 2011b; Galindo-Castañeda et al., 2018), root cortical senescence (Schneider et al., 2017a), root hairs (Miguel, 2004; Lynch, 2011), and adventitious roots (Miller et al., 2003; Lynch and Ho, 2005). Axial roots, the primary structural framework of root systems, have particular importance in P acquisition (Lynch, 2011, 2013). Results from greenhouse and field studies showed that common bean (Phaseolus vulgaris) with greater basal root whorl number had shallower rooting and greater root length, and thereby improved topsoil foraging, resulting in greater P acquisition and shoot biomass in low-P soil (Lynch and Brown, 2012; Miguel et al., 2013). However, little information is available regarding how axial root number in Poaceae species responds to P stress and affects P capture.
Axial roots of annual dicot and monocot crop species have important differences that may affect the costs and benefits of axial root production for P capture. They are morphologically and developmentally distinct, as the majority of axial roots in monocots arise from shoot tissue, whereas axial roots in dicots consist primarily of the primary root and dominant lateral roots arising from it. For example, the rate of secondary development in axial roots varies among genotypes of common bean, a dicot species, and genotypes with reduced secondary development have greater P capture from low-P soils (Strock et al., 2017). This adaptation to low P availability is not possible in monocot species, which lack secondary growth. The lack of secondary growth in monocots means that their axial roots are not as protected against biotic stress, which affects root longevity and, therefore, resource capture (Lynch, 2018). Because monocots lack secondary growth, their root cortical tissue is more persistent than in monocots, which has implications for P capture. For example, the formation of root cortical aerenchyma in axial roots is more advantageous for P capture in maize than in common bean (Postma and Lynch, 2011a). Root cortical senescence also may increase P capture in monocot species (Schneider et al., 2017a), but this process is not known to occur in dicots. The persistence of the root cortex also affects mycorrhizal symbioses, which are important for P capture but require living cortex as habitat (Schneider et al., 2017b; Galindo-Castañeda et al., 2018). Many dicot species have a dominant primary root, which with its laterals comprise the basic architectural phenotype. In contrast, in monocot species, axial roots are continually produced from shoot nodes at or above the soil surface, which descend downward over time. These contrasting architectural strategies have important implications for the spatiotemporal dynamics of topsoil foraging and, thereby, for the acquisition of P. The formation of new roots in dicots generally takes place as laterals of existing roots in deeper soil domains, which may have low P availability, whereas in monocots, new roots form at or above the soil surface from shoot nodes, so that the topsoil is explored continuously throughout phenology. In addition, axial roots of monocot crops generally produce fewer root exudates capable of solubilizing P pools in the rhizosphere (Hinsinger et al., 2011; Li et al., 2014) and have fewer mycorrhizal symbioses (Shen et al., 2011) than dicot crops. These factors can affect the costs and benefits of axial root production for P capture, resulting in different strategies to improve P acquisition. A survey of seven major crop species in response to P limitation found that monocots generally have root morphological adaptations (i.e. total root surface area, root biomass, total root length, root-shoot ratio, specific root length, and specific root surface area) to P stress, while dicots, and especially legumes, primarily showed root physiological adaptations to P stress, such as root exudate production (Lyu et al., 2016). Therefore, the utility of axial root number of Poaceae species for P capture in low-P soil is uncertain, and whether the changes in axial root production could improve topsoil exploration, and thereby P acquisition and yield, merits investigation.
The production of axial roots is a key element of root phenotypes and is particularly important for the balance between the capture of mobile and immobile resources (Lynch, 2013). Results from mesocosms and the field showed that small CN was beneficial for N and water acquisition in conditions of suboptimal N or water availability (Saengwilai et al., 2014b; Gao and Lynch, 2016). This can be attributed to the fact that the formation of a small number of crown roots can decrease interplant competition for soil resources, reduce metabolic costs, and allocate extra metabolic resources for root elongation, thereby improving subsoil foraging for mobile resources like N and water, whose availability is greater in the subsoil in most agroecosystems (Saengwilai et al., 2014b; Lynch, 2015; Gao and Lynch, 2016). However, this may be disadvantageous for the capture of immobile soil resources like P, because of greater P bioavailability in shallow soil strata (Lynch, 2011, 2013). On the other hand, the production of a large number of crown roots can increase the sink strength of root systems, promote the development of root length, and thereby improve soil resource acquisition, especially for P, whose acquisition occurs mostly less than 1 mm from the surface of a root and the intraplant and interplant competition is quite small (Nye and Tinker, 1977; Varney and Canny, 1993; Miguel et al., 2013; Postma et al., 2014). Following the economic paradigm of plant resource allocation (Lynch and Ho, 2005), root construction and maintenance requires metabolic investment, which can exceed 50% of daily photosynthesis (Lambers et al., 2002). Thus, the metabolic costs of root construction and maintenance for a larger CN may potentially weaken the elongation of crown roots into deep soil strata and increase the root distribution in surface soil strata (Gao and Lynch, 2016). While this may be disadvantageous for the capture of mobile soil resources like water and N (Saengwilai et al., 2014b; Zhan and Lynch, 2015; Zhan et al., 2015; Gao and Lynch, 2016), it could facilitate topsoil foraging for immobile resources like P. Therefore, an intermediate number of crown roots may be ideal to cooptimize the acquisition of mobile and immobile resources, and the optimum range of CN is likely to be greater in soils of low P availability (Lynch, 2013), although this hypothesis has not been tested empirically.
The objective of this study was to test the hypothesis that maize genotypes with a large number of crown roots (Supplemental Fig. S1) will have greater topsoil exploration and, therefore, better P acquisition under suboptimal P availability, resulting in better plant growth and yield. To test this hypothesis, we compared the performance of maize recombinant inbred lines (RILs) sharing a common genetic background but having contrasting CN under contrasting P availability in greenhouse mesocosms and the field.
RESULTS
P Stress Effects on Soil P Availability
P distribution in greenhouse mesocosms was stratified, and soil P availability (mg kg−1 dry soil) in the topsoil (0–20 cm) 35 d after planting (DAP) was significantly greater than in the subsoil (20–140 cm), regardless of P regime. Compared with high P, P availability under low P was reduced by 92% in the topsoil and 64% in the subsoil (Supplemental Fig. S2A). In the field, soil P availability at 0 to 10 and 10 to 20 cm was 42.7 and 21.9 mg kg−1, respectively, under high P and 7.3 and 4.4 mg kg−1, respectively, under low P. No significant difference was found between high- and low-P treatments in the subsoil (20–60 cm), with soil P availability varying from 0.5 to 1.1 mg kg−1 (Supplemental Fig. S2B).
P Stress Effects on CN
CN was significantly affected by P availability, phenotype, and their interactions (Supplemental Tables S1 and S2). For both greenhouse and field experiments, CN, especially under high P, was significantly greater in large-CN phenotypes than in small-CN phenotypes (Fig. 1). When the comparison was done within each population, the large-CN genotypes had significantly greater CN than the small-CN genotypes under P deficiency, except IBM133 and NYH57 in the greenhouse and IBM133 and OHW170 in the field (Supplemental Fig. S3). P stress dramatically reduced CN for both phenotypes, by an average of 22% at 35 DAP in greenhouse mesocosms and 26% at anthesis in the field (Fig. 1). The effect of P availability on CN by nodal position differed in mesocosms and field conditions. In mesocosms, P stress did not influence the number of crown roots in the first, second, and third nodes but significantly reduced the number of crown roots of the large-CN phenotype in the fourth node, reduced the number of crown roots of both phenotypes in the fifth node, and there was no sixth node development under P deficiency for either phenotype. The large-CN phenotype had significantly greater CN than the small-CN phenotype in the fifth node under both P regimes and in the sixth node under high P (Fig. 2A). In the field, CN of the third, fifth, sixth, and seventh nodes of the large-CN phenotype was reduced significantly by P stress, while that of the small-CN phenotype was reduced dramatically from the second to the sixth node. Under high P, the small-CN phenotype had significantly less CN in the sixth node and no seventh node development relative to the large-CN phenotype; under low P, CN of the small-CN phenotype was significantly less than that of the large-CN phenotype in the second and fourth nodes, and no seventh node developed for either phenotype (Fig. 2B).
Figure 1.
Effects of P stress on CN. CN of maize is shown at 35 d after planting in greenhouse mesocosms (A) and at anthesis in the field (B) under high and low P. The data shown are means ± se of four replications of four genotypes in each phenotypic class of either large CN or small CN. Different letters represent significant differences compared within a graph at the level of α = 0.05 with Duncan’s multiple range test. HP = high P, LP = low P, LCN = large CN, SCN = small CN.
Figure 2.
Effects of P stress on CN per node. CN per node of maize is shown at 35 d after planting in greenhouse mesocosms (A) and at anthesis in the field (B) under high and low P. The data shown are means ± se of four replications of four genotypes in each phenotypic class of either large CN or small CN. Different letters represent significant differences (P ≤ 0.05) compared within each node. HP = high P, LP = low P, LCN = large CN, SCN = small CN.
CN Effects on Topsoil Root Length Density and Rooting Depth
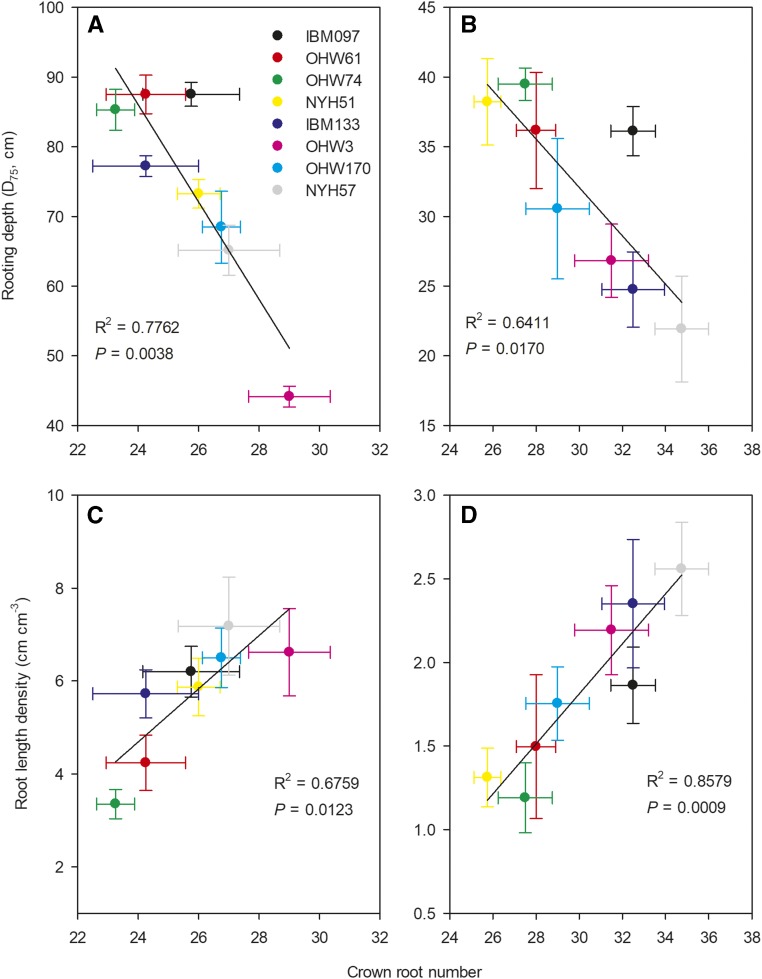
Under P deficiency, the large-CN phenotype proliferated more roots in the topsoil (mesocosms, 0–20 cm, by 32%; field, 0–10 cm, by 51%) and had shallower rooting depth (D75, the depth above which 75% of total root length is located in the soil profile) by 24% in mesocosms and 32% in the field, as compared with the small-CN phenotype (Fig. 3, B and D). However, there was no significant difference between large- and small-CN phenotypes in topsoil root length density or D75 under high P (Fig. 3, A and C). Under low P, CN was associated significantly with rooting depth (mesocosms, R2 = 0.7762, P = 0.0038; field, R2 = 0.6411, P = 0.0170) and root length density in the topsoil (mesocosms, R2 = 0.6759, P = 0.0123; field, R2 = 0.8579, P = 0.0009; Fig. 4).
Figure 3.
Effects of CN and P stress on root length density. Root length density (cm cm−3) of maize is shown at 35 d after planting in greenhouse mesocosms under high P (A) and low P (B) and at anthesis in the field under high P (C) and low P (D). The data shown are means ± se of four replications of four genotypes in each phenotypic category. The average values of D75 for four replications of four large-CN and four small-CN genotypes are shown in each graph and indicated by the dashed lines. *, P ≤ 0.05 and **, P ≤ 0.01. LCN = large CN, SCN = small CN.
Figure 4.
Relationships between CN and rooting depth and root length density. Correlations were determined between CN and rooting depth (D75; cm) and root length density (cm cm−3) from 0- to 20-cm soil depth of maize at 35 d after planting in greenhouse mesocosms (A and C) and from 0- to 10-cm soil depth at anthesis in the field (B and D) under low-P conditions. Each point is the mean ± se of four replications of each genotype.
CN Effects on Leaf Photosynthesis and Root Respiration
P availability, phenotype, and their interactions had significant effects on leaf photosynthetic rate, stomatal conductance, and root respiration (Supplemental Tables S1 and S2). Regardless of CN phenotype, P deficiency significantly reduced leaf photosynthetic rate, stomatal conductance, and root respiration. Under low P, phenotypes with large CN had 48% (greenhouse) and 18% (field) greater leaf photosynthesis, 33% (greenhouse) and 21% (field) greater stomatal conductance, 48% (greenhouse) greater root respiration per unit of root length, and 73% (greenhouse) greater root respiration of the crown root system than phenotypes with small CN. However, no significant difference was found between large-CN and small-CN phenotypes in leaf photosynthetic rate, stomatal conductance, or root respiration under high P (Fig. 5; Supplemental Fig. S4).
Figure 5.
Effects of P stress and CN on leaf photosynthesis, stomatal conductance, and root respiration. Leaf photosynthesis (μmol CO2 m−2 s−1) and leaf stomatal conductance (mmol water m−2 s−1) of maize at 35 DAP in greenhouse mesocosms (A and C) and at anthesis in the field (B and D), as well as root respiration (nmol CO2 cm−1 s−1) of maize at 35 DAP in greenhouse mesocosms (E), under high and low P are shown. The data shown are means ± se of four replications of four genotypes in each phenotypic category. Different letters represent significant differences compared within a graph at the level of α = 0.05. HP = high P, LP = low P, LCN = large CN, SCN = small CN.
CN Effects on Tissue P Concentration, P Acquisition, and P Acquisition Efficiency
Leaf P concentration, plant P acquisition, and P acquisition efficiency of both phenotypes were reduced dramatically by P stress (Figs. 6 and 7; Supplemental Tables S1 and S2). Under P deficiency, P concentration, P acquisition, and P acquisition efficiency of all large-CN genotypes were significantly greater than those of small-CN genotypes (Supplemental Figs. S5 and S6). Under low P, the large-CN phenotype had 37% (greenhouse) and 44% (field) greater leaf P concentration, 97% (greenhouse) greater P acquisition, and 86% (greenhouse) greater P acquisition efficiency than the phenotype with small CN (Figs. 6 and 7; Supplemental Tables S1 and S2). Leaf P concentration (mesocosm, R2 = 0.5796, P = 0.0282; field, R2 = 0.5672, P = 0.0310), plant P acquisition (R2 = 0.6105, P = 0.0220), and P acquisition efficiency (R2 = 0.4834, P = 0.0556) under P deficiency were closely associated with CN (Figs. 8 and 9). However, there was no significant difference in leaf P concentration, P acquisition, and P acquisition efficiency between the two phenotypes under high P (Figs. 6 and 7).
Figure 6.
Effects of P stress and CN on leaf P concentration. Leaf P concentration (mg g−1) of maize is shown at 35 d after planting in greenhouse mesocosms (A) and at anthesis in the field (B) under high- and low-P conditions. The data shown are means ± se of four replications of four genotypes in each phenotype category. Different letters represent significant differences within a graph at the level of α = 0.05. HP = high P, LP = low P, LCN = large CN, SCN = small CN.
Figure 7.
Effects of P stress and CN on P acquisition and acquisition efficiency. P acquisition (mg plant−1; A) and P acquisition efficiency (μg m−1 root length plant−1; B) of maize are shown at 35 d after planting in greenhouse mesocosms under high- and low-P conditions. The data shown are means ± se of four replications of four genotypes in each phenotype category. Different letters represent significant differences within a graph at the level of α = 0.05. HP = high P, LP = low P, LCN = large CN, SCN = small CN.
Figure 8.
Relationships between CN and leaf P concentration. Correlations were determined between CN and leaf P concentration (mg g−1) of maize at 35 d after planting in greenhouse mesocosm (A) and at anthesis in the field (B) under low-P conditions. Each point is the mean ± se of four replicates of each genotype.
Figure 9.
Relationships between CN and P acquisition and acquisition efficiency. Correlations were determined between CN and P acquisition (mg plant−1; A) and P acquisition efficiency (μg m−1 root length plant−1; B) of maize at 35 d after planting in greenhouse mesocosms under low-P conditions. Each point is the mean ± se of four replicates of each genotype.
CN Effects on Shoot Biomass and Grain Yield
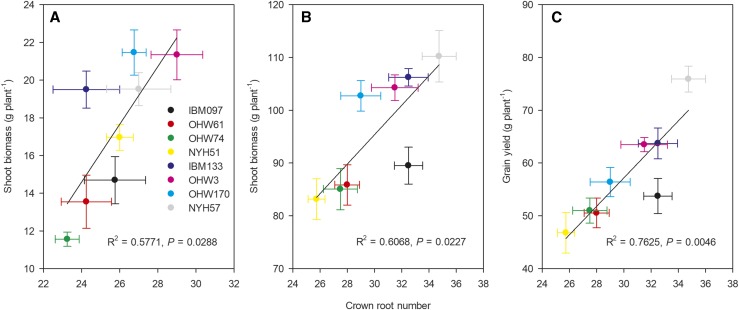
P stress reduced shoot biomass and grain yield, and the reductions in the small-CN phenotype (shoot biomass, 51% in mesocosms and 35% in the field; yield, 46%) were greater than those in the large-CN phenotype (shoot biomass, 32% in mesocosms and 24% in the field; yield, 33%; Fig. 10; Supplemental Tables S1 and S2). Under P deficiency, large-CN genotypes had greater shoot biomass and grain yield than small-CN genotypes, except for NYH51 in mesocosms (Supplemental Fig. S7), and when comparing between phenotypes, the large-CN phenotype had 44% (greenhouse) and 23% (field) greater shoot biomass and 28% greater grain yield than the small-CN phenotype (Fig. 10; Supplemental Tables S1 and S2). Both shoot biomass (mesocosm, R2 = 0.5771, P = 0.0288; field, R2 = 0.6068, P = 0.0227) and grain yield (R2 = 0.7625, P = 0.0046) under P deficiency were closely associated with CN (Fig. 11).
Figure 10.
Effects of P stress and CN on shoot biomass and grain yield. Shoot biomass (g plant−1) of maize is shown at 35 d after planting in greenhouse mesocosms (A) and at anthesis in the field (B), and grain yield (g plant−1) is shown at maturity in the field (C), under high- and low-P conditions. Data shown are means ± se of four replications of four genotypes in each phenotype category. Different letters represent significant differences within a graph at the level of α = 0.05. HP = high P, LP = low P, LCN = large CN, SCN = small CN.
Figure 11.
Relationships between CN and shoot biomass and grain yield. Correlations were determined between CN and shoot biomass of maize at 35 d after planting in greenhouse mesocosms (A) and at anthesis in the field (B), and between CN and grain yield at maturity in the field (C), under low-P conditions. Each point is the mean ± se of four replicates of each genotype.
DISCUSSION
Our results support the hypothesis that a large number of crown roots improves P acquisition by maize under P stress by increasing topsoil exploration (Figs. 3, 4, and 6–9). Phenotypes differed in their CN under P stress (Figs. 1 and 2); lines with many crown roots had shallower rooting depth (Figs. 3 and 4), greater root length density in the topsoil (Figs. 3 and 4), greater P acquisition efficiency, and greater P acquisition (Figs. 6–9). This led to greater leaf photosynthesis and stomatal conductance (Fig. 5), biomass production, and yield (Figs. 10 and 11) than lines with fewer crown roots.
Lynch (2011) proposed that a greater number of axial roots may improve topsoil foraging and optimize P capture from low-P soils. Our results support the inclusion of large CN as an effective phene state for improved soil exploration and P acquisition under suboptimal P availability (Figs. 3, 4, and 6–9). We obtained comparable results from P stress treatments in two distinct environments, greenhouse mesocosms and the field. In the greenhouse, we used mesocosms to create P-stratified environments comparable to conditions in agricultural soils. Mesocosms are simplified, controlled environments, allowing detailed investigations of root physiological traits and root distribution by depth, since entire root systems can be excavated more easily than in field studies. Field experiments include many environmental factors that may affect results. For example, mycorrhizal symbioses can increase P acquisition, extending soil exploration via the formation of mycorrhizal hyphae (Shen et al., 2011). The physical properties of soil, such as mechanical impedance, structure, and texture, can independently or interactively influence root growth and, thereby, nutrient acquisition and yield production (Jin et al., 2013). We used RILs, which are particularly suited for the analysis of phenotypic traits governed by multiple genes, as is the case for CN in maize (Burton et al., 2014; Zhang et al., 2018). RILs within a population share a common genetic background (i.e. they descend from the same two parents) with no artificially induced mutations or transformation events, and the comparison of several RILs from multiple populations enables the analysis of a phenotype in distinct genomes, which can minimize the risk of confounding effects from pleiotropy, epistasis, or other genetic interactions (Zhu and Lynch, 2004). Our results show that IBM133 and NYH57 in the greenhouse and IBM133 and OHW170 in the field had intermediate CN under P deficiency (Supplemental Fig. S3), but this did not substantially affect the results: regardless of whether these genotypes were classified as having large CN, small CN, or were excluded entirely from the analyses, category means for large-CN and small-CN phenotypes under P deficiency were comparable for photosynthetic rate, stomatal conductance, root respiration, root length density in the topsoil, rooting depth, leaf P concentration, plant P acquisition, P acquisition efficiency, shoot biomass, and grain yield (Supplemental Table S3). The fact that results from two distinct environments with three different sets of RILs are in agreement with each other is noteworthy and indicates that the utility of CN for P capture is independent of potentially confounding factors of any given environment and the specific genotypic context.
Plant strategies to acquire P are oriented around two basic themes: soil exploration and mobilization of P from poorly available P pools in the rhizosphere (Lynch, 2011). P mobilization depends mainly on root exudation of P-mobilizing compounds, such as protons, organic acids, and phosphatases (Hinsinger, 2001; Shen et al., 2011). Root architecture, the spatial configuration of the root system, determines the exploration and exploitation of localized P resources by the plant, the distribution of roots relative to their neighbors within and among root systems, as well as the placement and functional benefit of root exudates in specific soil domains, and therefore is particularly important for P acquisition (Lynch, 1995, 2011; Lynch and Brown, 2001; Miguel et al., 2013). In maize, crown roots are the majority of axial roots in the root system, contribute 60% to 80% of root biomass, and form the primary structural framework from which lateral roots emerge. CN, a central feature of maize root architecture, is an important regulator in soil resource capture by lateral roots and root symbionts (Lynch, 2013; Saengwilai et al., 2014b; Gao and Lynch, 2016).
The three primary soil resources that limit plant growth in most soils are N, P, and water. Water and N in the form of nitrate are highly mobile and tend to localize in deeper soil strata over time (Lynch and Wojciechowski, 2015; Thorup-Kristensen and Kirkegaard, 2016), whereas P is highly immobile and has greatest bioavailability in the topsoil (Lynch, 2011). It has been shown previously that reduced CN in maize is beneficial for the capture of water (Gao and Lynch, 2016) and N (Saengwilai et al., 2014b) by reducing the metabolic costs of soil exploration, resulting in greater rooting depth and, thus, greater capture of deep soil resources by remaining axial roots. The data presented here support the hypothesis that, in contrast to water and N, P capture is improved by maize phenotypes with larger CN. This is a clear tradeoff: the optimal CN phenotype for P capture is opposite to the optimal CN phenotype for the capture of water and N. Tradeoffs for the capture of mobile and immobile resources are evident for several root architectural phenes in maize. Shallow root growth angles favor topsoil foraging and P capture (Lynch, 2011), whereas steep root growth angles favor subsoil foraging and the capture of water (Ho et al., 2005) and N (Trachsel et al., 2013; Dathe et al., 2016). Dense lateral root branching promotes P capture (Postma et al., 2014), whereas sparse lateral branching promotes the capture of water (Zhan et al., 2015) and N (Zhan and Lynch, 2015). Anatomical phenes that reduce the volume of living cortical parenchyma, like root cortical aerenchyma, root cortical senescence, and reduced cortical cell file number, are beneficial for water capture but may reduce symbiotic P capture by reducing mycorrhizal habitat. Anatomical phenes that reduce the metabolic cost of soil exploration should have benefits for the capture of both mobile and immobile resources. For example, root cortical aerenchyma is beneficial for the capture of the mobile resources water (Zhu et al., 2010; Chimungu et al., 2015) and N (Saengwilai et al., 2014a) while also being beneficial for the capture of the immobile resources P and K (Postma and Lynch, 2011a, 2011b; Galindo-Castañeda et al., 2018). Root hairs are useful for the capture of P (Bates and Lynch, 2000a; Miguel et al., 2015) as well as water (Carminati et al., 2017) while incurring little direct metabolic cost (Bates and Lynch, 2000b). These tradeoffs in root form and function may account for the large phenotypic variation among crop genotypes and suggest that, for crop breeding programs, optimal root phenotypes should be identified for specific agroecologies (Lynch, 2018).
Results from greenhouse mesocosms and the field showed that root length density in the subsoil, where most water and N are distributed, was increased significantly under drought and N-deficient conditions, resulting in improved water and N acquisition and plant growth (Zhan et al., 2015; Zhan and Lynch, 2015; Gao and Lynch, 2016). In this study, the large-CN phenotype had 32% (greenhouse) and 51% (field) greater root length density in surface soil strata, where P availability is greatest, than the small-CN phenotype under P stress (Figs. 3 and 4), which dramatically increased topsoil exploration and, thereby, improved P acquisition, shoot biomass, and grain yield (Figs. 6–11).
Plants under P stress cannot simply grow more roots throughout the soil profile without regard for the costs of root growth and exploration (Lynch and Ho, 2005; Miguel et al., 2013; Lynch, 2015) but need to balance the metabolic allocations and tradeoffs among roots to optimize plant growth (Walk et al., 2006; Rubio and Lynch, 2007; Gao and Lynch, 2016). Walk et al. (2006) found that adventitious rooting of common bean reduced the growth of tap and basal lateral roots yet improved P acquisition by up to 10% in stratified soil. The removal of a specific root class induced an increase in the growth of the remaining root classes (Rubio and Lynch, 2007). In maize, under drought or N stress, reduced production of crown roots can conserve internal plant resources by reducing intraplant root competition and metabolic costs, and thereby promote the remaining crown root axes to elongate rapidly, resulting in greater root depth, increased subsoil foraging for water or N, and thus improved plant growth (Saengwilai et al., 2014b; Lynch, 2015; Gao and Lynch, 2016). In this study, the large-CN phenotype had significantly more crown roots than the small-CN phenotype under P stress, and the differences originated mainly from the fifth node in the greenhouse and from the second and fourth nodes in the field (Figs. 1 and 2), indicating that CN in later maturing nodes plays crucial roles in plant P acquisition and yield production in late vegetative and reproductive growth. With increasing CN, the metabolic costs of root construction and maintenance were significantly greater (Fig. 5; Supplemental Fig. S4), and thus the resources available for axial root elongation were probably reduced (Lynch, 2013; Saengwilai et al., 2014b; Gao and Lynch 2016). Therefore, the large-CN phenotype had shallower rooting depth and less root length density in subsoil than the small-CN phenotype under P deficiency (Figs. 3 and 4).
Accumulating evidence indicates that plants with shallow rooting depth have growth advantages in P acquisition and yield production over deep-rooted cultivars under P stress (Lynch and Beebe, 1995; Bonser et al., 1996; Ge et al., 2000; Liao et al., 2001; Lynch and Brown, 2001; Ho et al., 2005; Zhu et al., 2005; Heppell et al., 2015), and topsoil foraging is one of the most important ways to improve plant fitness under suboptimal P availability (Lynch and Brown, 2001; Zhu et al., 2005; Lynch, 2011). In this study, results from both mesocosms and the field clearly showed that the shallowness of rooting depth of the large-CN phenotype was associated with improved P capture and, thereby, plant growth and yield in low-P soils (Figs. 3, 4, and 6–11). Our results agree with previous studies that showed that the increased number of basal roots in common bean reduced the internal resources available to individual basal root axes, slowed root elongation into deeper soil domains, and thus improved P acquisition and plant growth in low-P soils (Walk et al., 2006; Rubio and Lynch, 2007; Miguel et al., 2013). Therefore, a large number of crown roots is a positive adaption to P stress in maize.
The rhizoeconomic paradigm indicates that plant fitness under water- and nutrient-limiting conditions is influenced by the balance between the benefits and the costs of root traits as direct metabolic costs, tradeoffs, opportunity costs, and increased risks (Lynch and Ho, 2005; de Kroon and Mommer, 2006; Lynch, 2015), and the metabolic costs of root construction and maintenance are substantial (Lambers et al., 2002; Zhu et al., 2005). Previous studies have shown that reduced formation of crown roots can significantly reduce metabolic costs for root construction and maintenance, and more metabolic resources can be conserved for root elongation and water and N capture (Saengwilai et al., 2014b; Gao and Lynch, 2016). However, in the case of suboptimal P availability, greater investment in axial root production slows axial root elongation, which is useful, since P is immobile and enriched in the surface soil strata. As shown in this study, although the increased production of crown roots increased root respiration (Fig. 5; Supplemental Fig. S4), the large-CN phenotype was superior to the small-CN phenotype in adapting to P stress (Figs. 6–11). Metabolic resources allocated to root growth and elongation into deep soil were reduced significantly, and root length density in the subsoil was decreased while that in the topsoil was increased significantly (Figs. 3 and 4), resulting in improved topsoil exploration and P acquisition (Figs. 7 and 9). P is one of the important elements influencing photosynthesis, and P acquisition improvement can significantly increase the net rate of photosynthesis, which is positively associated with the growth and yield of crop plants (Terry and Ulrich, 1973; Raghothama, 1999; Gastal and Lemaire, 2002). Therefore, the large-CN phenotype had substantially improved leaf photosynthesis, shoot biomass, and grain yield, although the metabolic costs were increased (Figs. 5–11).
CN in maize varies greatly among genotypes and resource levels from five to 62 (Bayuelo-Jiménez et al., 2011; Gaudin et al., 2011; Trachsel et al., 2011; Burton et al., 2013; Saengwilai et al., 2014b; York et al., 2015; Gao and Lynch, 2016), and our range of CN (24–52) falls in the medium to high range of phenotypic variation observed. Moreover, CN is a heritable trait (Hetz et al., 1996; Burton et al., 2014), and genes affecting CN expression have been identified (Hetz et al., 1996; Taramino et al., 2007; Muthreich et al., 2013), making CN a feasible target for plant breeding. Although this study focused on maize, we suggest that the phenotype of large CN would improve P capture in other Poaceae species, such as sorghum (Sorghum bicolor), whose root system architecture is similar to that of maize (Lynch, 2013). Other graminaceous species, such as wheat (Triticum aestivum), rice (Oryza sativa), barley (Hordeum vulgare), and oat (Avena sativa), have the same basic root structure as maize and also may benefit from the optimal CN, although the greater density of nodal roots in tillering species may change the relationship of nodal root occupancy and resource capture. This merits investigation. Our results are entirely consistent with the hypothesis that large-CN phenotypes have shallower rooting depth and greater root length density in topsoil (Figs. 3 and 4), resulting in greater P acquisition from topsoil and improved growth and yield under P deficiency (Figs. 6–11). Therefore, we suggest that CN merits consideration as a potential trait to improve plant tolerance to suboptimal P availability in crop breeding programs.
MATERIALS AND METHODS
Plant Materials
Eight genotypes of maize (Zea mays) were selected from three RIL populations: RILs IBM133 and IBM097 from the intermated population of B73 × Mo17 (IBM); OHW3, OHW61, OHW74, and OHW170 from the RIL population of Oh43 × W64a (OHW); and NYH51 and NYH57 from the RIL population of Ny821 × H99 (NYH). Previous studies show that these genotypes presented contrasting CN: large CN in IBM133, OHW3, OHW170, and NYH57 and small CN in IBM097, OHW61, OHW74, and NYH51 (Bayuelo-Jiménez et al., 2011; Gaudin et al., 2011; Burton et al., 2013; Saengwilai et al., 2014b; York et al., 2015; Gao and Lynch, 2016). All seeds were obtained from Dr. Shawn Kaeppler (University of Wisconsin, Madison).
Greenhouse Mesocosm Study
Experimental Design
The greenhouse experiment was a randomized complete block design with four replications. The factors were two P regimes (high- and low-P conditions) and eight genotypes. Planting was staggered 7 d between replicates with time of planting treated as a block effect.
Growth Conditions
Plants were grown from May 16 to June 20, 2016, in a greenhouse located on the campus of Pennsylvania State University in University Park (40°48′N, 77°51′W), with a photoperiod of 14/10 h (light/darkness) at 28°C/24°C, 1,200 μmol photons m−2 s−1 maximum photosynthetically active radiation (PAR), and 40% to 70% relative humidity. Seeds of uniform size were surface sterilized in 0.05% NaOCl (v/v) for 15 min and imbibed for 24 h in aerated 1 mm CaSO4, then placed in darkness at 28°C ± 1°C for 2 d. Seedlings of similar size were transplanted to mesocosms consisting of PVC cylinders 15.7 cm in diameter and 155 cm in height. The cylinders were lined inside with plastic sleeves made of 4 mil (0.116 mm) transparent high-density polyethylene film, which were used to facilitate root sampling. The growth medium consisted of (by volume) 50% medium-size (0.5–0.3 mm) commercial grade sand, 30% horticultural size 3 vermiculite, 5% perlite, and 15% sieved low-P topsoil. The topsoil was collected from the Russell E. Larson Agricultural Research Center (fine, mixed, semiactive, mesic Typic Hapludalf, pH 6.7, silt loam, P availability [Mehlich], 5.56 mg kg−1). A uniform volume (29 L) of the mixture was used in each cylinder to ensure a consistent bulk density of the medium. Each cylinder was filled with medium to 5 cm from the surface and stratified into two layers, which were separated at 25 cm depth from the surface of the cylinder, with the upper layer 20 cm thick (5–25 cm depth) and the bottom layer 130 cm thick (25–155 cm depth). In the upper layer, 0.232 and 3.096 g P were added for the low- and high-P treatments, respectively, by mixing the medium with triple superphosphate [whose main component is Ca(H2PO4)2·H2O, with P content of about 20.1%] fertilizer, and P availability was maintained at 12.8 and 164 μg P g-1, respectively; for the bottom layer, no P was applied in either P treatment.
One day before transplanting, each mesocosm was saturated with 4 L of a nutrient solution adjusted to pH 6 and consisting of (in μm): N (16,000), K (6,000), Ca (4,000), S (1,000), Mg (1,000), Cl (50), B (25), Mn (2), Zn (2), Cu (0.5), Mo (0.5), and EDTA-Fe (50). Three plants were transplanted to each cylinder and thinned to one after 5 d. Following transplanting, plants were irrigated with 300 mL of the nutrient solution per mesocosm every 2 d for the first 10 d via drip irrigation using a DI-16 Dosatron fertilizer injector (Dosatron International), and 300 mL of nutrient solution was applied daily thereafter.
Sampling and Measurements
Plants were harvested 5 weeks after transplanting. Two days before harvest, the net photosynthesis rate of the youngest fully expanded leaf was measured with a LI-6400 Infrared Gas Analyzer (Li-Cor Biosciences) using a red-blue light at PAR intensity of 1,200 μmol photons m−2 s−1, constant CO2 concentration of 400 µL L−1, 25°C leaf temperature, and 40% relative humidity.
The youngest fully expanded leaf was sampled and oven dried. Tissue samples were ashed at 495°C for 15 h, dissolved in 8 mL of 100 mm HCl, and then analyzed for P concentration spectrophotometrically (Murphy and Riley, 1962). Shoots were severed at the soil surface and then oven dried at 70°C for 72 h for biomass determination. Roots were extracted by rinsing the medium with water. All nodal roots emerging belowground were classified as crown roots. CN in each nodal whorl was counted manually.
Root respiration of three 10-cm root segments from the second whorl of crown roots (8 cm from the base) was measured. Excised root samples were patted dry and placed in a 40-mL custom chamber connected to the LI-6400 Infrared Gas Analyzer. The temperature of the chamber was maintained at 25°C ± 1°C using a water bath while respiration was measured. CO2 evolution from the root segments was recorded every 5 s for 180 s.
Root length distribution was measured by cutting the root system into eight segments in 20-cm-depth increments. Roots from each increment (preserved in 75% ethanol) were spread in a 5-mm layer of water in transparent plexiglass trays and imaged with a flatbed scanner equipped with top lighting at a resolution of 23.6 pixels mm−1 (600 dots per inch). The total root length for each segment was quantified using WinRhizo Pro (Regent Instruments). Following scanning, the roots were dried at 70°C for 72 h and weighed. To summarize the vertical distribution of the root length density, we used D75.
Field Experiment
Growth Conditions and Experimental Design
The field experiment was conducted during May to September 2016 at the Russell E. Larson Agricultural Research Center of Pennsylvania State University (40°43′N, 77°56′W). The soil was a Hagerstown silt loam (fine, mixed, mesic Typic Hapludalf). Based on soil analysis at the beginning of the cropping season, P fertilizers were applied at the rate of 78.5 kg P ha−1 for high-P plots, while low-P plots received no P fertilizer. Other nutrients were adjusted to meet the requirements for maize production as determined by soil tests. Pest control and irrigation were carried out as needed.
A randomized complete block design with a split-plot arrangement of treatments was employed. The main plot was high and low P levels, and the subplot was treated with eight genotypes. There were four biological replications for each treatment. Each plot consisted of three rows, and each row had 18 plants grown with 0.76-m interrow spacing and 0.23-m within-row spacing, resulting in a plant population of 57,000 plants ha−1.
Sampling and Measurements
Shoots and roots were harvested at anthesis (approximately 80 d after planting). Two days before harvest, the net photosynthesis rate of the ear leaf was measured as described above, except PAR was set to 1,800 μmol photons m−2 s−1, with a constant CO2 concentration of 400 µL L−1, leaf temperature of 25°C, and relative humidity of 40%.
Two adjacent plants were randomly selected in the central row per replicate. The ear leaves were sampled, oven dried, and then ground for tissue P analysis. Shoots were severed at the soil surface and oven dried at 70°C for 72 h before dry weight determination. Roots were excavated by removing a soil cylinder of approximately 40 cm diameter and 25 cm depth with the plant base as the horizontal center of the soil cylinder. A large portion of soil was removed from roots by careful shaking. The remaining soil was removed by soaking the roots in diluted commercial detergent followed by vigorously rinsing with water. Because two representative root crowns within a plot usually appear to be homogenous, only one clean root crown was selected for phenotyping. CN in each nodal whorl was measured by counting.
Root distribution was measured by soil coring (Giddings Machine). One soil core of 5 cm diameter and 60 cm length was taken midway between plants within a row in each plot. Each soil core was subdivided into 10-cm segments, and roots were extracted from each segment and washed. Subsequently, the washed roots were scanned with the image-processing software WinRhizo Pro (Regent Instruments) to obtain root length in each soil depth. Root distribution in the soil profiles was calculated as described above, and roots were then oven dried at 70°C for 80 h and dry weight was determined.
At physiological maturity (approximately 127 d after planting), ears were collected from six plants per plot, and grain yield was calculated at zero water content after drying at 75°C for 100 h.
Data Analysis
Statistical analyses were performed using SPSS 17.0 (SPSS). Normality and homogeneity of variances were tested for all the data with Shapiro-Wilk tests. Two-way ANOVA was used to assess the effects of high- and low-CN lines, P levels, and their interactions, with block as a random factor. Duncan’s multiple range test was used for multiple comparisons. Differences of soil P availability in the same soil depth between high P and low P and root length density in the same soil depth between high-CN and low-CN genotypes were analyzed by Student’s t test. Linear regressions and correlations were carried out by Sigmaplot 12.5 (Systat Software). The significance level was set at P ≤ 0.05.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Image of the root systems of small-CN and large-CN maize genotypes at anthesis in the field.
Supplemental Figure S2. Soil P availability from 0 to 140 cm depth in greenhouse mesocosms and from 0 to 60 cm depth in the field under high- and low-P conditions.
Supplemental Figure S3. Crown root number of maize at 35 d after planting in greenhouse mesocosms and at anthesis in the field under high- and low-P conditions.
Supplemental Figure S4. Root respiration of the crown root system (nmol CO2 s−1 plant−1) of maize at 35 d after planting in greenhouse mesocosms under high and low P.
Supplemental Figure S5. Leaf P concentration (mg g−1) of maize at 35 d after planting in greenhouse mesocosms and at anthesis in the field under high- and low-P conditions.
Supplemental Figure S6. Phosphorus acquisition (mg plant−1) and P acquisition efficiency (μg m−1 root length plant−1) of maize at 35 d after planting in greenhouse mesocosms under high- and low-P conditions.
Supplemental Figure S7. Shoot biomass (g plant−1) of maize at 35 d after planting in greenhouse mesocosms and at anthesis in the field, and grain yield (g plant−1) at maturity in the field under high- and low-P conditions.
Supplemental Table S1. Summary of ANOVA for crown root number, leaf photosynthesis, leaf stomatal conductance, root respiration, leaf phosphorus concentration, plant P uptake, P uptake efficiency, and shoot biomass of maize at 35 d after planting in greenhouse mesocosms as influenced by soil phosphorus regime, crown root phenotype, and their interactions.
Supplemental Table S2. Summary of ANOVA for crown root number, leaf photosynthesis, leaf stomatal conductance, leaf phosphorus concentration, shoot biomass of maize at anthesis, grain yield at physiological maturity in the field as influenced by soil phosphorus regime, crown root phenotype, and their interactions.
Supplemental Table S3. Analysis of the effect of plasticity of IBM133 and NYH57 in greenhouse mesocosms and IBM133 and OHW170 in the field under low-P conditions.
Acknowledgments
We thank Xucun Jia and Robert Snyder for technical assistance.
Footnotes
B.R.S. was supported by the China Scholarship Council and the National Key Basic Research Program of China (2016YFC0500703). Research costs were supported by the Agriculture and Food Research Initiative of the USDA National Institute of Food and Agriculture competitive grant 2014-67013-2157 to J.P.L.
Articles can be viewed without a subscription.
References
- Azeez J, Adetunji M, Lagoke S (2006) Response of low-nitrogen tolerant maize genotypes to nitrogen application in a tropical Alfisol in northern Nigeria. Soil Tillage Res 91: 181–185 [Google Scholar]
- Bates TR, Lynch JP (2000a) Plant growth and phosphorus accumulation of wild type and two root hair mutants of Arabidopsis thaliana (Brassicaceae). Am J Bot 87: 958–963 [PubMed] [Google Scholar]
- Bates TR, Lynch JP (2000b) The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am J Bot 87: 964–970 [PubMed] [Google Scholar]
- Bayuelo-Jiménez JS, Gallardo-Valdéz M, Pérez-Decelis VA, Magdaleno-Armas L, Ochoa I, Lynch JP (2011) Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Res 121: 350–362 [Google Scholar]
- Bonser AM, Lynch J, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132: 281–288 [DOI] [PubMed] [Google Scholar]
- Burton AL, Brown KM, Lynch JP (2013) Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Sci 53: 1042–1055 [Google Scholar]
- Burton AL, Johnson JM, Foerster JM, Hirsch CN, Buell CR, Hanlon MT, Kaeppler SM, Brown KM, Lynch JP (2014) QTL mapping and phenotypic variation for root architectural traits in maize (Zea mays L.). Theor Appl Genet 127: 2293–2311 [DOI] [PubMed] [Google Scholar]
- Carminati A, Passioura JB, Zarebanadkouki M, Ahmed MA, Ryan PR, Watt M, Delhaize E (2017) Root hairs enable high transpiration rates in drying soils. New Phytol 216: 771–781 [DOI] [PubMed] [Google Scholar]
- Chimungu J, Maliro M, Nalivata P, Kanyama-Phiri G, Brown K, Lynch J (2015) Utility of root cortical aerenchyma under water limited conditions in tropical maize (Zea mays L.). Field Crops Res 171: 86–98 [Google Scholar]
- Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19: 292–305 [Google Scholar]
- Dathe A, Postma JA, Postma-Blaauw MB, Lynch JP (2016) Impact of axial root growth angles on nitrogen acquisition in maize depends on environmental conditions. Ann Bot 118: 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon H, Mommer L (2006) Root foraging theory put to the test. Trends Ecol Evol 21: 113–116 [DOI] [PubMed] [Google Scholar]
- Desnos T. (2008) Root branching responses to phosphate and nitrate. Curr Opin Plant Biol 11: 82–87 [DOI] [PubMed] [Google Scholar]
- Fan MS, Zhu JM, Richards C, Brown KM, Lynch JP (2003) Physiological roles for aerenchyma in phosphorus-stressed roots. Funct Plant Biol 30: 493–506 [DOI] [PubMed] [Google Scholar]
- Galindo-Castañeda T, Brown KM, Lynch JP (2018) Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant Cell Environ (in press) [DOI] [PubMed] [Google Scholar]
- Gao Y, Lynch JP (2016) Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J Exp Bot 67: 4545–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastal F, Lemaire G (2002) N uptake and distribution in crops: an agronomical and ecophysiological perspective. J Exp Bot 53: 789–799 [DOI] [PubMed] [Google Scholar]
- Gaudin ACM, McClymont SA, Holmes BM, Lyons E, Raizada MN (2011) Novel temporal, fine-scale and growth variation phenotypes in roots of adult-stage maize (Zea mays L.) in response to low nitrogen stress. Plant Cell Environ 34: 2122–2137 [DOI] [PubMed] [Google Scholar]
- Ge Z, Rubio G, Lynch JP (2000) The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant Soil 218: 159–171 [DOI] [PubMed] [Google Scholar]
- Grassini P, Eskridge KM, Cassman KG (2013) Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat Commun 4: 2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppell J, Talboys P, Payvandi S, Zygalakis KC, Fliege J, Withers PJ, Jones DL, Roose T (2015) How changing root system architecture can help tackle a reduction in soil phosphate (P) levels for better plant P acquisition. Plant Cell Environ 38: 118–128 [DOI] [PubMed] [Google Scholar]
- Hetz W, Hochholdinger F, Schwall M, Feix G (1996) Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J 10: 845–857 [Google Scholar]
- Hinsinger P. (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237: 173–195 [Google Scholar]
- Hinsinger P, Betencourt E, Bernard L, Brauman A, Plassard C, Shen J, Tang X, Zhang F (2011) P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol 156: 1078–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP (2005) Root architectural trade-offs for water and phosphorus acquisition. Funct Plant Biol 32: 737–748 [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D (2004) Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Ann Bot 93: 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe DC, McCully ME, Wenzel CL (1986) The nodal roots of Zea: their development in relation to structural features of the stem. Can J Bot 64: 2524–2537 [Google Scholar]
- Jin K, Shen J, Ashton RW, Dodd IC, Parry MAJ, Whalley WR (2013) How do roots elongate in a structured soil? J Exp Bot 64: 4761–4777 [DOI] [PubMed] [Google Scholar]
- Lambers H, Atkin OK, Millenaar FF (2002) Respiratory patterns in roots in relation to their functioning. In Waisel Y, Eshel A, Kafkaki K, eds, Plant Roots, Hidden Half, Ed 3 Marcel Dekker, New York, pp 521–552 [Google Scholar]
- Li L, Tilman D, Lambers H, Zhang FS (2014) Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol 203: 63–69 [DOI] [PubMed] [Google Scholar]
- Liao H, Rubio G, Yan X, Cao A, Brown KM, Lynch JP (2001) Effect of phosphorus availability on basal root shallowness in common bean. Plant Soil 232: 69–79 [PubMed] [Google Scholar]
- Lynch J. (1995) Root architecture and plant productivity. Plant Physiol 109: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. (2007) Roots of the second Green Revolution. Aust J Bot 55: 493–512 [Google Scholar]
- Lynch JP. (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156: 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. (2015) Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant Cell Environ 38: 1775–1784 [DOI] [PubMed] [Google Scholar]
- Lynch JP. (2018) Rightsizing root phenotypes for drought resistance. J Exp Bot (in press) [DOI] [PubMed] [Google Scholar]
- Lynch JP, Beebe SE (1995) Adaptation of beans (Phaseolus vulgaris L.) to low phosphorus availability. HortScience 30: 1165–1171 [Google Scholar]
- Lynch JP, Brown KM (2001) Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil 237: 225–237 [Google Scholar]
- Lynch JP, Brown KM (2012) New roots for agriculture: exploiting the root phenome. Philos Trans R Soc Lond B Biol Sci 367: 1598–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Deikman J (1998) Phosphorus in Plant Biology: Regulatory Roles in Molecular, Cellular, Organismic, and Ecosystem Processes. American Society of Plant Physiologists, Rockville, MD [Google Scholar]
- Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269: 45–56 [Google Scholar]
- Lynch JP, Wojciechowski T (2015) Opportunities and challenges in the subsoil: pathways to deeper rooted crops. J Exp Bot 66: 2199–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR, Shen J (2016) Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front Plant Sci 7: 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manske GGB, Ortiz-Monasterio JI, Van Ginkel M, Gonzalez RM, Rajaram S, Molina E, Vlek PLG (2000) Traits associated with improved P-uptake efficiency in CIMMYT’s semidwarf spring bread wheat grown on an acid Andisol in Mexico. Plant Soil 221: 189–204 [Google Scholar]
- Miguel M. (2004) Genotypic variation in root hairs and phosphorus efficiency in common bean (Phaseolus vulgaris L.). MSc thesis. Pennsylvania State University, University Park, PA [Google Scholar]
- Miguel MA, Postma JA, Lynch JP (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167: 1430–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel MA, Widrig A, Vieira RF, Brown KM, Lynch JP (2013) Basal root whorl number: a modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Ann Bot 112: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP (2003) Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Funct Plant Biol 30: 973–985 [DOI] [PubMed] [Google Scholar]
- Murphy J, Riley J (1962) A modified single solution method for the determination of phosphorus in natural waters. Anal Chim Acta 27: 31–36 [Google Scholar]
- Muthreich N, Majer C, Beatty M, Paschold A, Schützenmeister A, Fu Y, Malik WA, Schnable PS, Piepho HP, Sakai H, et al. (2013) Comparative transcriptome profiling of maize coleoptilar nodes during shoot-borne root initiation. Plant Physiol 163: 419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye PH, Tinker PB (1977) Solute Movement in the Soil-Root System. Blackwell Scientific Publishers, Oxford [Google Scholar]
- Postma JA, Dathe A, Lynch JP (2014) The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol 166: 590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP (2011a) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Ann Bot 107: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP (2011b) Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol 156: 1190–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, et al. (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349: 121–156 [Google Scholar]
- Rubio G, Lynch JP (2007) Compensation among root classes in Phaseolus vulgaris L. Plant Soil 290: 307–321 [Google Scholar]
- Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP (2014a) Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166: 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch JP (2014b) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider HM, Postma JA, Wojciechowski T, Kuppe C, Lynch JP (2017a) Root cortical senescence improves growth under suboptimal availability of N, P, and K. Plant Physiol 174: 2333–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider HM, Wojciechowski T, Postma JA, Brown KM, Lücke A, Zeisler V, Schreiber L, Lynch JP (2017b) Root cortical senescence decreases root respiration, nutrient content and radial water and nutrient transport in barley. Plant Cell Environ 40: 1392–1408 [DOI] [PubMed] [Google Scholar]
- Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156: 997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock CF, Morrow de la Riva L, Lynch J (2017) Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiol 176: 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramino G, Sauer M, Stauffer JL Jr, Multani D, Niu X, Sakai H, Hochholdinger F (2007) The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J 50: 649–659 [DOI] [PubMed] [Google Scholar]
- Terry N, Ulrich A (1973) Effects of phosphorus deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol 51: 43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup-Kristensen K, Kirkegaard J (2016) Root system-based limits to agricultural productivity and efficiency: the farming systems context. Ann Bot 118: 573–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292: 281–284 [DOI] [PubMed] [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341: 75–87 [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2013) Maize root growth angles become steeper under low N conditions. Field Crops Res 140: 18–31 [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Varney GT, Canny MJ (1993) Rates of water uptake into the mature root system of maize plants. New Phytol 123: 775–786 [Google Scholar]
- Walk T, Jaramillo RE, Lynch J (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 279: 347–366 [Google Scholar]
- Worku M, Bänziger M, Erley GSA, Friesen D, Diallo AO, Horst WJ (2007) Nitrogen uptake and utilization in contrasting nitrogen efficient tropical maize hybrids. Crop Sci 47: 519–528 [Google Scholar]
- York LM, Galindo-Castañeda T, Schussler JR, Lynch JP (2015) Evolution of US maize (Zea mays L.) root architectural and anatomical phenes over the past 100 years corresponds to increased tolerance of nitrogen stress. J Exp Bot 66: 2347–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM, Lynch JP (2015) Intensive field phenotyping of maize (Zea mays L.) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. J Exp Bot 66: 5493–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, White PJ, Hochholdinger F, Li C (2014) Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta 240: 667–678 [DOI] [PubMed] [Google Scholar]
- Zhan A, Lynch JP (2015) Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J Exp Bot 66: 2055–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan A, Schneider H, Lynch JP (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168: 1603–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ma W, Ji Y, Fan M, Oenema O, Zhang F (2008) Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in China. Nutr Cycl Agroecosyst 80: 131–144 [Google Scholar]
- Zhang Z, Zhang X, Lin Z, Wang J, Xu M, Lai J, Yu J, Lin Z (2018) The genetic architecture of nodal root number in maize. Plant J 93: 1032–1044 [DOI] [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP (2005) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays). Funct Plant Biol 32: 749–762 [DOI] [PubMed] [Google Scholar]
- Zhu J, Lynch JP (2004) The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays L.) seedlings. Funct Plant Biol 31: 949–958 [DOI] [PubMed] [Google Scholar]