Magnaporthe oryzae (Guy11) infection induces osa-miR319 expression, which stalls host jasmonic acid signaling and intensifies its pathogenicity.
Abstract
MicroRNAs play crucial roles in plant responses to pathogen infections. The rice blast disease, caused by the fungus Magnaporthe oryzae, is the most important disease of rice (Oryza sativa). To explore the microRNA species that participate in rice immunity against the rice blast disease, we compared the expression of small RNAs between mock- and M. oryzae-treated rice. We found that infection by M. oryzae strain Guy11 specifically induced the expression of rice miR319 and, consequently, suppressed its target gene TEOSINTE BRANCHED/CYCLOIDEA/PROLIFERATING CELL FACTOR1 (OsTCP21), which encodes a transcription factor. Using transgenic rice that overexpresses miR319b (OE) or expresses OsTCP21-Res (which is resistant to miR319-mediated silencing), we found that OsTCP21 is a positive regulator of the rice defense response against the blast disease. When wild-type and miR319b-OE rice were infected by Guy11, multiple jasmonic acid (JA) synthetic and signaling components were suppressed, indicating that Guy11 suppresses JA signaling through inducing miR319. In particular, we found that LIPOXYGENASE2 (LOX2) and LOX5 were specifically suppressed by miR319 overexpression or by Guy11 infection. LOXs are the key enzymes of JA synthesis, which catalyze the conversion of α-linoleic acid to hydroperoxy-octadecadienoic acid. The application of α-linoleic acid rescued disease symptoms on the OsTCP21-Res rice but not wild-type rice, supporting our hypothesis that OsLOX2 and OsLOX5 are the key JA synthesis genes hijacked by Guy11 to subvert host immunity and facilitate pathogenicity. We propose that induced expression of OsLOX2/5 may improve resistance to the rice blast disease.
Small RNAs (sRNAs) are a novel group of short, noncoding RNA molecules ranging from 20 to more than 40 nucleotides in length (Yu et al., 2017; Niu et al., 2018). sRNAs act as master modulators that negatively regulate gene expression at either the transcription or posttranscription level (known as gene silencing; Iwakawa and Tomari, 2015; Jonas and Izaurralde, 2015). By recognizing sequence reverse complementary to sRNA, genes containing conserved sequences are silenced coordinately, thus offering sRNA a tremendous regulating capacity on genes with conserved structures (and related functions derived from conserved structures). For example, in plants, such as Medicago spp., Nicotiana benthamiana, tomato (Solanum lycopersicum), and Arabidopsis (Arabidopsis thaliana), the sequences encoding the Toll/IL-1 receptor (TIR) domain of nucleotide-binding Leu-rich repeat resistance proteins are targeted by several groups of sRNAs, which offers these sRNAs great regulatory potentials on plant innate immunity (Zhai et al., 2011; Li et al., 2012; Shivaprasad et al., 2012; Boccara et al., 2014).
According to their biogenesis and mode of function, sRNA consists of small interfering RNA (siRNA) and microRNA (miRNA), both of which are capable of regulating numerous biological processes associated with plant growth, development, and reproduction (Shivaprasad et al., 2012; Niu et al., 2015). sRNAs participating in plant responses to biotic stresses have received more attention during the past decade (Navarro et al., 2006; Katiyar-Agarwal and Jin, 2007; Zhai et al., 2011; Khraiwesh et al., 2012; Li et al., 2012, 2017; Shivaprasad et al., 2012). For example, miR393 was the first identified miRNA involved in plant innate immune regulation. miR393 suppresses the expression of TRANSPORT INHIBITOR RESPONSE1 (TIR1) that encodes the receptor of the auxin signaling pathway, which is a negative regulator of plant defense response. By suppressing TIR1, miR393 enhances the plant defense response against Pseudomonas syringae infection (Navarro et al., 2006). Later, more miRNA regulatory modules associated with plant innate immunity were identified (Li et al., 2014; Jin and Wu, 2015; Wu et al., 2015), highlighting the master role these sRNAs may play in plant innate immunity.
Rice (O. sativa) endogenous miRNAs play important roles between rice and associated pathogens. For example, miR160a and miR398b participate in modulating rice innate immunity against the blast fungus M. oryzae. miR160 targets ARF16, whose Arabidopsis homolog is associated with immune responses against bacterial pathogens (Zhang et al., 2011; Li et al., 2014). miR398 targets SUPEROXIDE DISMUTASE2 that maintains the dynamic balance of superoxide anion and hydrogen peroxide (H2O2) metabolism during M. oryzae infection. Overexpression of miR160a and miR398b enhances rice resistance against the blast disease, confirming their contribution to rice defense against M. oryzae infection (Li et al., 2014). miR168 is a critical player in the response against infection with Rice stripe virus (RSV) and Rice dwarf virus, in which it may function through incorporation into the Argonaute1 (AGO1)-associated RNA-induced silencing complex. When miR168 is loaded into other AGOs, such as AGO18, which is triggered by virus infection, the defense responses are suppressed (Wu et al., 2015), manifesting a critical role of miR168 in regulating broad-spectrum resistance against viral diseases in rice. miR444 is a positive regulator of the RNA-Dependent RNA Polymerase1-dependent RNA-silencing pathway. Upon induction by RSV infection, miR444 silences specific viral RNAs and host genes and contributes to defense against RSV (Wang et al., 2016). Also in rice infected with RSV, it was found that the reduced miR171b expression contributed to the viral stunting and yellowing symptoms on RSV-infected rice plants (Tong et al., 2017). A different group showed that miR169 acted as a negative regulator in rice immunity against M. oryzae by repressing the expression of NUCLEAR FACTOR Y-A genes (Li et al., 2017b). Taken together, rice endogenous miRNAs are active immune regulators against various pathogen infections.
The miR319 family is an ancient miRNA group that is widely conserved in plants (Talmor-Neiman et al., 2006; Fattash et al., 2007; Cuperus et al., 2011). The target genes of miR319 include TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR1 (TCP), which encodes transcription factors regulating many plant growth and developmental processes (Martín-Trillo and Cubas, 2010; Lopez et al., 2015). TCPs are important growth regulators controlling leaf morphogenesis (Martín-Trillo and Cubas, 2010; Lopez et al., 2015). In addition, the miR319/TCP module also participates in plant responses to biotic and abiotic stresses. For example, overexpressing rice miR319 or suppressing the corresponding target genes, such as OsPCF5, OsPCF6, OsPCF8, and OsTCP21, led to enhanced tolerance to cold stress (Yang et al., 2013; Wang et al., 2014). In tomato, sl-miR319 behaves both as a systemic signal and a regulatory factor of the defense response to root-knot nematodes (Zhao et al., 2015).
The jasmonic acid (JA) signaling pathway is a key regulator of plant defense responses against both hemibiotrophic and necrotrophic pathogens (Thaler et al., 2012; Tamaoki et al., 2013; Zhang et al., 2016). Ath-miR319 regulates the expression of a key JA synthetic component, LIPOXYGENASE2 (LOX2), through silencing TCP4 and TCP20 (Schommer et al., 2008; Danisman et al., 2012). In rice infected by Rice ragged stunt virus (RRSV), the induced expression of miR319 is associated with promoted viral infection, suppression of OsTCP21, down-regulation of JA synthetic genes, and reduced JA accumulation, indicating that RRSV may manipulate endogenous JA levels through miR319 (Zhang et al., 2016). Therefore, it is suggested that miR319/TCP may manipulate the plant innate response against RRSV by affecting JA synthesis and signaling. However, whether similar regulatory machinery is conserved in the rice immune system against M. oryzae is unknown.
Rice is the staple food crop worldwide, which makes the rice-M. oryzae interaction of great significance at both scientific and practical levels (Liu et al., 2013). To identify more miRNAs involved in the regulation of rice innate immunity against the blast disease, we compared and analyzed differentially expressed sRNAs upon M. oryzae infection. We show that miR319 is induced specifically by the M. oryzae strain Guy11, which leads to the suppression of genes, such as OsTCP21, and subsequent pathways regulated by this transcription factor. Our results show that M. oryzae infection blocks the conversion of α-linoleic acid (LnA) to hydroperoxy-octadecadienoic acid (HPODE), which is a critical step of JA synthesis. We further confirm the involvement of miR319 in the key JA biosynthetic step by genetic approaches and an external LnA application assay. Our study indicates that the M. oryzae pathogen can intensify its pathogenicity by blocking the key steps of the host JA biosynthetic and signaling pathway. Our study identifies critical molecular components affecting the rice immune response against the blast disease, which may lead to engineered rice varieties with enhanced resistance.
RESULTS
M. oryzae Infection Induces the Expression of miR319
To identify endogenous blast disease-responsive miRNAs that are potentially involved in rice immunity against M. oryzae infection, we infected rice (cv Nipponbare) with the M. oryzae strain Guy11 for different times (0, 24, 48, and 120 h). Total RNA from each sample was used for sRNA library construction as described previously (Zhang et al., 2011). As shown in Table I, 14,588,511, 15,931,384, 15,695,043, and 13,275,491 total reads were generated from each library, representing the different time points, respectively. By mapping to the rice genome (Rice Genome Annotation Project; http://rice.plantbiology.msu.edu/), 10,638,183, 10,840,688, 11,977,454, and 8,505,278 rice sRNA reads were obtained, corresponding to 925,406, 1,552,456, 977,547, and 805,238 unique reads, respectively. These reads were further searched against the Rfam database (http://xfam.org/) to remove known sRNAs, such as rRNAs, tRNAs, and small nucleolar RNAs. In the end, 7,376,173, 6,516,391, 6,815,060, and 4,637,845 reads were retrieved from each library and were considered as sRNAs originated from rice. We classified total rice sRNAs into three categories according to their origination and structural features: (1) conserved miRNAs that have a stem-loop precursor structure and are listed in the miRNA database (http://www.mirbase.org/); (2) novel rice miRNAs that have a stem-loop precursor structure but are not listed in the miRNA database; and (3) rice siRNAs that do not have a stem-loop precursor structure. According to these criteria, we retrieved 3,948, 5,721, 3,598, and 3,467 unique conserved rice miRNAs (osa-miRNAs) in each library, respectively (Table I; Supplemental Table S1). In addition, we obtained 2,155, 3,542, 2,080, and 1,820 novel rice miRNAs and 271,594, 410,909, 312,402, and 252,439 rice siRNAs, which will be discussed in separate articles.
Table I. Distribution of sRNAs among different categories in each library.
A rice sRNA deep-sequencing summary is shown. The abundance in different libraries was normalized to library 0 hpi, and sRNAs were calculated as reads per million.
| Types | Normalized Reads | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 hpi | 24 hpi | 48 hpi | 120 hpi | |||||
| reads | % | reads | % | reads | % | reads | % | |
| Total sRNA | 14,588,511 | 100.00 | 15,931,384 | 100.00 | 15,695,043 | 100.00 | 13,275,491 | 100.00 |
| Mapped sRNA | 10,638,183 | 72.92 | 10,840,688 | 68.05 | 11,977,454 | 76.31 | 8,505,278 | 64.07 |
| Unique reads | 925,406 | 1,552,456 | 977,547 | 805,238 | ||||
| Rfam | 3,262,010 | 22.69 | 4,324,297 | 27.44 | 5,162,394 | 33.07 | 3,867,433 | 29.48 |
| rRNA | 2,523,283 | 17.30 | 34,90,529 | 21.91 | 4,144,215 | 26.40 | 2,859,734 | 21.54 |
| tRNA | 713,233 | 4.89 | 789,733 | 4.96 | 955,329 | 6.09 | 974,162 | 7.34 |
| snRNA | 2,498 | 0.35 | 2,487 | 0.31 | 1,792 | 0.19 | 3,666 | 0.38 |
| Small nucleolar RNA | 22,996 | 0.16 | 41,548 | 0.26 | 61,058 | 0.39 | 29,871 | 0.23 |
| Rice sRNA | 7,376,173 | 50.23 | 6,516,391 | 40.60 | 6,815,060 | 43.25 | 4,637,845 | 34.59 |
| Conserved miRNA (total) | 131,480 | 0.90 | 247,126 | 1.55 | 81,347 | 0.52 | 84,275 | 0.63 |
| Conserved miRNA (unique) | 3,948 | 5,721 | 3,598 | 3,467 | ||||
| Novel miRNA (total) | 11,179 | 0.08 | 22,391 | 0.14 | 10,398 | 0.07 | 9,198 | 0.07 |
| Novel miRNA (unique) | 2,155 | 3,542 | 2,080 | 1,820 | ||||
| trans-acting siRNA (total) | 6,512 | 0.04 | 16,197 | 0.10 | 6,864 | 0.04 | 6,438 | 0.05 |
| trans-acting siRNA (unique) | 401 | 643 | 312 | 354 | ||||
| siRNA (total) | 7,227,002 | 49.54 | 6,230,677 | 39.11 | 6,716,451 | 42.79 | 4,537,934 | 34.18 |
| siRNA (unique) | 271,594 | 410,909 | 312,402 | 252,439 | ||||
To identify the osa-miRNAs responsive to blast infection, we compared miRNA expression between Guy11- and mock-treated rice. Osa-miRNAs both abundantly and significantly differentially expressed were identified by employing the following criteria: (1) total reads ≥ 150; and (2) [treated/mock] ≥ 2 or [treated/mock] ≤ 0.5 in each stage. At the end, we found 11 significantly differentially expressed conserved osa-miRNAs (Table II). By using reverse-cDNA fragments as probes, we validated our bioinformatic prediction by northern-blot assay (there is only a one-nucleotide difference between miR169a and miR169h, between miR166k-3p and miR166j-3p, and between miR1317-3p, miR5150-3p, miR169a, miR319b, and their corresponding family members; therefore, detection using probes reverse complementary to the aforementioned sRNAs represents the expression of the family). In the six northern blots that were used to detect the up-regulated miRNAs, miR1317-3p, miR166k-3p, miR169 (Fig. 1A), and miR319 (Fig. 1C) showed discernible up-regulation; miR162a showed weak expression with no noticeable expression variation. Surprisingly, miR5150-3p showed a significant reduction on expression, which is opposite to the bioinformatic prediction (Fig. 1A). For the three down-regulated miRNAs, miR156f-3p and miR435 were expressed under the detectable level. miR444d.3 showed an obvious reduction at 24 and 120 hpi (Fig. 1B).
Table II. List of some candidate miRNAs involved in rice immunity against M. oryzae in each library.
Expression variations were analyzed. Osa-miRNAs with significant changes (greater than 2-fold) and more than 150 reads in four libraries were selected.
| miRNA Identifier | Normalized Reads | Fold Change | |||||
|---|---|---|---|---|---|---|---|
| 0 hpi | 24 hpi | 48 hpi | 120 hpi | 24 hpi/0 hpi | 48 hpi/0 hpi | 120 hpi/0 hpi | |
| reads mg−1 | |||||||
| Up-regulated | |||||||
| osa-miR169a | 63.22 | 418.67 | 879.59 | 532.46 | 6.62 | 13.91 | 8.42 |
| osa-miR1317-3p | 12,512.79 | 29,328.42 | 139,549.83 | 62,645.91 | 2.34 | 11.15 | 5.01 |
| osa-miR169h | 109.21 | 231.20 | 338.30 | 348.15 | 2.12 | 3.10 | 3.19 |
| osa-miR162a | 5,402.86 | 10,966.52 | 51,230.29 | 15,308.21 | 2.03 | 9.48 | 2.83 |
| osa-miR5150-3p | 557.53 | 1,212.25 | 3,473.24 | 1,546.18 | 2.17 | 6.23 | 2.77 |
| osa-miR166j-3p | 425.33 | 915.44 | 2,954.51 | 983.00 | 2.15 | 6.95 | 2.31 |
| osa-miR319b | 137.95 | 306.19 | 304.47 | 286.71 | 2.22 | 2.21 | 2.08 |
| osa-miR166k-3p | 7,443.30 | 18,580.60 | 34,619.64 | 14,898.63 | 2.50 | 4.65 | 2.00 |
| Down-regulated | |||||||
| osa-miR444d.3 | 959.87 | 484.28 | 112.77 | 399.34 | 0.50 | 0.12 | 0.42 |
| osa-miR435 | 488.56 | 240.58 | 135.32 | 204.79 | 0.49 | 0.28 | 0.42 |
| osa-miR156f-3p | 827.67 | 187.46 | 112.77 | 215.03 | 0.23 | 0.14 | 0.26 |
Figure 1.
Expression patterns of miRNAs upon M. oryzae infection. Two-week-old seedlings were inoculated with Guy11 spores (1 × 105 mL−1), and total RNA was extracted from leaves at the indicated time points. A, Detection of the up-regulated osa-miRNAs using RNA blotting. A total of 100 μg of total RNA was loaded. RNA blots were hybridized with DNA oligonucleotide probes complementary to the indicated miRNAs. U6 was used as a loading control. Values below each section represent the relative abundance of miRNA normalized to U6. B, Detection of the down-regulated osa-miRNAs using RNA blotting. C, RNA-blotting detection of mR319b and quantitative reverse transcription-PCR (RT-qPCR) analyses of the mR319b precursor at the indicated time points upon Guy11 infection. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance of differences between 0 h post infection (hpi) and the indicated time points. Asterisks indicate significant differences (**, P < 0.01). The RT-qPCR experiments were repeated three times with similar results.
In these miRNAs that showed significant up- or down-regulated expression, miR162a, miR156f-3p, and miR435 were expressed at relatively low levels and were not studied further. miR166 is known for its development-related function (Fujioka et al., 2008). miR169a was identified as a rice blast-responsive sRNA in a previous study (Li et al., 2014). miR1317 was predicted to target a gene encoding a transposon protein (LOC_Os10g20480), which is less likely to be involved directly in the plant defense response. miR5150-3p was predicted to target a receptor-like protein kinase (LOC_Os01g70260); miR444d.3 could target OsMADS57 (LOC_Os02g49840), which is a MADS-box family gene. Investigation of these two positive immune regulators will be discussed in a separate article.
The induced expression of miR319 also was observed at the transcriptional level, which was confirmed by the examination of the miR319b precursor using real-time PCR (Fig. 1C). Interestingly, when inoculated with the avirulent strain 2539, the expression of miR319 was not obviously affected (Supplemental Fig. S1). Strain 2539 is an avirulent strain with a moderately pathogenic phenotype to many rice genotypes, which is mediated by an avirulence gene, AVR1-CO39 (Smith and Leong, 1994; Zheng et al., 2011). Therefore, our observation indicates that the elicitation of miR319 expression is Guy11 specific. miR319 is predicted to target OsTCP21 (LOC_Os07g05720) and a GA MYB gene (OsGAmyb [LOC_Os01g59660]; Fig. 2A; Supplemental Table S2), both of which are transcription factors. Transcription factors are key regulators of gene expression in response to various developmental and environmental cues (Danisman, 2016; Samad et al., 2017). Therefore, we focused on the functional study of miR319 in rice immune responses against the blast disease.
Figure 2.
miR319b targets OsTCP21 and OsGAmyb. A, Western-blot analysis of OsTCP21 and OsGAmyb expression by the transient expression system. Agrobacterium tumefaciens-mediated transient coexpression of OsTCP21 or OsGAmyb with miR319b or an irrelevant miRNA (miR169a) in Nicotiana benthamiana for 48 h is shown. The expression levels of OsTCP21 and OsGAmyb were normalized to β-actin. B, RT-qPCR analyses of OsTCP21 and OsGAmyb expression levels at the indicated time points upon Guy11 infection. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance of differences between 0 hpi and the indicated time points. Asterisks indicate significant differences (**, P < 0.01). Similar results were obtained from three biological replicates.
To validate whether OsTCP21 and OsGAmyb are authentic target genes regulated by miR319, both the miRNA and the Flag tag-fused OsTCP21 (35S::Flag:OsTCP21) and OsGAmyb (35S::Flag:OsGAmyb) were transiently coexpressed in N. benthamiana. When detected by an anti-Flag antibody, both OsTCP21 and OsGAmyb were obviously suppressed by coexpression with miR319, but not in the control assay or by an irrelevant miRNA (such as miR169a, a miRNA without reverse complementarity to both target genes; Fig. 2A). This result suggests that miR319 silences the expression of both OsTCP21 and OsGAmyb in vivo. The expression of OsTCP21 and OsGAmyb also was checked in rice infected by Guy11 (Fig. 2B). The transcription of both OsTCP21 and OsGAmyb declined obviously after 48 hpi, which corresponded to the induced expression of miR319 by Guy11 infection (Fig. 1C). Taken together, we concluded that OsTCP21 and OsGAmyb are authentic target genes of miR319. OsGAmyb is a transcription factor involved in many developmental processes, such as regulating the expression of α-amylase in the aleurone layer of rice seed; it also plays a role in pollen development (Liu et al., 2010; Sutoh et al., 2015). By contrast, OsTCP21 function is related to rice responses to biotic and abiotic stresses. For example, OsTCP21 is involved in the synthesis and signaling of JA in response to cold stress and virus infection (Wang et al., 2014; Zhang et al., 2016). Therefore, we concentrated our study on the miR319/OsTCP21 regulatory module in rice defense responses against the blast disease.
miR319b Overexpression Causes Rice Susceptibility to the Blast Disease
To investigate the involvement of miR319 in rice immunity against the blast disease, we employed a miR319b overexpression line (miR319b-OE) and an OsTCP21 mutant line (OsTCP21-Res) in which the OsTCP21 gene is relieved from regulation by miR319b due to engineered synonymous mismatches (Zhang et al., 2016). When disease responses against Guy11 infection were compared among wild-type, miR319b-OE, and OsTCP21-Res rice, miR319b-OE showed obviously stronger disease symptoms than the wild-type rice, such as heavily developed lesions, obvious chlorosis (Fig. 3A), and significantly more hyphae accumulation (biomass; Fig. 3B). In contrast, OsTCP21-Res plants showed relatively resistant phenotypes against Guy11 infection, manifested by fewer visible lesions, less severe chlorosis (Fig. 3A), and reduced hyphae accumulation (Fig. 3B). We further checked lesion numbers and quantitatively analyzed the composition of lesion types in both wild-type and transgenic plants (Fig. 3, C and D). In miR319b-OE plants, more lesions were visible (8.7 cm−2), and more type 4 and type 5 lesions (26.8% in total) were observed than in the wild-type plants (5.1 cm−2; 13.1% in total). In contrast, OsTCP21-Res plants exhibited fewer lesions on the leaf surface (2 cm−2), and no type 4 or type 5 lesions formed.
Figure 3.
Overexpressing miR319b results in elevated susceptibility to M. oryzae in the cv Nipponbare (NPB) background. Two-week-old seedlings of the wild type (cv Nipponbare) and transgenic lines (miR319b-OE and OsTCP21-Res) were inoculated with Guy11 spores (1 × 105 mL−1). A, Rice pathogenicity assays. Leaf phenotypes were observed at 120 hpi. B, RT-qPCR analyses of M. oryzae biomass (expression level of MGG03982.6) on the indicated rice at 120 hpi. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance of differences between the wild type and the indicated lines. C and D, Count of lesion numbers (per 1 cm2) and composition of lesion types of wild-type and transgenic rice. Lesions in eight diseased leaves were analyzed at 120 hpi. Values in C represent means ± sd of three biological replicates. Student’s t test was used to determine the significance of differences between wild-type cv Nipponbare and the indicated rice lines. E, RT-qPCR analyses of OsTCP21 expression levels in mock- or Guy11-infected wild-type and transgenic rice. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance of differences between the mock- and Guy11-infected rice. Asterisks indicate significant differences (**, P < 0.01). Similar results were obtained in three independent experiments.
Notably, in both the wild-type and the miR319b-OE plants, the OsTCP21 expression level was reduced significantly by Guy11 infection, but not in the OsTCP21-Res mutant (Fig. 3E). This is an indication that an integral miR319/OsTCP21 regulatory module is functional in the wild-type and the miR319b-OE plants. Furthermore, the reduced expression of OsTCP21 in miR319b-OE and the elevated expression in OsTCP21-Res plants appeared coincidentally with the observed disease symptoms. These results implicate a tight association between the OsTCP21 expression level and rice resistance against the blast disease.
To further confirm that miR319 has a negative regulatory role in rice resistance against the blast disease, miR319b-OE and OsTCP21 silencing mutant (OsTCP21-RNAi) lines from a different background (cv Kongyu 131) also were tested (Wang et al., 2014). The wild-type cv Kongyu 131 is almost completely immune to Guy11 infection, which developed nearly no visible lesions at 120 hpi (Fig. 4A); on miR319b-OE plants, prominent lesion formation and hyphae accumulation were observed (Fig. 4B). Quantitative examination of lesion numbers and the composition of lesion types revealed that both miR319b-OE and OsTCP21-RNAi plants developed significantly more lesions (6.5 and 4.5 cm−2, respectively) than the wild-type plants (1.4 cm−2) as well as more prominent type 4 and 5 lesions (22.7% and 11.4%, respectively; Fig. 4, C and D), indicating that the elevated miR319 expression corresponds to the decreased resistance. OsTCP21-RNAi plants also showed reduced resistance to Guy11 infection, but to a lesser degree in comparison with the miR319b-OE plants (Fig. 4, A–D), which may be due to partial suppression in the RNAi plants (Wang et al., 2014). A similar observation was made in the cv Kongyu 131 background plants, whereby OsTCP21 expression was significantly reduced upon Guy11 infection in the wild-type or mutant plants (Fig. 4E). Therefore, we conclude that the OsTCP21 expression is positively associated with rice resistance against the blast disease resistance.
Figure 4.
Overexpressing miR319b results in elevated susceptibility to M. oryzae in the cv Kongyu 131 background. Two-week-old seedlings of wild-type (cv Kongyu 131) and transgenic (miR319b-OE and OsTCP21-RNAi) rice were inoculated with Guy11 spores (1 × 105 mL−1). A, Rice pathogenicity assays. Leaf phenotypes were observed at 120 hpi. B, RT-qPCR analyses of M. oryzae biomass (expression level of MGG03982.6) on the indicated lines at 120 hpi. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance of differences between the wild type and the indicated lines. C and D, Comparison of disease lesion number (per 1 cm2) and composition of disease lesion types of wild-type and transgenic rice. Lesions in eight diseased leaves were analyzed at 120 hpi. Values in C represent means ± sd of three biological replicates. Student’s t test was used to determine the significance of differences between the wild type and the indicated lines. E, RT-qPCR analyses of OsTCP21 expression levels in mock- or Guy11-infected wild-type and transgenic rice. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance of differences between the mock- and Guy11-infected plants. Asterisks indicate significant differences (**, P < 0.01). Similar results were obtained in three independent experiments.
Taken together, our results confirmed the association between the expression level of OsTCP21 and enhanced resistance against M. oryzae infection in different rice varieties. Our results suggest that OsTCP21 may play a role in the rice defense response against the blast disease, which is regulated by miR319-mediated gene silencing.
OsTCP21 Overexpression Leads to Early Activation of Defense Responses
To validate the involvement of miR319/OsTCP21 in rice resistance against the blast disease, we examined reactive oxygen species (ROS) accumulation in both wild-type and mutant plants. ROS is produced when plants are under stress conditions and is one of the early responses against pathogen infection (Lamb and Dixon, 1997; Thaler et al., 2012). A recent report showed that OsTCP21 could enhance cellular ROS levels and contribute to plant tolerance against cold stress (Yang et al., 2013; Wang et al., 2014). In mock-treated wild-type rice plants (cv Nipponbare), H2O2 accumulation was negligible, indicating that a low ROS level was produced in rice without M. oryzae infection (Fig. 5A). When infected by Guy11, wild-type plants accumulated measurable levels of H2O2, which could be detected by diaminobenzidine (DAB) staining. In miR319b-OE plants, where the expression of OsTCP21 is suppressed, no measurable H2O2 accumulation was detected in either mock- or Guy11-infected rice; in contrast, in the OsTCP21-Res plants, H2O2 accumulated to a significantly higher level than in the mock-treated plants (Fig. 5A). We further tested H2O2 accumulation in wild-type and OsTCP21-RNAi plants in the cv Kongyu 131 background. Consistent with its resistance to the blast disease, wild-type cv Kongyu 131 accumulated high levels of H2O2 when infected with Guy11. However, in either the miR319b-OE or the OsTCP21-RNAi plants, significantly less H2O2 accumulation than that in the wild-type cv Kongyu 131 was observed (Fig. 5B). Taken together, the co-occurrence between cellular H2O2 levels and the in vivo OsTCP21 expression levels suggests an association of this gene with the early immune response against the blast disease in rice.
Figure 5.
The expression of OsTCP21 results in the activation of early defense responses. Two-week-old leaves of wild-type and transgenic lines were dipped in a Guy11 spore suspension (1 × 105 mL−1). A, Sustained expression of OsTCP21 results in the accumulation of H2O2 in the cv Nipponbare (NPB) background. B, Silencing of OsTCP21 blocks H2O2 accumulation in the cv Kongyu 131 background. To detect the accumulation of H2O2, representative rice leaves from the indicated lines (48 hpi) were stained with DAB to show the accumulation of H2O2; mock-treated wild-type cv Nipponbare and cv Kongyu 131 were used as controls. Expression levels were quantified using ImageJ as instructed. Similar results were obtained from three biological replicates. Values represent means ± sd of three biological replicates. Student’s t test was used to determine the significance of differences between the indicated lines and treatments.
miR319 Impedes JA Synthesis and Signaling Elicited by M. oryzae Infection
JA is a phytohormone that plays a critical role in defense responses against necrotrophic and hemibiotrophic pathogens (Thaler et al., 2012; Tamaoki al., 2013; Zhang et al., 2016). It has been reported that, in both Arabidopsis and rice, some TCP transcription factors are involved in the JA signaling pathway (Schommer et al., 2008; Zhang et al., 2016). Therefore, we hypothesized that the miR319-mediated regulation of OsTCP21 might affect rice resistance against the blast disease by manipulating JA signaling.
To validate our hypothesis, we examined the expression levels of JA signaling pathway reporter genes, such as PATHOGENESIS-RELATED PROTEIN1B (OsPR1b), JA synthetic component genes, such as PHOSPHOLIPASE D α1 (OsPLDα1), OsLOX2, OsLOX5, and ALLENE OXIDE SYNTHASE2 (OsAOS2), and JA signaling component genes, such as CORONATINE INSENSITIVE1B (OsCOI1b), OsCOI2, and JASMONATE ZIM-DOMAIN PROTEIN8 (OsJAZ8; Chini et al., 2007; Thines et al., 2007; Katsir et al., 2008; Yamada et al., 2012; Lee et al., 2013).
In wild-type rice, both the JA signaling reporter gene, OsPR1b, and JA synthesis and signaling components were induced by Guy11 infection at the early stage but declined at the late stage (Supplemental Fig. S2). This unique expression pattern suggests a close association between the up-regulated JA signaling pathway and the progress of pathogen infection. Moreover, in plants in which the miR319/OsTCP21 regulatory module was perturbed, the expression of JA signaling pathway components changed. For example, after Guy11 infection, the expression of OsPR1b was suppressed in miR319b-OE rice but was highly induced in the OsTCP21-Res rice (Fig. 6A), indicating that the JA signaling pathway is affected by the miR319/OsTCP21 regulatory module. Additionally, the expression of components involved in JA synthesis also was affected. Specifically, genes involved in the conversion from membrane lipids to LnA (OsPLDa1) and from LnA to HPODE (OsLOX2/5) were both suppressed, while the gene involved in the conversion of HPODE to 12-oxo-phytodienoic acid (OsAOS2) was induced (Fig. 6B). These genes showed reversed expression patterns in the OsTCP21-Res rice. Genes involved in JA signaling (OsCOI1b, OsCOI2, and OsJAZ8) also varied in expression when miR319b was overexpressed or suppressed. In particular, the expression of OsCOI1 and OsCOI2 was suppressed in the miR319b-OE rice but induced in the OsTCP21-Res rice, while OsJAZ8 showed a reversed pattern (Fig. 6B). These results demonstrated that both JA synthesis and signaling are affected by the miR319/OsTCP21 regulatory module.
Figure 6.
miR319b impedes JA synthesis and signaling. Two-week-old seedlings of the wild type (WT; cv Nipponbare [NPB]) and transgenic lines (miR319b-OE and OsTCP21-Res) were used for the assays. A, RT-qPCR analyses of the JA reporter gene OsPR1b in the indicated lines upon Guy11 infection at 24 hpi. B, RT-qPCR analyses of the key JA synthesis and signaling components in the indicated lines. C, RT-qPCR analyses of the key SA synthesis and signaling components in the indicated lines. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance of differences between the wild type and the indicated lines. Asterisks indicate significant differences (**, P < 0.01). The RT-qPCR experiments were repeated three times with similar results. OPDA, 12-Oxo-phytodienoic acid.
Similarly, we also checked the expression of key genes involved in the salicylic acid (SA) signaling pathway. The results showed that neither synthesis (OsICS1 and OsPAL1) nor signaling (OsPAD4 and OsEDS1) of the SA pathway was affected noticeably by miR319/OsTCP21 (Fig. 6C). Taken together, our results demonstrated that the miR319/OsTCP21 module regulates multiple key factors in the JA signaling pathway, among which OsLOX genes (OsLOX2 and OsLOX5) are severely affected. We speculated that, in Guy11-infected rice, JA synthesis is weakened due to the jeopardized LOX activities.
Guy11 Infection Induces miR319b and Inhibits JA-Mediated Defense Responses
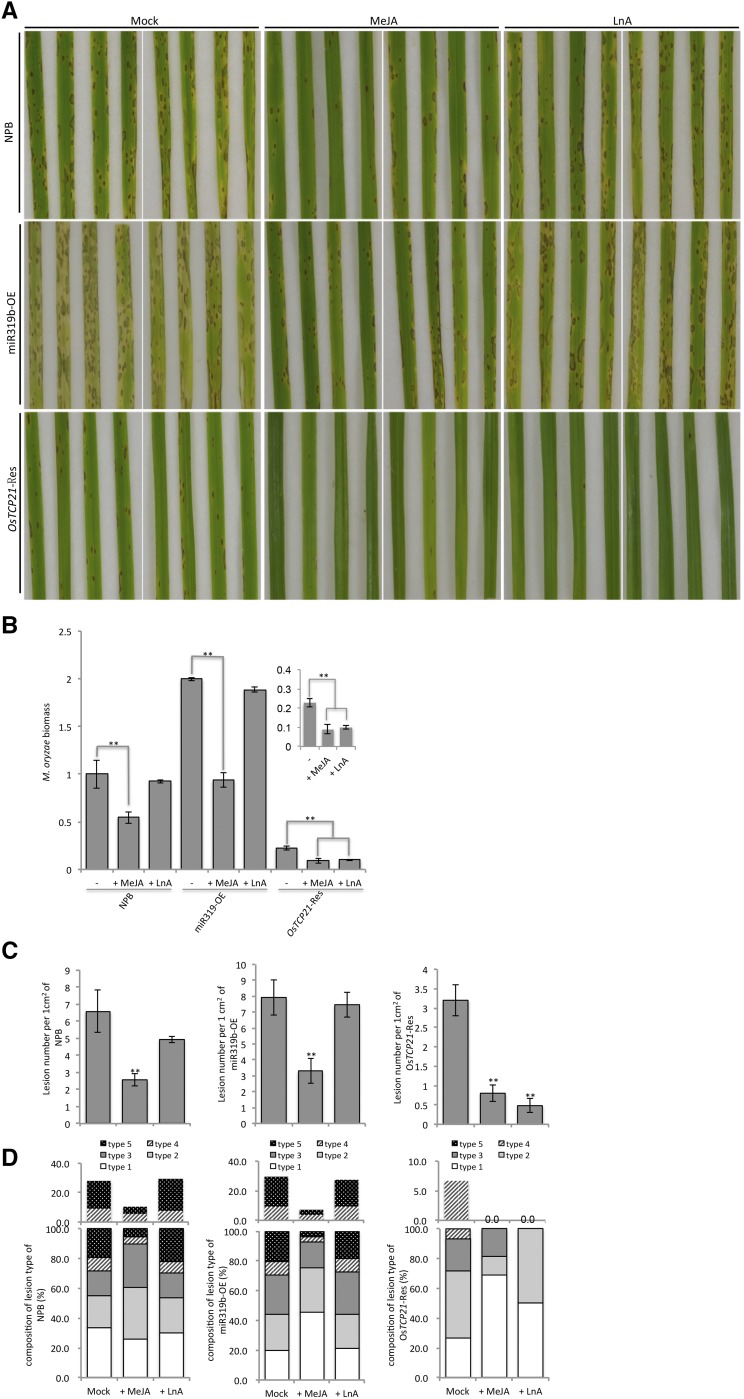
To validate our hypothesis, we applied both methyl jasmonate (MeJA) and LnA to wild-type and mutant rice. We speculated that MeJA would demonstrate a disease-rescue effect on both wild-type and transgenic rice, while LnA could only complement the disease symptoms in plants in which the LOX activities are not inhibited.
On both wild-type (cv Nipponbare) and miR319b-OE rice, but not OsTCP21-Res rice, Guy11 infection caused apparent disease symptoms at 120 hpi. Application of MeJA on both wild-type and miR319b-OE rice obviously rescued the disease susceptibility (Fig. 7A). Specifically, MeJA-treated plants displayed much less severe disease symptoms, demonstrated by fewer lesions (about 60% less), less hyphae accumulation (about 50% less), and about 70% fewer type 4 and 5 lesions than the mock-treated rice (Fig. 7, B and C). In contrast, LnA treatment displayed no discernible effects on either wild-type or miR319b-OE rice, suggesting that MeJA treatment, but not LnA, could rescue the reduced resistance on these plants (Fig. 7A). Similarly, the effects of MeJA and LnA were tested on OsTCP21-Res rice. In OsTCP21-Res rice, which was relatively resistant to Guy11 infection, the application of MeJA and LnA showed no visible difference at 120 hpi. However, when quantitative examinations were employed, both MeJA-treated and LnA-treated rice showed significantly less Guy11 infection than the mock-treated plants (Fig. 7B). Specifically, the MeJA-treated rice formed 50% fewer lesions and accumulated 75% fewer hyphae than the mock-treated rice; the LnA-treated rice formed 50% fewer lesions and accumulated 85% fewer hyphae than the mock-treated rice. Neither MeJA-treated nor LnA-treated rice formed any type 4 or 5 lesions, while 6.7% of the lesions formed on the mock-treated rice were type 4 or 5 (Fig. 7, C and D). We also measured the expression levels of miR319b and OsTCP21 in both MeJA- and LnA-treated rice. In both miR319-OE and OsTCP21-Res plants, the expression of miR319b and OsTCP21 was not affected by the application of hormones, indicating that the effects of MeJA or LnA are genetically downstream of OsTCP21 (Supplemental Fig. S3). These results confirmed our hypothesis that the elevated disease susceptibility in wild-type or miR319b-OE rice is due to the blocking of JA synthesis at the LnA step, which consequently disrupted JA-mediated disease resistance against the blast disease. Our results demonstrated that the LnA-to-HPODE conversion is the key JA synthesis step that is manipulated by the blast fungus.
Figure 7.
Overexpressing miR319b inhibits JA-mediated defense responses. Two-week-old seedlings of wild-type (cv Nipponbare [NPB]) and transgenic (miR319b-OE and OsTCP21-Res) rice were treated with mock, MeJA, or LnA. Two days later, the plants were inoculated with Guy11 spores (1 × 105 mL−1). A, Rice pathogenicity assays. Leaf phenotypes in the indicated rice upon mock, JA, or LnA treatment were observed at 120 hpi. B, RT-qPCR analyses of M. oryzae biomass (expression level of MGG03982.6) on the indicated lines upon the different treatments at 120 hpi. C and D, Count of lesion number (per 1 cm2) and composition of lesion types on the indicated lines upon the different treatments. Lesions in eight diseased leaves were analyzed at 120 hpi. Values represent means ± sd of three biological replications. Student’s t test was used to determine the significance of differences in the mock and the indicated treatments. Asterisks indicate significant differences (**, P < 0.01). Similar results were obtained in three independent experiments.
M. oryzae Induces miR319 Expression through cis-Elements in Its Promoter Region
To investigate how the infection signals are perceived by potential cis-elements in the miR319 promoter, we analyzed the 1,452-bp sequence located immediately upstream of the miR319 stem-loop region by using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; Lescot et al., 2002). Some stress/hormone-responding cis-elements, such as the fungal elicitor-responsive element (Box-W1), defense/stress-responding element (TC-rich repeats), abscisic acid-responding element (ABRE), MeJA-responsive element (CGTCA motif), and SA-responsive element (TCA element) were identified (Supplemental Fig. S4; Supplemental Table S3). We fused a GFP reporter gene to either the wild type or different deletion mutants of the miR319 promoter (Fig. 8A). When transiently expressed in rice protoplasts with and without elicitor (Guy11 cell wall extraction) treatment (Forlani et al., 2011), GFP fluorescence was under the detectable level, indicating that the miR319 promoter is not a strong promoter. When driven by the wild-type promoter, the GFP gene was expressed significantly higher in the elicitor-treated samples than in the mock-treated samples, indicating that the protoplast transient expression assay is a good system for our promoter analysis (Fig. 8B). Under the mock-treated condition, the reporter driven by either the wild-type or the mutant promoter was expressed at a similar level, indicating that none of the cis-elements tested (namely TC-rich repeats, Box-W1, ABRE, CGTCA motif, and TCA element) affect the expression of miR319 without elicitor treatment. However, when treated with elicitors, deletion of either the TC-rich repeats or the Box-W1 motif caused significantly reduced expression of the GFP gene, while mutation in other motifs (ABRE, CGTCA motif, and TCA element) did not cause measurable change (Fig. 8B). When both the TC-rich repeats and the Box-W1 motif were deleted, the expression of the reporter gene was similar to that of the single mutation, indicating that these two cis-elements do not function redundantly. Therefore, our results indicated that the TC-rich repeats and the Box-W1 motif are critical for the Guy11-induced expression of the miR319 precursor gene.
Figure 8.
Identification of M. oryzae-responsive cis-elements. A, Schematic maps of the cis-elements in the miR319b promoter sequence and corresponding deletion mutations. B, RT-qPCR analyses of the reporter gene in the wild type and the indicated mutants, which were transiently expressed in rice protoplasts with or without elicitor treatment. Values represent means ± sd of three independent samples. Student’s t test was used to determine the significance between the wild-type and mutant promoter sequences. Different letters at the top of each column indicate significant differences (P < 0.01). The RT-qPCR experiments were repeated three times with similar results.
In summary, we found that rice endogenous miR319b is induced specifically by M. oryzae strain Guy11 infection via recognizing conserved motifs, such as the TC-rich repeats or Box-W1, in the promoter region. miR319b suppresses OsTCP21 and some key JA synthetic component genes, such as OsPLDα1, OsLOX2, and OsLOX5, which specifically interrupt JA synthesis at two early steps (from membrane lipids to LnA, then to HPODE). Stalled JA synthesis, as well as signaling transduction, led to reduced defense responses, including ROS accumulation and the production of pathogenesis-related proteins. The involvement of miR319a/b in cold tolerance also was reported, which involves OsPCF5, OsPCF6, OsPCF8, and OsTCP21, and ROS elimination in rice cells (Yang et al., 2013; Wang et al., 2014). Therefore, the miR319/OsTCP21 regulatory module may serve as a signaling hub mediating rice responses to both biotic and abiotic stresses (Fig. 9). Whether there is any interplay between resistance to the blast disease and tolerance to cold needs further investigation.
Figure 9.
A model of miR319/OsTCP21 modulates the rice and M. oryzae interaction through affecting the JA pathway. Guy11 infection induces miR319b expression, which diminishes the stimulative roles of OsTCP21 on key JA synthesis and signaling components (such as OsPLDa, OsLOXs, and OsCOL). Disruption of JA signaling leads to reduced defense responses, including reduced ROS accumulation and PR1 expression. Transgenic rice expressing LOXs in an induced manner (miR319::LOXs) could be a potential resistance mechanism for the blast disease. Cold stress suppresses miR319 expression in rice, which results in induced expression of TCPs, weakened ROS elimination, and, consequently, reduced tolerance to cold stress. A potential cross talk between rice resistance to biotic stresses and tolerance to abiotic stresses is suggested. OPDA, 12-Oxo-phytodienoic acid.
DISCUSSION
As a master modulator of gene expression, miRNA-mediated gene silencing is a potent regulatory mechanism involved in plant responses against pathogen infection (Padmanabhan et al., 2009; Ding, 2010). In the rice-M. oryzae interaction, both compatible and incompatible infections trigger specific gene expression variation on endogenous sRNAs, either initiated by pathogen infection or by the host innate immunity (Li et al., 2014, 2017). As a consequence, gene expression regulated by these sRNAs may facilitate disease development (Li et al., 2017a) or contribute to disease resistance (Li et al., 2014). Therefore, identification of sRNAs involved in the interaction between M. oryzae and rice and investigation of the target gene functions are crucial in discovering critical rice immune components against the blast disease. Previous studies have shown that miR160a and miR398b participate in rice innate immunity against the blast disease, overexpression of which significantly enhances disease resistance (Li et al., 2014, 2017). In this study, we found that miR319 is a novel factor that determines the relationship between rice and M. oryzae. Moreover, the blast pathogen can manipulate rice immune responses by blocking JA signaling. This blockage is accomplished by the miR319-mediated silencing of a transcription factor gene (OsTCP21) and its target genes (such as OsLOX2 and OsLOX5 that encode key synthetic components of JA). By repressing the rice JA signaling pathway, the blast pathogen facilitates its infection, growth, and propagation in the host plant. Our discovery identified a potential focal point that could be used for efficient control of the blast disease.
Researchers have shown that members of the miR319 family are involved in plant responses against a variety of biotic stresses (Zhang et al., 2011; Shen et al., 2014). Although a common resistant mechanism has not been identified, a lot of evidence points to a potential association with the modulation of JA signaling. The JA signaling pathway is a potent mechanism employed by plants that can defend against necrotrophic and hemibiotrophic pathogens, including the blast pathogen (Thaler et al., 2012; Tamaoki et al., 2013; Zhang et al., 2016). In tomato, miR319 behaves as a systemic signal and a negative regulator of defense responses against root-knot nematodes through decreasing the JA level (Zhao et al., 2015). In rice, RRSV infection induces miR319 expression. The resultant suppressed expression of TCP inhibits plant defense responses and promotes RRSV infection and disease symptom development (Zhang et al., 2016). In our study, JA signaling was induced at the early stage but declined at the late stage (Supplemental Fig. S2), which matched the expression pattern of miR319 very well (Fig. 1C). The fluctuating JA signaling is a good indicator of an enhanced immune effort at the early stage of M. oryzae infection, which was later suppressed by the pathogen as the infection proceeded.
Target genes of miR319 include OsTCPs, which encode transcription factors that participate in JA synthesis and homeostasis (Palatnik et al., 2003; Schommer et al., 2008). In Arabidopsis, OsTCP promotes OsLOX2 expression at the transcriptional level (Schommer et al., 2008; Hao et al., 2012). By using multiple transgenic and mutant rice lines, our results built a strong connection between miR319 expression and JA signaling. For instance, the miR319b-OE and OsTCP21-RNAi plants were more susceptible to Guy11 infection, while the OsTCP21-Res plants were more resistant (Figs. 3 and 4). Both the OsTCP21-OE and the OsTCP21-Res lines showed similar resistant phenotypes to Guy11 infection, suggesting that the function of miR319 is through manipulating the expression of OsTCP21 (Supplemental Fig. S5). Further observations showed that enhanced H2O2 accumulation was observed in OsTCP21-Res but not in miR319b-OE rice (Fig. 5), indicating an enhanced early defense response when OsTCP21 expression is elevated. Many JA synthetic component genes possess OsTCP21-binding sites (GTGGNCCC, TGGGCY, and GGACCA) in the promoter regions, through which the in vivo JA level is tightly controlled (Zhang et al., 2016). Therefore, the suppression of miR319-mediated JA signaling may be a counter defense mechanism that is conserved among many plant/pathogen systems, which is worthy of more attention.
Despite multiple observations suggesting that the JA signaling pathway is a potent defense machinery against many pathogens (including M. oryzae; Mei et al., 2006; Thaler et al., 2012; Tamaoki et al., 2013; Zhang et al., 2016), the detailed molecular mechanism regarding how this regulation is carried out, and how JA signaling is maneuvered in the interaction between rice and the blast pathogen, is not clear. Our results showed that, in miR319b-OE rice, key JA synthesis components, such as OsPLDα1, OsLOX2, and OsLOX5, were specifically suppressed (Fig. 6). This observation suggests that Guy11 infection can suppress the expression of these genes and evade the JA-mediated rice defense response. This is manifested by the MeJA and LnA spray-application assay on both wild-type and transgenic plants. MeJA boosted the rice defense response against Guy11 in both wild-type and transgenic plants (miR319b-OE and OsTCP21-Res), while LnA was only effective on OsTCP21-Res rice (Fig. 7). Therefore, we propose that, although the JA signaling pathway is an effective defense mechanism against the M. oryzae pathogen, some strains have evolved successful machinery to ward off the host defense and invade its host. In the case of Guy11, through the miR319-mediated silencing of OsTCP21, key JA synthetic components are suppressed, which sabotages host immunity by stalling JA signaling (Fig. 9). Puccinia striiformis uses a similar strategy to break down wheat (Triticum aestivum) resistance (Wang et al., 2017). Therefore, our discovery may reveal a common host resistance-suppressing strategy employed by various pathogens, which is of great significance in understanding the pathogenicity mechanism. Our discovery potentializes engineered rice harboring a chimeric gene with the LnA-encoding gene driven by the miR319b promoter. This engineered rice should express LnA upon blast pathogen infection (and maybe other pathogens as well) and could restore the stalled JA signaling caused by infection.
Intrigued by the inducing mechanism of Guy11 infection, we analyzed the miR319b promoter sequence and identified several cis-elements in the miR319b promoter region, including potential fungal elicitor-responding elements, defense-responding elements, and hormone-responding elements (Supplemental Fig. S4; Supplemental Table S3). Deletion analysis indicated that the Box-W1 and TC-rich repeats regions are required for response to Guy11 infection (Fig. 8). Box-W1 has been identified as an elicitor-responsive element that is critical for the elicitor-induced expression of the parsley (Petroselinum crispum) PR1 genes and the Arabidopsis cytosolic thioredoxin h5 gene (Rushton et al., 1996, 2002; Laloi et al., 2004). The TC-rich repeats regions are conserved defense-responsive motifs that play an important role in the response to Erysiphe necator (Diaz-De-Leon et al., 1993; Wen et al., 2017). Interestingly, the expression of miR319b was not affected by an avirulent M. oryzae strain (strain 2539), either at the precursor transcript or at the mature miRNA level (Supplemental Fig. S1), indicating that the induced expression of miR319b is Guy11 specific. Thus, our results favor a scenario that some unidentified Guy11 effector(s) may be secreted to the rice cells and bind directly or indirectly to these cis-elements, which triggers the induced expression of the miR319b precursor. By using these cis-elements as bait molecules, potential Guy11 effectors may be identified in future studies.
Our results further suggest a potential cross talk between rice resistance against biotic and abiotic stresses. Overexpression of miR319a/b or reduced expression of endogenous target genes (such as OsPCF5, OsPCF6, OsPCF8, and OsTCP21) could enhance plant cold tolerance through adjusting the in vivo ROS level (Yang et al., 2013; Wang et al., 2014). This suggests that miR319 is responsive to both cold stress and fungal infection. Cold stress suppresses miR319 expression in rice, which results in induced expression of OsTCPs and weakened ROS elimination. The correlation between OsTCPs and ROS also is observed in rice infected by Guy11. It would be interesting to investigate how tolerance to cold and resistance to M. oryzae are reconciled by miR319.
MATERIALS AND METHODS
Pathogen Infection and sRNA Library Construction
Rice (O. sativa japonica ‘Nipponbare’) was grown in a growth room maintained at 26°C and 70% relative humidity with a 12-h/12-h light/dark photoperiod. M. oryzae strain Guy11 was used for rice infection. Briefly, three-leaf-stage plants were spray inoculated with gelatin or the indicated conidial suspensions (1 × 105 spores mL−1 in 0.2% gelatin). The inoculated plants were kept in darkness at 80% relative humidity for 24 h before they were transferred to a growth chamber at 25°C, 80% relative humidity, and a 12-h/12-h light/dark photoperiod. Seedlings from at least 15 to 20 rice plants from the same treatment were pooled for RNA extraction, library construction, and RT-qPCR analysis. Plants that received the same treatment and were maintained under the same conditions were used as biological replicates.
sRNA library construction and Illumina sequencing were performed as described (Mi et al., 2008). The sRNA reads with length over 16 nucleotides were mapped to the rice nuclear, chloroplast, and mitochondrial genomes (http://rice.plantbiology.msu.edu/; version 6.0) and M. oryzae genomes (http://www.broadinstitute.org). The perfect genome-matched sRNAs were analyzed following a previous report (Wu et al., 2009). The normalized abundance of sRNAs was calculated as reads per million.
Northern-Blot Analysis
RNA-blot analyses for miRNAs from total extracts were performed as described previously (Katiyar-Agarwal and Jin, 2007). The inoculated plants were used for RNA extraction at 0, 24, 48, and 120 hpi. Total RNA was extracted using TRIzol reagent (Takara) following the manufacturer’s instructions. RNA was resolved on a 14% denaturing 8 m urea-PAGE gel and then transferred and chemically cross-linked onto a Hybond N+ membrane (GE Healthcare Life Sciences) using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride. miRNA probes were end labeled with [γ-32P]ATP by T4 polynucleotide kinase (New England Biolabs). Expression levels were quantified using ImageJ as instructed. Probes used for RNA blotting are listed in Supplemental Table S4.
RT-qPCR
Total RNA was extracted from rice leaves or rice protoplasts using TRIzol reagent (Takara). A NanoDrop-1000 was used to detect the quality and quantity of RNA (NanoDrop). The RNA was then reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (Takara). RT-qPCR was performed in 15 μL of reaction mixture consisting of 1.5 μL of 1× SYBR Green (Invitrogen), 1.5 μL of PCR buffer, 0.3 μL of 10 mm deoxyribonucleotide triphosphates (Takara), 0.3 μL of Taq, 0.3 μL of ROX DYE2 (Vazyme), 1.5 μL of 2 mm each primer, and 2 μL of appropriate diluted cDNA. The conditions for RT-qPCR were as follows: 94°C for 3 min, then 40 cycles at 94°C for 30 s and 58°C for 30 s, followed by 72°C for 35 s for PCR amplification. Transcript levels of each gene were measured by the Applied Biosystems 7500 system according to the manufacturer’s instructions. 18s rRNA was used as a quantitative control in the RT-qPCR analysis. Primers used in this study are listed in Supplemental Table S4.
Transient Expression Analysis in Nicotiana benthamiana
To generate the construct for the miR319b lines, the primary amiR319b construct was engineered from pRS300 (miR319a) Arabidopsis (Arabidopsis thaliana) with the WMD3 online tool (http://wmd3.weigelworld.org) and was cloned into a pEarleyGate (pEG202) destination vector using LR clonase II (Invitrogen). To generate the overexpression lines, the OsTCP21 and OsGAmyb coding sequences were cloned into the pEG202 vector.
Transient coexpression assays in N. benthamiana were performed by infiltrating 3-week-old N. benthamiana plants with Agrobacterium tumefaciens GV3101 (OD600 = 1) harboring constructs containing miR319b (pEG202) with A. tumefaciens GV3101 (OD600 = 1) containing target gene coding sequences (pEG202), or with miR169a (pEG202) as a control. Leaf tissue was collected 48 hpi, and protein expression was detected by western blot.
Western Blot
The tissue was ground in liquid nitrogen, and total proteins were extracted using 2× SDS loading buffer. The samples were resolved on a 12% SDS-PAGE gel and transferred onto an Amersham Hybond-P PVDF membrane (GE Healthcare) using a Tris-Gly transfer buffer. Membranes were blocked with 5% milk in 0.05% TBS-plus Tween 20 (TBST) for 40 min and incubated overnight at 4°C with 1:5,000 dilutions of primary antibodies of mouse anti-Flag conjugated to horseradish peroxidase (Abmart), washed three times with TBST, then incubated overnight at 4°C with 1:1,000 dilutions of secondary antibodies (Beytime). Membranes were washed three times with TBST. Detection was performed using the ECL Plus Western Blotting Detection Reagents (GE Healthcare) and ChemiDoc Touch Imager (Bio-Rad). Image data were analyzed with Image Lab Software (Bio-Rad) and assembled in Adobe Photoshop CS6.
Pathogenicity Assays and Chemical Treatments
The pathogenicity test was performed by spraying a conidial suspension at a concentration of 1 × 105 spores mL−1 onto the leaves of 2-week-old rice. The inoculated plants were kept in darkness at 80% relative humidity for 24 h before they were transferred to a growth chamber at 25°C, 80% relative humidity, and a 12-h/12-h light/dark photoperiod. Leaf phenotypes were observed at 120 h after fungal inoculation. The blast disease lesions were examined and classified into six types. In brief, 0 = no disease lesions observed; 1 = small pinpoint-like disease lesions between two small vascular bundles; 2 = lesions with diameter 0.5 to 1 mm that developed over the two small vascular bundles but did not reach the big vascular bundles; 3 = disease lesions with diameter about 1 to 3 mm that developed between the two big vascular bundles; 4 = disease lesions with diameter about 3 to 4 mm that developed over the two big vascular bundles; and 5 = disease lesions with diameter over 4 mm that developed over the main vein.
Chemical treatments of 2-week-old rice plants with 100 μm MeJA (in 10% ethanol) or LnA (in 1% methanol; Sigma) were sprayed on the leaves. Distilled water containing 10% ethanol and 1% methanol was used as a control treatment. After 24 h, control-treated, JA-treated, and LnA-treated plants were subjected to pathogenicity assays. Leaf phenotypes were observed at 120 h after fungal inoculation. Image data were assembled in Adobe Photoshop CS6.
Detection of H2O2 Accumulation
To detect the accumulation of H2O2, rice leaves from at least three different plants were stained with 1 mg of DAB (pH 3.8) per 1 mL for 8 h in the dark at 25°C to 28°C. The DAB solution was then supplemented with 50 mm ascorbic acid, a scavenger of H2O2, to ensure the specificity of staining. Subsequently, rice leaves were cleared with 96% (v/v) ethanol and were preserved in 50% (v/v) ethanol. DAB staining visualizes H2O2 as red-brown precipitate using the light microscope. Image data were assembled in Adobe Photoshop CS6. Expression levels were quantified using ImageJ as instructed.
Protoplast Isolation and Transformation
Rice protoplasts were isolated as described previously (Bart et al., 2006). In each tube, 1 mL of protoplasts was gently mixed with 10 μg of plasmid DNA and 1 mL of 40% PEG. After incubation at 28°C for 15 to 20 min, each tube was added with 4 mL of W5 solution (154 mm NaCl, 125 mm CaCl2, 5 mm KCl, and 2 mm MES [pH 5.7]) and gently mixed. The tubes were spun at 200 rpm for 3 min, and the pellet was resuspended with 200 μL of W5 solution and then incubated at 28°C in the dark overnight. The pXZP008 vector was used to overexpress miR319b and OsTCP21 in rice protoplasts. The amiR319b and full-length cDNA fragment were cloned into the pXZP008 vector with the Ubiquitin promoter with the homologous recombination method using the ClonExpress II One Step Cloning Kit (Vazyme). Primers used in this study are listed in Supplemental Table S4.
Promoter Cloning and Analysis
The 1,452-bp region upstream of miR319b was cloned by PCR and then cloned into the pXZP008 vector with the homologous recombination method using the ClonExpress II One Step Cloning Kit (Vazyme). The putative regulatory elements were predicted using the PlantCARE program (Lescot et al., 2002; http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). We obtained a single-site mutant of the putative regulatory elements (Box-W1 and TC-rich repeats) by overlap PCR and then cloned them into a pXZP008 vector with the homologous recombination method. All three constructs were transformed into rice protoplasts as described previously. Primers used in this study are listed in Supplemental Table S3.
Accession Numbers
Sequence data from this study can be found in the Rice Genome Database (http://rice.plantbiology.msu.edu) and GenBank (https://www.ncbi.nlm.nih.gov/genbank) under the following accession numbers: OsTCP21 (LOC_Os07g05720), OsGAmyb (LOC_Os01g59660), OsPLDα1 (LOC_Os01g07760), OsLOX5 (LOC_Os02g10120), OsLOX2 (LOC_Os03g08220), OsAOS2 (LOC_Os03g12500), OsCOI1b (LOC_Os05g37690), OsCOI2 (LOC_Os03g15880), OsJAZ8 (LOC_Os09g23650), OsPR1b (LOC_Os01g28450), OsICS1 (LOC_Os09g19734), OsPAD4 (LOC_Os11g09010), OsPAL1 (LOC_Os02g41630), and OsEDS1 (AK100117).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Expression of miR319b is not induced by the avirulent strain 2539.
Supplemental Figure S2. There is a close association between the JA pathway and the progress of pathogen infection.
Supplemental Figure S3. The expression levels of miR319b and OsTCP21 in MeJA- and LnA-treated rice lines.
Supplemental Figure S4. Schematic location maps of the cis-elements in the miR319b promoter sequence.
Supplemental Figure S5. The phenotype of different transgenic lines upon the Guy11 infection.
Supplemental Table S1. List of identified miRNAs differentially expressed in M. oryzae-infected rice.
Supplemental Table S2. miRNA target genes.
Supplemental Table S3. Identified M. oryzae-responding cis-elements.
Supplemental Table S4. Primer information.
Acknowledgments
We thank Dr. Yanming Zhu and Dr. Xiaoli Sun for kindly providing the Kongyu 131 seeds. We also thank Tanzeela Zia for critical proofreading of the article.
Footnotes
This work was supported by a key project of the National Natural Science Foundation of China (31530063), the Fundamental Research Funds for the Central Universities (KYTZ201403), and an Innovation Team Program for Jiangsu Universities (2017) to H.Z.
Articles can be viewed without a subscription.
References
- Bart R, Chern M, Park CJ, Bartley L, Ronald PC (2006) A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara M, Sarazin A, Thiébeauld O, Jay F, Voinnet O, Navarro L, Colot V (2014) The Arabidopsis miR472-RDR6 silencing pathway modulates PAMP- and effector-triggered immunity through the post-transcriptional control of disease resistance genes. PLoS Pathog 10: e1003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Cuperus JT, Fahlgren N, Carrington JC (2011) Evolution and functional diversification of MIRNA genes. Plant Cell 23: 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danisman S. (2016) TCP transcription factors at the interface between environmental challenges and the plant’s growth responses. Front Plant Sci 7: 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danisman S, van der Wal F, Dhondt S, Waites R, de Folter S, Bimbo A, van Dijk AD, Muino JM, Cutri L, Dornelas MC, et al. (2012) Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol 159: 1511–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-De-Leon F, Klotz KL, Lagrimini LM (1993) Nucleotide sequence of the tobacco (Nicotiana tabacum) anionic peroxidase gene. Plant Physiol 101: 1117–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SW. (2010) RNA-based antiviral immunity. Nat Rev Immunol 10: 632–644 [DOI] [PubMed] [Google Scholar]
- Fattash I, Voss B, Reski R, Hess WR, Frank W (2007) Evidence for the rapid expansion of microRNA-mediated regulation in early land plant evolution. BMC Plant Biol 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlani G, Occhipinti A, Bossi S, Bertea CM, Varese C, Maffei ME (2011) Magnaporthe oryzae cell wall hydrolysate induces ROS and fungistatic VOCs in rice cell cultures. J Plant Physiol 168: 2041–2047 [DOI] [PubMed] [Google Scholar]
- Fujioka T, Kaneko F, Kazama T, Suwabe K, Suzuki G, Makino A, Mae T, Endo M, Kawagishi-Kobayashi M, Watanabe M (2008) Identification of small RNAs in late developmental stage of rice anthers. Genes Genet Syst 83: 281–284 [DOI] [PubMed] [Google Scholar]
- Hao J, Tu L, Hu H, Tan J, Deng F, Tang W, Nie Y, Zhang X (2012) GbTCP, a cotton TCP transcription factor, confers fibre elongation and root hair development by a complex regulating system. J Exp Bot 63: 6267–6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakawa HO, Tomari Y (2015) The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol 25: 651–665 [DOI] [PubMed] [Google Scholar]
- Jin W, Wu F (2015) Characterization of miRNAs associated with Botrytis cinerea infection of tomato leaves. BMC Plant Biol 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S, Izaurralde E (2015) Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16: 421–433 [DOI] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Jin H (2007) Discovery of pathogen-regulated small RNAs in plants. Methods Enzymol 427: 215–227 [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiwesh B, Zhu JK, Zhu J (2012) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta 1819: 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld JP (2004) The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol 134: 1006–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Lee HY, Seo JS, Cho JH, Jung H, Kim JK, Lee JS, Rhee S, Do Choi Y (2013) Oryza sativa COI homologues restore jasmonate signal transduction in Arabidopsis coi1-1 mutants. PLoS ONE 8: e52802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30: 325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Pignatta D, Bendix C, Brunkard JO, Cohn MM, Tung J, Sun H, Kumar P, Baker B (2012) MicroRNA regulation of plant innate immune receptors. Proc Natl Acad Sci USA 109: 1790–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Castillo-González C, Yu B, Zhang X (2017a) The functions of plant small RNAs in development and in stress responses. Plant J 90: 654–670 [DOI] [PubMed] [Google Scholar]
- Li Y, Lu YG, Shi Y, Wu L, Xu YJ, Huang F, Guo XY, Zhang Y, Fan J, Zhao JQ, et al. (2014) Multiple rice microRNAs are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiol 164: 1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao SL, Li JL, Hu XH, Wang H, Cao XL, Xu YJ, Zhao ZX, Xiao ZY, Yang N, et al. (2017b) Osa-miR169 negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. Front Plant Sci 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu J, Ning Y, Ding B, Wang X, Wang Z, Wang GL (2013) Recent progress in understanding PAMP- and effector-triggered immunity against the rice blast fungus Magnaporthe oryzae. Mol Plant 6: 605–620 [DOI] [PubMed] [Google Scholar]
- Liu Z, Bao W, Liang W, Yin J, Zhang D (2010) Identification of gamyb-4 and analysis of the regulatory role of GAMYB in rice anther development. J Integr Plant Biol 52: 670–678 [DOI] [PubMed] [Google Scholar]
- Lopez JA, Sun Y, Blair PB, Mukhtar MS (2015) TCP three-way handshake: linking developmental processes with plant immunity. Trends Plant Sci 20: 238–245 [DOI] [PubMed] [Google Scholar]
- Martín-Trillo M, Cubas P (2010) TCP genes: a family snapshot ten years later. Trends Plant Sci 15: 31–39 [DOI] [PubMed] [Google Scholar]
- Mei C, Qi M, Sheng G, Yang Y (2006) Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact 19: 1127–1137 [DOI] [PubMed] [Google Scholar]
- Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, Wu L, Li S, Zhou H, Long C, et al. (2008) Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133: 116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312: 436–439 [DOI] [PubMed] [Google Scholar]
- Niu D, Wang Z, Wang S, Qiao L, Zhao H (2015) Profiling of small RNAs involved in plant-pathogen interactions. Methods Mol Biol 1287: 61–79 [DOI] [PubMed] [Google Scholar]
- Niu D, Zhang X, Song X, Wang Z, Li Y, Qiao L, Wang Z, Liu J, Deng Y, He Z, et al. (2018) Deep sequencing uncovers rice long siRNAs and its involvement in immunity against Rhizoctonia solani. Phytopathology 108: 60–69 [DOI] [PubMed] [Google Scholar]
- Padmanabhan C, Zhang X, Jin H (2009) Host small RNAs are big contributors to plant innate immunity. Curr Opin Plant Biol 12: 465–472 [DOI] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425: 257–263 [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Reinstädler A, Lipka V, Lippok B, Somssich IE (2002) Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell 14: 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE (1996) Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J 15: 5690–5700 [PMC free article] [PubMed] [Google Scholar]
- Samad AFA, Sajad M, Nazaruddin N, Fauzi IA, Murad AMA, Zainal Z, Ismail I (2017) MicroRNA and transcription factor: key players in plant regulatory network. Front Plant Sci 8: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schommer C, Palatnik JF, Aggarwal P, Chételat A, Cubas P, Farmer EE, Nath U, Weigel D (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6: e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Suhrkamp I, Wang Y, Liu S, Menkhaus J, Verreet JA, Fan L, Cai D (2014) Identification and characterization of microRNAs in oilseed rape (Brassica napus) responsive to infection with the pathogenic fungus Verticillium longisporum using Brassica AA (Brassica rapa) and CC (Brassica oleracea) as reference genomes. New Phytol 204: 577–594 [DOI] [PubMed] [Google Scholar]
- Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BACM, Baulcombe DC (2012) A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. Plant Cell 24: 859–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Leong SA (1994) Mapping of a Magnaporthe grisea locus affecting rice (Oryza sativa) cultivar specificity. Theor Appl Genet 88: 901–908 [DOI] [PubMed] [Google Scholar]
- Sutoh K, Washio K, Imai R, Wada M, Nakai T, Yamauchi D (2015) An N-terminal region of a Myb-like protein is involved in its intracellular localization and activation of a gibberellin-inducible proteinase gene in germinated rice seeds. Biosci Biotechnol Biochem 79: 747–759 [DOI] [PubMed] [Google Scholar]
- Talmor-Neiman M, Stav R, Frank W, Voss B, Arazi T (2006) Novel micro-RNAs and intermediates of micro-RNA biogenesis from moss. Plant J 47: 25–37 [DOI] [PubMed] [Google Scholar]
- Tamaoki D, Seo S, Yamada S, Kano A, Miyamoto A, Shishido H, Miyoshi S, Taniguchi S, Akimitsu K, Gomi K (2013) Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal Behav 8: e24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17: 260–270 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Tong A, Yuan Q, Wang S, Peng J, Lu Y, Zheng H, Lin L, Chen H, Gong Y, Chen J, et al. (2017) Altered accumulation of osa-miR171b contributes to rice stripe virus infection by regulating disease symptoms. J Exp Bot 68: 4357–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Sun Y, Song N, Zhao M, Liu R, Feng H, Wang X, Kang Z (2017) Puccinia striiformis f. sp. tritici microRNA-like RNA 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. New Phytol 215: 338–350 [DOI] [PubMed] [Google Scholar]
- Wang H, Jiao X, Kong X, Hamera S, Wu Y, Chen X, Fang R, Yan Y (2016) A signaling cascade from miR444 to RDR1 in rice antiviral RNA silencing pathway. Plant Physiol 170: 2365–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ST, Sun XL, Hoshino Y, Yu Y, Jia B, Sun ZW, Sun MZ, Duan XB, Zhu YM (2014) MicroRNA319 positively regulates cold tolerance by targeting OsPCF6 and OsTCP21 in rice (Oryza sativa L.). PLoS ONE 9: e91357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Yao L, Singer SD, Muhammad H, Li Z, Wang X (2017) Constitutive heterologous overexpression of a TIR-NB-ARC-LRR gene encoding a putative disease resistance protein from wild Chinese Vitis pseudoreticulata in Arabidopsis and tobacco enhances resistance to phytopathogenic fungi and bacteria. Plant Physiol Biochem 112: 346–361 [DOI] [PubMed] [Google Scholar]
- Wu J, Yang Z, Wang Y, Zheng L, Ye R, Ji Y, Zhao S, Ji S, Liu R, Xu L, et al. (2015) Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. eLife 4: e05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhang Q, Zhou H, Ni F, Wu X, Qi Y (2009) Rice microRNA effector complexes and targets. Plant Cell 21: 3421–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Kano A, Tamaoki D, Miyamoto A, Shishido H, Miyoshi S, Taniguchi S, Akimitsu K, Gomi K (2012) Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol 53: 2060–2072 [DOI] [PubMed] [Google Scholar]
- Yang C, Li D, Mao D, Liu X, Ji C, Li X, Zhao X, Cheng Z, Chen C, Zhu L (2013) Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant Cell Environ 36: 2207–2218 [DOI] [PubMed] [Google Scholar]
- Yu Y, Jia T, Chen X (2017) The ‘how’ and ‘where’ of plant microRNAs. New Phytol 216: 1002–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Jeong DH, De Paoli E, Park S, Rosen BD, Li Y, González AJ, Yan Z, Kitto SL, Grusak MA, et al. (2011) MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev 25: 2540–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ding Z, Wu K, Yang L, Li Y, Yang Z, Shi S, Liu X, Zhao S, Yang Z, et al. (2016) Suppression of jasmonic acid-mediated defense by viral-inducible microRNA319 facilitates virus infection in rice. Mol Plant 9: 1302–1314 [DOI] [PubMed] [Google Scholar]
- Zhang W, Gao S, Zhou X, Chellappan P, Chen Z, Zhou X, Zhang X, Fromuth N, Coutino G, Coffey M, et al. (2011) Bacteria-responsive microRNAs regulate plant innate immunity by modulating plant hormone networks. Plant Mol Biol 75: 93–105; erratum Zhang W, Gao S, Zhou X, Chellappan P, Chen Z, Zhou XF, Zhang XM, Fromuth N, Coutino G, Coffey M, et al. (2011) Plant Mol Biol 76: 205–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Li Z, Fan J, Hu C, Yang R, Qi X, Chen H, Zhao F, Wang S (2015) Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J Exp Bot 66: 4653–4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Zheng W, Lin F, Zhang Y, Yi Y, Wang B, Lu G, Wang Z, Wu W (2011) AVR1-CO39 is a predominant locus governing the broad avirulence of Magnaporthe oryzae 2539 on cultivated rice (Oryza sativa L.). Mol Plant Microbe Interact 24: 13–17 [DOI] [PubMed] [Google Scholar]