Aequorin-based probes targeted to the thylakoid lumen and membrane reveal an integrated role for thylakoids in Ca2+ homeostasis and modulation of chloroplast Ca2+ signals.
Abstract
Chloroplasts require a fine-tuned control of their internal Ca2+ concentration, which is crucial for many aspects of photosynthesis and for other chloroplast-localized processes. Increasing evidence suggests that calcium regulation within chloroplasts also may influence Ca2+ signaling pathways in the cytosol. To investigate the involvement of thylakoids in Ca2+ homeostasis and in the modulation of chloroplast Ca2+ signals in vivo, we targeted the bioluminescent Ca2+ reporter aequorin as a YFP fusion to the lumen and the stromal surface of thylakoids in Arabidopsis (Arabidopsis thaliana). Thylakoid localization of aequorin-based probes in stably transformed lines was confirmed by confocal microscopy, immunogold labeling, and biochemical analyses. In resting conditions in the dark, free Ca2+ levels in the thylakoid lumen were maintained at about 0.5 μm, which was a 3- to 5-fold higher concentration than in the stroma. Monitoring of chloroplast Ca2+ dynamics in different intrachloroplast subcompartments (stroma, thylakoid membrane, and thylakoid lumen) revealed the occurrence of stimulus-specific Ca2+ signals, characterized by unique kinetic parameters. Oxidative and salt stresses initiated pronounced free Ca2+ changes in the thylakoid lumen. Localized Ca2+ increases also were observed on the thylakoid membrane surface, mirroring transient Ca2+ changes observed for the bulk stroma, but with specific Ca2+ dynamics. Moreover, evidence was obtained for dark-stimulated intrathylakoid Ca2+ changes, suggesting a new scenario for light-to-dark-induced Ca2+ fluxes inside chloroplasts. Hence, thylakoid-targeted aequorin reporters can provide new insights into chloroplast Ca2+ storage and signal transduction. These probes represent novel tools with which to investigate the role of thylakoids in Ca2+ signaling networks within chloroplasts and plant cells.
Intracellular calcium signaling is universal among eukaryotes, mediating a wide range of physiological processes. Plant Ca2+ homeostasis and signaling reflect both structures and organelles unique to the plant cell as well as differences in the growth and development of plants compared with eukaryotes that lack plastids. In plants, Ca2+ is involved in environmental sensing, and a wide variety of abiotic and biotic stimuli (such as osmotic stress, oxidative stress, and pathogen attack) can induce transient changes in intracellular free Ca2+ concentration ([Ca2+]) with unique spatiotemporal patterns, thus enabling specific stimulus-response coupling (Dodd et al., 2010). Several intracellular compartments of the plant cell participate in Ca2+ homeostasis, including the vacuole, endoplasmic reticulum, mitochondria, and plastids. However, the mobilization of Ca2+ from the different organelles and their potential interplay in plant Ca2+ signaling networks are still under investigation (Stael et al., 2012).
The vacuole is the main intracellular Ca2+ reservoir in plant cells, in terms of both function and volume, with a prominent role in overall ion homeostasis in mature cells. It is a major intracellular stimulus-releasable Ca2+ store in the signal transduction pathways triggered by cold shock (Knight et al., 1996) as well as drought and salinity (Knight et al., 1997). The vacuolar cation channel TPC1 (Peiter et al., 2005), the structure of which was reported recently (Guo et al., 2016; Kintzer and Stroud, 2016), was shown to be involved in mediating systemic Ca2+ signaling upon salt stress (Choi et al., 2014), wounding, and herbivory (Kiep et al., 2015) and in the generation of highly localized cytosolic Ca2+ elevations during aphid feeding (Vincent et al., 2017).
Chloroplasts also have been shown to participate in Ca2+ homeostasis and signaling. Numerous studies indicate that chloroplasts require a fine-tuned control of the organellar Ca2+ concentration. It is known that free Ca2+ modulates crucial aspects of photosynthesis, including the assembly and function of PSII, the regulation of stromal enzymes of the Calvin cycle, as well as other plastid-localized processes such as the import of nucleus-encoded proteins and organelle division (Rocha and Vothknecht, 2012; Nomura and Shiina, 2014; Hochmal et al., 2015). However, little is known about the mechanisms that underlie the generation and dissipation of Ca2+ transients and the transduction of environmental signals within plastids or their localization inside the organelle.
To better understand how chloroplasts are integrated into the plant Ca2+ signaling network, specific high-resolution tools are required to monitor and quantify plastid Ca2+ dynamics. In early studies, the bioluminescent Ca2+ reporter aequorin was targeted to the chloroplast stroma, highlighting the induction of stromal Ca2+ fluxes upon light-to-dark transition (Johnson et al., 1995; Sai and Johnson, 2002). Studies on the thylakoid-localized Ca2+-sensing receptor CAS showed that chloroplasts modulate intracellular Ca2+ signals by controlling external Ca2+-induced cytosolic Ca2+ transients during stomatal closure (Nomura et al., 2008; Weinl et al., 2008). Stromal Ca2+ signals also have been implicated in the activation of plant immunity (Nomura et al., 2012; Stael et al., 2015). Constructs encoding YFP-aequorin chimeras targeted to the stroma as well as outer and inner membranes of the chloroplast envelope are available to investigate Ca2+ dynamics in these compartments (Mehlmer et al., 2012). Plastid-targeted aequorin probes also were used recently to determine differential stimulus-specific Ca2+ responses between amyloplasts and chloroplasts (Sello et al., 2016). However, information about free Ca2+ levels and their changes during signal transduction on thylakoid membranes and inside the thylakoid lumen is lacking.
Here, we present the targeting of YFP-fused aequorin probes to the thylakoid lumen and the stromal surface of the thylakoid membrane. Arabidopsis (Arabidopsis thaliana) lines stably expressing these aequorin chimeras were used in Ca2+ measurement assays in response to different abiotic and biotic stimuli, revealing the occurrence of stimulus-specific thylakoid Ca2+ signals. This novel toolkit of thylakoid-targeted Ca2+ reporters advances the current understanding of Ca2+ regulation in chloroplasts and paves the way for future investigations on plant organellar Ca2+ signaling.
RESULTS
Generation of Arabidopsis Transgenic Lines Stably Expressing YFP-Aequorin Chimeras Targeted to Thylakoids
To monitor Ca2+ dynamics in Arabidopsis thylakoids, we expressed YFP-aequorin (YA) chimeras targeted to the thylakoid lumen (TL-YA) and the stromal surface of the thylakoid membrane (TM-YA). YA was fused to the first 92 amino acids of the thylakoid lumen protein TLP18.3 (Sirpiö et al., 2007; Wu et al., 2011) to obtain TL-YA and to the N-terminal transmembrane domain of the thylakoid protein kinase STN7 to obtain TM-YA (Supplemental Table S1). In the latter, the transmembrane domain anchors the protein in an orientation that exposes the C-terminal tag to the stroma (Bellafiore et al., 2005; Tikkanen et al., 2012). Aequorin expression was confirmed with reverse transcription (RT)-PCR and immunoblot analyses on transgenic Arabidopsis plants expressing these constructs under the control of the UBI10 promoter (Fig. 1). Aequorin activity was measured in the T2 generation by screening five independent TL-YA and TM-YA sublines for aequorin-based total luminescence. In vivo discharge gave positive results for all sublines, with luminescence levels ranging from 105 to 2 × 106 counts per seedling. The established thylakoid-targeted aequorin sensor lines (T3 generation) did not exhibit any significant difference in either phenotype or photosynthetic activity when compared with wild-type plants. Moreover, the ultrastructural organization of chloroplasts was well preserved (Fig. 2).
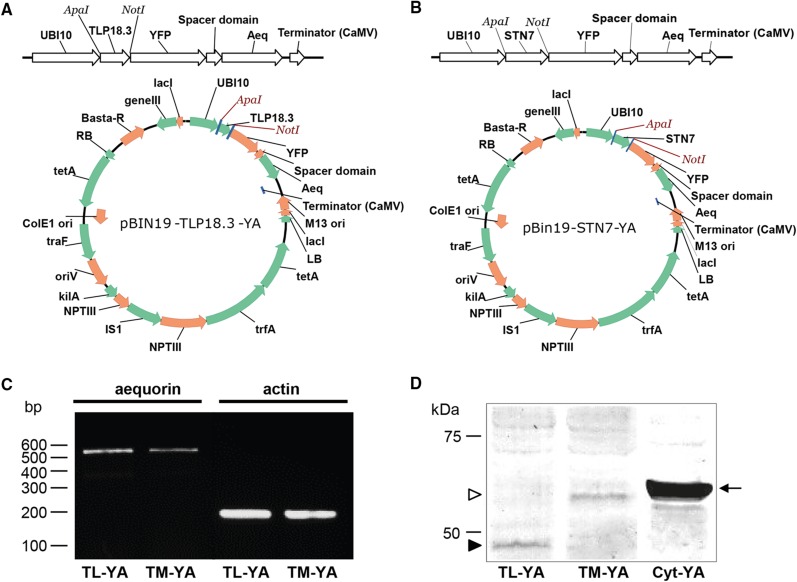
Figure 1.
Overview of the cloning strategy for the creation of expression vectors targeting YA to the thylakoid lumen and thylakoid membrane, and analysis of aequorin expression in Arabidopsis transgenic lines. A and B, The targeting sequences for the thylakoid lumen (A) and for the thylakoid membrane (B) were cloned into an expression cassette in front of YA using the restriction enzymes ApaI and NotI. The complete expression cassettes were transferred subsequently into the binary vector pBIN19 carrying Basta resistance. C and D, Analysis of aequorin expression by RT-PCR (C) and immunoblotting (D) in Arabidopsis transgenic lines stably transformed with the constructs encoding TL-YA and TM-YA. For RT-PCR analyses, actin was used as a housekeeping gene. For immunoblot analyses, total protein extracts (50 μg) were separated by 10% SDS-PAGE, transferred to PVDF, and incubated with an anti-aequorin antibody (diluted 1:10,000). Black and white arrowheads indicate the YA chimeras targeted to the thylakoid lumen and the thylakoid membrane, respectively. The black arrow indicates the YA targeted to the cytosol, excluding the nucleus, in the Arabidopsis line CPK17G2A-NES-YA (Cyt-YA), used here as a positive control.
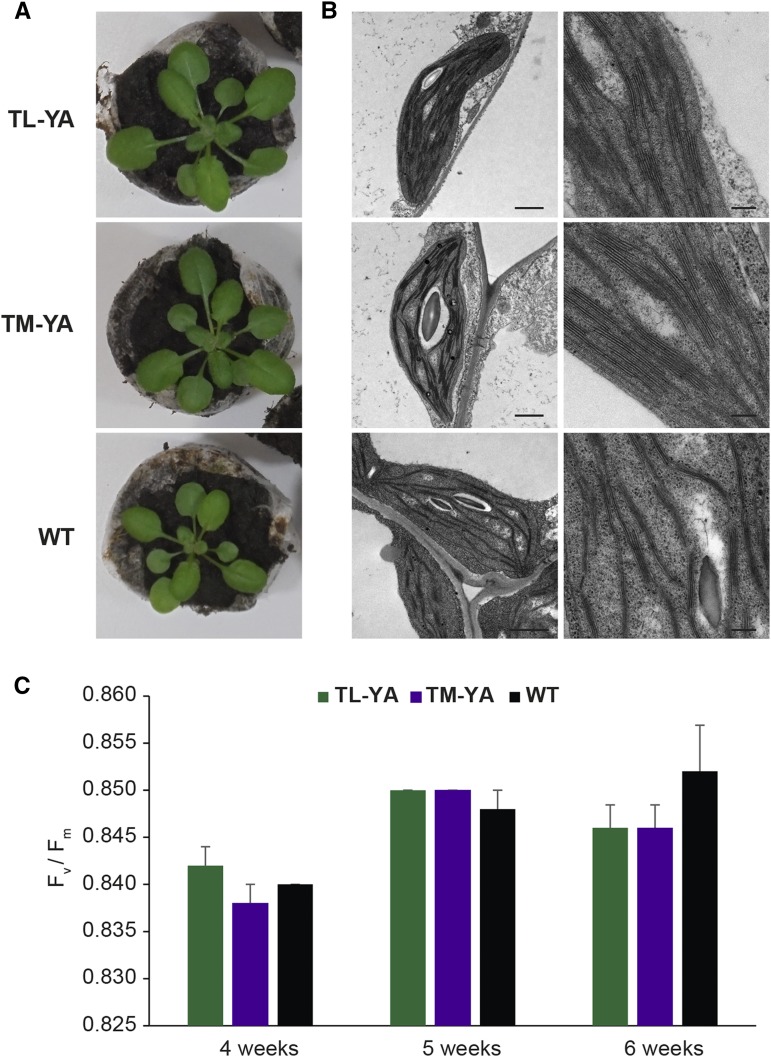
Figure 2.
Phenotype, chloroplast ultrastructure, and photosynthetic activity of Arabidopsis transgenic lines stably expressing TL-YA and TM-YA. A, Representative photographs of 4-week-old seedlings of TL-YA, TM-YA, and wild-type (WT) lines. B, Transmission electron microscopy (TEM) observations of chloroplast ultrastructure, showing good preservation of thylakoids. Bars = 1 µm (left column) and 250 nm (right column). C, Pulse amplitude modulation imaging analyses of Arabidopsis seedlings at 4, 5, and 6 weeks. Data are means ± se of five independent experiments. No significant differences were observed (Student’s t test).
Subcellular Localization of YA Fusion Proteins
Confocal microscopy observations of leaves from the TL-YA and TM-YA lines showed that the fluorescent signal of YFP overlapped perfectly with the red chlorophyll fluorescence of mesophyll cells, indicating that the recombinant proteins indeed reside inside chloroplasts (Fig. 3A). Fluorescence was not detected in roots, except a faint signal in the external cell layers. This was likely due to the light exposure of roots growing on the surface of the agar-containing medium for confocal microscopy experiments (Supplemental Fig. S1A). Conversely, roots of dark-grown seedlings from both TL-YA and TM-YA lines exhibited no fluorescence (Supplemental Fig. S1B).
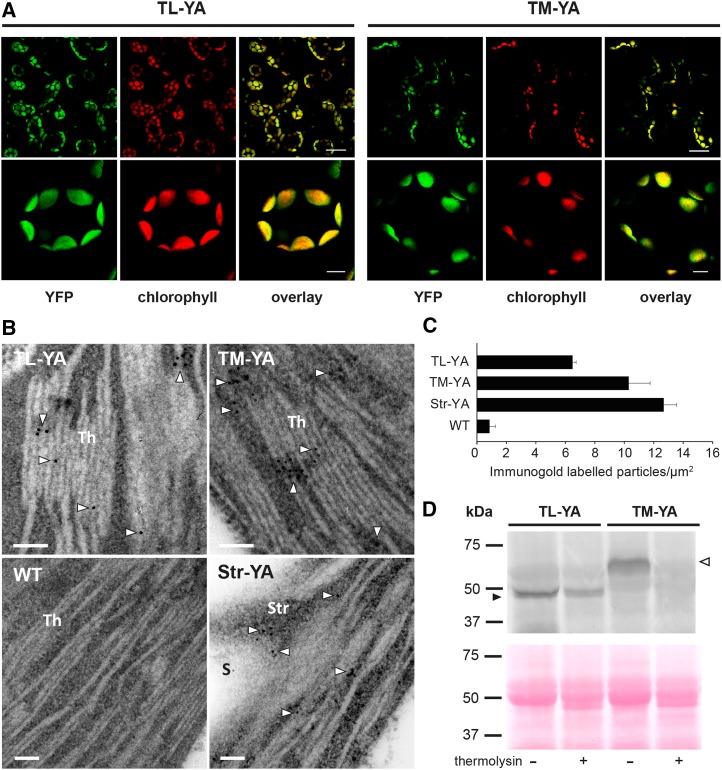
Figure 3.
Confocal microscopy analyses, immunogold labeling, and biochemical analyses of Arabidopsis seedlings of the TL-YA and TM-YA lines. A, Fluorescence microscopy images of mesophyll cells of Arabidopsis seedling leaves with a YFP filter and a chlorophyll filter. An overlay of the two channels also is shown. Bars = 25 µm (top row) and 5 µm (bottom row). B, Immunocytochemical analyses of aequorin subcellular localization. The wild-type (WT) line and a line expressing YA in the stroma (Str-YA) were used as negative and positive controls, respectively. White arrowheads indicate gold particles. Bars = 100 nm. S, Starch granule; Str, stroma; Th, thylakoids. C, Quantitative analyses of immunogold-labeled particles. Data are means ± se of 40 different fields from three biological replicates. D, Immunoblot analyses of isolated thylakoids, incubated in the absence (−) or presence (+) of 0.1 µg µL−1 thermolysin, as indicated. Isolated thylakoids corresponding to 50 µg of protein were probed with an anti-aequorin antibody (top gel). Equal loading was confirmed by Ponceau Red staining of the blot membrane (bottom gel). Black and white arrowheads indicate YA chimeras targeted to the thylakoid lumen and the thylakoid membrane, respectively.
TEM after immunogold labeling with an anti-aequorin antibody showed the presence of electron-dense gold particles on thylakoids in both the TL-YA and TM-YA lines (Fig. 3, B and C). The wild-type negative control lacked labeling anywhere in the cell. As a positive control, the Str-YA line (Mehlmer et al., 2012) was used, revealing gold particles in the chloroplast stroma (Fig. 3, B and C). Furthermore, intact thylakoids were isolated from Arabidopsis leaves of the TL-YA and TM-YA transgenic lines, with half of the preparation incubated for 20 min with the proteolytic enzyme thermolysin (0.1 μg μL−1) to remove all proteins exposed on the membrane surface. Immunoblot analyses showed that aequorin targeted to the thylakoid lumen was protected from proteolysis, indicating that the chimeric TL-YA protein resides inside the thylakoid lumen. For the TM-YA line, the signal from chimeric protein targeted to the thylakoid membrane was removed by the proteolytic treatment, indicating its expected exposure on the stromal thylakoid surface (Fig. 3D). Together, these data document the correct subcellular localization and topology of the thylakoid lumen- and thylakoid membrane surface-targeted YA fusion proteins for the subsequent Ca2+ signaling study.
Stimulus-Specific Ca2+ Signals Are Triggered in the Thylakoid Lumen and at the Thylakoid External Surface by Different Environmental Stimuli
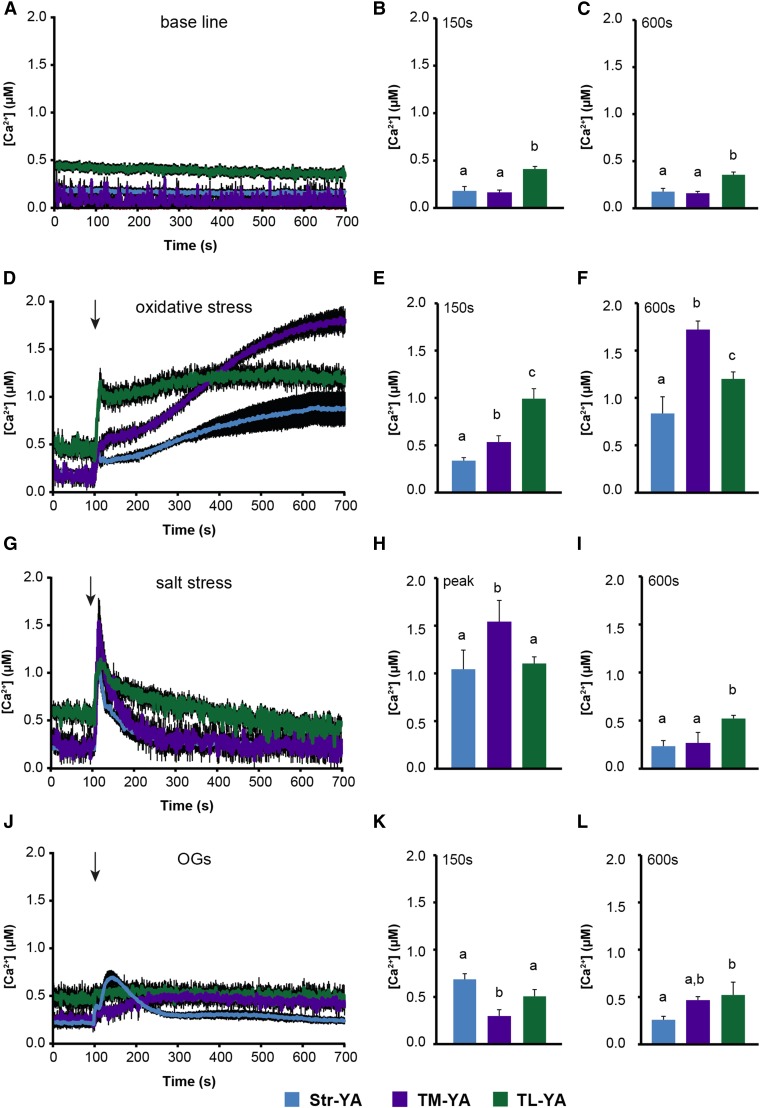
Arabidopsis photosynthetic cell lines expressing the thylakoid-targeted YA probes were established (Supplemental Fig. S2, A–C) by using a recently developed procedure (Sello et al., 2017) and assayed in Ca2+ measurement experiments to elucidate potential changes in free [Ca2+] in thylakoids. The Str-YA line also was used for comparison (Sello et al., 2016). In this first validation stage, photosynthetic cell suspension cultures were used instead of seedlings to avoid the detection of Ca2+ signals originating from the stroma of nongreen plastids that lack thylakoids, such as amyloplasts in roots (Sello et al., 2016). Aequorin protein levels in the TL-YA and TM-YA lines were suitable to perform reliable Ca2+ assays, even though these levels were lower than in the Str-YA lines (Supplemental Fig. S2D). This was expected based on the level of aequorin-derived total luminescence observed in Ca2+ assays after the discharge step. In resting conditions in the dark, free [Ca2+] in the thylakoid lumen was maintained around 0.5 µm. This concentration is 3- to 5-fold higher than the level recorded in the bulk stroma (∼0.1–0.15 µm) as well as in the stromal region just outside the thylakoid membrane (Fig. 4, A–C). Next, suspension-cultured cells were exposed to different environmental cues, which were demonstrated previously to induce both cytosolic and stromal Ca2+ signals (Sello et al., 2016). In particular, 10 mm H2O2 and 0.3 m NaCl were applied as abiotic stimuli simulating oxidative and salt stress, respectively (Price et al., 1994; Knight et al., 1997; Ranf et al., 2008; Martí et al., 2013; Abdul-Awal et al., 2016). Plus, 20 µg mL−1 oligogalacturonides (OGs) with degree of polymerization 10 to 15 were applied as elicitors that mimicked a pathogen attack (Navazio et al., 2002; Moscatiello et al., 2006). These treatment doses did not affect cell viability, as demonstrated by Evans blue-based assays (Supplemental Fig. S3), and touch-control experiments ruled out that the stress application itself caused remarkable changes in [Ca2+] within the chloroplasts (Supplemental Fig. S4).
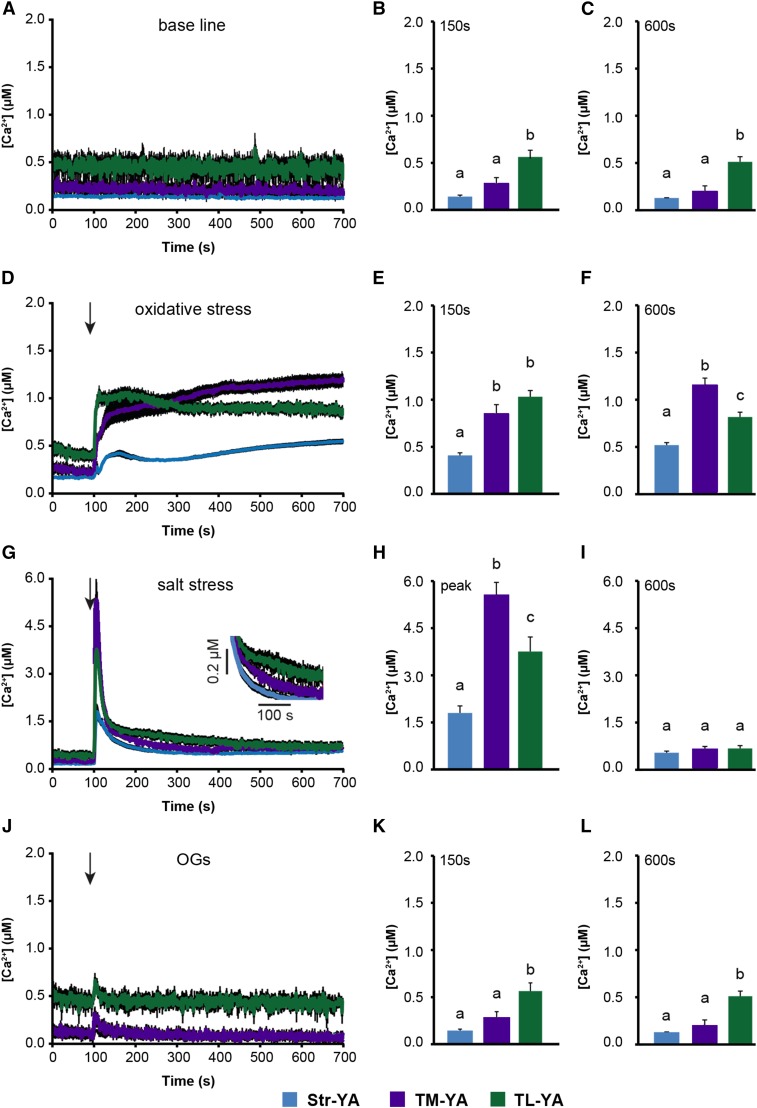
Figure 4.
Monitoring of subchloroplast free [Ca2+] in Arabidopsis cell suspension cultures in response to environmental stimuli. Ca2+ measurements were conducted in Str-YA (blue traces), TM-YA (violet traces), and TL-YA (green traces) lines. A to C, In resting conditions. D to F, In response to 10 mm H2O2. G to I, In response to 0.3 m NaCl. J to L, In response to 20 µg mL−1 OGs. In A, D, G, and J, data are presented as means ± se (black shading) of 15 traces obtained from 15 aliquots of suspension-cultured cells derived from five independent growth replicates. Arrows indicate the time of stimulation (100 s). B, C, E, F, H, I, K, and L show statistical analyses of Ca2+ levels recorded in the different transgenic lines at two different time points: after 150 s (B, E, and K) and 600 s (C, F, I, and L). In H, the maximum [Ca2+] (at the peak) is reported. Bars labeled with different letters differ significantly (P < 0.05, Student’s t test).
Oxidative stress triggered a gradual [Ca2+] increase to well over 1.5 µm at the external surface of the thylakoid membrane that followed the trend recorded in the bulk stroma, although characterized by a higher magnitude. Ca2+ dynamics in the thylakoid lumen started from a higher resting level and quickly reached a plateau at an intermediate level of about 1 µm (Fig. 4, D–F). In the case of salt stress, NaCl triggered a very rapid and transient [Ca2+] increase in all three chloroplast subcompartments. However, distinct differences in the amplitude and shape of the transient could be observed. Notably, a microdomain of high [Ca2+] was found to be transiently established in proximity to the thylakoid membrane surface, whose dissipation temporally coincided with the sustained [Ca2+] increase in the thylakoid lumen (Fig. 4, G–I). These data suggest that the thylakoid lumen may be involved in dissipating Ca2+ gradients across the thylakoid membrane and, thus, shaping stromal Ca2+ signals. By contrast, OGs induced a transient and relatively small Ca2+ change in the bulk stroma and on the thylakoid surface, although characterized by slower dynamics. However, no detectable Ca2+ changes were triggered in the thylakoid lumen (Fig. 4, J–L). Altogether, these data strongly indicate that the intrachloroplast [Ca2+] changes measured by the TL-YA and TM-YA lines are specific, stimulus-induced responses.
Aequorin-based Ca2+ assays in response to the same stimuli were conducted subsequently in entire seedlings (Fig. 5; Supplemental Fig. S4), reinforcing the involvement of thylakoids in Ca2+-mediated responses to environmental stimuli. Resting Ca2+ levels and Ca2+ responses to oxidative stress were found to be very similar in seedlings (Fig. 5, A–F) and cultured cells (Fig. 4, A–F). The sustained chloroplast Ca2+ changes induced by 10 mm H2O2 did not dissipate within 1 h (Supplemental Fig. S5, A–C), indicating a prolonged organellar Ca2+ response. However, a direct effect of H2O2 on coelenterazine oxidation to coelenteramide independent of aequorin was ruled out by comparing luminescence levels in seedlings of the TL-YA, TM-YA, and Str-YA lines with those in wild-type (nontransformed) seedlings preincubated with coelenterazine (Supplemental Fig. S5, D–F).
Figure 5.
Monitoring of subchloroplast free [Ca2+] in Arabidopsis seedlings in response to environmental stimuli. Ca2+ measurements were conducted in Str-YA (blue traces), TM-YA (violet traces), and TL-YA (green traces) lines. A to C, In resting conditions. D to F, In response to 10 mm H2O2. G to I, In response to 0.3 m NaCl. J to L, In response to 20 µg mL−1 OGs. In A, D, G, and J, data are presented as means ± se (black shading) of 15 traces obtained from 15 different plants derived from five independent growth replicates. Arrows indicate the time of stimulation (100 s). In the inset in G, a magnification of the region between 150 and 400 s is shown. B, C, E, F, H, I, K, and L show statistical analyses of Ca2+ levels recorded after 150 s (B, E, and K), after 600 s (C, F, I, and L), and at the peak (H). Bars labeled with different letters differ significantly (P < 0.05, Student’s t test).
For the salt stress treatment, the magnitude of chloroplast [Ca2+] change was found to be higher in entire seedlings (Fig. 5, G–I) than in cultured cells (Fig. 4, G–I), possibly as a consequence of systemic Ca2+ signaling in plants in toto (Choi et al., 2014). The different Ca2+ dynamics observed in the three intrachloroplast subcompartments suggested a role of thylakoids in switching off stromal Ca2+ signals (Fig. 5G, inset), as observed previously in cultured cells (Fig. 4G). On the other hand, exposing the seedlings to OG elicitors caused only modest and transient chloroplast Ca2+ changes (Fig. 5, J–L). These data indicate that subtle chloroplast Ca2+ signals, such as those triggered by OGs, may be amplified in homogenous cell populations (i.e. suspension cell cultures) compared with the in vivo situation, where Ca2+ signals may be limited only to a particular tissue or cell type (Moscatiello et al., 2013). These findings demonstrate that the thylakoid lumen- and thylakoid membrane surface-targeted Ca2+ probes can effectively monitor Ca2+ changes elicited by specific stimuli in the respective chloroplast subcompartments.
Unique Ca2+ Dynamics in the Thylakoid Lumen Are Induced by the Transition from Light to Dark
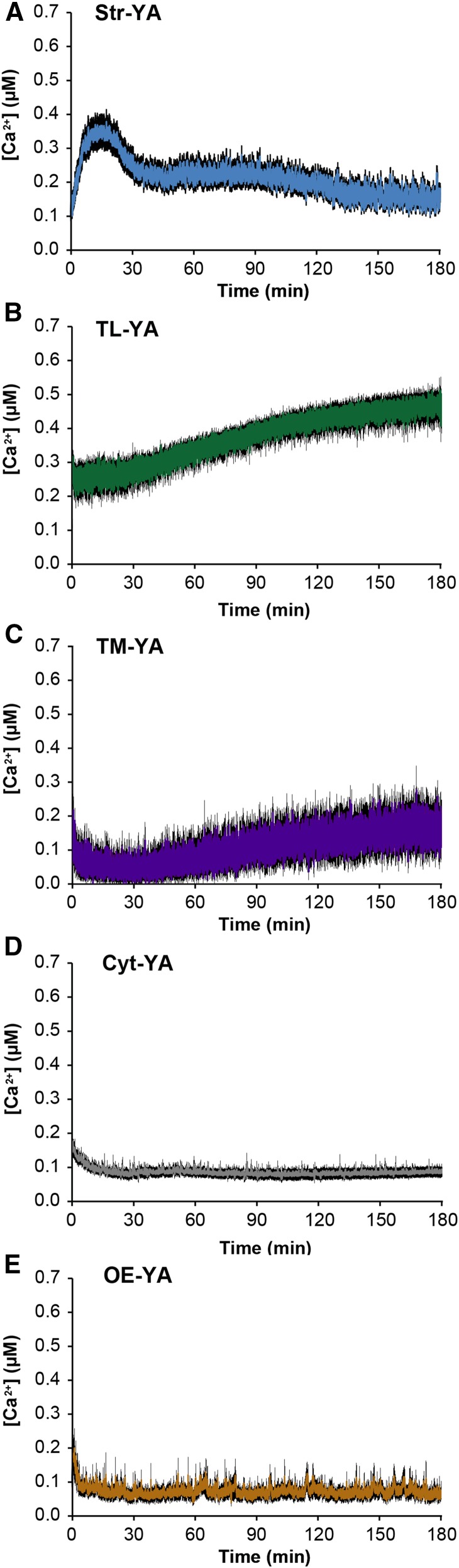
Light is one of the most important factors for plant growth and development. Therefore, Arabidopsis lines stably expressing the thylakoid-targeted bioluminescent Ca2+ reporter were used to evaluate the effect of light-to-dark transition on chloroplast Ca2+ dynamics. In this set of experiments, whole seedlings were used (instead of plant cell suspension cultures), since previous studies had shown a transient stromal Ca2+ signal increase in response to the light-to-dark transition in chloroplasts but not in amyloplasts (Sello et al., 2016). Hence, the presence of roots should not affect the readout. Moreover, analyses of the organism in toto may more closely mimic the actual in vivo situation, especially if the overall Ca2+-mediated response entails systemic integration (Gilroy et al., 2014). In agreement with previous reports (Nomura et al., 2012), the lights-off stimulus was found to trigger a transient [Ca2+] increase in the stroma, which peaked at about 0.35 µm after 15 min and slowly decayed to the basal level within 3 h (Fig. 6A). Concurrently in the thylakoid lumen, [Ca2+] was found to increase gradually from a basal level of about 0.25 µm to about 0.45 µm (Fig. 6B). The Ca2+ trace recorded at the thylakoid membrane exposed to the stroma differed from the Ca2+ dynamics recorded in the whole stroma, being characterized by a gradual, moderate Ca2+ increase; this suggests that the stromal Ca2+ increase likely occurred in microdomain(s) far from the thylakoid surface (Fig. 6C). In addition to Str-YA, TL-YA, and TM-YA, Ca2+ measurement assays in response to light-to-dark transition were performed in previously established Arabidopsis lines with aequorin localized in the cytosol (Cyt-YA) and on the cytosolic surface of the outer membrane of the plastid envelope (OE-YA; Mehlmer et al., 2012). Lights-off-induced Ca2+ transients were not observed in the cytosol or at the cytosolic surface of the plastid envelope (Fig. 6, D and E), confirming that the cytosol does not seem to be involved in Ca2+ changes in response to light-to-dark transition, as suggested previously (Nomura et al., 2012). Control measurements of luminescence values in either aequorin-expressing lines that were not reconstituted with coelenterazine or wild-type (nontransformed) seedlings incubated with coelenterazine (Supplemental Fig. S6) suggested that the initial decrease of [Ca2+] commonly observed upon light-to-dark transition (Fig. 6) is actually due to chlorophyll fluorescence emission.
Figure 6.
Measurement of Ca2+ fluxes in response to light-to-dark transition. Ca2+ assays were conducted in Arabidopsis seedlings stably expressing YA chimeras in different subcellular locations: stroma (A; blue trace), thylakoid lumen (B; green trace), thylakoid membrane (C; violet trace), cytosol (D; gray trace), and cytosolic surface of the plastid outer envelope (E; brown trace). Data are presented as means ± se (black shading) of 15 traces obtained from 15 different plants derived from five independent growth replicates. In all graphs, the lights-off stimulus starts at time 0.
Ca2+ assays in seedlings that had been exposed to 6 h of light and then moved rapidly to darkness confirmed that [Ca2+] is maintained around 0.2 μm in the thylakoid lumen during the light phase and that the lights-off stimulus induces a gradual increase in thylakoid [Ca2+] (Supplemental Fig. S7A). Moreover, at the end of the dark transition, lumen [Ca2+] persisted at high levels (∼0.5 µm), and this concentration remained unchanged when plants were kept in the dark at the beginning of the following day (Supplemental Fig. S7B). The high level of Ca2+ recorded in the thylakoid lumen at the end of light-to-dark experiments (Fig. 6B; Supplemental Fig. S7) corresponded to the thylakoid luminal [Ca2+] detected in resting conditions in the dark (Figs. 4A and 5A).
To test if the activity of a putative H+/Ca2+ antiporter localized at the thylakoid membrane could be involved in the dark-induced Ca2+ fluxes into the thylakoid lumen, Arabidopsis seedlings were pretreated with 5 µm nigericin (an H+/K+ ionophore able to dissipate ΔpH without affecting the Δψ component of the proton motive force). Upon disruption of the H+ gradient across the thylakoid membrane, the gradual increase of luminal [Ca2+] was abolished, suggesting that the slow dark-induced Ca2+ uptake into the thylakoid lumen requires a pH gradient across the membrane (Supplemental Fig. S8, A–C). Notably, in the presence of nigericin, a rapid and transient Ca2+ rise was recorded in the lumen, peaking at about 1 µm after ∼3 min and dissipating promptly within 30 min (Supplemental Fig. S8, A–C). A luminal Ca2+ increase was observed at a smaller amplitude when nigericin was added 45 min after the onset of darkness, when the relaxation of the Ca2+ signal in the stroma had already begun (Supplemental Fig. S8A, inset). The transient [Ca2+] increase, activated in response to dark in the bulk stroma, displayed a higher amplitude in the presence of 5 µm nigericin and maintained a higher steady-state level at the end of light-to-dark experiments without falling to the basal level observed in control conditions (Supplemental Fig. S8, D–F). A similar Ca2+ signature, although characterized by a slightly higher and delayed Ca2+ peak, was observed at the stromal surface of the thylakoid membrane (Supplemental Fig. S8, G–I). These data suggest that the rise in dark-induced stromal Ca2+ may be amplified as a consequence of the inhibition of Ca2+ uptake in the thylakoid lumen, indirectly triggered by the proton-translocating uncoupler nigericin.
DISCUSSION
The participation of chloroplasts, and plastids in general, in the cellular Ca2+ response and their ability to generate their own Ca2+ transients have long been debated. Previous studies of chloroplast Ca2+ dynamics provided information only on stromal [Ca2+] (Johnson et al., 1995; Sai and Johnson, 2002; Manzoor et al., 2012; Nomura et al., 2012; Loro et al., 2016; Sello et al., 2016; Stephan et al., 2016). A novel set of YA chimeras were targeted recently to the outer and inner surfaces of the chloroplast envelope membranes as well as the stroma (Mehlmer et al., 2012). However, information on thylakoid lumen [Ca2+] was lacking.
In this work, TL-YA and TM-YA chimeras were engineered, and Arabidopsis lines stably transformed with the above constructs were generated for in vivo Ca2+ measurements. The thylakoid lumen and outer thylakoid surface localizations of TL-YA and TM-YA were confirmed by laser scanning confocal microscopy, plus immunocytochemical and biochemical analyses. Since aequorin is largely insensitive to pH (Brini, 2008), it was used as a reliable probe to monitor thylakoid lumen [Ca2+], where the pH can drop to 5.5 (Szabó et al., 2005). Its bioluminescent properties, high signal-to-noise ratio, and lack of damaging excitation light permit Ca2+ measurement in chlorophyll-containing tissues for long time intervals, an advantage over fluorescent Ca2+ reporters (Martí et al., 2013; Xiong et al., 2014).
Monitoring of [Ca2+] in transgenic Arabidopsis lines resulted in [Ca2+] values measured just outside the thylakoid surface and within the thylakoid lumen. Thylakoid-targeted aequorin revealed that lumen Ca2+ levels in the dark are 3- to 5-fold higher than in the stroma. Oxidative and salt stresses triggered chloroplast Ca2+ signals in both the chloroplast stroma and inside the thylakoid lumen, characterized by specific kinetic parameters. On the other hand, exposure to OGs elicited a moderate Ca2+ increase only in the stroma and only when suspension-cultured cells were used (i.e. homogenous cell populations), compared with entire seedlings. Because the basal [Ca2+] in the thylakoid lumen in the dark is high, it is possible that the extent of the transient stromal [Ca2+] increase was not sufficient to induce elevated [Ca2+] in the lumen, potentially mediated by passive Ca2+ transporters located at the thylakoid membrane. Further work will be required to clarify whether the thylakoid lumen may have a role in Ca2+ responses to other biotic stresses.
The proteins mediating Ca2+ flux into and out of the thylakoid lumen are not known. Although a Ca2+-permeable ion channel was detected in the thylakoid membrane by patch clamp (Enz et al., 1993) and compelling evidence for the presence of Ca2+/H+ exchange activity has been provided (Ettinger et al., 1999), genes encoding these proteins are still unidentified (Carraretto et al., 2016). A putative chloroplast-localized Ca2+/H+ antiporter was identified recently in Arabidopsis (Wang et al., 2016). The same protein has been proposed to function instead as a transporter for Mn2+ and to be located in the thylakoid membrane (Schneider et al., 2016).
The effect of light on chloroplast [Ca2+] is known to encompass a lights-off stimulus that induces a slow, long-lasting [Ca2+] increase in the stroma (Sai and Johnson, 2002; Nomura et al., 2012). This dark-induced Ca2+ transient also was observed in our stroma-targeted aequorin line. The lack of any detectable [Ca2+] variation in either the bulk cytosol or the cytosolic microdomain close to the chloroplast envelope is consistent with earlier reports indicating that the cytosol does not participate in the generation of the stromal [Ca2+] change in response to light-to-dark transition (Nomura et al., 2012). However, [Ca2+] measured in the thylakoid lumen revealed unexpected Ca2+ dynamics in response to darkness. In particular, the light-to-dark transition induced a long-lasting and sustained [Ca2+] increase in the thylakoid lumen. After 16 h of light, thylakoid lumen [Ca2+] was maintained at about 0.25 μm (a slightly higher concentration than in the stroma). After lights off, [Ca2+] in the thylakoid lumen showed a progressive increase to about 0.5 µm after 3 h of darkness. This high [Ca2+] remained unchanged during the night and correlates well with the dark resting level observed in both autotrophic cell cultures and seedlings. Due to the lack of any detectable [Ca2+] changes either in the cytosol or on the chloroplast surface, the dark-induced intrachloroplast Ca2+ fluxes likely originate from inside the organelle. Since the lights-off stimulus was found to induce increases of [Ca2+] in both stroma and thylakoid lumen, a possibility for the origin of these Ca2+ fluxes is the release of the ion from thylakoid membrane proteins. Since there was a lack of evident high-[Ca2+] microdomains close to the thylakoid membrane, the involvement of soluble Ca2+-binding/buffering proteins potentially located in the chloroplast stroma cannot be ruled out. At later time points, the [Ca2+] peak in the stroma seemed to dissipate by the continuous Ca2+ uptake into the thylakoid lumen. This was responsible for the generation of the high intraluminal [Ca2+] established progressively during the dark phase. The finding that transient stromal Ca2+ signals in response to light-to-dark transition occurred only in chloroplasts (but not in nongreen plastids of Arabidopsis cell cultures [Sello et al., 2016], as confirmed recently by in vivo Ca2+ imaging based on stroma-targeted cameleon [Loro et al., 2016]) is consistent with our current data suggesting a modulation of the lights-off-induced stromal Ca2+ transient by thylakoids.
As mentioned above, nigericin was reported to dissipate ΔpH without affecting the Δψ component of the proton motive force in higher plants (Joliot and Johnson, 2011). Abolishment of the dark-induced Ca2+ uptake into the thylakoid lumen observed after nigericin addition suggests that the ΔpH built up during illumination is necessary for the dark-phase return of stromal Ca2+ to the lumen. In the presence of nigericin, a sustained increase in thylakoid luminal Ca2+ was not observed. Instead, stromal Ca2+ was significantly higher over prolonged time scales. The presence of a residual ΔpH maintained across the thylakoid membrane in the dark (Bailleul et al., 2015) may provide a driving force for the uptake of Ca2+ into the lumen via a Ca2+/H+ antiporter suggested previously to be localized at thylakoid membranes (Ettinger et al., 1999). Interestingly, in the presence of nigericin during the light phase (30 min before the shift to dark), aequorin-based Ca2+ measurements revealed an immediate, fast, and unexpected Ca2+ increase in the thylakoid lumen upon transition to dark, which then dissipated just as quickly. These rapid changes may be mediated by still unidentified channel proteins that would allow the flux of calcium ions according to the actual electrochemical gradient, depending on both the concentration gradient for Ca2+ and the membrane potential. One hypothesis is that indirect inhibition of a Ca2+/H+ exchange activity might cause a transient microdomain of high [Ca2+] just outside the thylakoid membrane, leading to a rapid and transient opening of a still unidentified Ca2+-permeable thylakoid channel. This is supported by the nigericin experiments conducted with the TM-YA line. However, the identities of Ca2+-permeable channels/transporters in the plastid envelope and thylakoid membranes remain unknown (Pottosin and Dobrovinskaya, 2015; Xu et al., 2015; Carraretto et al., 2016). Unless the molecules responsible for intraplastidial Ca2+ transport and their ion specificity are clarified, only a tentative interpretation of these results can be provided. Moreover, we cannot exclude that nigericin also may affect H+ gradients across the inner envelope membrane (Checchetto et al., 2016), thus participating in the observed increase in stromal [Ca2+] upon addition of the K+/H+ ionophore. Furthermore, proton gradients in additional intracellular membranes (such as the inner membrane of mitochondria and the tonoplast) could be affected by nigericin, and these changes might a priori contribute indirectly to the observed intrachloroplast Ca2+ transients. In summary, the determination of the exact role of transmembrane pH gradients in the regulation of chloroplast Ca2+ fluxes requires further exploration. Unfortunately, neither aequorin (a bioluminescent probe) nor cameleon (a fluorescent probe) can be used for direct [Ca2+] measurements under white light conditions, such that the luminal [Ca2+] response to lights-on stimuli cannot be easily measured.
In conclusion, the new toolkit of aequorin reporters targeted to thylakoids has provided estimates of the thylakoid luminal [Ca2+] and has shown that thylakoids have an active role in the modulation of chloroplast Ca2+ responses to environmental cues. The involvement of chloroplasts in switching off stromal Ca2+ signals, such as those triggered by salt stress, may be essential to quickly restore low basal stromal [Ca2+] after a Ca2+ signaling event that traverses into the organelle from the cytosol without prolonging the transient in that compartment via a back flux. For the light-to-dark transition, thylakoids may play a key role in shaping the stroma-located transient Ca2+ increase. This latter change in stromal [Ca2+] has been suggested to regulate the activity of some Calvin cycle enzymes, resulting in a further inhibition of CO2 fixation during the night (Sai and Johnson, 2002; Johnson et al., 2006) in addition to that provided by the well-studied, light-driven thioredoxin system.
Thylakoid-targeted aequorin probes provide new tools to analyze the role of thylakoid Ca2+ signals in modulating the many processes specific to the thylakoid lumen (for review, see e.g. Finazzi et al., 2015; Hochmal et al., 2015). This toolkit should initiate the identification and elucidation of the functions of protein(s) that may mediate Ca2+ fluxes across thylakoids (e.g. the Ca2+[Mn2+]/H+ transporter) under different light and stress conditions.
MATERIALS AND METHODS
Molecular Cloning and Construction of Expression Plasmids
The nucleotide sequences encoding the first 92 amino acids of TLP18.3 (At1G54780.1) and the first 137 amino acids (including the transmembrane domain) of the thylakoid protein kinase STN7 (At1G68830) were used as targeting sequences for the thylakoid lumen and the stromal surface of the thylakoid membrane, respectively. These were cloned into vectors carrying the coding sequence for YA to create expression cassettes driven by the UBI10 promoter (Supplemental Table S1). Afterward, expression cassettes were cloned into pBIN19 vectors carrying Basta resistance via the ApaI and NotI restriction sites as described previously (Mehlmer et al., 2012), creating the final constructs pBIN19-TLP18.3-YA and pBIN19-STN7-YA (Fig. 1). For cloning of TLP18.3, the primers 5′-AATAACCATGGAGACCCTTCTCTCCCCTCG-3′ and 5′-ATTATTAGCGGCCGCCATCGTTGAGGATATTGAAC-3′ were used. For cloning of STN7, the primers 5′-TTATAAGGGCCCATGGCTACAATATCTCCGGG-3′ and 5′-TAATTATGCGGCCGCTCCCAACAACAAAATCATCC-3′ were used.
Generation of Transgenic Arabidopsis Lines
Arabidopsis (Arabidopsis thaliana) ecotype Columbia plants were transformed with the floral dip method (Clough and Bent, 1998) with the constructs pBIN19-TLP18.3-YA and pBIN19-STN7-YA to generate lines called TL-YA and TM-YA, respectively. Primary T1 transformants were identified by Basta (0.25%, w/v) spraying on soil. Successful transformation and expression of the constructs were confirmed by the analysis of YFP fluorescence. Selected plants were transferred into single pots, and the T2 generation was assayed for aequorin expression and activity.
Analysis of Aequorin Expression
RT-PCR analyses were carried out using Arabidopsis leaves from 1-month-old Basta-resistant plants. Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen) and reverse transcribed with SuperScript III (Thermo Fisher Scientific) according to the manufacturer’s instructions. Primers designed on the cDNA sequence of aequorin (forward, 5′-TCGACAACCCAAGATGGATTGGA-3′; reverse, 5′-TGATAGCATGCGAATTCATCAGTGTTTTAT-3′) were used to analyze aequorin gene expression. The coding sequence of actin was used as a control (forward, 5′-GGTTGCACCGCCAGAGAGAAAATAC-3′; reverse, 5′-AACAAACTCACCACCACGAACCAGA-3′). Immunoblot analyses were carried out on total protein extracts obtained from leaves of 1-month-old Arabidopsis plants as described previously (Zonin et al., 2011). Aequorin fusion proteins were decorated with a polyclonal anti-aequorin antibody (Abcam) diluted 1:10,000. An Arabidopsis line stably expressing aequorin in the cytosol (CPK17G2A-NES-YA; Mehlmer et al., 2012) was used as a positive control.
Analysis of Aequorin Activity
For the initial screening of the T2 generation of Arabidopsis TL-YA and TM-YA lines, luminescence levels were monitored in planta with a custom-built luminometer (Electron Tubes) containing a 9893/350A photomultiplier (Thorn EMI). Arabidopsis seeds were surface sterilized for 60 s in a 70% (v/v) ethanol and 0.05% (v/v) Triton X-100 solution, 60 s in 100% (v/v) ethanol, and then dried on an autoclaved Whatman paper disc for at least 10 min. Seeds were subsequently plated on one-half-strength Murashige and Skoog (MS) medium (Duchefa Biochemie) containing 0.8% (w/v) plant agar supplemented with 1.5% (w/v) Suc and 50 µm Basta. After 48 h of stratification in darkness at 4°C, MS plates were incubated in a growth chamber at 21°C with a 16-h/8-h light/dark cycle. Five 11- to 14-d-old seedlings from all sublines were reconstituted overnight with 5 µm coelenterazine (Prolume) and kept in the dark. The next day, seedlings were placed singly in the luminometer chamber containing 300 µL of water, and total aequorin was discharged by injection of an equal volume of the discharge solution (30% [v/v] ethanol and 1 m CaCl2). Total luminescence values from every plant line were pooled, and the average luminescence was calculated. All subsequent analyses were carried out in the T3 generation.
Setup of Autotrophic Cell Suspension Cultures from the Transformed Arabidopsis Lines
Arabidopsis seeds (T3 generation) from the TL-YA and TM-YA sublines that revealed the highest aequorin activity were used to establish photosynthetic cell cultures, as described recently (Sello et al., 2017). Briefly, surface-sterilized seeds were plated on MS medium containing 3% (w/v) Suc, 0.8% (w/v) agar, pH 5.5, 0.5 µg mL−1 2,4-dichlorophenoxyacetic acid, 0.25 µg mL−1 6-benzylaminopurine, and 50 µm Basta. After exactly 3 weeks, well-developed green calli formed at the hypocotyl. Those showing the highest YFP fluorescence were separated from roots and cotyledons, cut in small pieces with a sterilized scalpel, and transferred into liquid MS medium containing 2% (w/v) Suc with the same concentrations of phytohormones and herbicide as described above. Cell suspension cultures were maintained at 24°C with a 16-h/8-h light/dark cycle under an illumination rate of 110 µmol photons m−2 s−1 on a rotary shaker at 80 rpm. They were subcultured every week by transferring 1 packed cell volume of cell culture in 20 mL of fresh medium containing decreasing Suc concentration (up to 0.5%, w/v) to stimulate photosynthetic activity (Hampp et al., 2012). The expression of thylakoid-targeted aequorin in the cell cultures was confirmed by western-blot analyses of total protein extracts from midexponential phase cultured cells. Densitometric analysis was carried out using Quantity One software (Bio-Rad). Aequorin protein levels were normalized against calreticulin (by using an anti-spinach calreticulin antibody diluted 1:2,000; Navazio et al., 1995) and compared with a cell line stably expressing aequorin in the stroma (Sello et al., 2016).
Fluorescence and Confocal Microscopy Analyses
Developing calli were observed with a Leica MZ16F fluorescence stereomicroscope equipped with a GFP filter (excitation at 450/490 nm and emission at 500/550 nm) and a chlorophyll filter (excitation at 460/500 nm and emission above 605 nm). Images were acquired with a Leica DFC480 digital camera using the Leica Application Suite software. Arabidopsis seedlings (14-d-old) grown either under a 16-h-light/8-h-dark cycle or in the dark (etiolated seedlings) in vertical square petri dishes (100 × 100 × 20 mm) and suspension-cultured cells (4-d-old) were observed with a Leica TCS SP5 II confocal laser scanning system mounted on a Leica DMI6000 inverted microscope. Excitation with the argon laser was carried out at 488 nm, and the emitted fluorescence was detected at 505 to 530 nm for YFP and at 680 to 720 nm for chlorophyll.
Pulse Amplitude Modulation Analyses
Arabidopsis seedlings and exponentially growing cell suspension cultures were placed in a Closed FluorCam 800 MF (Photon Systems Instruments), and the photosynthetic activity was measured as Fv/Fm (where Fv is the difference between the maximal [Fm] and the basal [F0] fluorescence of chlorophyll). Chlorophyll fluorescence images were recorded using a CCD camera, and data analysis was conducted using the FluorCam 7 software (Photon Systems Instruments).
Immunocytochemistry and TEM Analyses
Leaf fragments from 2-week-old Arabidopsis plants were fixed overnight at 4°C in 4% (v/v) paraformaldehyde and 0.5% (v/v) glutaraldehyde in 0.1 m cacodylate buffer, pH 7. After three washes in cacodylate buffer, dehydration in a graded ethanol series was performed. Samples were then embedded progressively in medium-grade London Resin White (PolySciences). Ultrathin sections (500 Å) were obtained on a Reichert-Jung ultramicrotome and mounted on uncoated nickel grids. For immunogold labeling, grids were incubated for 45 min in 0.1% (v/v) Tween 20, 1% (w/v) BSA in TBS, and then for 1 h with rabbit anti-aequorin antibody at a 1:1,000 dilution. After three washes with 0.1% (v/v) Tween 20 in TBS, samples were incubated for 1 h with an anti-rabbit secondary antibody conjugated with colloidal gold particles of 10 nm diameter (Sigma-Aldrich) diluted 1:100. After two washes as above and one wash in distilled water, samples were exposed to osmium tetroxide vapors overnight. After extensive washing with distilled water, samples were counterstained with uranyl acetate and lead citrate and observed using a Tecnai 12-BT transmission electron microscope (FEI) operating at 120 kV and equipped with a Tietz camera. Arabidopsis wild-type plants and plants stably expressing aequorin in the chloroplast stroma (Mehlmer et al., 2012) were used as negative and positive controls, respectively. TEM analyses of chloroplast ultrastructure were carried out as described by Zuppini et al. (2004).
Chloroplast Isolation and Thermolysin Treatment
Chloroplasts were isolated from leaves of 1-month-old Arabidopsis plants using the procedure described by Aronsson and Jarvis (2011). Chloroplasts were resuspended in wash buffer (0.3 m sorbitol, 50 mm HEPES/KOH, 3 mm MgCl2, and 1 mm CaCl2, pH 7.6), vigorously diluted with 10 volumes of osmotic shock buffer (50 mm HEPES/KOH, 3 mm MgCl2, and 1 mm CaCl2, pH 7.6), and then centrifuged at 48,000g for 10 min at 4°C to isolate thylakoids. The thylakoid-containing pellet was then resuspended in wash buffer. Treatment with the proteolytic enzyme thermolysin was carried out as described by Mehlmer et al. (2012). Briefly, the thylakoid suspension (50 μg of protein) was incubated with 0.1 µg µL−1 thermolysin (Sigma-Aldrich) for 20 min on ice, and the reaction was stopped by the addition of 5 mm EDTA. Immunoblot analyses were conducted as described above.
Aequorin-Based Ca2+ Measurement Assays
For Ca2+ measurement assays in the Arabidopsis cell lines stably expressing aequorin targeted to the thylakoid system, exponentially growing (4-d-old) autotrophic cell suspension cultures were reconstituted overnight with 5 µm coelenterazine. On the following day, after extensive washing, 50 µL of cells was placed in the luminometer chamber and exposed to different environmental stimuli, as described by Sello et al. (2016). Oxidative stress (10 mm H2O2), salt stress (0.3 m NaCl), and pathogen attack (20 µg mL−1 OGs with degree of polymerization from 10 to 15; Moscatiello et al., 2006) were simulated by injection into the cell suspension culture of an equal volume of a 2-fold-concentrated stimulant dissolved in plant cell culture medium. Touch controls were performed by injecting an equal volume of plant cell culture medium. Ca2+ dynamics were recorded for a total of 700 s before the injection of 100 μL of the discharge solution (30% v/v ethanol and 1 m CaCl2). The light signal was collected and converted offline into Ca2+ concentration values using a computer algorithm based on the Ca2+ response curve of aequorin (Brini et al., 1995). In parallel experiments, an Arabidopsis autotrophic cell line stably expressing aequorin in the chloroplast stroma (Sello et al., 2016) was used. Cell viability was determined, after 1 h of treatment with the above stimuli, by the Evans blue method (Baker and Mock, 1994).
To perform Ca2+ measurements in aequorin-expressing seedlings in toto, seeds (T3 generation) were surface sterilized and sown on agarized (0.8% [w/v] plant agar) one-half-strength MS medium supplemented with 1.5% (w/v) Suc to germinate under a 16-h/8-h light/dark cycle at 21°C. After overnight reconstitution with 5 µm coelenterazine, 7- to 14-d-old seedlings were exposed to the same stimuli used for the in vitro cell cultures, as described above. For the monitoring of Ca2+ dynamics in response to light-to-dark transition, Arabidopsis lines stably expressing aequorin targeted to the cytosol and outer surface of the plastid envelope (Mehlmer et al., 2012) also were used. At the beginning of the dark phase, reconstituted seedlings were quickly transferred into the luminometer chamber containing 300 µL of water, and Ca2+ dynamics were monitored for a total of 3 h. In some experiments, seedlings were pretreated with 5 µm nigericin (Sigma-Aldrich) for 30 min before the end of the light phase or for 45 min after the onset of darkness. The final concentration of ethanol was 0.1% (v/v).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: NP_564667 (Arabidopsis TLP18.3, At1G54780.1), NP_564946 (Arabidopsis STN7, At1G68830), NP_565954 (Arabidopsis NTRC, At2G41680), NP_190810 (Arabidopsis OEP7, At3G52420) and NP_196779 (Arabidopsis CPK17, At5G12180).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Confocal microscopy analyses of roots of Arabidopsis seedlings of the TL-YA and TM-YA lines.
Supplemental Figure S2. Setup of Arabidopsis photosynthetic cell suspension cultures from the TL-YA and TM-YA lines.
Supplemental Figure S3. Effect of the treatment with different stimuli on Arabidopsis cell viability.
Supplemental Figure S4. Monitoring of subchloroplast free [Ca2+] in Arabidopsis transgenic lines in response to touch.
Supplemental Figure S5. Monitoring of subchloroplast free [Ca2+] in Arabidopsis transgenic lines in response to oxidative stress over 1 h, and comparison of luminescence levels in aequorin-expressing seedlings versus wild-type seedlings.
Supplemental Figure S6. Control measurements of luminescence levels upon light-to-dark transition in Arabidopsis seedlings.
Supplemental Figure S7. Monitoring of thylakoid luminal [Ca2+] in Arabidopsis seedlings that were transferred to the luminometer chamber after 6 h of dark or at the end of the dark phase.
Supplemental Figure S8. Effect of the H+-translocating uncoupler nigericin on dark-induced Ca2+ dynamics in the thylakoid lumen, stroma, and stromal surface of thylakoids.
Supplemental Table S1. Amino acid and corresponding nucleotide sequences used for the targeting of the YA fusion proteins to the thylakoid lumen and thylakoid membrane.
Acknowledgments
We thank M. Brini (University of Padova, Italy) for fruitful discussion on aequorin-based measurements of organellar Ca2+ dynamics and W. Martin (University of Düsseldorf, Germany) for critical comments on an earlier version of the article. We also thank the Electron Microscopy Service of the Department of Biology, University of Padova, for skillful technical assistance.
Footnotes
This work was supported by Progetti di Ricerca di Ateneo (prot. CPDA127210) and Dotazione Ordinaria della Ricerca Dipartimentale 2016-2017 to L.N., the EU within the Marie-Curie ITN CALIPSO (FP7) (project no. 607607) to U.C.V., and the Human Frontier Science Program (HFSP0052) to I.S.
References
- Abdul-Awal SM, Hotta CT, Davey MP, Dodd AN, Smith AG, Webb AA (2016) NO-mediated [Ca2+]cyt increases depend on ADP-ribosyl cyclase activity in Arabidopsis. Plant Physiol 171: 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsson H, Jarvis RP (2011) Rapid isolation of Arabidopsis chloroplasts and their use for in vitro protein import assays. Methods Mol Biol 774: 281–305 [DOI] [PubMed] [Google Scholar]
- Bailleul B, Berne N, Murik O, Petroutsos D, Prihoda J, Tanaka A, Villanova V, Bligny R, Flori S, Falconet D, et al. (2015) Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524: 366–369 [DOI] [PubMed] [Google Scholar]
- Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disk assays using Evans blue. Plant Cell Tissue Organ Cult 39: 7–12 [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Brini M. (2008) Calcium-sensitive photoproteins. Methods 46: 160–166 [DOI] [PubMed] [Google Scholar]
- Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R (1995) Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c): a critical evaluation. J Biol Chem 270: 9896–9903 [DOI] [PubMed] [Google Scholar]
- Carraretto L, Teardo E, Checchetto V, Finazzi G, Uozumi N, Szabo I (2016) Ion channels in plant bioenergetic organelles, chloroplasts and mitochondria: from molecular identification to function. Mol Plant 9: 371–395 [DOI] [PubMed] [Google Scholar]
- Checchetto V, Teardo E, Carraretto L, Leanza L, Szabo I (2016) Physiology of intracellular potassium channels: a unifying role as mediators of counterion fluxes? Biochim Biophys Acta 1857: 1258–1266 [DOI] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Enz C, Steinkamp T, Wagner R (1993) Ion channels in the thylakoid membrane (a patch-clamp study). Biochim Biophys Acta 1143: 67–76 [Google Scholar]
- Ettinger WF, Clear AM, Fanning KJ, Peck ML (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119: 1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi G, Petroutsos D, Tomizioli M, Flori S, Sautron E, Villanova V, Rolland N, Seigneurin-Berny D (2015) Ions channels/transporters and chloroplast regulation. Cell Calcium 58: 86–97 [DOI] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19: 623–630 [DOI] [PubMed] [Google Scholar]
- Guo J, Zeng W, Chen Q, Lee C, Chen L, Yang Y, Cang C, Ren D, Jiang Y (2016) Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 531: 196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp C, Richter A, Osorio S, Zellnig G, Sinha AK, Jammer A, Fernie AR, Grimm B, Roitsch T (2012) Establishment of a photoautotrophic cell suspension culture of Arabidopsis thaliana for photosynthetic, metabolic, and signaling studies. Mol Plant 5: 524–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmal AK, Schulze S, Trompelt K, Hippler M (2015) Calcium-dependent regulation of photosynthesis. Biochim Biophys Acta 1847: 993–1003 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A (1995) Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269: 1863–1865 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Shingles R, Ettinger W (2006) Regulation and role of calcium fluxes in the chloroplast. In Wise RR, Hoober JK, eds, The Structure and Function of Plastids. Springer, Dordrecht, The Netherlands, pp 403–416 [Google Scholar]
- Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiep V, Vadassery J, Lattke J, Maaß JP, Boland W, Peiter E, Mithöfer A (2015) Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol 207: 996–1004 [DOI] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM (2016) Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 531: 258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Loro G, Wagner S, Doccula FG, Behera S, Weinl S, Kudla J, Schwarzländer M, Costa A, Zottini M (2016) Chloroplast-specific in vivo Ca2+ imaging using Yellow Cameleon fluorescent protein sensors reveals organelle-autonomous Ca2+ signatures in the stroma. Plant Physiol 171: 2317–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor H, Chiltz A, Madani S, Vatsa P, Schoefs B, Pugin A, Garcia-Brugger A (2012) Calcium signatures and signaling in cytosol and organelles of tobacco cells induced by plant defense elicitors. Cell Calcium 51: 434–444 [DOI] [PubMed] [Google Scholar]
- Martí MC, Stancombe MA, Webb AAR (2013) Cell- and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Plant Physiol 163: 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmer N, Parvin N, Hurst CH, Knight MR, Teige M, Vothknecht UC (2012) A toolset of aequorin expression vectors for in planta studies of subcellular calcium concentrations in Arabidopsis thaliana. J Exp Bot 63: 1751–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatiello R, Baldan B, Navazio L (2013) Plant cell suspension cultures. Methods Mol Biol 953: 77–93 [DOI] [PubMed] [Google Scholar]
- Moscatiello R, Mariani P, Sanders D, Maathuis FJM (2006) Transcriptional analysis of calcium-dependent and calcium-independent signalling pathways induced by oligogalacturonides. J Exp Bot 57: 2847–2865 [DOI] [PubMed] [Google Scholar]
- Navazio L, Baldan B, Dainese P, James P, Damiani E, Margreth A, Mariani P (1995) Evidence that spinach leaves express calreticulin but not calsequestrin. Plant Physiol 109: 983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Moscatiello R, Bellincampi D, Baldan B, Meggio F, Brini M, Bowler C, Mariani P (2002) The role of calcium in oligogalacturonide-activated signalling in soybean cells. Planta 215: 596–605 [DOI] [PubMed] [Google Scholar]
- Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T (2008) Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J 53: 988–998 [DOI] [PubMed] [Google Scholar]
- Nomura H, Komori T, Uemura S, Kanda Y, Shimotani K, Nakai K, Furuichi T, Takebayashi K, Sugimoto T, Sano S, et al. (2012) Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat Commun 3: 926. [DOI] [PubMed] [Google Scholar]
- Nomura H, Shiina T (2014) Calcium signaling in plant endosymbiotic organelles: mechanism and role in physiology. Mol Plant 7: 1094–1104 [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434: 404–408 [DOI] [PubMed] [Google Scholar]
- Pottosin I, Dobrovinskaya O (2015) Ion channels in native chloroplast membranes: challenges and potential for direct patch-clamp studies. Front Physiol 6: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6: 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S, Wünnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P (2008) Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J 53: 287–299 [DOI] [PubMed] [Google Scholar]
- Rocha AG, Vothknecht UC (2012) The role of calcium in chloroplasts: an intriguing and unresolved puzzle. Protoplasma 249: 957–966 [DOI] [PubMed] [Google Scholar]
- Sai J, Johnson CH (2002) Dark-stimulated calcium ion fluxes in the chloroplast stroma and cytosol. Plant Cell 14: 1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Steinberger I, Herdean A, Gandini C, Eisenhut M, Kurz S, Morper A, Hoecker N, Rühle T, Labs M, et al. (2016) The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. Plant Cell 28: 892–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sello S, Moscatiello R, La Rocca N, Baldan B, Navazio L (2017) A rapid and efficient method to obtain photosynthetic cell suspension cultures of Arabidopsis thaliana. Front Plant Sci 8: 1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sello S, Perotto J, Carraretto L, Szabò I, Vothknecht UC, Navazio L (2016) Dissecting stimulus-specific Ca2+ signals in amyloplasts and chloroplasts of Arabidopsis thaliana cell suspension cultures. J Exp Bot 67: 3965–3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirpiö S, Allahverdiyeva Y, Suorsa M, Paakkarinen V, Vainonen J, Battchikova N, Aro EM (2007) TLP18.3, a novel thylakoid lumen protein regulating photosystem II repair cycle. Biochem J 406: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stael S, Kmiecik P, Willems P, Van Der Kelen K, Coll NS, Teige M, Van Breusegem F (2015) Plant innate immunity: sunny side up? Trends Plant Sci 20: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M (2012) Plant organellar calcium signalling: an emerging field. J Exp Bot 63: 1525–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AB, Kunz HH, Yang E, Schroeder JI (2016) Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc Natl Acad Sci USA 113: E5242–E5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó I, Bergantino E, Giacometti GM (2005) Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation. EMBO Rep 6: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M, Gollan PJ, Suorsa M, Kangasjärvi S, Aro EM (2012) STN7 operates in retrograde signaling through controlling redox balance in the electron transfer chain. Front Plant Sci 3: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent TR, Avramova M, Canham J, Higgins P, Bilkey N, Mugford ST, Pitino M, Toyota M, Gilroy S, Miller AJ, et al. (2017) Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 29: 1460–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Xu W, Jin H, Zhang T, Lai J, Zhou X, Zhang S, Liu S, Duan X, Wang H, et al. (2016) A putative chloroplast-localized Ca2+/H+ antiporter CCHA1 is involved in calcium and pH homeostasis and required for PSII function in Arabidopsis. Mol Plant 9: 1183–1196 [DOI] [PubMed] [Google Scholar]
- Weinl S, Held K, Schlücking K, Steinhorst L, Kuhlgert S, Hippler M, Kudla J (2008) A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol 179: 675–686 [DOI] [PubMed] [Google Scholar]
- Wu HY, Liu MS, Lin TP, Cheng YS (2011) Structural and functional assays of AtTLP18.3 identify its novel acid phosphatase activity in thylakoid lumen. Plant Physiol 157: 1015–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong TC, Ronzier E, Sanchez F, Corratgé-Faillie C, Mazars C, Thibaud JB (2014) Imaging long distance propagating calcium signals in intact plant leaves with the BRET-based GFP-aequorin reporter. Front Plant Sci 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Martinoia E, Szabo I (2015) Organellar channels and transporters. Cell Calcium 58: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonin E, Moscatiello R, Miuzzo M, Cavallarin N, Di Paolo ML, Sandonà D, Marin O, Brini M, Negro A, Navazio L (2011) TAT-mediated aequorin transduction: an alternative approach for effective calcium measurements in plant cells. Plant Cell Physiol 52: 2225–2235 [DOI] [PubMed] [Google Scholar]
- Zuppini A, Baldan B, Millioni R, Favaron F, Navazio L, Mariani P (2004) Chitosan induces Ca2+-mediated programmed cell death in soybean cells. New Phytol 161: 557–568 [DOI] [PubMed] [Google Scholar]