Polyamines play an important role in strawberry fruit ripening by integration of multiple plant hormones, including abscisic acid, auxin, and ethylene.
Abstract
Polyamines (PAs) participate in many plant growth and developmental processes, including fruit ripening. However, it is not clear whether PAs play a role in the ripening of strawberry (Fragaria ananassa), a model nonclimacteric plant. Here, we found that the content of the PA spermine (Spm) increased more sharply after the onset of fruit coloration than did that of the PAs putrescine (Put) or spermidine (Spd). Spm dominance in ripe fruit resulted from abundant transcripts of a strawberry S-adenosyl-l-Met decarboxylase gene (FaSAMDC), which encodes an enzyme that generates a residue needed for PA biosynthesis. Exogenous Spm and Spd promoted fruit coloration, while exogenous Put and a SAMDC inhibitor inhibited coloration. Based on transcriptome data, up- and down-regulation of FaSAMDC expression promoted and inhibited ripening, respectively, which coincided with changes in several physiological parameters and their corresponding gene transcripts, including firmness, anthocyanin content, sugar content, polyamine content, auxin (indole-3-acetic acid [IAA]) content, abscisic acid (ABA) content, and ethylene emission. Using isothermal titration calorimetry, we found that FaSAMDC also had a high enzymatic activity with a Kd of 1.7 × 10−3 m. In conclusion, PAs, especially Spm, regulate strawberry fruit ripening in an ABA-dominated, IAA-participating, and ethylene-coordinated manner, and FaSAMDC plays an important role in ripening.
Fleshy fruit ripening involves complicated changes in sugar, texture, color, flavor, and aroma and is controlled by plant hormones. Based on studies in the model plant tomato (Solanum lycopersicum), climacteric fruit ripening is known to be controlled by ethylene (Alexander and Grierson, 2002). By contrast, the ripening of strawberry (Fragaria ananassa), a model nonclimacteric plant, is complex (Shen and Rose, 2014) and controlled by abscisic acid (ABA; Li et al., 2011), auxin (indole-3-acetic acid [IAA]; Given et al., 1988), gibberellic acid (GA; Csukasi et al., 2011), ethylene (Merchante et al., 2013), jasmonate (JA; Concha et al., 2013), and brassinosteroids (Chai et al., 2013). Polyamines (PAs) have been previously reported to be involved in strawberry fruit development (Tilak and Raymond, 1996), but whether PAs play a role in ripening is unknown.
PAs, which are ubiquitous aliphatic amines and biogenic regulators, are involved in many physiological and developmental processes, including plant growth, senescence, and stress (Tabor and Tabor, 1984; Minocha, 1988; Mariani et al., 1989; Nambeesan et al., 2008). In plants, putrescine (Put) is converted to spermidine (Spd) and then spermine (Spm) by Spd synthase (SPDS)- and Spm synthase (SPMS)-catalyzed sequential additions of amino propyl residues. Decarboxylated S-adenosyl-l-Met is required for the reactions and is a product of SAM and the catalyst SAM decarboxylase (SAMDC). Thus, SAMDC is a rate-limiting step for Spd and Spm synthesis (Mehta et al., 2002; Wei Hu et al., 2006).
Notably, SAM acts as a common precursor for both PA and ethylene biosynthesis (Apelbaum et al., 1981; Minocha, 1988; Larsson et al., 1997; Martin-Tanguy, 2001). Although SAM is transformed preferentially into PAs (Khan and Singh, 2010; de Dios et al., 2006), ethylene and PA biosynthesis do not compete (Kushad et al., 1988). SAM is homeostatically regulated, so as to maintain higher rates in the simultaneous production of ethylene and PA (Van de Poel et al., 2013; Lasanajak et al., 2014). Overexpression of the gene encoding Orn decarboxylase (ODC) promotes Put, Spd, and Spm synthesis, inhibits ethylene emission, respiration and water loss, and delays ripening but enhances tomato fruit quality (Gupta et al., 2013; Pandey et al., 2015). Overexpression of the SPDS gene in tomato fruits promotes carotenoid and lycopene accumulation, as well as ethylene production (Neily et al., 2011). Notably, the increased expression of a yeast SAMDC (ySAMDC) gene in tomato fruits promotes conversion of Put to Spd and Spm, and influences multiple cell pathways and broad gene expression levels, thereby increasing lycopene content, prolonging shelf life, and enhancing fruit juice quality (Mehta et al., 2002; Mattoo et al., 2006, 2007; Nambeesan et al., 2010; Kolotilin et al., 2011). High Spd and Spm contents exhibit strong effects on various metabolic processes, including amino acid, sugar, and energy, and regulate ripening rate and extent in tomato (Tassoni et al., 2006; Fatima et al., 2016). Changes in the contents of ripening-related physiological parameters, including sugar, ethylene, and lycopene, are positively related to Spd and Spm contents but negatively related to Put content, emphasizing that individual biogenic amines have differential roles in developing tomato fruits (Handa and Mattoo, 2010).
In addition to their roles in tomato fruit, PAs have been reported to function in other fruits. PAs play important roles in grape (Vitis vinifera) berry ripening, including cell expansion and aroma development (Agudelo-Romero et al., 2013, 2014; Fortes et al., 2015). Exogenous PAs strongly inhibit peach (Prunus persica) fruit ripening, especially softening (Bregoli et al., 2002; Ziosi et al., 2006; Torrigiani et al., 2012), while higher PA concentrations coincide with fruit enlargement during oil-palm (Elaeis guineensis) maturation (Teh et al., 2014). PAs also take part in raspberry (Rubus occidentalis) and date (Phoenix dactylifera) fruit ripening (Diboun et al., 2015; Simpson et al., 2017). The pre- and postharvest application of PAs delays ripening and extends shelf lives in mango (Mangifera indica), peach (Prunus persica), plum (Prunus domestica), and apple (Malus domestica) fruit, while controversial conclusions on the roles of PAs in ripening have also been reported (Law et al., 1991; Wang et al., 1993; Escribano and Merodio, 1994; Mattoo et al., 2002; Pérez-Amador et al., 2002; Torrigiani et al., 2004; Neily et al., 2011). Thus, more research is required to further understand the roles of PAs in fleshy fruit ripening.
Given that strawberry is regarded as an ideal model plant for studying nonclimacteric fruit ripening (Li et al., 2011), we examined the roles of PAs during strawberry fruit ripening to better understand the molecular mechanisms of nonclimacteric fruit development. We first measured PA contents in developing strawberry fruits and investigated the effects of PAs on ripening. Next, we cloned the strawberry SAMDC gene and identified its functions during ripening using virus-induced gene silencing (VIGS) and overexpression (OE). Finally, we analyzed the enzymatic activity of SAMDC by HPLC and isothermal titration calorimetry. Our results demonstrated that FaSAMDC positively regulates strawberry fruit ripening, and PAs, especially Spm, play important roles in this process.
RESULTS
PA Contents in Developing Strawberry Fruits
According to a previous report (Jia et al., 2011), the developing fruit of strawberry (F. ananassa cv ‘Sweet Charlie’) can be divided into seven stages: small green (SG), large green (LG), degreening (DG), white (Wt), initial red (IR), partial red (PR; namely, turning), and full red (FR; Fig. 1). HPLC analysis of Put, Spd, and Spm contents is shown in Figure 1. Put contents gradually decreased from SG to FR stages and reached the lowest levels in FR fruits. Spd content was low from the SG to PR stages but increased slightly in FR fruits. Notably, Spm contents declined slightly in green fruits but increased sharply after the Wt stage and reached the highest concentration in FR fruits. These results suggest that PAs, especially Spm, play roles in strawberry fruit ripening.
Figure 1.
Polyamine contents in seven developmental stages of strawberry fruit. Polyamine contents examined using HPLC. Three fruits were used for detection in each stage. Error bars represent se (n = 3).
Effects of PAs and a SAMDC Inhibitor on Strawberry Fruit Ripening
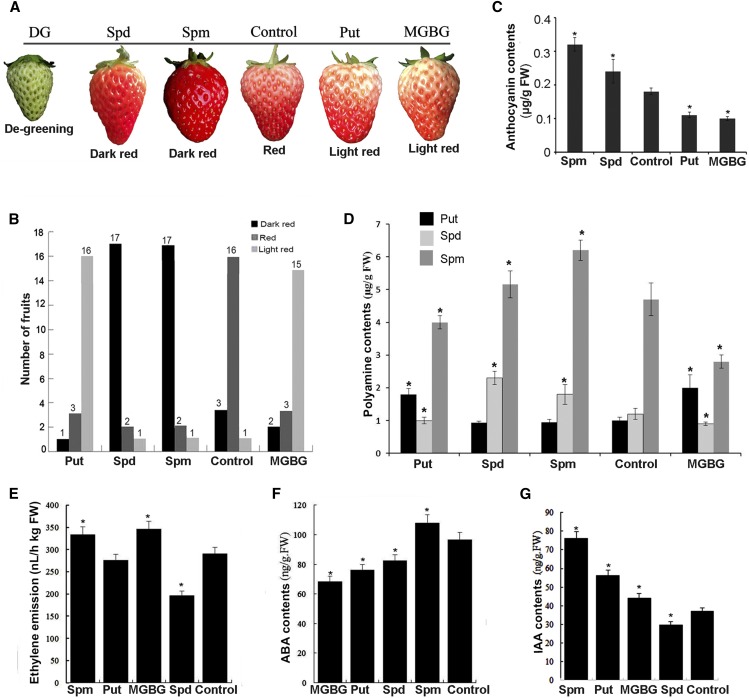
To further determine the role of PAs in strawberry fruit ripening, we immersed 20 DG fruits still attached to plants in Put, Spd, and Spm, as well as methylgloxal bis(guanylhydrazine) (MGBG), a SAMDC inhibitor (Fig. 2A). Sterile water was used as a control. Eight days after treatment, we observed the ratio of dark-red, red, and light-red fruits. Spm-, Spd-, Put-, MGBG-, and water-treated fruits showed ratios of 17:2:1, 17:2:1, 1:3:16, 2:3:15, and 3:16:1, respectively (Fig. 2B). Thus, exogenous Spm and Spd promoted coloration, while MGBG and Put inhibited coloration compared to the control, which was supported by anthocyanin contents (Fig. 2C). Analysis of PA contents in these treated fruits showed that exogenous Spm and Spd significantly promoted endogenous Spm or Spd accumulation but not Put contents; in contrast, exogenous Put and MGBG significantly inhibited endogenous Spm or Spd accumulation, but significantly promoted Put accumulation compared to the control (Fig. 2D). These results demonstrate that Spm and Spd promote ripening, while Put inhibits ripening.
Figure 2.
Effects of polyamines and an inhibitor on strawberry fruit coloration. Twenty degreening strawberry fruits still attached to the plants were used for each independent treatment and immersed in 100 µm Put, Spd, Spm, or MGBG (n = 20, one replication). Water was used as the control. Three fruits representative of the Put-, Spm-, Spd-, and MGBG-treated phenotypes were used for detection of the physiological parameters (n = 3, three replications). A, Fruit phenotypes before (DG) and after treatment. B, The number of fruits per phenotype within each treatment. C, Anthocyanin contents. D, Polyamine contents. E, Ethylene emission rates. F, ABA contents. G, IAA contents. Error bars represent se (n = 3).The asterisk in same-colored columns indicates a statistically significant difference compared to the control (P < 0.05) after an ANOVA followed by Duncan’s multiple range tests.
To understand the effects of PAs on coloration, contents of several ripening-related plant hormones, including ethylene, ABA, and IAA, were investigated. The results showed that in comparison to the control, exogenous Spm, Put, and MGBG promoted ethylene emission, while Spd inhibited ethylene emission (Fig. 2E); exogenous Spm promoted ABA accumulation, while Spd, Put, and MGBG inhibited ABA accumulation (Fig. 2F); exogenous Spm, Put, and MGBG promoted IAA accumulation, while Spd inhibited IAA accumulation (Fig. 2G). These results suggest that the effects of PAs on strawberry fruit coloration might be related to ABA, ethylene, and IAA.
RNA-Seq, Annotation, and Plant Hormone Signal Transduction Pathway Analyses
To maximize the transcriptional data obtained from ripening strawberries, we used cDNA libraries from four stages (mixed and separate), i.e. LG, Wt, IR, and PR fruit, for RNA-seq. Approximately 5 to 9 billion raw reads distributed among each library and could be used for further analyses (Supplemental Table S1; NCBI, SUB3724936). A total of 98,859 unigenes were considered valid (Supplemental Table S2) and were annotated using 25 KOGs (eukaryotic orthologous groups; Supplemental Fig. S1). The top five pathways with more abundant differentially expressed genes (DEGs) were involved in ribosome processing, spliceosome processing, protein processing, plant-pathogen interaction, and plant hormone signal transduction (Supplemental Table S3). Based on Gene Ontology analysis, a total of 31,792 unigenes were classified into three groups: “biological process,” “cellular component,” and “molecular function” (Supplemental Fig. S2).
To analyze the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of unigenes, we used the KEGG Automatic Annotation Server to obtain KEGG ortholog numbers, which are mapped to corresponding KEGG pathways. Based on log2 expression level, a total of 301 pathways with 14,025 unigenes were annotated. Based on DEG analysis between library pairs (LG-Wt, Wt-IR, and IR-PR), we found that the total number of DEGs declined rapidly from LG to PR. More DEGs were downregulated between LG and Wt than were upregulated, and more DEGs were upregulated from Wt to IR than were downregulated. Notably, IR and PR had similar quantities of up- and down-regulated DEGs (Fig. 3A). These results suggest that metabolism transitions occurred between green-white-red stages, so white fruit is a distinct stage.
Figure 3.
DEGs during the onset of strawberry fruit ripening as determined by RNA-seq. Fruit cDNA libraries from four developmental stages, including LG, Wt, IR, and PR, were used for RNA-seq. A, The number of upregulated and downregulated DEGs between library pairs. B, Heat map and cluster analysis of the DEGs encoding proteins in plant hormone signaling pathways. The data for gene expression levels were normalized to a z-score with the formula log10 (FPKM+1) by color key and density plot. Green represents low expression and red represents high expression during ripening. IAA, auxin-responsive protein/transcription factor; LUX, auxin transporter 2; JAZ, jasmonate ZIM (zinc-finger inflorescence meristem) domain; PP2C37/ABI37, 2C-type protein phosphatase 37/ ABA-insensitive 37; EBF1, EIN3 (ethylene insensitive 3) binding F-box 1; GID1, gibberellin receptor 1; ACO1, 1-aminocyclopropane-1-carboxylic acid oxidase 1; SnRK2, Ser/Thr-protein kinase 2.
Next, we focused on screening the DEGs involved in plant hormone signal transduction around the onset of ripening and found several important signaling DEGs, including genes related to IAA pathway (IAA2, ARF11, IAA16, IAA27, and LUX2), ABA pathway (PP2C37 and SnRK2), ethylene pathway (ACO1 and EBF1), JA (JAZ), and GA pathway (GID1; Fig. 3B). Notably, in conjunction with degreening and coloration, expression of IAA2, IAA16, IAA27, AUX2, and JAZ declined gradually, while ARF11 increased continually. Expression of PP2C and EBF1 increased during degreening but decreased during coloration. ACO1 expression increased during coloration whereas GID1 expression increased during degreening, decreased during initial coloration, and finally increased in the ripening fruits (Fig. 3B). The fact that the most abundant hormone-related DEGs during ripening were related to IAA (five unigenes), ABA (two unigenes), and ethylene (two unigenes) suggests that ABA, IAA, and ethylene may play important roles in strawberry fruit ripening.
Roles of FaSAMDC in Strawberry Fruit Ripening
PA contents in developing fruit (Fig. 1) and pharmacological experiments (Fig. 2) suggest an important role of SAMDC, which is necessary for Spd and Spm biosynthesis (Mehta et al., 2002; Wei Hu et al., 2006), in ripening. We investigated strawberry homologs of SAMDC in our RNA-seq data and found eight SAMDC-like transcripts, including their complementary sequences. We divided these transcripts into four groups, of which comp72320_c0_seq1 and its complementary strand, comp73015_c0_seq2, had substantially higher mRNA expression levels than the others (Fig. 4A). This transcript encoded a protein with the SAM-decarbbox domain (Supplemental Fig. S3) and thus was named FaSAMDC (GenBank no. MG266892). Based on log2 expression levels, the transcripts of FaSAMDC were highly expressed in the LG fruits, declined during degreening, and increased rapidly during coloration (Fig. 4A). This trend of FaSAMDC expression levels was further confirmed by quantitative PCR (qPCR) analysis (Fig. 4B). These results suggest a role of FaSAMDC in ripening.
Figure 4.
Transcripts of FaSAMDC during strawberry fruit ripening as determined by RNA-seq and qPCR expression analysis. A, Eight SAMDC gene-like contigs were found in the transcriptomic data from LG, Wt, IR, and PR fruits. B, The mRNA expression levels of FaSAMDC in six fruit stages, including LG, DG, Wt, IR, PR, and FR. Actin mRNA was used as an internal control. Columns with different letters (a–f) indicate statistically significant differences (P < 0.05) after an ANOVA followed by Duncan’s multiple range tests. Error bars represent se (n = 3).
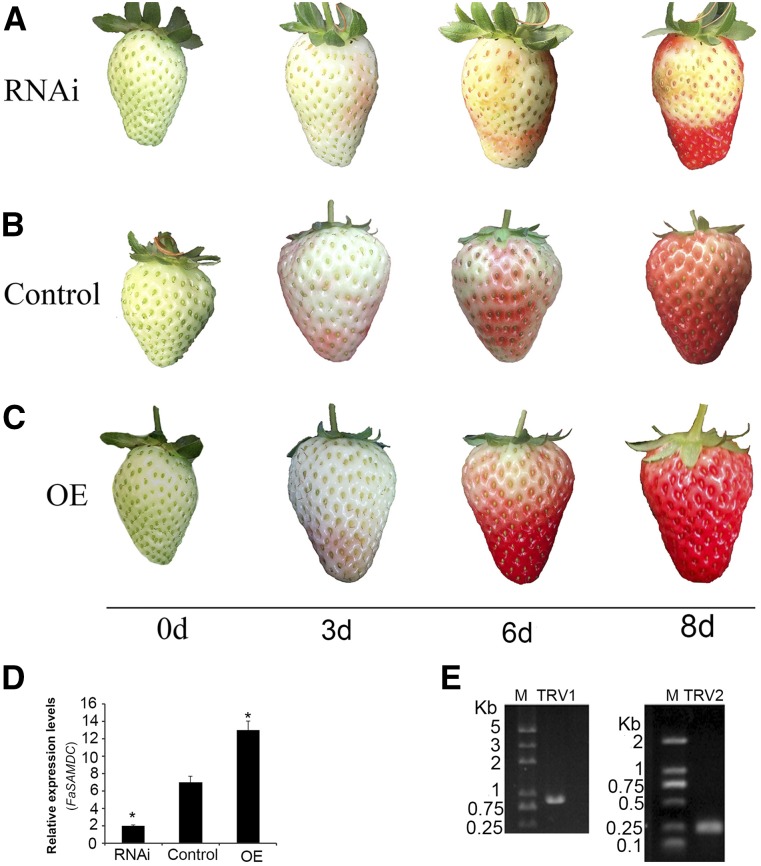
We then used RNA interference (RNAi) and OE techniques (Jia et al., 2011, 2013) to determine the function of FaSAMDC in ripening. Eight days after inoculation, RNAi-treated fruits had a chimeric phenotype (Fig. 5A), while most of the OE-treated fruits turned dark red (18 dark red and 2 red; Figure 5C); in contrast, control fruits remained light red (Fig. 5B). qPCR analyses showed that the mRNA expression levels of FaSAMDC significantly decreased and increased in the RNAi and OE fruits compared to the control fruits (Fig. 5D), respectively. In addition, both vectors were detected in infected sections (Fig. 5E). These results demonstrate that FaSAMDC plays a role in strawberry fruit ripening.
Figure 5.
Silencing and overexpression of FaSAMDC in developing strawberry fruit. Twenty DG fruit still attached to the plant were used for inoculations. A to C, Eight days after inoculation, FaSAMDC-VIGS (RNAi) fruit (A) showed chimeric phenotypes compared to the control (B), while the FaSAMDC-OE fruits (C) appeared dark red. D, FaSAMDC transcripts in RNAi and OE fruit compared to the control. E, TRV vector detection in VIGS fruit. Actin mRNA was used as an internal control. The asterisk in the columns indicates statistically significant differences (P < 0.05) compared to the control after an ANOVA followed by Duncan’s multiple range tests. Error bars represent se (n = 3).
Manipulation of FaSAMDC Expression Affects Several Physiological Parameters and Corresponding Gene Transcripts
Based on previous reports (Jia et al., 2013; Zhao et al., 2017; Zhang et al., 2017) and our RNA-seq data, the expression levels of various genes and their corresponding physiological parameters, including firmness (PG1 and PL1), anthocyanin content (CHS and DFR), sugar content (SUT1 and SS), polyamine content (ADC, ODC, SPMS, and SPDM), IAA levels and signaling (IAA2, ARF11, and LUX2), ABA levels and signaling (ABI37, SnRK2, and NCED1), and ethylene emissions and signaling (ACO1 and EBF1), were analyzed in the RNAi and OE fruits, in which FaSAMDC expression levels were down- and up-regulated over 80% compared with the mixed control fruits. The results showed that in comparison to the control, both Spm and Spd contents were higher in OE fruits and lower in the RNAi fruits, but Put contents were lower in the OE fruits and higher in the RNAi fruits (Fig. 6A). Ethylene emission rates decreased in both RNAi and OE fruits (Fig. 6B). IAA (Fig. 6C) and anthocyanin (Fig. 6E) contents declined in RNAi fruits but increased in OE fruits. The contents of ABA (Fig. 6C) and soluble sugars (Fig. 6F) increased in the OE fruits but decreased in the RNAi fruits. Compared to the control, the expression of most genes, including PG1, PL1, CHS, DFR, SUT1, SS, ADC, ODC, SPDS, SPMS, ARF11, SnRK2, NCED1, and EBF1, was upregulated in the OE fruits and downregulated in the RNAi fruits, while the expression of ABI37, IAA2, and AUX2 was downregulated in the OE fruits and upregulated in the RNAi fruits. Notably, ACO1 expression was downregulated in both OE and RNAi fruits. Taken together, manipulation of FaSAMDC expression affected both synthesis-related PA gene transcript levels and PA contents. As a result, altered FaSAMDC expression also affected ABA, ethylene, and IAA levels and signaling, ripening-related gene expression levels, and fruit ripening.
Figure 6.
Manipulation of FaSAMDC expression affects several physiological parameters and relevant gene transcripts. The FaSAMDC transgenic fruits, in which the target gene was down (RNAi)- and up (OE)-regulated by more than 80% compared with the mixed control fruits, were used for analysis. A, Polyamine contents including Put, Spd, and Spm. B, Ethylene emissions. C, ABA and IAA contents. D, Fruit firmness. E, Anthocyanin contents. F, Soluble sugar contents. G, Ripening-related gene expression levels, including firmness (polygalacturonase [PG1] and pectate lyase [PL1]), anthocyanin (chalcone synthase [CHS] and dihydroflavonol 4-reductase [DFR]), sugar (Suc transporter 1 [SUT1] and Suc synthase [SS]), polyamines (Arg decarboxylase [ADC], Orn decarboxylase [ODC], Spd synthase [SPDS], and Spm synthase [SPMS]), IAA (auxin-responsive protein 2 and factor 11 [IAA2 and ARF11], and auxin transporter 2 [LUX2]), ABA (protein phosphatase 2C 37 [ABI37], Ser/Thr-protein kinase SRK2 [SnRK2], and 9-cis-epoxycarotenoid dioxygenase [NCED1]), and ethylene (1-aminocyclopropanecarboxylate synthase 1 [ACO1] and EIN3-binding F-box protein [EBF1]) genes. Actin mRNA was used as an internal control. The asterisk in the same color or gene columns indicates statistically significant differences (P < 0.05) compared to the control after an ANOVA followed by Duncan’s multiple range tests. Error bars represent se (n = 3).
Prokaryotic Expression and Enzymatic Activity of Recombinant FaSAMDC Protein
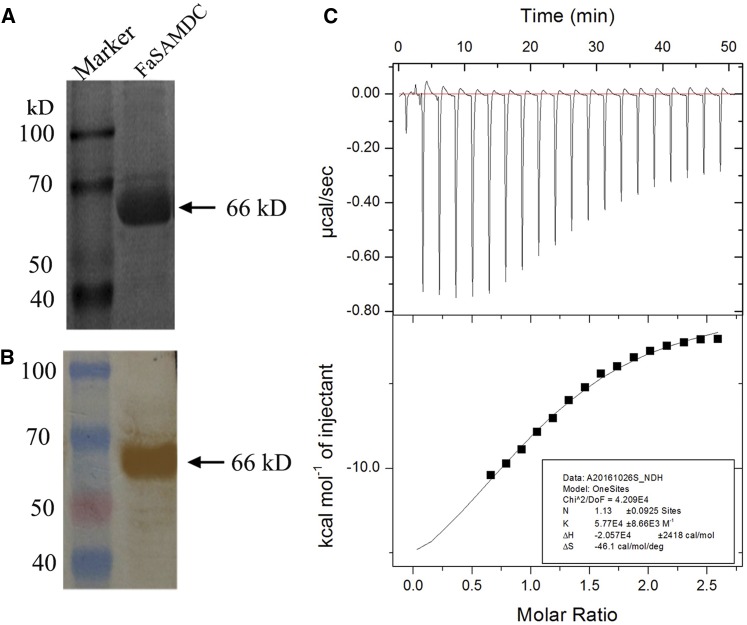
To investigate the activity of FaSAMDC, we expressed the coding sequence of FaSAMDC in Escherichia coli cells. We purified the ∼66-kD FaSAMDC protein (Fig. 7A) and identified the protein with an anti-C-terminal GST antibody (Fig. 7B), resulting in a FaSAMDC protein with 97.1% purity and a concentration of 3.2 mg/mL. The combination of chemical reactions and HPLC analysis revealed that the enzymatic activity of FaSAMDC reached 1.88 units/mg based on the standard curve analysis (Supplemental Fig. S4). The SAM-buffer-FaSAMDC titration reactions in ITC200 indicated that one purified FaSAMDC molecule could bind one molecule of SAM molecule, and the average Kd was 1.7 × 10−3 m (Fig. 7C). These results demonstrated that strawberry SAMDC protein has a high enzymatic activity.
Figure 7.
Purification, identification, and enzymatic activity of FaSAMDC protein. A, Purification of the 66-kD recombinant FaSAMDC protein. B, Immunoblot identification of the recombinant FaSAMDC. C, Measurement of the affinity between SAM and the purified FaSAMDC protein using isothermal titration calorimetry. A typical and specific saturation curve with stoichiometry (N) of 1:1 was obtained, suggesting that one SAM molecule could bind per purified protein molecule with a dissociation constant of 1.7 × 10−3 m.
DISCUSSION
PAs, in Particular Spm, Play a Role in Strawberry Fruit Ripening
Polyamines do not simply act as protective molecules, but rather as signals in plant stress tolerance (Pál et al., 2015). Although much progress has been made in flesh fruit ripening (Alexander and Grierson 2002; Adams-Phillips et al., 2004; Prasanna et al., 2007; Jia et al., 2011; Li et al., 2011; Osorio et al., 2013; Seymour et al., 2013; Kumar et al., 2014; Shen and Rose, 2014; Liu et al., 2015; Gallusci et al., 2016), the role of PAs in the ripening of strawberry, a model nonclimacteric plant, is not fully understood.
In this study, we found that exogenous Put inhibited ripening, while exogenous Spm and Spd both promoted ripening (Fig. 2). Given that Spm content was highest in the ripening fruits (Fig. 1), we speculated that Spm may play an important role in ripening. Since SAMDC is needed for Spd and Spm biosynthesis (Mehta et al., 2002; Wei Hu et al., 2006), we manipulated its expression in strawberry fruits, finding that up- and down-regulation of FaSAMDC expression affected the abundance of ADC, ODC, SPDS, and SPDM transcripts, which led to lower and higher (Spd + Spm)/Put ratio in the RNAi and OE fruits, inhibiting and promoting ripening, respectively (Fig. 5). In addition, strawberry FaSAMDC was shown to have a high enzymatic activity with a Kd of 1.7 × 10−3 m (Fig. 7). These results demonstrate that PAs, in particular Spm, play important roles in strawberry fruit ripening.
In tomato, enhanced expression of the gene encoding SAMDC in fruits increases the conversion of Put to Spd and Spm, promoting ripening. However, the ripening fruits of such transgenic plants have more Spd than Spm (Lasanajak et al., 2014). In contrast, the transgenic ripening strawberry fruits contained higher levels of Spm than Spd (Fig. 6). Given that tomato and strawberry represent two different types of fruit ripening, climacteric and nonclimacteric, respectively, it is likely that the roles of PA components vary with different types of fruits during ripening.
Understanding Polyamine Mechanisms through ABA, Ethylene, and IAA during Strawberry Fruit Ripening
Ripening of strawberry fruits is not only controlled by ABA (Chai et al., 2011; Li et al., 2011; Jia et al., 2011, 2013; Daminato et al., 2013; Li et al., 2013; Han et al., 2015), but also by several other hormones, including IAA (Given et al., 1988), GA (Csukasi et al., 2011), ethylene (Merchante et al., 2013), JA (Concha et al., 2013), and brassinosteroids (Chai et al., 2013). Although strawberry fruits do not show a peak in ethylene emission during ripening (Given et al., 1988), this hormone plays a role in ripening (Sun et al., 2013). A gradual increase in ABA content is coupled with a decrease in IAA in developing strawberry fruit, suggesting that the ABA/IAA ratio serves as a signal to trigger ripening (Perkins-Veazie, 1995). IAA stimulates early receptacle expansion but inhibits later ripening (Given et al., 1988), while ABA is a key inducer of ripening (Jia et al., 2011). Our pharmacological tests revealed that PA regulation of strawberry fruit ripening was involved in ABA, ethylene, and IAA (Fig. 2). Consistently, our RNA-seq data indicated that the hormone-related DEGs were also involved in IAA, ABA, and ethylene pathways (Fig. 3). In support of this, up- and down-regulation of FaSAMDC expression promoted and inhibited ABA accumulation and inhibited and promoted IAA accumulation, respectively, while both inhibited ethylene emission in the RNAi and OE fruits (Figs. 5 and 6). These results demonstrate that PA regulation of strawberry fruit ripening is closely related to ABA, ethylene, and IAA.
Given that SAM serves as a common precursor in the synthesis of PAs and ethylene (Apelbaum et al., 1981; Minocha, 1988; Larsson et al., 1997; Martin-Tanguy, 2001), there may be an equilibrium between PA and ethylene in strawberry fruit that is disrupted by the manipulation of FaSAMDC, thus inhibiting ethylene emission (Fig. 6). We also note that the enhanced expression of FaSAMDC in strawberry fruits resulted in an increase in conversion of Put to Spd and then Spm and a high ratio of (Spm + Spd)/Put with the following consequences: (1) Expression levels were promoted in the NCED1 (key for ABA synthesis gene; Jia et al., 2011) and SnRK2 (a positive regulator gene of ABA signaling; Jia et al., 2013) genes but inhibited in the ABI37 (a negative regulator gene of ABA signaling; Jia et al., 2013) gene. This synergistic regulation in ABA synthesis and signaling contributes to a maximized role of ABA in ripening. (2) Expression levels were inhibited in the LUX2 gene (key for IAA transport gene; Carrier et al., 2008), but separately inhibited and promoted in the IAA2 (a negative regulator gene of IAA signaling; Liu et al., 2011) and ARF11 (a positive regulator gene of IAA signaling; Estrada-Johnson et al., 2017) genes, showing that IAA levels and IAA signaling are not synergistic in ripening. (3) Expression levels were inhibited in the ACO1 (key for ethylene synthesis gene; Liu et al., 1999) gene, but promoted in the EBF1 (a negative regulator gene of ethylene signaling; Yang et al., 2010) gene, showing that ethylene synthesis and signaling were inhibited in ripening. Based on these results, we propose a model for PA-mediated regulation of strawberry fruit ripening involving ABA, ethylene, and IAA: In the developmental strawberry fruit, the high Put contents inhibit ripening and contribute to early fruit growth. With the onset of fruit ripening, the high expression levels of SAMDC promote conversion of Put to Spd and then Spm through synergistic expression of ODC, ADC, SPDS, and SPDM. As a result, the higher Spm contents promote ABA accumulation and signaling and inhibit both IAA and ethylene accumulation, while promoting IAA signaling but inhibiting ethylene signaling. This leads to increased expression of softening-, anthocyanin-, and sugar-related genes, which promotes ripening (Fig. 8). In conclusion, PAs, especially Spm, regulate strawberry fruit ripening in an ABA-dominated, IAA-participating, and ethylene-coordinated manner.
Figure 8.
A model for PA regulation of strawberry fruit ripening through ABA, IAA, and ethylene. In developing strawberry fruit, the biosynthesis of polyamines (Put, Spd, and Spm) depends on ODC, ADC, SAMDS, SPDS, and SPDM, of which SAMDC is a rate-limiting step for Spd and Spm synthesis. The up-regulation of SAMDC expression positively regulates the expression of ODC, ADC, SPDS, and SPDM, which promotes Spd and, especially, Spm accumulation while inhibiting Put accumulation. A high ratio of (Spd + Spm)/Put accelerates ABA synthesis and signaling by promoting the expression of NCED1 (key to ABA synthesis) and SnRK2 (a positive regulator of ABA signaling) and inhibiting the expression of ABI37 (a negative regulator of ABA signaling).The high (Spd + Spm)/Put ratio also differently regulates IAA transport and signaling by inhibiting the expression of AUX2 (key to ABA transport) and IAA2 (a negative regulator of IAA signaling) and promoting the expression of ARF11 (a positive regulator of ABA signaling). However, the high ratio inhibits ethylene synthesis and signaling by inhibiting the expression of ACO1 (key to ethylene synthesis) gene and promoting the expression of EBF1 (a negative regulator of ethylene signaling) gene. Thus, PAs regulate strawberry fruit ripening in an ABA-dominated, IAA-participating, and ethylene-coordinated manner to promote ripening-related gene expression levels, including those for firmness (PG1 and PL1), anthocyanin content (CHS and DFR), and sugar content (SUT1 and SS). The graphic symbols (arrow, t-bar, and arrow with bar) represent promote, inhibit, and no cooperation. Red arrows indicate promotion.
MATERIALS AND METHODS
Plant Material
Strawberry (Fragaria ananassa) plants (‘Sweet Charlie’) were grown in the greenhouse during the spring season from 2015 to 2016. In total, 300 SG fruit on 50 plants were tagged after flowering. Seven stages (SG, LG, DG, Wt, IR, PR, and FR) were collected at 7, 15, 20, 23, 25, 27, and 30 d after flowering, respectively. At every stage, 40 uniformly sized fruit were sampled and frozen in liquid nitrogen (−80°C) until further use.
RNA-Seq, cDNA Synthesis, and Data Analysis
Three fruits (n = 3) of each stage were randomly selected for RNA isolation and cDNA synthesis. RNA was extracted from the receptacles of DG, Wt, IR, and PR fruits using the RNeasy plant mini kit (Qiagen). DNase digestion was performed by RNase-Free DNase (Qiagen), and cDNA synthesis was conducted using the RNA library prep kit (New England Biolabs). RNA-seq and data analysis were carried out using Illumina HiSeq 2000 by the Yuanyi Gene Company as previously described (Mortazavi et al., 2008; Ming et al., 2012; Wang et al., 2010; Benjamini and Yekutieli, 2001; Langmead and Salzberg, 2012) and using available genetic databases, including the NCBI nonredundant protein database, SWISS-PROT, TrEMBL, Cdd, pfam, KOG database, KEGG, and Gene Ontology based on an E-value of 1e−5 and identity with 30%. The experiment was repeated three times.
FaSAMDC Cloning
The obtained cDNA was used as a template for amplifying FaSAMDC with primers (GenBank MG266892, not released; forward, 5′-ATGGCTGTACCGGTTTCTGC-3′; reverse, 5′-CTAGTTCTTCATGCTCAAAC-3′). PCR was performed with an annealing temperature of 58°C.The PCR fragment was inserted into a T1 simple vector and then transformed into Escherichia coli DH5α (BioTeke). Positive colonies were sequenced by the Huada Company.
Construction of Recombinant Plasmids and Transfection of Strawberry by Agroinfiltration
pTRV1 and pTRV2 were used for VIGS (Liu et al., 2002). A 650-bp cDNA fragment of FaSAMDC was amplified (primers: sense, 5′-GGAATTCGTAGCCCTGAC-3′; antisense, 5′-GGGGTACCGTTCTTCATGC-3′) and inserted into pTRV2 using restriction enzymes EcoRI and KpnI. Agrobacterium tumefaciens strain GV3101 containing pTRV1, pTRV2, or pTRV2-FaSAMDC was infiltrated into strawberry fruits. To generate the FaSAMDC overexpression construct, the full-length cDNA of FaSAMDC was obtained by PCR (primers: forward, 5′-GAATTCATGGCT GTACCGGTTTCTGC-3′, EcoRI site underlined; reverse, 5′-GGTACCTAGTTCTTCATGCTCAAACTC-3′, KpnI site underlined). The cDNA was inserted into pCAMBIA1304 using EcoRI and KpnI. The A. tumefaciens suspension was injected into 20 DG strawberry fruits attached to the plants as previously described (Fu et al., 2005; Jia et al., 2011). The experiment was repeated one time.
Detection of TRV Vectors
Three infected fruits (n = 3) were used. The primers for TRV1 (sense, 5′-TGCTCCTGAAAGTATGTTAGTGG-3′; antisense, 5′-CATCTCGGATGTCTCGACG-3′) and TRV2 (sense, 5′-CCGACTCATTGTCTTACCATAG-3′; antisense, 5′-TCTCCCGTTTCGTCCTTT-3′) were used to detect TRV vectors in the infiltrated strawberry fruits as previously described (Chai et al., 2011). The experiment was repeated three times.
qPCR
Three transgenic fruits (n = 3) were randomly selected from each stage for RNA isolation and cDNA synthesis as above. Gene expression levels were analyzed by real-time qPCR. The qPCR was performed using a Light Cycler 96 real-time PCR system (Bio-Rad). The reactions (20 μL) contained 10 μL of SYBR Premix ExTaq (TaKaRa), 0.4 μL of 10 μm forward-specific primer, 0.4 μL of 10 μm reverse-specific primer, and 2 μL of 1 μm cDNA templates. qPCR was conducted with three biological replicates, and each sample was analyzed at least in triplicate and normalized using Actin (Han et al., 2015) as an internal control for relative expression levels confirmed by the form 2–ΔΔCT (Livak and Schmittgen, 2001). The primers used for real-time PCR are shown in Supplemental Table S4. The experiment was repeated three times.
Effects of PAs and a SAMDC Inhibitor on Strawberry Fruit Ripening in Vivo
Twenty LG stage fruits still attached to plants were selected (n = 20) and immersed independently into 100 µM Put, Spd, Spm, and MGBG for 20 s, respectively. Sterile water was used as a control. A total of four immersions were performed for every treatment at 1-d intervals. Ten days after treatment, phenotypes were observed. The experiment was repeated one time.
Expression and Purification of FaSAMDC Recombinant Proteins
Expression and purification of FaSAMDC were done in an E. coli expression system. The coding sequence of FaSAMDC was amplified by PCR (forward, 5′-GAATTCATGGCTGTACCGGTTTCTG-3′, EcoRI site underlined; reverse, 5′-GCGGCCGCCTAGTTCTTCATGCTCAAACTC-3′, NotI site underlined) and inserted into expression vector PGEX-4T1 in frame with the N-terminal GST-tag fusion (BioTeke). The recombinant plastids were transformed into E. coli for selection of transformants on LB plates with ampicillin (100 μg/mL). The FaSAMDC fusion protein was expressed and purified using BeaverBeads GST, and immunoblotting was performed with an antibody of the C-terminal His tag using a one-step western kit HRP (anti-Mouse; Kangwei Company) following the manufacturer’s protocols.
Analysis of Enzyme Activity
The activity of FaSAMDC was assayed by HPLC using an enzyme activity standard curve. SAM was formulated at concentrations with 1.0, 0.8, 0.5, 0.4, and 0.1 mg/mL for construction of a SAM standard curve. To investigate enzymatic activity, the reaction of FaSAMDC protein (2 mg/mL) and SAM (25 mM) was carried out in a buffer (50 mm Tris-HCl, pH 8.0) at 30°C for 10 min. After centrifugation, the supernatant (1 mL) was filtered with a 0.22-µm filter, and the free SAM content was determined by HPLC. The C18-column (Zorbax Eclipse XDB-C18, 4.6 × 150 mm, 5 µm; Agilent) was used at 30°C for 10 min at a flow rate of 0.6 mL per min, and the mobile phase was 0.01 mm ammonium formate:methanol (v:v, 97:3). Effluent absorbance was monitored at 260 nm. The injection volume was 10 µL. The experiment was repeated three times.
Determination of PA, Anthocyanin, and Soluble Sugar Contents
Three fruits (n = 3) were used for determination of PA contents by HPLC after the preparation and derivation of standard products. Based on Redmond and Tseng (1979), the improved method of acetylation of benzene was used. Samples (0.1 g) of individual PAs, including Put, Spd, and Spm, were dissolved with ultrapure water to 10 mL in volumetric flasks as standards. The 20-µL solutions of Put, Spd, and Spm, individually, were placed into 10-mL centrifugal tubes, benzoyl chloride (10 µL) and 2 mol/L sodium hydroxide (1 mL) were added, and the mixture was shaken for 20 s using a vortex mixer and then incubated at 37°C for 20 min. Saturated sodium chloride solution was added, and the solution was extracted with diethyl ether (2 mL). After centrifugation at 4°C at 1,500g for 5 min to separate the layers, the upper organic phase was removed and evaporated. The residue was dissolved in methanol (1 mL) and filtered with a 0.45-µm filter. Strawberry fruit (1 g, n = 3) was ground in liquid nitrogen to powder and then placed in a centrifuge tube (10 mL). Precooled perchlorate (volume fraction 5%) was added to the centrifuge tubes, and the mixture was shaken using a vortex mixer and then extracted in an ice bath for 1 h. After extraction, the mixtures were centrifuged at 4°C at 1,500g for 30 min. The PA extraction from the supernatant (500 µL) was added to centrifuge tube (10 mL). The derivation of the supernatant was also as described above. The PA contents were examined by HPLC using the C18-column (Zorbax Eclipse XDB-C18, 4.6 × 250 mm, 5 µm; Agilent) at 30°C. The mobile phase was methanol:water (v:v, 64:36) for 20 min at a flow rate of 0.7 mL per min. Effluent absorbance was monitored at 230 nm. The injection volume was 10 µL. The experiment was repeated three times.
Three fruits (n = 3) were used for detection of anthocyanin and soluble sugar contents by HPLC using a Zorbax Eclipse XDB-C18 column (4.6 × 150 mm, 5 µm; Agilent) and Agilent Technologies 1200 Series, 6.5 × 300-mm Sugar-Pak column (Waters), respectively, as previously described (Jia et al., 2011). The experiment was repeated three times.
Determination of ABA, IAA, and Ethylene Contents
Three strawberry fruits (n = 3) were used for ABA or IAA analysis. For ABA or IAA extractions, 1 g of receptacle was ground and homogenized in a solution (80% methanol, v/v) and then centrifuged at 10,000g for 20 min. The supernatants were eluted by the Sep-Pak C18 cartridge (Waters), after removal of polar compounds, then used for immune assays as described by Zhang et al. (2009).
Three uniform strawberry fruits (n = 3) were selected and placed in 200-mL glass jars for 2 h at 25°C, then 1 mL of gas from the jar headspace was withdrawn and injected into a gas chromatograph with a flame ionization detector and an activated alumina column (model 6890 N; Agilent) as previously described (Sun et al., 2013). The experiment was performed with three replications.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: Actin, AB116565; SAMDC, MG266892; PG1, AF380299; PL1, DQ076239; SUT1, JX013937; SS, AB275666; CHS, AY997297; DFR, AY695813; SPMS, XM_011471922; SPDS, XM_004297595; ADC, XM_004290251; ODC, XM_011464632; NCED1, HQ290318; ACO1, AJ851828; IAA2, XM_009340458; ARF11, XM_011459741; LUX2, XM_004302600; ABI37, XM_004307422; SnRK2, KJ748362; and EBF1, XM_004287259.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. KOG function classification of consensus sequence.
Supplemental Figure S2. Gene Ontology classification.
Supplemental Figure S3. Putative conserved domains in the FaSAMDC protein.
Supplemental Figure S4. The S-adenosyl-Met (SAM) standard curve.
Supplemental Table S1. Raw reads and clean reads from RNA-seq.
Supplemental Table S2. Summary of Illumina transcriptome assembly for strawberry.
Supplemental Table S3. The top five pathways annotated with KEGG pathway analysis.
Supplemental Table S4. Primers used for qPCR.
Acknowledgments
We thank Dr. Yu-Le Liu (Qinghua University) for the pTRV vectors and Dr. Qing Zhang (Beijing Key Laboratory for Agricultural Application and New Technique) for isothermal titration calorimetry analysis.
Footnotes
Articles can be viewed without a subscription.
References
- Adams-Phillips L, Barry C, Giovannoni J (2004) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9: 331–338 [DOI] [PubMed] [Google Scholar]
- Agudelo-Romero P, Ali K, Choi YH, Sousa L, Verpoorte R, Tiburcio AF, Fortes AM (2014) Perturbation of polyamine catabolism affects grape ripening of Vitis vinifera cv. Trincadeira. Plant Physiol Biochem 74: 141–155 [DOI] [PubMed] [Google Scholar]
- Agudelo-Romero P, Bortolloti C, Pais MS, Tiburcio AF, Fortes AM (2013) Study of polyamines during grape ripening indicate an important role of polyamine catabolism. Plant Physiol Biochem 67: 105–119 [DOI] [PubMed] [Google Scholar]
- Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53: 2039–2055 [DOI] [PubMed] [Google Scholar]
- Apelbaum A, Burgoon AC, Anderson JD, Lieberman M (1981) Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol 68: 453–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29: 1165–1188 [Google Scholar]
- Bregoli AM, Scaramagli S, Costa G, Sabatini E, Ziosi V, Biondi S, Torrigiani P (2002) Peach (Prunus persica) fruit ripening: aminoethoxyvinylglycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol Plant 114: 472–481 [DOI] [PubMed] [Google Scholar]
- Carrier DJ, Bakar NT, Swarup R, Callaghan R, Napier RM, Bennett MJ, Kerr ID (2008) The binding of auxin to the Arabidopsis auxin influx transporter AUX1. Plant Physiol 148: 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai YM, Jia HF, Li CL, Dong QH, Shen YY (2011) FaPYR1 is involved in strawberry fruit ripening. J Exp Bot 62: 5079–5089 [DOI] [PubMed] [Google Scholar]
- Chai YM, Zhang Q, Tian L, Li CL, Xing Y, Qin L, Shen YY (2013) Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul 69: 63–69 [Google Scholar]
- Concha CM, Figueroa NE, Poblete LA, Oñate FA, Schwab W, Figueroa CR (2013) Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol Biochem 70: 433–444 [DOI] [PubMed] [Google Scholar]
- Csukasi F, Osorio S, Gutierrez JR, Kitamura J, Giavalisco P, Nakajima M, Fernie AR, Rathjen JP, Botella MA, Valpuesta V, Medina-Escobar N (2011) Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytol 191: 376–390 [DOI] [PubMed] [Google Scholar]
- Daminato M, Guzzo F, Casadoro G (2013) A SHATTERPROOF-like gene controls ripening in non-climacteric strawberries, and auxin and abscisic acid antagonistically affect its expression. J Exp Bot 64: 3775–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dios P, Matilla AJ, Gallardo M (2006) Flower fertilization and fruit development prompt changes in free polyamines and ethylene in damson plum (Prunus insititia L.). J Plant Physiol 163: 86–97 [DOI] [PubMed] [Google Scholar]
- Diboun I, Mathew S, Al-Rayyashi M, Elrayess M, Torres M, Halama A, Méret M, Mohney RP, Karoly ED, Malek J, Suhre K (2015) Metabolomics of dates (Phoenix dactylifera) reveals a highly dynamic ripening process accounting for major variation in fruit composition. BMC Plant Biol 15: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano M, Merodio C (1994) The Relevance of polyamine levels in cherimoya (Annona cherimola Mill.) fruit ripening. J Plant Physiol 143: 207–212 [Google Scholar]
- Estrada-Johnson E, Csukasi F, Pizarro CM, Vallarino JG, Kiryakova Y, Vioque A, Brumos J, Medina-Escobar N, Botella MA, Alonso JM, et al. (2017) Transcriptomic analysis in strawberry fruits reveals active auxin biosynthesis and signaling in the ripe receptacle. Front Plant Sci 8: 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima T, Sobolev AP, Teasdale JR, Kramer M, Bunce J, Handa AK, Mattoo AK (2016) Fruit metabolite networks in engineered and non-engineered tomato genotypes reveal fluidity in a hormone and agroecosystem specific manner. Metabolomics 12: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes AM, Teixeira RT, Agudelo-Romero P (2015) Complex interplay of hormonal signals during grape berry ripening. Molecules 20: 9326–9343 [DOI] [PMC free article] [PubMed]
- Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB (2005) Virus-induced gene silencing in tomato fruit. Plant J 43: 299–308 [DOI] [PubMed] [Google Scholar]
- Handa AK, Mattoo AK (2010) Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol Biochem 48: 540–546 [DOI] [PubMed] [Google Scholar]
- Gallusci P, Hodgman C, Teyssier E, Seymour GB (2016) DNA methylation and chromatin regulation during fleshy fruit development and ripening. Front Plant Sci 7: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given NK, Venis MA, Gierson D (1988) Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta 174: 402–406 [DOI] [PubMed] [Google Scholar]
- Gupta A, Pal RK, Rajam MV (2013) Delayed ripening and improved fruit processing quality in tomato by RNAi-mediated silencing of three homologs of 1-aminopropane-1-carboxylate synthase gene. J Plant Physiol 170: 987–995 [DOI] [PubMed] [Google Scholar]
- Han Y, Dang R, Li J, Jiang J, Zhang N, Jia M, Wei L, Li Z, Li B, Jia W (2015) FaSnRK2.6, an ortholog of open stomata 1, is a negative regulator of strawberry fruit development and ripening. Plant Physiol 167: 915–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AS, Singh Z (2010) Pre-harvest application of putrescine influences Japanese plum fruit ripening and quality. Food Sci Technol Int 16: 53–64 [DOI] [PubMed] [Google Scholar]
- Kolotilin I, Koltai H, Bar-Or C, Chen L, Nahon S, Shlomo H, Levin I, Reuveni M (2011) Expressing yeast SAMdc gene confers broad changes in gene expression and alters fatty acid composition in tomato fruit. Physiol Plant 142: 211–223 [DOI] [PubMed] [Google Scholar]
- Kushad MM, Yelenosky G, Knight R (1988) Interrelationship of polyamine and ethylene biosynthesis during avocado fruit development and ripening. Plant Physiol 87: 463–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Khurana A, Sharma AK (2014) Role of plant hormones and their interplay in development and ripening of fleshy fruits. J Exp Bot 65: 4561–4575 [DOI] [PubMed] [Google Scholar]
- Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157: 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HF, Lu D, Sun JH, Li CL, Xing Y, Qin L, Shen YY (2013) Type 2C protein phosphatase ABI1 is a negative regulator of strawberry fruit ripening. J Exp Bot 64: 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed]
- Lasanajak Y, Minocha R, Minocha SC, Goyal R, Fatima T, Handa AK, Mattoo AK (2014) Enhanced flux of substrates into polyamine biosynthesis but not ethylene in tomato fruit engineered with yeast S-adenosylmethionine decarboxylase gene. Amino Acids 46: 729–742 [DOI] [PubMed] [Google Scholar]
- Larsson J, Rasmuson-Lestander A (1997) Cloning, mapping and mutational analysis of the S-adenosylmethionine decarboxylase gene in Drosophila melanogaster. Mol Gen Genet 256: 652–660 [DOI] [PubMed] [Google Scholar]
- Law DM, Davies PJ, Mutschler MA (1991) Polyamine-induced prolongation of storage in tomato fruits. Plant Growth Regul 10: 283–290 [Google Scholar]
- Li C, Jia H, Chai Y, Shen Y (2011) Abscisic acid perception and signaling transduction in strawberry: a model for non-climacteric fruit ripening. Plant Signal Behav 6: 1950–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ji K, Sun Y, Luo H, Wang H, Leng P (2013) The role of FaBG3 in fruit ripening and B. cinerea fungal infection of strawberry. Plant J 76: 24–35 [DOI] [PubMed] [Google Scholar]
- Liu DJ, Chen JY, Lu WJ (2011) Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Mol Biol Rep 38: 1187–1193 [DOI] [PubMed] [Google Scholar]
- Liu M, Pirrello J, Chervin C, Roustan JP, Bouzayen M (2015) Ethylene control of fruit ripening: revisiting the complex network of transcriptional regulation. Plant Physiol 169: 2380–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shiomi S, Nakatsuka A, Kubo Y, Nakamura R, Inaba A (1999) Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol 121: 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Mariani P, Dorazi D, Bagni N (1989) Polyamines in primary walls of carrot cells: endogenous content and interactions. J Plant Physiol 135: 508–510 [Google Scholar]
- Martin-Tanguy J. (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34: 135–148 [Google Scholar]
- Mattoo A, Cassol T, Mehta R, Handa A, Ali N, Abdul-Baki A (2002) Genetic engineering of tomato fruit for sustained accumulation of polyamines during ripening to study their physiological role(s). Acta Hortic 575: 157–161 [Google Scholar]
- Mattoo AK, Sobolev AP, Neelam A, Goyal RK, Handa AK, Segre AL (2006) Nuclear magnetic resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions. Plant Physiol 142: 1759–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Chung SH, Goyal RK, Fatima T, Solomos T, Srivastava A, Handa AK (2007) Overaccumulation of higher polyamines in ripening transgenic tomato fruit revives metabolic memory, upregulates anabolism-related genes, and positively impacts nutritional quality. J AOAC Int 90: 1456–1464 [PubMed] [Google Scholar]
- Mehta RA, Cassol T, Li N, Ali N, Handa AK, Mattoo AK (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotechnol 20: 613–618 [DOI] [PubMed] [Google Scholar]
- Merchante C, Vallarino JG, Osorio S, Aragüez I, Villarreal N, Ariza MT, Martínez GA, Medina-Escobar N, Civello MP, Fernie AR, Botella MA, Valpuesta V (2013) Ethylene is involved in strawberry fruit ripening in an organ-specific manner. J Exp Bot 64: 4421–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming YL, Gui RQ, Jing J, Hui QY, Li HX, Jin ZX, Ren YZ (2012) Transcriptome sequencing and de novo analysis for bamboo using the illumina platform. PLoS One 7: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha SC. (1988) Relationship between polyamine and ethylene biosynthesis in plants and its significance for morphogenesis in cell cultures. Adv Exp Med Biol 250: 601–616 [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628 [DOI] [PubMed] [Google Scholar]
- Nambeesan S, Datsenka T, Ferruzzi MG, Malladi A, Mattoo AK, Handa AK (2010) Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J 63: 836–847 [DOI] [PubMed] [Google Scholar]
- Nambeesan S, Mattoo AK, Handa AK (2008) Polyamines and regulation of ripening and senescence. In G Paliyath, DP Murr, AK Handa, S Lurie, eds, Postharvest Biology and Technology of Fruits, Vegetables, and Flowers. Wiley-Blackwell, Ames, IA, pp 319–340 [Google Scholar]
- Neily MH, Matsukura C, Maucourt M, Bernillon S, Deborde C, Moing A, Yin YG, Saito T, Mori K, Asamizu E, et al. (2011) Enhanced polyamine accumulation alters carotenoid metabolism at the transcriptional level in tomato fruit over-expressing spermidine synthase. J Plant Physiol 168: 242–252 [DOI] [PubMed] [Google Scholar]
- Osorio S, Scossa F, Fernie AR (2013) Molecular regulation of fruit ripening. Front Plant Sci 4: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál M, Szalai G, Janda T (2015) Speculation: Polyamines are important in abiotic stress signaling. Plant Sci 237: 16–23 [DOI] [PubMed] [Google Scholar]
- Pandey R, Gupta A, Chowdhary A, Pal RK, Rajam MV (2015) Over-expression of mouse ornithine decarboxylase gene under the control of fruit-specific promoter enhances fruit quality in tomato. Plant Mol Biol 87: 249–260 [DOI] [PubMed] [Google Scholar]
- Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130: 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins-Veazie P. (1995) Growth and ripening of strawberry fruit. Hortic Rev (Am Soc Hortic Sci) 17: 267–297 [Google Scholar]
- Prasanna V, Prabha TN, Tharanathan RN (2007) Fruit ripening phenomena--an overview. Crit Rev Food Sci Nutr 47: 1–19 [DOI] [PubMed] [Google Scholar]
- Redmond JW, Tseng A (1979) High-press liquid chromatographic determination of putrescine,cadaverine,spermidine and spermine. J Chromatogr A 170: 479–481 [Google Scholar]
- Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64: 219–241 [DOI] [PubMed] [Google Scholar]
- Shen YY, Rose JKC (2014) ABA metabolism and signaling in fleshy fruits. In DP Zhang, ed, Abscisic Acid: Metabolism, Transport and Signaling, Springer, New York, NY, pp 271–286 [Google Scholar]
- Simpson CG, Cullen DW, Hackett CA, Smith K, Hallett PD, McNicol J, Woodhead M, Graham J (2017) Mapping and expression of genes associated with raspberry fruit ripening and softening. Theor Appl Genet 130: 557–572 [DOI] [PubMed] [Google Scholar]
- Sun JH, Luo JJ, Tian L, Li CL, Xing Y, Shen YY (2013) New evidence for the role of ethylene in strawberry fruit ripening. Plant Growth Regul 32: 461–470 [Google Scholar]
- Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53: 749–790 [DOI] [PubMed] [Google Scholar]
- Tassoni A, Watkins CB, Davies PJ (2006) Inhibition of the ethylene response by 1-MCP in tomato suggests that polyamines are not involved in delaying ripening, but may moderate the rate of ripening or over-ripening. J Exp Bot 57: 3313–3325 [DOI] [PubMed] [Google Scholar]
- Teh HF, Neoh BK, Wong YC, Kwong QB, Ooi TE, Ng TL, Tiong SH, Low JY, Danial AD, Ersad MA, Kulaveerasingam H, Appleton DR (2014) Hormones, polyamines, and cell wall metabolism during oil palm fruit mesocarp development and ripening. J Agric Food Chem 62: 8143–8152 [DOI] [PubMed] [Google Scholar]
- Tilak P, Raymond MA (1996) Polyamines in normal and auxin-induced strawberry fruit development. Physiol Plant 98: 447–454 [Google Scholar]
- Torrigiani P, Bressanin D, Ruiz KB, Tadiello A, Trainotti L, Bonghi C, Ziosi V, Costa G (2012) Spermidine application to young developing peach fruits leads to a slowing down of ripening by impairing ripening-related ethylene and auxin metabolism and signaling. Physiol Plant 146: 86–98 [DOI] [PubMed] [Google Scholar]
- Torrigiani P, Bregoli AM, Ziosi V, Scaramagli S, Ciriaci T, Rasori A, Biondi S, Costa G (2004) Pre-harvest polyamine and aminoethoxyvinylglycine (AVG) applications modulate fruit ripening in Stark Red Gold nectarines (Prunus persica L. Batsch). Postharvest Biol Technol 33: 293–308 [Google Scholar]
- Van de Poel B, Bulens I, Oppermann Y, Hertog ML, Nicolai BM, Sauter M, Geeraerd AH (2013) S-adenosyl-L-methionine usage during climacteric ripening of tomato in relation to ethylene and polyamine biosynthesis and transmethylation capacity. Physiol Plant 148: 176–188 [DOI] [PubMed] [Google Scholar]
- Wang CY, Conway WS, Abbott JA, Kramer GF, Sams CE (1993) Postharvest infiltration of polyamines and calcium influences ethylene production and texture changes in ‘Golden Delicious’ apples. J Am Soc Hortic Sci 118: 801–806 [Google Scholar]
- Wang Z, Fang B, Chen J, Zhang X, Luo Z, Huang L, Chen X, Li Y (2010) De novo assembly and characterization of root transcriptome using Illumina paired-end sequencing and development of cSSR markers in sweet potato (Ipomoea batatas). BMC Genomics 11: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Hu W, Gong H, Pua EC (2006) Modulation of SAMDC expression in Arabidopsis thaliana alters in vitro shoot organogenesis. Physiol Plant 128: 740–750 [Google Scholar]
- Yang Y, Wu Y, Pirrello J, Regad F, Bouzayen M, Deng W, Li Z (2010) Silencing Sl-EBF1 and Sl-EBF2 expression causes constitutive ethylene response phenotype, accelerated plant senescence, and fruit ripening in tomato. J Exp Bot 61: 697–708 [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60: 1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SH, Hou BZ, Chai L, Yang AZ, Yu XY, Shen YY (2017) Sigma factor FaSigE positively regulates strawberry fruit ripening by ABA. Plant Growth Regul 83: 417–427 [Google Scholar]
- Zhao C, Hua LN, Liu XF, Li YZ, Shen YY, Guo JX (2017) Sucrose synthase FaSS1 plays an important role in the regulation of strawberry fruit ripening. Plant Growth Regul 81: 175–181 [Google Scholar]
- Ziosi V, Bregoli AM, Bonghi C, Fossati T, Biondi S, Costa G, Torrigiani P (2006) Transcription of ethylene perception and biosynthesis genes is altered by putrescine, spermidine and aminoethoxyvinylglycine (AVG) during ripening in peach fruit (Prunus persica). New Phytol 172: 229–238 [DOI] [PubMed] [Google Scholar]