Abstract
Our objective was to determine if progesterone pretreatment could ameliorate the detrimental effects of lipopolysaccharide (LPS)-induced inflammation on cortical neurogenesis. Timed pregnant mouse dams (n = 8) were given intraperitoneal injections of progesterone (42 mg/kg) or vehicle on embryonic day 17.5. Two hours later, mice were given intraperitoneal LPS (140 μg/kg) or vehicle. Mice were sacrificed 16 hours later on embryonic day 18. Two-color immunofluorescence was performed with primary antibodies T-box transcription factor 2 (Tbr2), ionized calcium binding adapter molecule 1 (Iba1), cleaved caspase 3 (CC3), and 5-bromo-2′-deoxyuridine (BrdU). Cells were counted, and statistical analysis was determined using analysis of variance and Tukey-Kramer method. The Tbr2 intermediate neural progenitor cell density decreased after LPS exposure (P = .0022). Pre-exposure to progesterone statistically increased Tbr2 intermediate neural progenitors compared to LPS treatment alone and was similar to controls (P = .0022). After LPS exposure, microglia displayed an activated phenotype, and cell density was increased (P < .001). Cell death rates were low among study groups but was increased in LPS exposure groups compared to progesterone alone (P = .0015). Lipopolysaccharide-induced systemic inflammation reduces prenatal neurogenesis in mice. Pre-exposure with progesterone is associated with increased neurogenesis. Progesterone may protect the preterm brain from defects of neurogenesis induced by inflammation.
Keywords: inflammation, microglia, neurogenesis, prematurity, progesterone
Introduction
Preterm birth complicates 15 million pregnancies annually worldwide and is the leading cause of childhood mortality.1 One of every 4 preterm births is the result of a microbial-derived inflammatory stimulus.1 Premature infants are at risk for neurologic injury which is dependent on the gestational age of the fetus at the time of injury and the presence of infection, inflammation, or ischemic injury.1–6 Both prematurity and inflammation can reduce neurogenesis in critical neural progenitor groups, leading to reduced cortical growth and increased risk of adverse neurodevelopmental outcomes.7,8 Perinatal inflammatory events such as chorioamnionitis affect the developing fetal brain through activation of the innate immune system.6,9 Microglia, the cerebral cortex’s resident macrophage, colonize the proliferating zone of the embryonic neocortex and may be mobilized by maternal inflammatory activation of the innate immune system, leading to local cytokine release, cell injury, and increased phagocytosis of neural precursor cells such as intermediate neural progenitors (INPs).7,10 Alterations in neural precursor populations may be an important mediator of the adverse neurodevelopmental outcomes seen in preterm infants.
New neurons are formed in the developing brain through direct and indirect neurogenesis.11 During the middle and later part of cortical neurogenesis, INP cells arise from radial glia cells.11 INPs may divide and ultimately give rise to 2 to 4 postmitotic neurons through indirect neurogenesis.11 Many factors, including sex steroids, play an important role in brain development and protection. For example, estrogen has been found to stimulate INP cell proliferation in the developing embryonic cerebral cortex.12 Estrogen and progesterone have been demonstrated to reduce brain injury following ischemic stroke and acute traumatic brain injury.13–15 The protective effect of sex steroids in the brain is likely modulated through various pathways, including membrane bound progesterone receptors and expression of steroid-converting enzymes on microglia.16,17 After a lipopolysaccharide (LPS)-induced inflammatory stimulus, steroid-converting enzyme expression on microglia is downregulated in the mouse brain, resulting in a reduction in endogenous steroid levels.17
Activated microglia have been implicated in altered embryonic neurogenesis in fetuses where maternal inflammation was induced by LPS.7 An LPS-induced inflammatory cascade enters the brain through interleukin 1B, which activates microglia, evidenced by a change in cell morphology and proinflammatory cytokine expression.7,17 Activated microglia increase local cytokine release and phagocytosis, reducing the number of neural progenitor cells in the prenatal brain.7 When microglia activity is inhibited, neural progenitor populations are increased.7 There is evidence that progesterone supplementation can shift proinflammatory microglia to an anti-inflammatory phenotype after hypoxia-ischemia–induced inflammation.18 There are no studies to date that have evaluated the effect of progesterone supplementation on microglia activity or cortical neurogenesis during an acute LPS-induced inflammatory event.
The primary aim of this study was to evaluate neurogenesis by measuring INP and microglia cell numbers in the embryonic mouse cerebral cortex after maternal intraperitoneal LPS exposure with or without progesterone pre-exposure. We hypothesized that intraperitoneal progesterone prevents INP cell loss after LPS exposure. The secondary aim was to describe microglia morphology after LPS and progesterone exposure, since morphology is a surrogate marker of cell phenotype, as well as quantify cell proliferation and cell death in the exposure groups.
Materials and Methods
Mouse Model of Intrauterine Inflammation
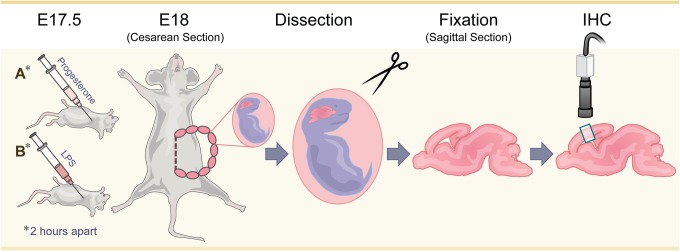
A total of 31 animals, including 8 pregnant female dams and 23 fetal pups, were used for this study. Animals were treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at Seattle Children’s Research Institute. C57BL6 timed pregnant dams (n = 8, Jackson Laboratory, California) were randomized into 4 groups of 2 each: control with sham, progesterone exposure only, LPS exposure only, or LPS plus progesterone pre-exposure. All pregnant dams on embryonic day (ED) 17.5 received intraperitoneal 5-bromo-2′-deoxyuridine (BrdU; 20 mg/kg, Sigma, Missouri), a thymidine analog that is incorporated into dividing cells; these animals simultaneously also received progesterone in oil (42 mg/kg, Sigma) or control oil vehicle. Two hours later, mice were given an intraperitoneal injection of LPS (140 µg/kg; from Escherichia coli 0111:B4, EMD Millipore, Calbiochem, California) or saline vehicle. The dose of LPS was adopted based on our laboratory’s experience with dosing that would achieve preterm labor, mimicking acute chorioamnionitis, as well as demonstrate brain pathology and a reduction in neurogenesis based on prior research.3,4,7 Based on our laboratories prior experience with LPS at this dose, dams routinely experienced preterm labor within 16 hours of LPS injection. Since LPS has been shown to reduce maternal progesterone levels, a pharmacologic dose of progesterone was chosen which was expected to counter the effect of LPS on maternal serum progesterone levels by at least 50%, without preventing preterm birth.19 On ED 18, 16 hours after LPS administration, dams were euthanized by cervical dislocation, and cesarean section was performed. Pups were dissected from the uterine horn. All pups were live born and once dissected were placed in cold phosphate buffered saline, followed by decapitation and immersion fixation in 4% paraformaldehyde in phosphate-buffered saline. Fetal weight and sex were not determined. Litter sizes for the control dams were 7 and 2, the smaller litter born from a younger and smaller dam compared to the other dams. Progesterone exposure litter sizes were 6 and 5, LPS exposure litters were 7 and 5, and the LPS and progesterone pre-exposure litters, 2 litters of 5 pups each. Brains and spinal cord were dissected, fixed for 24 hours in paraformaldehyde at 4°C and then transferred to 30% sucrose before being embedded in OCT (Tissue-Tek, Satura-Finetek, Torrance, California). Samples were stored at −80°C. Three pups representative of each litter were then selected for further study with the exception of only 2 pups available from the second control litter. A total of 6 pups were therefore analyzed from each exposure group with the exception of 5 pups in the control group. Embryonic brains were sectioned in sagittal section at 12 μm on a cryostat, mounted on Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, Pennsylvania) and stored at −80°C until immunohistochemistry was performed (Figure 1).
Figure 1.
Experimental design. On embryonic day 17.5 pregnant dams were randomized to control with sham; progesterone exposure only; LPS exposure only; or LPS plus progesterone pre-exposure. On embryonic day 18 mice were sacrificed and cesarean section was performed. Pups were dissected from the uterine horn and prenatal brains were fixed in sagittal section. Two-color immunofluorescence was preformed with selected markers and digital images obtained with a Zeiss LSM confocal microscope.
Intermediate neural progenitor cell (T-box transcription factor 2) and microglia (ionized calcium binding adapter molecule 1) cell immunohistochemistry
We analyzed INP cell populations using the T-box transcription factor 2 (Tbr2), one of a series of developmentally programed transcription factors sequentially expressed during glutamatergic neurogenesis in the developing neocortex.11 The INP cells primarily populate the ventricular (VZ) and subventricular (SVZ) zones in the mouse.11 Microglia cell density was evaluated using antibodies against ionized calcium binding adapter molecule 1 (Iba1) present on microglia.7,20 Two-color immunofluorescence was performed on the somatosensory cortex of ED 18 sagittal brain sections. Antigen enhancement was performed first on selected sections by heat treatment for 45 seconds in 10 mmol/L sodium citrate (pH 6.0). Slides were blocked for 30 minutes at room temperature in 5% normal donkey serum (D-9663-10 mL, Sigma) in 1× phosphate-buffered saline plus 0.3% triton-X and 1% bovine serum albumin (blocking solution). Primary antibodies were incubated at room temperature for 2 hours (rabbit anti-Tbr2 1:5008; goat anti-Iba1 1:200, ab5076, Abcam, Cambridge, Massachusetts). Conjugation with secondary antibodies Alexa Fluor 488 and 597 (A-212-6 and A-11057, Life Technologies, Chicago, Illinois) at 1:400 dilutions in blocking solution was performed at room temperature for 2 hours. Slides were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; D-1306-10mg, Life Technologies). Imaging was performed on a Zeiss LSM 710 confocal microscope.
Cell proliferation (BrdU) and cell death (cleaved caspase 3) immunohistochemistry
To further understand the role that microglia may have in the environment and with INPs, cell apoptosis and cell division were evaluated. BdrU was administered at the start of the experiment on ED 17.5 to label cells participating in cell division. Cleaved caspase 3 (CC3) is a marker of early cellular apoptosis.7 Two-color immunofluorescence was performed for CC3 and BrdU to evaluate cell death and division. Antigen enhancement was performed as stated earlier. Slides were blocked for 60 minutes at room temperature in 5% normal goat serum (G-9023-10mL, Sigma) in blocking solution. Primary antibodies were incubated overnight at 4°C (rat anti-BrdU 1:200, OBT0030G, Accurate, Westbury, New York; rabbit anti-CC3 1:400, 9579, Cell Signaling, Danvers, Massachusetts). Conjugation with secondary antibodies Alexa Fluor 488 and 568 (A-11006-7 & A-11011, Life Technologies, Chicago, IL) at 1:400 dilutions in blocking solution were performed at room temperature for 2 hours. Slides were counterstained with DAPI.
Cell counting and surface area measurements
Cell densities for Tbr2, Iba1, and BrdU were determined by conducting cell counts on 3 consecutive 12 μm sections from each animal studied, at the level of the somatosensory cortex, spanning the entire cortex from VZ to pia surface (5-6 animals per group). Images were obtained digitally using a Zeiss LSM 710 confocal microscope. Raw data images were then processed in Adobe Photoshop (Adobe Systems Inc, 2013, San Jose, CA) to optimize levels for high contrast and brightness. A plot and grid layer were overlaid to create 8 equal horizontal bins. Cells were individually marked and counted with a cell counter when high-level (suprathreshold) labeling was present. The area of each bin was measured using ImageJ (National Institutes of Health, Bethesda, MD). Cell scoring was performed primarily by a single researcher with slide review for quality control by a coscientist. Cell counts were done blind to treatment groups, except as unavoidable due to notations on slides. For all cells scored, the labeled antigen of interest and nuclear counterstain were visible above background thresholds and overlapping. Given the size and branching of microglia, only cells with labeled nuclei within the section were scored. Total cell numbers were divided by the total counting area to give the number of cells per millimeter squared. To compare cell death among our groups, CC3 labeled cells were quantified from the entire cerebral cortex but given the small number of cells which were labeled, scoring was done manually at 20× magnification in real time on the microscope. Microglia morphology was assessed based on previously published research which correlated morphology to immunohistochemistry markers of cell activity.7
Statistical analyses
Statistical analysis was performed using STATA 12.1, with analysis of variance and Tukey-Kramer post hoc analysis. Differences were considered statistically significant at P < .05. Power analysis predicted a sample size of 5 animals per exposure group to detect a 25% difference, power 0.8.
Results
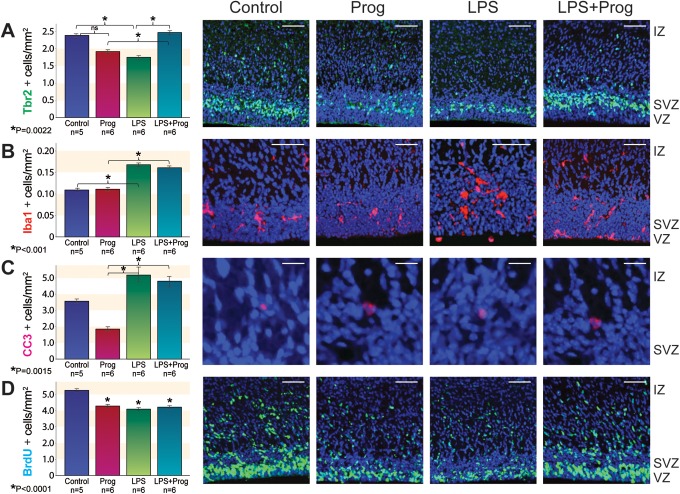
We evaluated embryonic neurogenesis by measuring Tbr2+ INPs under our exposure conditions in the somatosensory cortex (Figure 2A). The Tbr2+ INP cell density was not different between control and progesterone exposure groups. However, Tbr2+ cell density was reduced after LPS exposure compared to control (P = .0022). With progesterone pretreatment, LPS-treated animals had increased Tbr2+ INP cell density compared to LPS alone (P = .0022). The Tbr2+ cell density in control animals and animals exposed to progesterone or LPS plus progesterone was not statistically different. The Tbr2+ INP cell density in LPS plus progesterone exposure was significantly increased from progesterone pretreatment alone (Figure 2A).
Figure 2.
Immunohistochemistry treatment effect in the embryonic somatosensory cortex on ED 18 across exposure groups. At left, quantitative analysis by ANOVA with Tukey-Kramer. A, Tbr2 (green), (B) Iba1 (red), (C) CC3 (red), and (D) BrdU (green). Right, qualitative images from representative sections, control, Prog, LPS, and LPS + Prog. Scale bars: A, B, and D, 50 μm; and C, 10 μm. Nuclear counterstain, DAPI (blue). Error bar = standard error. *Significant difference by ANOVA with Tukey-Kramer; ns, not significant. Prog indicates progesterone; LPS, lipopolysaccharide; IZ, interventricular zone; SVZ, subventricular zone; VZ, ventricular zone; ED, embryonic day; ANOVA, analysis of variance; Tbr2, T-box transcription factor 2; Iba1, ionized calcium binding adapter molecule 1; CC3, cleaved caspase 3; BrdU, 5-bromo-2′-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
We observed that microglia are recruited to the embryonic cortex after LPS exposure (Figure 2B). Microglia cell density was not different between control and progesterone exposure. After LPS exposure, microglia cell density was significantly increased compared to control animals (P < .001) or animals pretreated with progesterone alone (P < .001). Microglia cell density was also increased among animals treated with LPS plus progesterone when compared to progesterone alone (P < .001). We also confirmed microglia are found within the somatosensory cortex from the VZ to the pia surface but were most predominant in the proliferative regions of the VZ and SVZ. Microglia were assessed based on morphologic characteristics. Microglia among control animals were in a resting phase, with smaller soma and multiple rami/processes (Figure 2B, control). In addition, cell morphology was similar between control and progesterone-exposed microglia (Figure 2B, control and Prog). After treatment with LPS, microglia were notably activated with larger cell bodies and fewer and shorter processes (Figure 2B, LPS). Clusters of microglia were observed in brains from the LPS-exposed animals, and these clusters were physically close to Tbr2 cells in the VZ and SVZ (Figure 2B, LPS). Findings were variable following LPS plus progesterone exposure, with more numerous microglia and a spectrum of both activated and resting morphologies (Figure 2B, LPS + Prog).
To determine if progesterone’s effect on neurogenesis is related to a reduction in apoptosis or change in cell proliferation, we performed immunofluorescence with CC3 and BrdU. We found that progesterone pretreatment did not significantly reduce apoptosis after LPS exposure. However, cell death was overall present in very low levels throughout the embryonic cortex which has been observed in embryonic primates under similar experimental conditions7 and mice at comparable gestational age.21 Other regions of the brain were not objectively assessed for changes in cell death after LPS or progesterone exposure. On average, approximately 3.5 cells per whole cerebral cortex labeled positive for CC3 in our control group. The control and progesterone groups did not differ significantly (Figure 2C). However, CC3 was significantly increased in both LPS and LPS plus progesterone animals compared to progesterone exposure alone (P = .0015). Progesterone did not significantly reduce apoptosis in LPS-treated animals (Figure 2C). Cell proliferation was compared among exposure groups by labeling cells participating in the cell cycle at the start of our experiment with BrdU. Compared to control animals, the density of cells proliferating in the progesterone, LPS, and LPS plus progesterone exposure groups was all statistically fewer (Figure 2D).
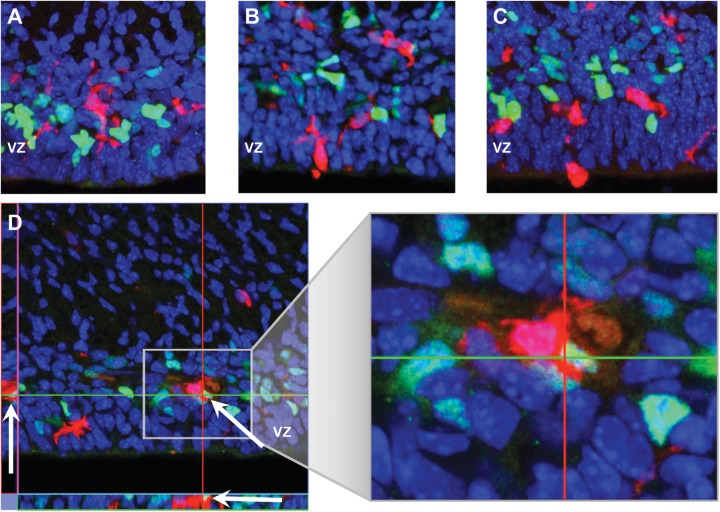
The morphology of microglia cells are a surrogate marker for the cells phenotype, resting or activated.7 As described earlier, microglia were morphologically more activated after LPS exposure compared to their appearance in controls (Figure 3A) or progesterone alone and were variably activated in the LPS plus progesterone exposure group (Figure 2B, LPS + Prog and Figure 3, B and C). Under all experimental conditions, microglia were predominately in the proliferative regions of the VZ and SVZ, where Tbr2+ INPs were located (Figures 2B and 3A-D). Additionally, microglia communicated with the VZ surface, through either their cell bodies or processes and many appeared to be in the process of either migrating into or out of the VZ (Figure 3B-C). We also surveyed the embryonic cortex for evidence of microglia ingesting Tbr2 + INPs (Figure 3D). The activated microglia shown is from an LPS exposed animal, and the microglia is seen surrounding and apparently phagocytizing a Tbr2 + INP through 3-dimensional imaging. Similar examples of morphologically activated microglia contacting Tbr2+ INPs were observed by 3-dimensional imaging in other cortical brain sections but this was an uncommon event and was limited to LPS and LPS plus progesterone-treated animals. Due to the rare occurrence of these events across all studied sections, statistical analysis was not performed.
Figure 3.
Image depicting the morphology of a resting Iba1+ microglia from a control fetal brain and proximity to Tbr2+ INPs. B and C. Iba1+ microglia with activated phenotypes from LPS treated fetal brain are located in close proximity to Tbr2+ INPs, the ventricular surface, and appear to be migrating into and out of the cortex (A, 40×, B and C 30×). D, Confocal slice from a stack with orthogonal views of activated microglia apparently phagocytizing a Tbr2+ INP at arrow and also communicating with adjacent Tbr2+ cells. 40× with orthogonal plane views. Iba1 (red)/Tbr2 (green)/DAPI (blue). Tbr2, T-box transcription factor 2; INPs, intermediate neurogenic progenitor; Iba1, ionized calcium binding adapter molecule 1; DAPI, 4′,6-diamidino-2-phenylindole. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
Discussion
Progesterone, a steroid hormone synthesized by the placenta in increasing amounts throughout gestation, is important in maintaining pregnancy and is also routinely used to reduce the risk of preterm birth in at risk women.1 This study is the first to evaluate the effect of progesterone pretreatment on INP cells and microglia in the developing mouse brain after an LPS-induced inflammatory event. We observed that Tbr2+ INP cell density was reduced 16 hours after LPS exposure in mice pups. The brains of embryonic pups pretreated with progesterone did not display a reduction in neurogenic progenitors after LPS exposure. In addition, progesterone treatment alone did not change Tbr2+ INP cell density compared to control but Tbr2+ INPs were unexpectedly lower than LPS plus progesterone (Figure 2A). In both the LPS and LPS plus progesterone exposure groups, microglia had a significant increase in cell density and displayed an activated morphology, particularly in the LPS exposure group (Figure 2B). The observed effect on INP neurogenesis could not be accounted for by changes in cell apoptosis since progesterone pretreatment did not reduce cell death after LPS exposure, and the total number of apoptotic cells was small (Figure 2C). Furthermore, cell proliferation was reduced across all exposure groups compared to control (Figure 2D) and therefore does not explain an increase in Tbr2+ INPs in the LPS plus progesterone exposure group compared to other groups. Our experiments suggest exposure to progesterone before LPS-induced inflammation effects proliferating INPs and reduces activated microglia morphology in the developing neocortex, a previously undescribed finding.
Reduced glutamatergic neurogenesis has been seen in the VZ and SVZ after preterm birth and inflammatory injury.7,8 After LPS-induced inflammation, Tbr2+ INPs are reduced, and Iba1 microglia are increased.7 Other studies have demonstrated that LPS-induced inflammation results in reduced neuronal dendritic processes, altered cell morphology and gene expression, dependent on gestational age and microglia, and may be further propagated by injured neurons themselves.2–4 Microglia activation has been seen throughout the brains of preterm neonates with diffuse white matter injury, including animal models where white matter injury and delayed cortical development were observed after LPS exposure increased microglia cell density.22 Interestingly, inhibition of microglia with doxycyline restored Tbr2+ INPs to control levels and reduced microglia density, further suggesting that microglia may be responsible for regulating neural progenitor populations.7
The sex steroids estrogen and progesterone can modulate neurogenesis and improve outcomes after brain injury.13,15,23 In embryonic mice, estradiol has the ability to simulate and increase INP cell division in the VZ and SVZ of the neocortex.12 Experimental models in rats and mice also show that progesterone dampens the proinflammatory process incited by LPS and propagated by microglia and restores neurogenesis by creating an anti-inflammatory and protective environment.18,20,24 Our data are the first to show that progesterone pretreatment alone can modify LPS-induced reduction in embryonic glutamatergic neurogenesis. This effect was seen without a reduction in microglia cell density.
While progesterone has not been previously shown to directly impact neurogenesis, there is evidence that it can influence the inflammatory microenvironment in the brain by promoting an anti-inflammatory microglia phenotype.20,24 Microglia are mononuclear phagocytes and resident immune cells of the central nervous system (CNS) that mediate brain immune response to acute injury.18 Lipopolysaccharide-induced cell signaling through toll-like receptor 4 can activate microglia and trigger a signal cascade that promotes cytokine release and results in damage to the surrounding developing neurons.2,20 However, the sex steroids estradiol and progesterone have been found to upregulate the genes Trem2 and Arg1, which favor an anti-inflammatory microglia phenotype, which in turn promotes tissue repair and modeling.24–26 In our study, we saw that prior to LPS exposure, pretreatment with progesterone resulted in Tbr2+ INP cell density similar to controls, and absence of progesterone supplementation lead to statistically fewer Tbr2+ INPs. One possible explanation is that progesterone pretreatment shifts a proinflammatory microglia phenotype to one that is primarily anti-inflammatory which in turn suppresses LPS-induced local brain inflammation and limits damage to Tbr2+ INP cell populations. This hypothesis is further supported by our assessment of microglia morphology among exposure groups. Despite an increase in microglia density, pretreatment with progesterone resulted in increased resting microglia morphology compared to LPS exposure alone (Figure 2B). Prior studies have similarly observed activated microglia morphology after LPS.7,20 Alternatively, progesterone could have a stimulatory effect on INP cell proliferation, similar to INPs exposed to the sex steroid estradiol.12 However, this was not seen in our statistical analysis of Tbr2+ INPs between our control and progesterone exposure group. Sex steroids could also alter cell differentiation or progesterone may directly affect the normal regulatory role microglia play in controlling the size of the neurogenic precursor pool in the developing cortex.
There is a growing body of research, suggesting that progesterone may offer protection to the vulnerable prenatal brain. Progesterone is a precursor for allopregnanolone, a highly abundant gamma-aminobutyric acid (GABAA) agonist in fetal development.27 High levels of allopregnanolone or its analogues have the ability to raise seizure threshold in animals but after birth, it falls abruptly and may increase susceptibility to brain injury involving excitotoxicity, particularly in the preterm neonate.27,28 Additionally, allopregnanolone is present in increasing amounts in the fetal brain after hypoxic stress, suggesting the fetal brain may have the capacity to independently upregulate steroid production and promote neuroprotection under certain stimuli.27 While perinatal infection can activate the fetal hypothalamic–pituitary–adrenal axis and raise sex steroid levels, evidence is mixed if this alters steroid levels in fetal circulation.27,29 The presence of membrane bound progesterone receptors in embryonic and adult brain supports a local role for progesterone.16,24 Additionally, immune modulating cells like microglia are dependent on circulating steroid precursors, have intrinsic steroid-converting enzymes, and can shift to an anti-inflammatory phenotype in the presence of progesterone.17,18,24 Differences in steroid hormone metabolism and signaling in the brain, including chemokine and cytokine signaling, shift during development and are dependent on fetal sex.24 However, while microglia express sex steroid-converting enzymes and sex hormone receptors, evidence is lacking if microglia in males and females differ in their expression of these receptors or response to sex hormones.24,30 It is biologically plausible that supplemental progesterone, given at the onset of a perinatal inflammatory event, could enhance neural steroid levels and dampen inflammation in the prenatal brain but this has not yet been reported in the literature.
This study tested a novel hypothesis that progesterone pretreatment alone might have a positive protective effect on fetal neurogenesis in the setting of an acute LPS-induced inflammatory exposure. As a result, we did not perform evaluations on long-term outcomes or exposure to chronic inflammation. Long-term measures of animal behavior would be critical to understanding the potential benefit progesterone therapy may offer after an acute or chronic inflammatory injury. The study design interval of 16 hours, while expected to be sufficient to detect pathology, could have also limited our ability to observe significant changes in cell apoptosis. However, previous studies have also observed low levels of apoptosis in the cortex at similarly studied ages.7,21 Additionally, the number of cells proliferating was reduced across all exposure groups and raises the question how long-term neural populations are effected by both progesterone and an LPS-induced inflammatory exposure. While estrogen has been observed to stimulate neurogenesis,12 progesterone alone does not appear to have the same effect. The mechanism in which progesterone affects cell proliferation is unclear but could be further evaluated by measuring other markers of cell differentiation and migration within the developing cortex, which are important for normal brain development.11 It is possible that progesterone creates an overly anti-inflammatory environment, which negatively impacts the normal regulatory mechanisms involved in neurogenesis in the absence of an inflammatory event. Additionally, chronic inflammation with and without recurrent doses of progesterone therapy prenatally could be evaluated to investigate the long-term effect s of an inflammatory environment on neurodevelopment. Characterization of fetal sex could also facilitate understanding neurodevelopmental differences existing between males and females in response to inflammation or with supplemental sex steroid administration, a limitation in our study. It is also important to recognize that this study was completed in a mouse model, which has distinct developmental differences from humans. However, prior studies between rodents and humans have shown conserved programs in neurogenesis, altered neurogenesis secondary to prematurity, and similar microglia activation following inflammation.7,11 Furthermore, there is compelling evidence to support that progesterone acts locally in the prenatal brain since both progesterone receptors and progesterone converting enzymes are present in rodents, sheep, and humans.24,27
Currently, there are few interventions available to reduce the risk of perinatal brain injury resulting from perinatal infection or more broadly, preterm birth.9 Only one study has evaluated postnatal therapy with sex steroids estrogen and progesterone.31,32 Five-year neurodevelopmental follow-up did suggest a trend toward fewer cases of cerebral palsy and spasticity, which was dependent on duration of treatment.32 Importantly, the authors demonstrated evidence that sex steroid therapy was not harmful.31
Conclusion
Our study has shown that progesterone may protect the preterm brain from defects of neurogenesis induced by inflammation. While the effect we describe is encouraging, large gaps still exist in our understanding of the mechanisms and pathways responsible for our observations. The studies reviewed above support a common theme that progesterone modifies microglia activation and may further promote an anti-inflammatory environment through cell-directed signaling. Further studies are needed to understand the long-term outcomes of progesterone treatment with perinatal inflammation.
Acknowledgements
I would like to acknowledge Dr Thomas Easterling, Dr Michael Gravett, Tony Scauzillo-Golden, and Jan Hamanishi for their contributions to this project.
Footnotes
Authors’ Note: Abstract presented at Society for Reproductive Investigation, San Francisco, CA, March 28, 2015.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded through the Thomas Benedetti Fund (to A.A.T), Washington State Obstetrical Association (to A.A.T), and R01 NS085081 from NIH (to R.F.H).
References
- 1. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breen K, Brown A, Burd I, Chai J, Friedman A, Elovitz MA. TLR-4-dependent and -independent mechanisms of fetal brain injury in the setting of preterm birth. Reprod Sci. 2012;19(8):839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burd I, Chai J, Gonzalez J, et al. Beyond white matter damage: fetal neuronal injury in a mouse model of preterm birth. Am J Obstet Gynecol. 2009;201(3):279 e271-e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29(6):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ofek-Shlomai N, Berger I. Inflammatory injury to the neonatal brain - what can we do? Front Pediatr. 2014;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29(6):551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33(10):4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malik S, Vinukonda G, Vose LR, et al. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33(2):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McAdams RM, Juul SE. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol Res Int. 2012;2012:561494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71(4):444–457. [DOI] [PubMed] [Google Scholar]
- 11. Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55(3):223–233. [DOI] [PubMed] [Google Scholar]
- 12. Martinez-Cerdeno V, Noctor SC, Kriegstein AR. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur J Neurosci. 2006;24(12):3475–3488. [DOI] [PubMed] [Google Scholar]
- 13. Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57(2):386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kipp M, Amor S, Krauth R, Beyer C. Multiple sclerosis: neuroprotective alliance of estrogen-progesterone and gender. Front Neuroendocrinol. 2012;33(1):1–16. [DOI] [PubMed] [Google Scholar]
- 15. Dang J, Mitkari B, Kipp M, Beyer C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav Immun. 2011;25(4):715–726. [DOI] [PubMed] [Google Scholar]
- 16. Meffre D, Labombarda F, Delespierre B, et al. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience. 2013;231:111–124. [DOI] [PubMed] [Google Scholar]
- 17. Gottfried-Blackmore A, Sierra A, Jellinck PH, McEwen BS, Bulloch K. Brain microglia express steroid-converting enzymes in the mouse. J Steroid Biochem Mol Biol. 2008;109(1-2):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Habib P, Beyer C. Regulation of brain microglia by female gonadal steroids. J Steroid Biochem Mol Biol. 2015;146:3–14. [DOI] [PubMed] [Google Scholar]
- 19. Aisemberg J, Vericelli CA, Bariani MV, Billi SC, Wolfson ML, et al. Progesterone Is Essential for Protecting against LPS-Induced Pregnancy Loss. LIF as a Potential Mediator of the Anti-inflammatory Effect of Progesterone. PLoS ONE. 2013;8(2):e56161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mouihate A. TLR4-mediated brain inflammation halts neurogenesis: impact of hormonal replacement therapy. Front Cell Neurosci. 2014;8:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hevner RF, Daza RAM, Englund C, Kohntz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward migration. Neuroscience. 2004;124:605–618. [DOI] [PubMed] [Google Scholar]
- 22. Baburamani AA, Supramaniam VG, Hagberg H, Mallard C. Microglia toxicity in preterm brain injury. Reprod Toxicol. 2014;48:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49(4):391–402, 402 e391-392. [DOI] [PubMed] [Google Scholar]
- 24. Habib P, Slowik A, Zendedel A, Johann S, Dang J, Beyer C. Regulation of hypoxia-induced inflammatory responses and M1-M2 phenotype switch of primary rat microglia by sex steroids. J Mol Neurosci. 2014;52(2):277–285. [DOI] [PubMed] [Google Scholar]
- 25. Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24(6):302–306. [DOI] [PubMed] [Google Scholar]
- 26. Bajramovic JJ. Regulation of innate immune responses in the central nervous system. CNS Neurol Disord Drug Targets. 2011;10(1):4–24. [DOI] [PubMed] [Google Scholar]
- 27. Hirst JJ, Palliser HK, Yates DM, Yawno T, Walker DW. Neurosteroids in the fetus and neonate: potential protective role in compromised pregnancies. Neurochem Int. 2008;52(4-5):602–610. [DOI] [PubMed] [Google Scholar]
- 28. Nguyen PN, Billiards SS, Walker DW, Hirst JJ. Changes in 5alpha-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr Res. 2003;53(6):956–964. [DOI] [PubMed] [Google Scholar]
- 29. Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol. 2000;182(6):1404–1413. [DOI] [PubMed] [Google Scholar]
- 30. Schwarz JM, Bilbo SD. Sex, Glia, and Development: interactions in health and disease. Horm Behav. 2012;62(3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trotter A, Maier L, Grill HJ, Kohn T, Heckmann M, Pohlandt F. Effects of postnatal estradiol and progesterone replacement in extremely preterm infants. J Clin Endocrinol Metab. 1999;84(12):4531–4535. [DOI] [PubMed] [Google Scholar]
- 32. Trotter A, Steinmacher J, Kron M, Pohlandt F. Neurodevelopmental follow-up at five years corrected age of extremely low birth weight infants after postnatal replacement of 17beta-estradiol and progesterone. J Clin Endocrinol Metab. 2012;97(3):1041–1047. [DOI] [PubMed] [Google Scholar]