Abstract
While live births resulting from assisted reproductive technology (ART) exceed 1% of total births annually, the effect of ART on fetal development is not well understood. Data have demonstrated that IVF leads to alterations in DNA methylation and gene expression in the placenta that may have long-term effects on health and disease. Studies have linked adverse neurodevelopmental outcomes to ART, although human studies are inconclusive. In order to isolate the peri-implantation environment and its effects on brain development, we utilized a mouse model with and without superovulation and examined the effect of adult behavior as well as adult cortical neuronal density. Adult offspring of superovulated dams showed increased anxiety-like behavior compared to offspring of naturally mated dams (P < .05). There was no difference in memory and learning tests between the 2 groups. The adult brains from offspring of superovulated recipients had fewer neurons per field compared to naturally mated control offspring (P < .05). In order to examine potential pathways leading to these changes, we measured messenger RNA and microRNA (miRNA) expression in fetal brains at E18.5. Microarray analysis found that miRNAs miR-122, miR-144, and miR-211, involved in regulation of neuronal migration and differentiation, were downregulated in brains of offspring exposed to a superovulated environment(P < .05). There was also altered expression of genes involved in neuronal development. These results suggest that the peri-implantation environment can affect neurodevelopment and can lead to behavioral changes in adulthood. Human studies with long-term follow-up of children from ART are necessary to further investigate the influence of ART on the offspring.
Keywords: superovulation, neurodevelopment, ART, epigenetics, neuronal differentiation, IVF
Introduction
While live births resulting from assisted reproductive technology (ART) and in vitro fertilization (IVF) exceed 1% of total US births annually, the effect of ART on fetal development is not well understood.1 The ART alters in utero environment at the earliest, and possibly most critical, time of development. Infants born following IVF are more likely to be born small for gestational age and may have metabolic changes later in life when compared to children born following natural conception.2,3 The link between ART and neurodevelopmental outcomes has been harder to demonstrate, though some studies have found associations between cognitive, motor, and behavior development and ART.4–9 Published data have been clouded by short follow-up time, small sample size, inadequate control groups, and the multiple adverse perinatal outcomes associated with IVF.
One intervention utilized ubiquitously in IVF is superovulation with gonadotropins, which creates serum concentrations of estradiol and other factors up to 10-fold higher than what is found in a natural cycle. These hormone levels remain elevated during the critical period of implantation and may affect the developing offspring. We recently demonstrated, in the mouse model, that transferring naturally conceived embryos into the uterus of superovulated recipients results in abnormal placentation and severely growth restricted pups.10 The effect of the peri-implantation milieu on the expression of genes important for neurodevelopment is not known.
Multiple studies have found that ART leads to epigenetic changes in the placenta and fetus.11–14 Specifically, ovulation induction with gonadotropins, also known as superovulation, is associated with epigenetic changes in the embryo which may result in phenotypic changes15,16 as well as imprinting syndromes such as Beckwidth-Wiedemann and Angelman.17 In our study, we hypothesize that the superovulation environment induces epigenetic changes, which not only affect fetal growth but also affect neuronal development and ultimately behavior, in the exposed offspring. We examine the effect of altering the hormonal milieu during embryo implantation on long-term cognitive development including spatial learning, memory, anxiety and depressive-like behavior in the mouse model and investigated changes in gene and microRNA (miRNA) expression in perinatal brain that may contribute to these neurodevelopmental outcomes.
Methods
All experiments and procedures were approved by the University of Pennsylvania and The Johns Hopkins University Institutional Animal Care and Use Committee Review Boards. All animal experiments were conducted at the University of Pennsylvania. Female CF-1 (Harlen Sprague Dawley), male C57BL/6J (Jackson Laboratories), and vasectomized C57Bl/6J males (Jackson Laboratories) were obtained and housed in a temperature- and light-controlled environment and fed ad libitum. To obtain blastocysts for transfer, females were naturally mated and mating was confirmed by the presence of a copulatory plug the morning following mating (0.5 days postcoitum). On postcoital day, 3.5 blastocysts were flushed from the uterine horns using standard technique18 and the embryos cultured in K Simplex Optimization Media (KSOM) + amino acids (Specialty Media; Millipore, Billerica, Massachusetts) under mineral oil at 37°C in an incubator for no more than 1 hour prior to transfer.
To obtain the experimental group of pseudo-pregnant females, a group of CF-1 (Harlen, Indianapolis, Indiana) females was superovulated with an intraperitoneal injection of 5 IU of equine chorionic gonadotropin followed by 5 IU of human chorionic gonadotropin 48 hours later and then mated with vasectomized C57BL/6J males (Jackson Laboratories). Mating was confirmed by the presence of a copulatory plug. Control recipients were obtained by mating CF-1 females with the vasectomized males, and mating was confirmed by the presence of a copulatory plug. On postcoital day 2.5, 10 blastocysts were transferred into a single horn of either a control or a superovulated recipient using the Non-surgical Embryo Transfer Device (Paratechs, Lexington, KY) per manufacturer’s protocol.
Behavioral Tests
A cohort of pregnant mice were allowed to deliver, and offspring were housed to 5 months of age, at which time behavioral testing was performed. All behavioral procedures were performed at the Neurobehavior Testing Core at the University of Pennsylvania. Mice received a battery of behavior tests to explore anxiety and depression-related phenotypes as well as tests for memory and cognition. At least 1 day of rest was given between the tests. Three days prior to tests of learning and memory, each mouse was handled for about 2 min/day to habituate them to the experimental manipulations and minimize the stress response.
Open field activity was used as a quantitative measure of general activity and exploratory behavior of the mice. Open field activity was assessed in a 40 × 40 × 38 cm arena fitted with photocells to detect motion (San Diego Instruments, San Diego, California) during a 10-minute trial. In addition to horizontal activity, rearing activity was also detected.
We utilized the Elevated Zero Maze as a standard test for anxiety-related behavior. Mice were individually placed on a 5.5 cm wide circular track with an external diameter of 45 cm, raised 40 cm above the floor (San Diego Instruments). The track had 2 open and 2 enclosed areas of equal dimensions. Mice were placed in an open segment to begin a 5-minute trial. All trials were recorded and graded for time in the open segments and transition between the open/closed areas by an observer blind to group designation. A mouse was scored as within a segment when all 4 paws were within that segment.
The Forced Swim Test (FST) was used to screen for depression-like behavior. Mice were individually placed in a 46 × 22 cm diameter glass cylinder filled to 20 cm with 22°C to 25°C water. All trials were digitally recorded and graded by an observer blind to group designation. The last 4 minutes of a 6-minute swim were graded for total time immobile. A mouse was considered immobile when it was motionless or exerted only enough activity to keep afloat.
The Novel Object Recognition (NOR) task was utilized to evaluate recognition memory. The NOR learning relies on the innate propensity of mice to explore their environment. Exploration is recorded as time spent sniffing, looking toward, rearing against, or whisker brushing objects in a 35 × 35 × 40 cm arena. During the training phase, mice are allowed to habituate to the empty arena for 10 minutes. After habituation, 2 similar objects are placed in the arena. Mice were allowed to explore the objects for 10 minutes over 3 trials. Twenty-four hours later, 1 of the training objects was replaced with a novel object. No difference in exploration of the objects was interpreted as a memory deficit. All trials were digitally recorded and graded by an observer blind to group designation.
Fear conditioning (FC) was used as a form of Pavlovian learning. Both contextual and cued learning require the amygdala, while the learned response to context recruits hippocampal circuitry. Mice were trained to associate a context (chamber) or cue (tone) with an aversive foot shock. If association was made between foot shock and context or cue, the mouse would demonstrate a freezing response upon reexposure. For our studies, mice were allowed to explore a unique chamber for 120 seconds and then a 30-second, 85-dB, 1200-Hz tone coterminating with a 2-second, 1.5-mA foot shock, followed by 30 additional seconds in the chamber. Twenty-four hours after training, the mice were placed back in the chamber to test for contextual learning. Forty-eight hours later, mice were placed in a context unique from the training chamber, and their response to a 3-minute, 85dB, 1200 Hz tone was tested to assess cued learning. Reexposures to the conditioned stimuli was digitally recorded and percentage of freezing was determined by FreezeScan software (CleverSys Inc, Reston, Virginia).
For behavioral studies, the data were expressed as mean ± standard error of the mean (SEM). All the data sets were analyzed for normality. To determine statistical significance, 2-tailed Student t test was performed for all normally distributed data, and a Mann-Whitney U test was performed for nonparametric data. A P < .05 was considered significant. Statistical analysis was performed using GraphPad Prism 5.0 software.
Microarray Analysis and miRNA Arrays
On e18.5, the recipient mice were killed, fetal brain was extracted, and flash frozen in liquid nitrogen. Messenger RNA (mRNA) and miRNA were isolated from mouse brains using Qiagen RNeasy and miRNeasy kits (Qiagen, the Netherlands), respectively, following the manufacturer’s standard protocol. Before sample processing, a Nanodrop spectrophotometer was utilized to determine sample concentration, and an Agilent Bioanalyzer Series II was used for sample quality.
The mRNA samples were amplified and labeled with the Ambion WT Expression kit following manufacturer’s protocol (Life Technologies Co, Carlsbad, California). Hybridization onto Affymetrix Mouse Gene 1.0 ST microarrays for 17 hours at 45°C and scanning on the Affymetrix GeneChip Scanner 3000 7G were performed as per manufacturer’s protocol (Affymetrix Inc, Santa Clara, Califonia). The miRNA samples were amplified and labeled with the Affymetrix FlashTag Biotin HSR RNA Labeling kit per manufacturer’s protocol. Hybridization onto Affymetrix GeneChip miRNA 3.0 microarrays for 16 hours at 48°C and scanning on the Affymetrix GeneChip Scanner 3000 7G were performed per manufacturer’s protocol. Scanning and raw analysis were performed with the Affymetrix Expression Command Console 1.2.
Data analyses began with the raw CEL files output by the Affymetrix Console for either mRNA or microRNA analyses. Data were extracted with the Partek Genome Suite 6.6 platform using robust multiarray analysis. RNA was normalized and transformed into log2 notation for quality control and further analyses. For the mRNA arrays, all probe sets were used, whereas for miRNA arrays, the extraction was limited to only the 2114 mouse-targeting probe sets so as to ensure proper normalization. Furthermore, the miRNA data were normalized to the 75th percentile as opposed to the mRNAs 50th percentile.
Superovulated samples were grouped and compared to naturally conceived samples for both mRNA and miRNA expression using the Student t test in Partek. Differentially expressed genes and microRNAs were selected based on the magnitude of the differential and the statistical significance of that change. Fold-change thresholds were selected based on the standard deviation of those changes’ Log2 values from 0.0 or no change. Cutoff points were thus determined by the expression results rather than arbitrarily defined, a priori, expectations. The miRNAs that passed a threshold of 6SD for the comparison of all superovulated offspring versus all naturally conceived offspring were then validated.
Potential microRNA targets, the genes whose protein expression may be altered, were derived with the ingenuity pathway analysis (IPA) platform on experimentally observed and targeting predictions extracted from the literature and databases such as TargetScan and TarBase. Gene Ontology analysis was performed on differentially expressed genes identified from the mRNA microarray to determine functional classification of genes affected by superovulation compared to control. Pathways analysis was performed using IPA software to identify significantly affected canonical pathways and gene interaction networks related to neurodevelopment.
Real-Time Quantitative Polymerase Chain ReactionValidation
Complementary DNA (cDNA) was synthesized from fetal brain RNA using the iScript cDNA Synthesis kit (Bio Rad, Hercules, California) according to the manufacturer’s protocol. Targets identified by microarray analysis were validated using real-time quantitative polymerase chain reaction (RT qPCR) and Taqman probes (Life Technologies Co) for miRNA expression or PrimeTime qPCR Assays (Integrated DNA Technologies, Inc, Coralville, Iowa) for mRNA expression. The standard statistical cutoff was P value <.05.
Histochemistry and Immunohistochemistry
Whole brains were harvested from the adult mice utilized in the behavioral studies following killing between 5 and 6 months of age. Brains were washed in 1× phosphate-buffered saline (PBS) for 30 minutes 5 times, followed by fixation in 4% paraformaldehyde at 4°C overnight. The samples were processed for histochemical and immunohistochemical (IHC) staining by immersing in 30% sucrose until saturation and cryosectioned at 20-μm thickness. Nissl staining for neuron-specific endoplasmic reticulum granular bodies was performed using cresyl violet. Briefly, sections were incubated with a 0.5% solution of cresyl violet (Chroma-Gesellschaft; Roboz, Inc) in sodium acetate buffer for 5 minutes followed by a brief rinse in tap water. The stain was differentiated in 1% glacial acetic acid in 95% ethanol until white matter tracts were visible from gray matter. After dehydration with gradient ethanol, the tissue was cleared in xyline and mounted with DPX (Fluka Chemical). For neural IHC, sections were incubated overnight at 4°C with a mouse NeuN (Millipore) antibody in a concentration of 1:50 in PBS containing 0.5% Triton X-100 (Sigma, St Louis, Missouri) and 3% horse serum (Life Technologies, Grand Island, New York). The next day, sections were rinsed with PBS and then incubated with fluorescent secondary donkey antimouse Alexa Fluor 568 (Life Technologies) antibody diluted in 1:500 for 3 hours at room temperature. At the end, sections were mounted with Fluromount-G (eBioscience, San Diego, California). Images were attained using Axioplan 2 Imaging system (Carl Zeiss, Thornwood, New York) attached to an EOS Rebel camera (Canon) through a 40× objective lens.
Neurons were counted (field of view) based on Nissl staining using Image J (v1.48, http://imagej.nih.gov/ij/, National Institute of Health, Bethesda, Maryland) on randomly chosen 5 to 6 fields in frontal cortex per animal. The neurons were identified by a large cell body or perikaryon containing a large, pale nucleus with a prominent dark nucleolus. The experiments were performed in triplicate for all groups. Data are presented as the mean ± SEM. Statistical significance was determined using Student unpaired t test.
Results
Embryo Transfer Experiments
We previously demonstrated that there is no difference in litter size between the control and the superovulated recipients.19 Although both fetal weight and placental weight were lower in the offspring of the superovulated recipients at E18.5,19 by 1 week of age, there was no difference in weight between the 2 groups.
Gene Expression
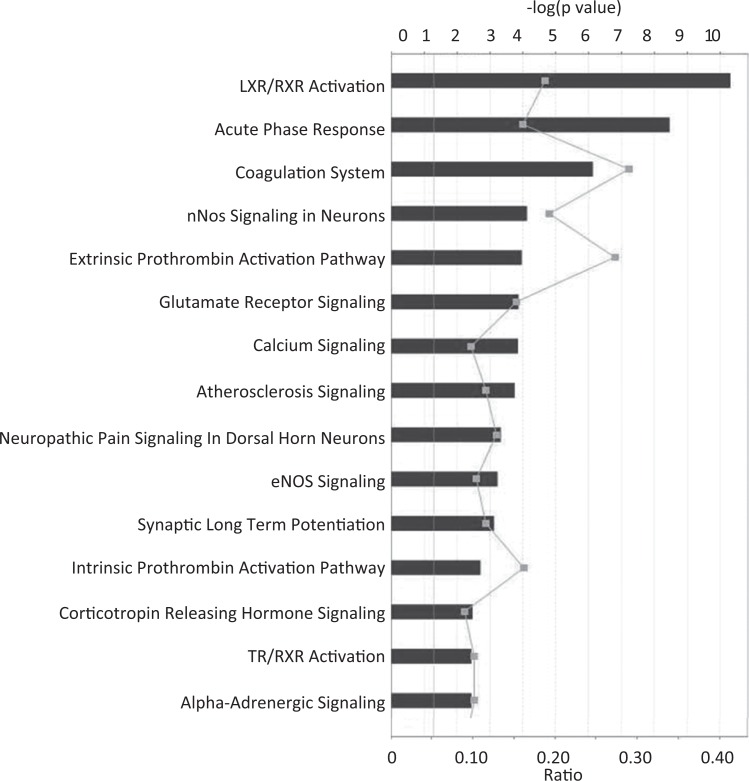
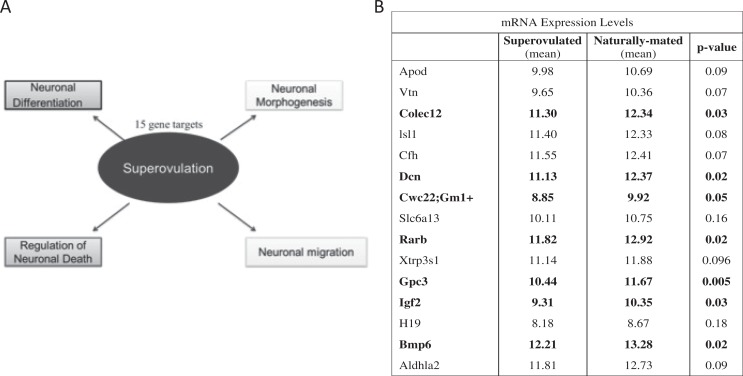
In order to identify whether changes in the peri-implantation environment can affect fetal brain development, microarray studies were formed on fetal brain at E18.5 from control and superovulated recipients. Gene ontology analysis and IPA results of mRNA microarray data identified gene expression changes in multiple pathways that impact neuronal development including neuronal nitric oxide synthase (nNOS) signaling, glutamate receptor signaling, calcium signaling, eNOS signaling, and synaptic long-term potentiation (Figure 1). We found altered expression of 15 genes involved in the processes of neuronal differentiation, neuronal morphogenesis, regulation of neuron cell death, and neuronal migration. Of these genes, 6 were confirmed by RT-qPCR validation studies and had significantly decreased gene expression greater than 1.5-fold: Colec12, Dcn, Rarb, Gpc3, Igf2, and Bmp6 (P < .05; Figure 2). The neuronal pathway most significantly affected by superovulation in the IPA analysis was the nNOS signaling pathway, with multiple genes in the nNOS pathway downregulated in the superovulated offspring compared to naturally mated offspring (Figure 3A). The RT-qPCR was utilized to validate these findings and expression of Grin1, Calm1, and Prkca were all significantly downregulated in the superovulated offspring (P < .05; Figure 3B).
Figure 1.
Ingenuity Pathway Analysis (IPA) results for gene expression changes induced by exposure to superovulation. Several pathways that could impact on neurodevelopment were affected, including neuronal nitric oxide synthase (nNOS) signaling, glutamate receptor signaling, calcium signaling, and synaptic long-term potentiation.
Figure 2.
Gene expression in neuronal regulation pathways. A, Gene ontology analysis and ingenuity pathway analysis (IPA) results of messenger RNA (mRNA) microarray data identified gene expression changes in several pathways that could impact neuronal development: neuronal differentiation, neuronal morphogenesis, regulation of neuron cell death, and neuronal migration. B, Of these genes, 6 had significantly decreased gene expression greater than 1.5-fold: Colec12, Dcn, Rarb, Gpc3, Igf2, and Bmp6 and confirmed significance (P < .05) on real-time quantitative polymerase chain reaction (RT-qPCR) validation studies.
Figure 3.
Gene Expression of neuronal nitric oxide synthase (nNOS) Pathway Targets. Significant downregulation of Grin1 (NMDAR), Calm1 (Calmodulin), and Prkca (PKC) identified by microarray and ingenuity pathway analysis (IPA) pathway analysis (A). Quantitative polymerase chain reaction (qPCR) was performed on the targets to validate the microarray data and verified decreased expression of Grin1, Calm1, and Prkca (B). Gene expression was normalized to Actin. (Control: n = 13; Superovulated: n = 17)
Expression of microRNA
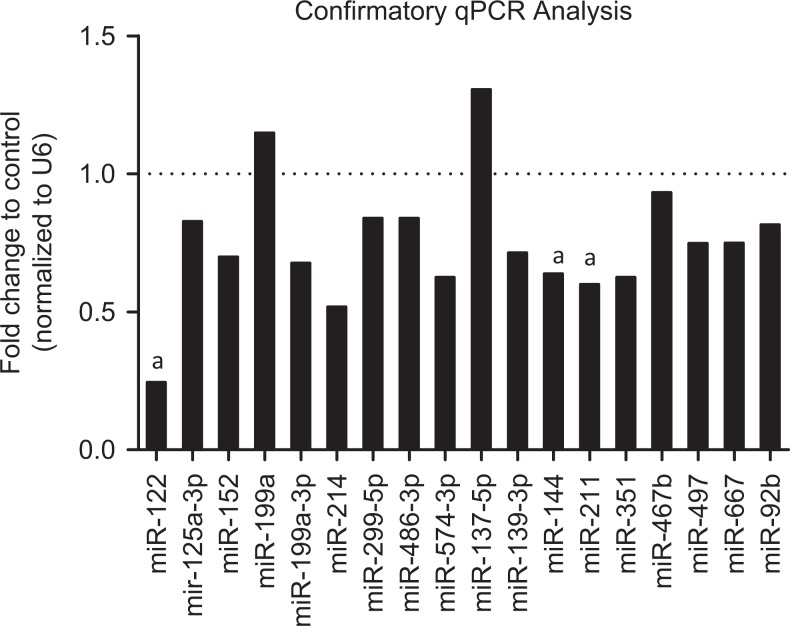
One method of epigenetic regulation of gene expression is miRNAs. Therefore, we examined miRNA expression by microarray in the fetal brains on control and superovulated recipients. Analysis of miRNA expression by microarray identified 18 miRNAs in the superovulated fetal brains that had expression changes greater than 6 standard deviations compared to control fetal brains. These targets were validated by RT-qPCR and confirmed significant expression changes in 3 miRNAs: miR-122, miR-144, and miR211 (Figure 4). In our subsequent IPA ontology analysis of miRNA array against mRNA array, the genes affected played roles in neuronal differentiation and migration.
Figure 4.
Confirmatory qPCR of miRNA microarray targets. Micro RNA (miRNA) targets identified by microarray were validated by quantitative polymerase chain reaction (qPCR). Of the 18 targets originally identified, only miR-122, miR211, and miR144 maintained statistical significance (bolded). a P < .05. (Control: n = 13; Superovulated: n = 17).
Behavioral Tests
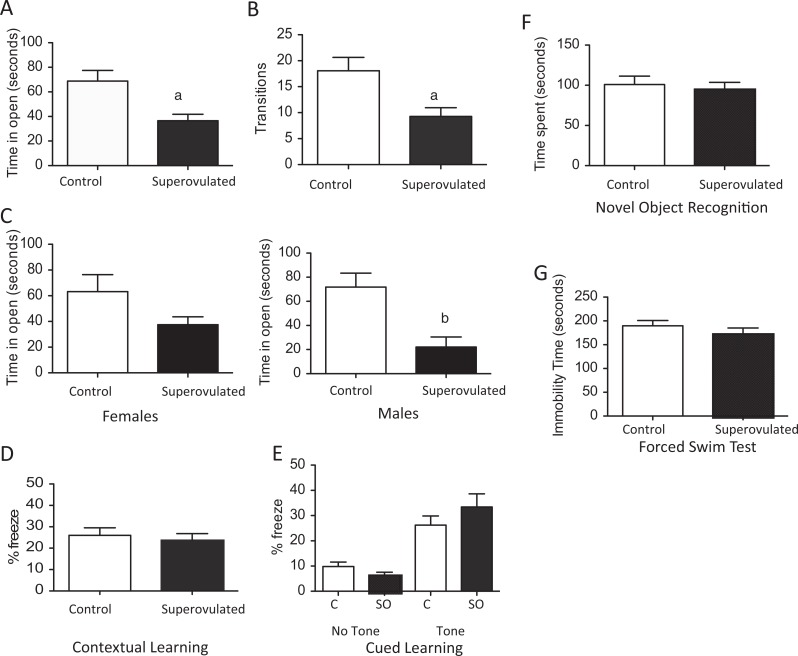
As our fetal studies suggested alterations in neuronal development during embryogenesis, we then utilized our transfer experiments and allowed a subset of litters to deliver. At five months of age, behavioral tests were performed to assess whether the expression changes during development led to alterations in adult behaviors. Anxiety behaviors were assessed using the elevated zero maze test. Mice born from superovulated recipients show increased anxiety-like behavior during the elevated zero maze test compared to offspring from naturally mated recipients. These mice spent significantly less time exploring the open arms (P < .01) and made fewer transitions to the open arm than control mice (P < .01; Figure 5 A and B). The mice from superovulated recipients had fewer transitions between the open and the closed areas, which can reflect decreased activity between the groups; this difference could also result from the altered in utero environment. These differences persisted for both male and female mice (1C), though for female mice the difference was not statistically significant. Open field testing is a less sensitive measure of anxiety-related behaviors. In the open field testing, offspring from the superovulated recipients also showed increased time in the periphery, although this difference was not statistically different (data not shown).
Figure 5.
Behavioral testing of adult offspring resulting from control recipient (white bar) or superouvlated recipient (black bar). Elevated zero maze testing showed offspring of superovulated recipients had significantly less time in open areas (A) and fewer transitions into the open area than offspring from control mice (B). This was true for both male and female mice (C). There was no difference in learning or memory between the groups by contextual or cued learning (D and E) or by novel object recognition (F). There was also no change in depressive behaviors by forced swim test (G). (Control: n = 25, 9 females and 16 males; Superovulated: n = 23, 16 females and 7 males) a P < .01 versus offspring of control recipients. b P < .05 versus offspring of control recipients. Data are expressed as mean ± standard error of the mean (SEM).
There was no difference in learning or memory. Offspring from both the control and the superovulated recipients showed similar associated learning by either cued or contextual FC (Figure 5D and E). Using novel object recognition testing, we found no difference in working memory between offspring from control or superovulated mice (Figure 5F). We also found no increase in depressive behavior in the offspring of the superovulated recipients compared to control during the FST (Figure 5G).
Cortical Neuron Density in Adult Offspring
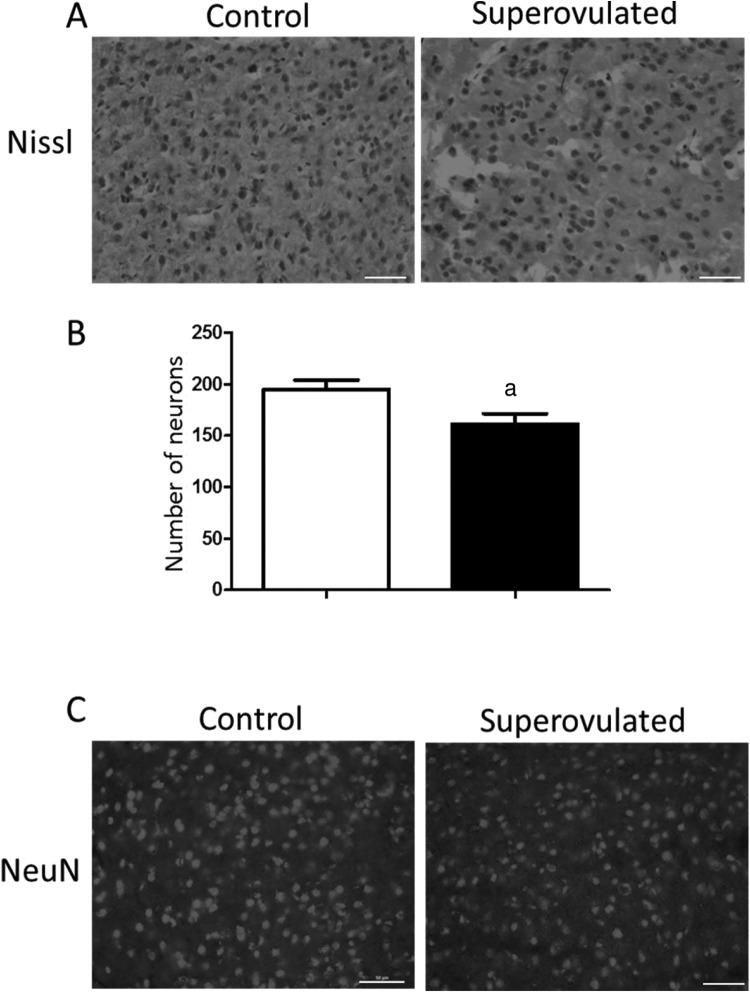
Cortical neuron density can be utilized as a surrogate measure of synaptic conductivity and has been associated with adverse sensorimotor outcomes.20,21 To examine the effect of fetal gene and miRNA expression changes on the adult brain, cortical neuron density was measured in Nissl-stained frontal cortex from the offspring at 5 to 6 months of age (Figure 6A). Superovulated offspring had fewer neurons per field compared to naturally mated control offspring (Figure 6B; P < .05). Immunohistochemical staining with NeuN confirmed the results from Nissl staining (Figure 6C).
Figure 6.
Embryonic exposure to superovulation affects neurons in adult cortex. (A) Histochemical Nissl staining in the cortex of mice between 5 and 6 months of age. (B) Neural quantification in cortex of adult offspring of natural recipients (white bar) and offspring of superovulated recipients (black bar; n = 3 in each group). (C) Confirmatory immunohistochemical staining of NeuN (neuronal staining) in cortex. Bar denotes 50μm, a P <.05. Data are expressed as mean ± standard error of the mean (SEM).
Discussion
In this study, we have demonstrated that hormonal milieu, at the time of embryo implantation, impacts fetal neuroprogramming and long-term behavioral phenotype. Our previous data in the mouse model suggest that altering the hormonal environment during implantation affects placentation and fetal growth in the mouse model. Corollary to that, epidemiologic data have suggested that both ART and growth restriction may be associated with adverse neurodevelopmental outcomes.4,6,22,23 In our study we identify aberrations in neuronal programming/cell death and migration that may be implicated in decrease in cortical neuronal mass as well as changes in the nNOS pathway as potential mechanisms by which the nonphysiologic peri-implantation environment can affect behaviors in the offspring
The nNOS catalyzes the production of nitric oxide, which acts as a neurotransmitter. The nNOS activity relies on calcium-mediated dimerization via calmodulin binding. Pathologic production of nitric oxide, such as during inflammation or ischemia, leads to excess formation of peroxynitrite, a highly reactive oxidant that contributes to neuronal excitotoxicity and oxidative stress.24,25 Polymorphisms, gene variants, and dysregulation of nNOS have been associated with schizophrenia, personality disorders, and anxiety.26–28 While the nNOS pathway was affected, ultimately, the level of nNOS was unchanged between the groups during the fetal period. However, offspring mice exposed to superovulation did demonstrate anxiety-like behaviors. We speculate that the alterations to the nNOS pathway in the perinatal pathway may have made it more vulnerable to further insults.
The miRNAs are important epigenetic regulators that affect gene expression through transcript silencing.29 Neuronal differentiation and migration has been shown to require proper miRNA function.30–32 The roles of several miRNAs in regulating neuronal development have been described.33–35 Our data indicate that exposure to superovulation in the first few days of life alters fetal neuroprogramming, and the aberrations in gene and miRNA expression involved in neuronal migration, differentiation, and cell death are not only confirmed by qPCR in perinatal period but also have lasting implications into adulthood, as there are fewer cortical neurons in the group subjected to superovulation.
The ART has been demonstrated to lead to epigenetic modifications in the placenta which affect fetal growth and may play a role in long-term health and disease.36–39 Our findings suggest that these epigenetic changes may extend beyond the placenta to alter fetal brain development and result in altered behaviors into adulthood. Indeed, our results show a decrease in the density of cortical neurons in brains of adult offspring exposed to a superovulation hormonal environment in utero and anxiety in the offspring.
These results demonstrate for the first time that the peri-implantation environment during a fresh IVF cycle not only affects placentation and fetal growth10 but also has effects on pathways critical to neurodevelopment and behavioral outcomes. Recent evidence suggesting that the hormonal environment created during fresh IVF cycles may be associated with increased adverse perinatal outcomes such as preeclampsia and small for gestational age infants40,41 has led to increased number of frozen embryo transfer cycles. Our data suggest that a more physiologic peri-implantation hormonal environment, such as that during a frozen embryo transfer cycle, may also be preferable for the neurologic health of the fetus. Long-term prospective human studies are necessary in order to understand the effect of ART, and specifically the peri-implantation hormonal environment, on the health and well-being of the resulting offspring.
Footnotes
Author’s Note: Monica Mainigi and Irina Burd were responsible for study design, data analysis, manuscript drafting, and critical discussion. Teri Ord performed embryo transfers and tissue dissections. Devvora Olalere performed the behavioral studies. Jason Rosenzweig, Virginia Mensah, Lauren Thomaier, Conover Talbot, and Rayyan Rozzah performed the miRNA and mRNA studies and performed data analysis. Jun Lei performed the immunohistochemistry studies and data analysis. Michael Johnston aided with study design and data analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Aramco Services Company Fellowship (JR), NICHD K08HD073315 (IB), Passano Foundation Grant (IB), Reproductive Scientists Development Program/NIH K12 HD000849-22 (MM), American Society for Reproductive Medicine (MM), and Society for Gynecologic Investigation.
References
- 1. Center for Disease Control and Prevention. Assisted Reproductive Technology (ART). 2014; Web site http://www.cdc.gov/art/. Accessed June 2, 2014.
- 2. Ceelen M, van Weissenbruch MM, Roos JC, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92(9):3417–3423. [DOI] [PubMed] [Google Scholar]
- 3. Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93(5):1682–1688. [DOI] [PubMed] [Google Scholar]
- 4. Hvidtjorn D, Schieve L, Schendel D, Jacobsson B, Svaerke C, Thorsen P. Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: a systematic review and meta-analysis. Arch Pediatr Adolescent Med. 2009;163(1):72–83. [DOI] [PubMed] [Google Scholar]
- 5. Conti E, Mazzotti S, Calderoni S, Saviozzi I, Guzzetta A. Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review. Hum Reprod. 2013;28(12):3316–3327. [DOI] [PubMed] [Google Scholar]
- 6. Hvidtjorn D, Grove J, Schendel D, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Commun Health. 2011;65(6):497–502. [DOI] [PubMed] [Google Scholar]
- 7. Knoester M, Helmerhorst FM, van der Westerlaken LA, Walther FJ, Veen S. Matched follow-up study of 5 8-year-old ICSI singletons: child behaviour, parenting stress and child (health-related) quality of life. Hum Reprod. 2007;22(12):3098–3107. [DOI] [PubMed] [Google Scholar]
- 8. Goldbeck L, Gagsteiger F, Mindermann I, Strobele S, Izat Y. Cognitive development of singletons conceived by intracytoplasmic sperm injection or in vitro fertilization at age 5 and 10 years. J Pediatr Psychol. 2009;34(7):774–781. [DOI] [PubMed] [Google Scholar]
- 9. Lyall K, Pauls DL, Spiegelman D, Santangelo SL, Ascherio A. Fertility therapies, infertility and autism spectrum disorders in the Nurses’ Health Study II. Paediatr Perinatal Epidemiol. 2012;26(4):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mainigi MA, Olalere D, Burd I, Sapienza C, Bartolomei M, Coutifaris C. Peri-implantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth. Biol Reprod. 2014;90(2):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katari S, Turan N, Bibikova M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18(20):3769–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turan N, Katari S, Gerson LF, et al. Inter- and intra-individual variation in allele-specific DNA methylation and gene expression in children conceived using assisted reproductive technology. PLoS Genet. 2010;6(7):e1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feuer SK, Liu X, Donjacour A, et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology. 2014;155(5):1956–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Waal E, Mak W, Calhoun S, et al. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod. 2014;90(2):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fortier AL, Lopes FL, Darricarrere N, Martel J, Trasler JM. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet. 2008;17(11):1653–1665. [DOI] [PubMed] [Google Scholar]
- 16. Laprise SL. Implications of epigenetics and genomic imprinting in assisted reproductive technologies. Mol Reprod Dev. 2009;76(11):1006–1018. [DOI] [PubMed] [Google Scholar]
- 17. Gosden R, Trasler J, Lucifero D, Faddy M. Rare congenital disorders, imprinted genes, and assisted reproductive technology. Lancet. 2003;361(9373):1975–1977. [DOI] [PubMed] [Google Scholar]
- 18. Nagy Aea. Manipulating the Mouse Embryo: A Laboratory Manual. New York, NY: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 19. Mainigi MA, Olalere D, Burd I, Sapienza C, Bartolomei M, Coutifaris C. Peri-implantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth. Biol Reprod. 2014;90(2):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carver AR, Tamayo E, Perez-Polo JR, Saade GR, Hankins GD, Costantine MM. The effect of maternal pravastatin therapy on adverse sensorimotor outcomes of the offspring in a murine model of preeclampsia. Int J Dev Neurosci. 2014;33:33–40. [DOI] [PubMed] [Google Scholar]
- 21. Carver AR, Andrikopoulou M, Lei J, et al. Maternal pravastatin prevents altered fetal brain development in a preeclamptic CD-1 mouse model. PLoS One. 2014;9(6):e100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larroque B, Bertrais S, Czernichow P, Leger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Pediatrics. 2001;108(1):111–115. [DOI] [PubMed] [Google Scholar]
- 23. Arcangeli T, Thilaganathan B, Hooper R, Khan KS, Bhide A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol. 2012;40(3):267–275. [DOI] [PubMed] [Google Scholar]
- 24. Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119(pt 2):339–349. [DOI] [PubMed] [Google Scholar]
- 25. Damy T, Ratajczak P, Robidel E, et al. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. FASEB J. 2003;17(13):1934–1936. [DOI] [PubMed] [Google Scholar]
- 26. Shinkai T, Ohmori O, Hori H, Nakamura J. Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Mol Psychiatry. 2002;7(6):560–563. [DOI] [PubMed] [Google Scholar]
- 27. Kurrikoff T, Lesch KP, Kiive E, et al. Association of a functional variant of the nitric oxide synthase 1 gene with personality, anxiety, and depressiveness. Dev Psychopathol. 2012;24(4):1225–1235. [DOI] [PubMed] [Google Scholar]
- 28. Tanda K, Nishi A, Matsuo N, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grishok A, Pasquinelli AE, Conte D, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106(1):23–34. [DOI] [PubMed] [Google Scholar]
- 30. McLoughlin HS, Fineberg SK, Ghosh LL, Tecedor L, Davidson BL. Dicer is required for proliferation, viability, migration and differentiation in corticoneurogenesis. Neuroscience. 2012;223:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hong J, Zhang H, Kawase-Koga Y, Sun T. MicroRNA function is required for neurite outgrowth of mature neurons in the mouse postnatal cerebral cortex. Front Cell Neurosci. 2013;7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andersson T, Rahman S, Sansom SN, et al. Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs. PLoS One. 2010;5(10):e13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Volvert ML, Prevot PP, Close P, et al. MicroRNA targeting of CoREST controls polarization of migrating cortical neurons. Cell Rep. 2014;7(4):1168–1183. [DOI] [PubMed] [Google Scholar]
- 34. Rago L, Beattie R, Taylor V, Winter J. miR379-410 cluster miRNAs regulate neurogenesis and neuronal migration by fine-tuning N-cadherin. EMBO J. 2014;33(8):906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cui Y, Xiao Z, Han J, et al. MiR-125b orchestrates cell proliferation, differentiation and migration in neural stem/progenitor cells by targeting Nestin. BMC Neurosci. 2012;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katari S, Turan N, Bibikova M, et al. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18(20):3769–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turan N, Ghalwash MF, Katari S, Coutifaris C, Obradovic Z, Sapienza C. DNA methylation differences at growth related genes correlate with birth weight: a molecular signature linked to developmental origins of adult disease? BMC Med Genomics. 2012;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lucifero D, Mann MR, Bartolomei MS, Trasler JM. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum Mol Genet. 2004;13(8):839–849. [DOI] [PubMed] [Google Scholar]
- 39. Denomme MM, Mann MRW. Genomic imprints as a model for the analysis of epigenetic stability during assisted reproductive technologies. Reproduction. 2012;144(4):393–409. [DOI] [PubMed] [Google Scholar]
- 40. Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95(2):548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Imudia AN, Awonuga AO, Kaimal AJ, Wright DL, Styer AK, Toth TL. Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obstetric outcomes: a preliminary study. Fertil Steril. 2013;99(1):168–173. [DOI] [PubMed] [Google Scholar]