Abstract
Objective:
To assess the impact of maternal obesity and excessive gestational weight gain (GWG) on maternal and neonatal iron status and to explore the possible mediating role of inflammation on hepcidin.
Methods:
This analysis included 230 pregnant adolescents (13-18 years) enrolled in either a longitudinal or a cross-sectional study. Prepregnancy body mass index (ppBMI) and GWG were obtained from medical records. Maternal iron status (hemoglobin, serum iron, ferritin, transferrin receptor, total body iron, and hepcidin) and inflammation (interleukin-6 [IL-6] and leptin) were assessed at midgestation (26.2 ± 3.3 weeks) in the longitudinal cohort and at delivery (39.8 ± 1.3 weeks) in both study cohorts. Cord blood was collected in both studies and analyzed for iron indicators.
Results:
Approximately 40% of the adolescents entered pregnancy overweight or obese. Multivariate analysis identified ppBMI as a negative predictor of serum iron at midgestation (P = .009) and a positive predictor of serum hepcidin at delivery (P = .02). None of the other maternal iron status indicators were significantly associated with ppBMI or GWG. Serum IL-6 was significantly positively associated with hepcidin at delivery (P = .0001) but not at midgestation. There was a positive relationship between ppBMI and cord hemoglobin (P = .03).
Conclusion:
These results suggest that adiposity-related inflammation does not override the iron-mediated signals that regulate hepcidin production during pregnancy, and in this adolescent cohort, there is no strong evidence for a detrimental effect of maternal obesity and excessive weight gain on iron status in the offspring at birth.
Keywords: iron, weight gain, pregnancy, hepcidin, obesity, inflammation
Introduction
Approximately 60% of US women of reproductive age are overweight or obese,1 and women are gaining excessive weight during pregnancy.2 Maternal obesity and excessive gestational weight gain (GWG) are associated with various adverse obstetric and neonatal outcomes including preeclampsia, stillbirth, cesarean delivery, congenital anomalies, and macrosomia.2,3 Although obesity and excessive GWG are common, iron deficiency (ID) remains a public health problem, with 30% of US pregnant women having depleted body iron in the third trimester.4
Epidemiological studies have long noted a link between obesity and low iron status across age-groups.5 Recent research suggests this association may be mediated by the proinflammatory cytokine interleukin 6 (IL-6), which directly stimulates production of the iron regulatory hormone hepcidin.6 Hepcidin induces the degradation of the cellular iron exporter ferroportin and limits iron mobilization from intestinal iron absorption, macrophage iron recycling, and liver iron stores.7 In addition to IL-6, the adipokine leptin has been shown to stimulate hepcidin transcription and may represent another explanation for the association between adiposity and low iron status.8
In support of the mediating role of inflammation in the obesity-hepcidin link, studies in children and nonpregnant women found higher circulating IL-6 and hepcidin in obese compared with normal-weight controls.9–11 It is unclear whether hepcidin responds similarly to adiposity and inflammation during pregnancy, but a recent study suggests this may be the case.5 In 30 lean and obese pregnant women, serum hepcidin was 2 times higher in the obese group, and serum iron was lower in neonates born to obese mothers. However, serum iron is not a sensitive marker of iron status, and possible confounders such as dietary iron intake and race were not controlled for. A recent study in 316 diabetic and nondiabetic pregnant women showed that maternal obesity was associated with lower neonatal serum ferritin (SF).12 However, this association appeared to be driven by maternal diabetes, as it was no longer significant in the nondiabetic group. In addition, this study did not measure hepcidin or inflammatory markers, thus, it is unclear whether the observed association between maternal obesity and neonatal iron status was mediated by inflammation-associated increase in maternal hepcidin. Concurrent assessment of hepcidin and inflammation in healthy patients is needed to clarify the association between adiposity and iron status in uncomplicated pregnancies.
In a large group of healthy pregnant adolescents, we aimed to (1) evaluate the impact of prepregnancy body mass index (ppBMI) and GWG on serum hepcidin and iron status indicators, (2) examine the relationships between IL-6 and leptin with hepcidin, and (3) examine the relationships between ppBMI and GWG with neonatal hepcidin and iron status at birth.
Participants and Methods
Study Design and Participants
A total of 255 pregnant adolescents (13-18 years) were enrolled in 2 studies undertaken in Rochester, New York, between 2005 and 2012. The first study (n = 158) was designed to characterize longitudinal changes in maternal bone in relation to fetal bone growth, and the second study (n = 144) aimed to examine cross-sectional relationships between maternal and neonatal iron status. Details regarding study design as well as the primary study results on vitamin D and bone growth13,14 and iron status15 have been published previously. A total of 47 teens participated in both studies, making the combined sample size 255. Both studies collected maternal and cord blood at delivery, and the longitudinal study collected an additional maternal blood sample at midgestation (26.2 ± 3.3 weeks). The 2 study cohorts were recruited using similar eligibility criteria (age ≤ 18 years and gestational age ≥ 12 weeks at study entry, singleton pregnancy, and no known medical conditions including diabetes, HIV, or malabsorption diseases), and the 2 cohorts did not differ in ppBMI, GWG, or maternal iron status at delivery. Of the 255 pregnant adolescents, 7 did not have self-reported ppBMI, 15 did not have recorded weight at delivery, 46 did not have delivery iron status measurement due to lack of blood sample associated with adverse birth outcomes (n = 22), and other miscellaneous situations such as study personnel were not notified about delivery or teens refused to give blood or were transferred to other hospitals for delivery. One participant with biologically implausible weight loss (14 kg) from prepregnancy to midgestation was excluded from all the analyses. The final study population consisted of 230 pregnant adolescents in whom complete data on ppBMI, GWG, and iron status assessment at midgestation (n = 144) and/or at delivery (n = 207) were available.
Weight Gain Determinations
Prepregnancy body mass index (kg/m2) was calculated using prepregnancy weight and height recorded in the medical chart. Body mass index categories were defined in accordance with Institute of Medicine (IOM) guidelines as underweight: body mass index (BMI) < 18.5; normal weight: BMI 18.5 to 24.9; overweight: BMI 25 to 29.9; or obese: BMI ≥ 30. In some analyses, we further classified obesity into class 1 (BMI 30-34.9), class 2 (BMI 35-39.9), and class 3 (BMI ≥ 40).16 Due to the small number of teens with class 3 obesity (n = 3), class 2 and class 3 obesity were collapsed into 1 group (n = 13). Total GWG was calculated as the difference between prepregnancy weight and weight recorded by clinical staff at delivery. Adequate total GWG was defined as a total weight gain of 12.5 to 18 kg for underweight women, 11.5 to 16 kg for normal-weight women, 7 to 11.5 kg for overweight women, and 5 to 9 kg for obese women.2 For participants in the longitudinal cohort, weight data were also available at midgestation corresponding to the time when the maternal blood samples were obtained. We determined the range of adequate weight gain specific to the gestational week at midgestation using ppBMI-specific recommendations for the first trimester and total GWG according to the method by Chmitorz et al.17
Biochemical Analyses
All whole blood samples were assessed for hemoglobin (Hb) by the hospital clinical laboratory as reported.18 Serum was stored at −80°C until analysis. Serum ferritin and soluble transferrin receptor (sTfR) were assessed using enzyme-linked immunosorbent assay (ELISA) kits (Ramco Laboratories, Stafford, Texas). Maternal total body iron was calculated from SF and sTfR using the equation by Cook et al.19 Anemia and ID were determined using established cutoffs and detailed in Supplemental Methods. Serum erythropoietin (EPO) and C-reactive protein were measured by the Immulite 2000 immunoassay system (Siemens, Los Angeles, California). Serum hepcidin was measured by Intrinsic LifeSciences (La Jolla, California) using a competitive ELISA with a lower limit of detection of 5 µg/L.20 Hepcidin values <5 µg/L were assigned a value of 2.5 µg/L for the purposes of data analysis.21 Serum iron was measured by graphic furnace atomic absorption spectrophotometry (Perkin Elmer AAnalyst 800, Norwalk, Connecticut) in samples without visual signs of hemolysis (81.8% of total available). Serum IL-6 was measured with a multiplex assay kit (Millipore, Billerica, Massachusetts). Serum leptin was analyzed by ELISA (Millipore).
Questionnaires About Dietary Iron Intake
Participants from both studies self-reported their frequency of prenatal supplement use and their current smoking status in a health questionnaire administered at the entry into the study (25.3 ± 7.3 weeks). Dietary iron intake was estimated by 24-hour dietary recall in both studies by a registered dietician using the Nutrition Data System for Research (University of Minnesota, Minneapolis, Minnesota). Dietary recalls were conducted up to 3 times during early, mid, and late gestation in conjunction with prenatal care visits. Dietary intake data from all recalls were averaged for each participant and used in all analyses.
Statistical Analyses
All variables were entered into a database by trained study personnel and checked by a member of the research team. Data were analyzed using JMP Pro 10 (SAS Institute, Cary, North Carolina). Two adolescents self-identified as American Indian. Excluding these 2 participants did not change any of the study results, and none of the race-specific analyses differed if these 2 adolescents were grouped with either the African American or the caucasian cohort. To meet the requirement of statistical assumptions and power considerations, data from the 2 adolescents were included within the African American race cohort. Variables with skewed distributions were log transformed (except for cord hepcidin, which was transformed using square root) before analysis. Differences in biochemical measures between ppBMI and GWG categories were assessed by analysis of variance and Tukey post hoc tests. Linear trends across increasing ppBMI categories were analyzed by contrast analysis of variance. Bivariate correlations were analyzed by Pearson correlation. The relationships between ppBMI and GWG with iron status were also analyzed in multivariate models controlling for maternal age, race, gestational age at blood draw, and dietary iron intake. For models of hepcidin at delivery, delivery mode (vaginal and cesarean) was also included, as a previous study showed differences in maternal hepcidin by mode of delivery.22 Data were reported as mean ± standard deviation (SD). P values < .05 were considered statistically significant, and P values < .1 were considered as marginally significant.
Results
Demographic characteristics of the study population (n = 230) stratified by ppBMI are presented in Table 1. Average age of the adolescents at enrollment was 17.2 ± 1.1 years, and 38% entered pregnancy overweight or obese. Among the 41 obese teens, 13 had class 2 obesity. A smaller percentage of overweight or obese teens had a spontaneous vaginal delivery when compared to the underweight or normal-weight teens (82.6% vs 91.3%, P = .051). More than half of the obese adolescents (58.3%) gained excessive weight by midgestation, and 92.5% exhibited excessive GWG at delivery. Among the 227 adolescents with glucose tolerance test data, 1 normal-weight participant had a confirmed diagnosis of gestational diabetes at 30 weeks of gestation. Excluding this participant did not change any of the study results. There were no differences in race, ethnicity, smoking status, prenatal supplement use, or daily dietary iron intake across ppBMI categories.
Table 1.
Characteristics of Pregnant Adolescents by ppBMI Categories.a
| Characteristics | ppBMI, kg/m2 | No. of Participantsb | |||
|---|---|---|---|---|---|
| Underweight < 18.5 | Normal Weight 18.5-24.9 | Overweight 25.0-29.9 | Obese ≥ 30.0 | ||
| Participants, n (%) | 16 (7.0) | 127 (55.2) | 46 (20.0) | 41 (17.8) | 230 |
| Age at enrollment, years | 17.0 ± 1.1 | 17.1 ± 1.0 | 17.1 ± 1.2 | 17.4 ± 1.0 | 230 |
| ppBMI, kg/m2 | 17.5 ± 0.8e | 21.9 ± 1.8f | 27.4 ± 1.4g | 34.0 ± 4.0h | 230 |
| Parity > 0, % | 12.5 | 15.0 | 13.0 | 26.8 | 230 |
| Smoking during pregnancy, % | 6.3 | 8.0 | 4.4 | 4.9 | 227 |
| Gestational age at midgestation, weeks | 26.4 ± 3.6 | 26.2 ± 3.3 | 26.9 ± 3.2 | 25.4 ± 3.5 | 144 |
| Gestational age at delivery, weeks | 38.4 ± 3.1 | 39.1 ± 2.4 | 39.9 ± 2.2 | 39.8 ± 3.2 | 227 |
| Preterm delivery, % | 18.8 | 8.9 | 4.4 | 2.4 | 227 |
| Delivery mode, % | 224 | ||||
| Vaginal delivery | 93.8 | 91.0 | 78.3 | 87.5 | |
| Daily prenatal supplement use, % | 50.0 | 39.5 | 60.9 | 45.0 | 226 |
| Dietary iron intake, mg/dc | 21.6 ± 24.0 | 17.5 ± 7.7 | 15.0 ± 6.5 | 16.6 ± 7.8 | 216 |
| Race, % | 230 | ||||
| African American | 68.7 | 70.9 | 73.9 | 65.5 | |
| Ethnicity, % | 230 | ||||
| Non-Hispanic | 81.2 | 74.8 | 78.3 | 65.8 | |
| Weight gain adequacy at midgestation, %d | 144 | ||||
| Inadequate | 30.0 | 36.6 | 14.3 | 29.2 | |
| Adequate | 30.0 | 31.7 | 17.9 | 12.5 | |
| Excessive | 40.0 | 31.7 | 67.9 | 58.3 | |
| Total gestational weight gain adequacy, %d | 224 | ||||
| Inadequate | 26.7 | 19.5 | 8.7 | 0.0 | |
| Adequate | 26.7 | 29.3 | 15.2 | 7.5 | |
| Excessive | 46.7 | 51.2 | 79.1 | 92.5 | |
| Infant weight, kg | 2.98 ± 0.79e | 3.12 ± 0.49e | 3.42 ± 0.49f | 3.40 ± 0.45f | 223 |
Abbreviations: ppBMI, prepregnancy body mass index; SD, standard deviation.
aValues are means ± SDs. Means in rows with different letters are significantly different.
bNumbers vary because of missing values for some variables.
cMean intakes from averaged 24-hour dietary recall data; up to 3 recalls per participant.
dGroup differences were significant, P < .05 (χ2 test).
Relationships Between ppBMI and Weight Gain With Inflammation Across Gestation
We examined ppBMI both as a categorical and a continuous variable in relation to cytokine levels across gestation. At midgestation, approximately 40.9% of the teens had elevated C-reactive protein (CRP; >5 mg/L). Serum leptin, CRP, and IL-6 were >2 times higher in obese compared with normal-weight teens (Table 2) and showed significant positive intercorrelations (P < .05 for all correlations). At delivery, leptin remained elevated in the overweight and obese groups, but IL-6 in the obese teens no longer differed from the normal-weight group. Bivariate analyses showed that ppBMI was positively associated with serum leptin (r = 0.58, P < .0001), CRP (r = 0.50, P < .0001), and IL-6 (r = 0.24, P = .004) at midgestation. At delivery, ppBMI was positively correlated with leptin (r = 0.48, P < .0001) but not with IL-6.
Table 2.
Prepregnancy Body Mass Index and Maternal and Neonatal Iron Status Indicators and Inflammation Markers.a
| ppBMI, kg/m2 | ||||
|---|---|---|---|---|
| Underweight < 18.5 | Normal Weight 18.5-24.9 | Overweight 25.0-29.9 | Obese ≥ 30 | |
| Maternal | ||||
| Midgestation | ||||
| Hemoglobin, g/dL | 10.9 ± 1.2 (9) | 11.1 ± 0.9 (62) | 11.3 ± 0.8 (22) | 11.4 ± 0.8 (21) |
| Anemia, % | 22.2 (2) | 8.1 (5) | 0.0 (0) | 9.5 (2) |
| Serum ferritin, µg/L | 14.8 ± 7.5 (10) | 22.4 ± 17.8 (82) | 24.3 ± 16.6 (28) | 28.4 ± 25.5 (24) |
| < 12 (%) | 30.0 (3) | 31.7 (26) | 17.9 (5) | 20.8 (5) |
| Serum sTfR, mg/L | 5.6 ± 2.5 (10) | 5.1 ± 3.6 (82) | 5.2 ± 6.8 (28) | 4.9 ± 2.2 (24) |
| > 8.5 (%) | 10.0 (1) | 6.1 (5) | 3.6 (1) | 12.5 (3) |
| Total body iron, mg/kg | 1.5 ± 4.4 (10) | 3.3 ± 3.7 (82) | 4.3 ± 3.5 (28) | 3.7 ± 5.0 (24) |
| <0 (%) | 20.0 (2) | 14.6 (12) | 10.7 (3) | 20.8 (5) |
| Serum iron, µg/dL | 94 ± 49b,c (7) | 127 ± 87b (72) | 97 ± 40b,c (25) | 77 ± 32c (20) |
| Serum EPO, mIU/mL | 38.3 ± 34.9 (9) | 31.7 ± 20.7 (79) | 30.7 ± 12.0 (28) | 36.7 ± 24.0 (24) |
| Serum hepcidin, µg/L | 18.7 ± 24.6 (10) | 27.3 ± 25.1 (81) | 35.6 ± 32.1 (27) | 29.8 ± 19.3 (24) |
| <5 (%) | 10.0 (1) | 6.2 (5) | 3.7 (1) | 0 (0) |
| C-reactive protein, µg/L | 2.0 ± 1.1b (8) | 4.2 ± 4.4b (61) | 10.7 ± 8.7c (21) | 9.0 ± 5.3c (20) |
| > 5 (%) | 0.0 (0) | 27.9 (17) | 66.7 (14) | 70.0 (14) |
| Serum leptin, µg/L | 17.9 ± 8.2b,c (10) | 20.3 ± 12.7b (79) | 34.4 ± 18.6c (25) | 54.9 ± 24.1d (23) |
| Serum IL-6, µg/L | 1.2 ± 1.2 (10) | 3.3 ± 8.9 (80) | 5.1 ± 10.3 (28) | 7.0 ± 22.0 (24) |
| Delivery | ||||
| Hemoglobin, g/dL | 10.9 ± 1.0 (14) | 11.6 ± 1.5 (102) | 11.4 ± 1.3 (41) | 11.5 ± 1.4 (41) |
| Anemia (%) | 21.4 (3) | 20.6 (21) | 14.6 (6) | 17.1 (7) |
| Serum ferritin, µg/L | 37.6 ± 69.8 (14) | 30.7 ± 30.2 (107) | 26.8 ± 30.9 (43) | 29.9 ± 18.6 (41) |
| <12 (%) | 28.6 (4) | 20.6 (22) | 25.6 (11) | 17.1 (7) |
| Serum sTfR, mg/L | 6.7 ± 3.6 (14) | 5.6 ± 2.9 (109) | 5.1 ± 2.2 (43) | 5.7 ± 4.4 (41) |
| >8.5 (%) | 21.4 (3) | 12.8 (14) | 9.3 (4) | 19.5 (8) |
| Total body iron, mg/kg | 2.6 ± 5.4 (14) | 3.9 ± 4.1 (107) | 3.6 ± 3.5 (43) | 4.4 ± 3.9 (41) |
| <0 (%) | 28.6 (4) | 15.0 (16) | 14.0 (6) | 9.8 (4) |
| Serum iron, µg/dL | 88.9 ± 43.4 (10) | 135.3 ± 149.0 (90) | 104.4 ± 67.7 (40) | 107.8 ± 67.7 (35) |
| Serum EPO, mIU/mL | 39.8 ± 44.9 (14) | 33.0 ± 24.7 (106) | 36.3 ± 31.1 (40) | 28.8 ± 23.9 (40) |
| Serum hepcidin, µg/L | 21.5 ± 18.4 (13) | 38.5 ± 42.0 (108) | 42.0 ± 41.6 (43) | 44.5 ± 33.3 (40) |
| <5 (%) | 7.7 (1) | 13.9 (15) | 4.7 (2) | 5.0 (2) |
| Serum leptin, µg/L | 23.4 ± 13.9b (9) | 29.0 ± 18.6b,c (68) | 41.6 ± 23.0c (24) | 57.8 ± 23.7c (25) |
| Serum IL-6, µg/L | 5.4 ± 5.2 (13) | 10.4 ± 19.5 (106) | 10.4 ± 14.3 (41) | 5.8 ± 8.8 (40) |
| Neonatal | ||||
| Hemoglobin, g/dL | 13.4 ± 2.4 (11) | 14.0 ± 2.8 (73) | 14.0 ± 2.3 (27) | 15.5 ± 2.4 (21) |
| Anemia (%) | 45.5 (5) | 27.4 (20) | 18.5 (5) | 9.5 (2) |
| Serum ferritin, µg/L | 198.2 ± 179.0 (14) | 147.4 ± 94.7 (99) | 133.3 ± 105.9 (40) | 144.6 ± 95.5 (36) |
| <76 (%) | 21.4 (3) | 19.2 (19) | 40.0 (16) | 25.0 (9) |
| Serum sTfR, mg/L | 6.9 ± 2.6 (14) | 7.9 ± 2.9 (101) | 9.0 ± 4.2 (40) | 8.7 ± 4.7 (36) |
| Body iron content, mg | 156.0 ± 34.5b,c (11) | 160.2 ± 31.2b (70) | 174.8 ± 28.8b,c (27) | 184.0 ± 26.6c (21) |
| Serum iron, µg/dL | 252.2 ± 95.5 (10) | 221.9 ± 110.0 (68) | 193.1 ± 60.2 (28) | 207.0 ± 60.0 (24) |
| Serum EPO, mIU/mL | 42.6 ± 35.7 (12) | 41.0 ± 42.8 (93) | 194.0 ± 862.1 (35) | 54.1 ± 55.3 (32) |
| Serum hepcidin, µg/L | 73.9 ± 48.5 (14) | 121.5 ± 84.2 (98) | 144.9 ± 121.2 (39) | 123.0 ± 76.4 (36) |
Abbreviations: EPO, erythropoietin; IL-6, interleukin 6; ppBMI, prepregnancy body mass index; SD, standard deviation; sTfR, soluble transferrin receptor.
aValues are mean ± SD (n). Means in rows with different letters are significantly different.
We also compared inflammatory markers between weight gain categories. At midgestation, serum leptin but not IL-6 was significantly higher in teens who gained above the recommended range compared with those gaining within or below the recommended range (Supplemenatary Table S1). At delivery, leptin remained elevated in teens with excessive GWG, but there was no difference in IL-6 between GWG groups.
Relationships Between ppBMI and Weight Gain With Maternal Iron Status Across Gestation
Maternal iron status indicators and hepcidin by ppBMI categories are shown in Table 2. None of the maternal iron status indicators at midgestation or at delivery differed significantly among ppBMI categories. Teens gaining lower than recommended GWG had lower hepcidin at delivery compared with those who gained within or greater than the recommended range (Supplementary Table S1). There was no difference in the prevalence of anemia and ID between ppBMI or GWG groups. Further dividing obesity categories into class 1 and class 2 or above revealed that serum hepcidin at midgestation in adolescents with class 2 and 3 obesity was 1.5 times higher than the normal-weight group (39.6 vs 27.3 µg/L, P = .1). Similar to hepcidin, SF at midgestation was significantly elevated in teens with ppBMI ≥ 35 compared with the normal-weight teens (46.0 vs 22.4 µg/L, P = .02).
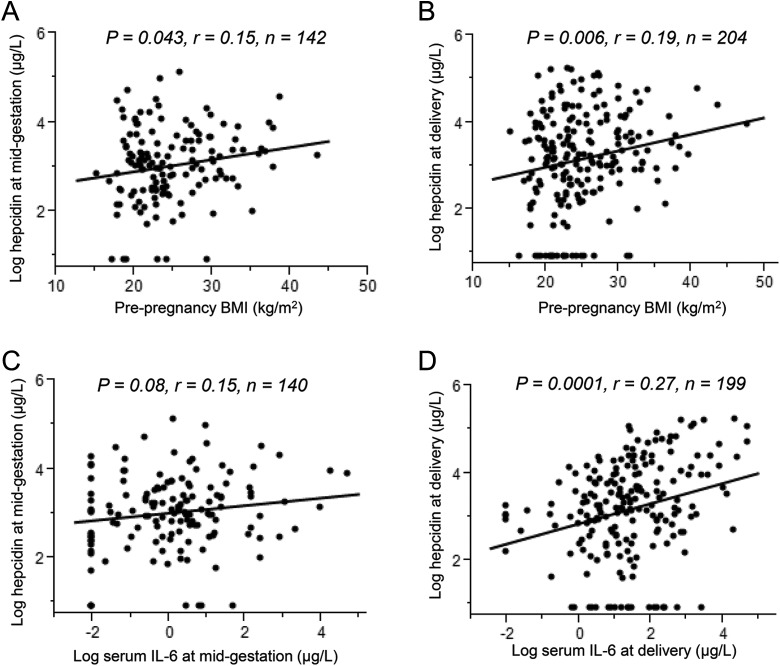
Possible relationships were explored with ppBMI and weight gain as continuous traits in relation to iron status and hepcidin across gestation. There was a weak positive correlation between ppBMI and serum hepcidin at midgestation (r = 0.17, P = .04; Figure 1A), but this relationship lost significance after controlling for maternal age, race, gestational age at midgestation, weight gain at midgestation, and dietary iron intake (Table 3). Serum hepcidin at delivery was positively correlated with ppBMI (r = .19, P = .006; Figure 1B), and ppBMI remained a significant positive predictor of maternal hepcidin at delivery after adjusting for age, race, gestational age at delivery, delivery mode, and dietary iron intake (Table 3). Serum iron at midgestation was inversely associated with ppBMI both in the bivariate analysis (r = −0.21, P = .02) and in the adjusted model (Table 3). Additional adjustment for smoking during pregnancy did not change the relationships between ppBMI with hepcidin or serum Fe. No relationship was evident between GWG and maternal iron status, except for a trend of a positive correlation between GWG and serum EPO at delivery (r = .12, P = .08).
Figure 1.
Correlations between prepregnancy body mass index (ppBMI) and serum interleukin 6 (IL-6) with serum hepcidin across gestation. Prepregnancy body mass index was positively associated with (A) serum hepcidin at midgestation (P = .043, r = 0.15, n = 142) and (B) serum hepcidin at delivery (P = .006, r = 0.19, n = 204). C, Serum IL-6 was insignificantly correlated with hepcidin at midgestation (P = .08, r = 0.15, n = 140) but was significantly correlated with hepcidin at delivery (P = .0001, r = 0.27, n = 199; D).
Table 3.
Significant Effects of ppBMI and Weight Gain on Maternal Iron Status Indicators.
| Variables | β | 95% CI | P | R 2 model |
|---|---|---|---|---|
| Midgestation serum irona | 0.09 | |||
| ppBMI, kg/m2 | −0.026 | −0.05 to −0.007 | .009 | |
| Weight gain at midgestation, kg | −0.005 | −0.02 to 0.01 | .58 | |
| Midgestation hepcidina | 0.07 | |||
| ppBMI, kg/m2 | 0.026 | −0.0004 to 0.05 | .054 | |
| Weight gain at midgestation, kg | −0.010 | −0.04 to 0.02 | .49 | |
| Delivery serum hepcidinb | 0.06 | |||
| ppBMI, kg/m2 | 0.035 | 0.005 to 0.06 | .024 | |
| Total gestational weight gain, kg | 0.0096 | −0.01 to 0.03 | .38 | |
Abbreviations: CI, confidence interval; ppBMI, prepregnancy body mass index.
aAdjusted for maternal age, race, gestational age at midgestation, and dietary iron intake during pregnancy.
bAdjusted for maternal age, race, gestational age at delivery, dietary iron intake during pregnancy, and delivery mode.
Relationships Between Hepcidin With IL-6 and Leptin Across Gestation
To explore a possible mediating role of inflammation in the relationship between ppBMI and hepcidin, we analyzed the relationships between serum IL-6 and leptin with hepcidin across pregnancy.
At midgestation, there was a trend for a positive correlation between IL-6 and hepcidin (r = 0.15, P = .08; Figure 1C). Serum IL-6 at delivery was on average 2.2 times higher than midgestation and was positively correlated with serum hepcidin (r = .27, P = .0001; Figure 1D). Leptin was not associated with hepcidin at midgestation or at delivery. Excluding participants with undetectable hepcidin at delivery further strengthened the positive correlation between hepcidin and IL-6 (r = .37, P < .0001). Serum IL-6 remained a significant positive predictor of hepcidin in multivariate analysis controlling for age, race, gestational age at delivery, and delivery mode (β = .23, P = .0001, r2 = .09). A model that included SF, EPO, and IL-6 at delivery explained 22% of the variation in hepcidin at delivery.
Relationships Between ppBMI and GWG With Neonatal Hepcidin and Iron Status
Neonatal iron status indicators in each maternal ppBMI category are presented in Table 2. Anemia was evident in 24% of the newborns at birth, and 25% of the neonates had SF < 76 µg/L, a cutoff that was previously associated with poor psychomotor performance at 5 years of age.23 Infants born to obese mothers had greater body iron content than those born to normal-weight mothers (184.0 mg vs 160.2 mg, P = .01). Neonates born to adolescents in higher ppBMI categories had higher cord Hb (P for trend = .03). None of the other neonatal iron status indicators including hepcidin differed among maternal ppBMI groups (Table 2). With the exception of body iron content, none of the neonatal iron status indicators showed significant differences between teens who gained excess weight compared to those who gained within the normal range (Supplemrntary Table S1).
Multivariate regression was used to assess the influence of ppBMI and GWG on neonatal iron status controlling for infant weight, sex, and gestational age at birth (Table 4). Neither ppBMI nor GWG was significantly associated with cord SF, sTfR, serum Fe, EPO, body iron content, or hepcidin. Higher GWG tended to be associated with lower cord hepcidin (β = −0.073, P = .07). Similar to the finding in categorical analysis, ppBMI was a positive predictor of cord Hb (β = .10, P = .03).
Table 4.
Associations Between Neonatal Iron Status Indicators and Maternal ppBMI and GWG.a
| Neonatal Iron Statusa | ppBMI, kg/m2 | GWG, kg | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | |
| Hemoglobin, g/dL | 0.10 | 0.01 to 0.19 | .028 | −0.0030 | −0.07 to 0.06 | .93 |
| Serum ferritin, µg/L | −0.011 | −0.03 to 0.01 | .33 | 0.0026 | −0.01 to 0.02 | .76 |
| Serum sTfR, mg/L | 0.0092 | −0.0005 to 0.02 | .064 | 0.0016 | −0.006 to 0.009 | .67 |
| Body iron content, mg | 0.76 | −0.05 to 1.6 | .065 | −0.019 | −0.6 to 0.6 | .95 |
| Serum hepcidin, µg/L | 0.036 | −0.07 to 0.14 | .51 | −0.073 | −0.15 to 0.006 | .070 |
| Serum iron, µg/dL | −0.0045 | −0.02 to 0.010 | .54 | −0.0021 | −0.01 to 0.009 | .70 |
| Serum EPO, mIU/mL | −0.0024 | −0.02 to 0.03 | .86 | 0.0048 | −0.01 to 0.02 | .61 |
Abbreviations: CI, confidence interval; EPO, erythropoietin; GWG, gestational weight gain; ppBMI, prepregnancy body mass index; sTfR, soluble transferrin receptor.
aAdjusted for infant sex, birth weight, and gestational age at delivery.
Discussion
This is the first study to examine the impact of both ppBMI and GWG on hepcidin and iron status in a large cohort of pregnant adolescents and their neonates. Concurrent assessment of inflammatory markers and hepcidin provided a unique opportunity to explore possible mediating factors in the relationship between obesity and iron status. We report for the first time different relationships between ppBMI, serum IL-6, and hepcidin at midgestation compared to those at delivery. Maternal obesity was associated with elevated serum IL-6 but not hepcidin at midgestation. Serum IL-6 at delivery was on average 2.2 times higher than midgestation and was positively correlated with serum hepcidin at delivery. With the exception of serum iron, obesity and excessive GWG did not negatively impact maternal iron status during pregnancy. In contrast to our hypothesis, maternal obesity had no negative impact on neonatal iron status and was positively associated with cord Hb concentrations. Collectively, our results suggest that adiposity-associated inflammation does not override the impact of low iron status in regulating hepcidin production during pregnancy, and there is little evidence for a detrimental effect of maternal obesity and excessive GWG on iron status in the offspring at birth.
Studies in nonpregnant women (n = 40)11 and pregnant adults (n = 29)5 demonstrated a 9 times and 2 times greater serum hepcidin, respectively, in the obese than the normal-weight group. In our study, serum hepcidin in obese pregnant adolescents was not significantly elevated compared with the normal-weight group. This discrepancy may reflect differences in the degree of adiposity between populations. Average BMIs of the obese participants in previous studies were above the cutoff for class 2 obesity, whereas the majority of obese teens in our study had class 1 obesity. When the obese group in our study was further stratified into different classes of obesity, we observed a 50% increase in hepcidin in adolescents with class 2 and class 3 obesity compared with the lean group. Although this increase did not reach statistical significance, our data are consistent with previous studies that showed higher hepcidin in very obese individuals and support the notion that a certain level of adiposity may be necessary to elicit significant elevations in hepcidin.24 The increased hepcidin in the high ppBMI group did not appear to be mediated by inflammation, as neither IL-6 nor leptin was associated with hepcidin at midgestation. It is possible that other hepcidin regulators, such as bone morphogenetic protein 625 and IL-22,26 contributed to the hepcidin increase in adolescents with the greatest obesity. The significance of these hepcidin modulators in severely obese populations warrants further investigation.
A negative association between ppBMI and serum iron was evident at midgestation, consistent with data in women and female adolescents.27–29 The link between low serum iron and adiposity is often attributed to inflammation-induced increases in hepcidin, but the lack of association between hepcidin and serum iron in our study and others30–32 does not support this explanation. An alternative explanation has been proposed27,28 and involves lipocalin 2, an iron binding protein highly expressed in adipose tissue.33,34 Adipose lipocalin 2 increases in obesity35 and may lead to iron sequestration in adipose tissue.
We assessed a potential mediating role of inflammation in the link between weight and hepcidin by measuring cytokines (IL-66 and leptin8) that have been shown to stimulate hepcidin production. Consistent with obesity-associated inflammation,36 IL-6, leptin, and CRP at midgestation were significantly elevated in obese compared to lean adolescents. However, neither IL-6 nor leptin was correlated with hepcidin during pregnancy. Positive correlations between IL-6 and hepcidin have been reported in patients with end-stage renal disease37 and sepsis38 but not in healthy premenopausal11 or pregnant women.5,32 The absence of positive relationships in individuals without severe inflammation may suggest that a threshold level of IL-6 must be reached to significantly impact hepcidin production.
The high prevalence of anemia in our study is consistent with previous reports in adolescent pregnancy.39,40 Common causes of anemia include nutritional deficiencies, chronic diseases, parasitic infections, and genetic hemoglobinopathies, with ID typically accounting for half of the cases with anemia.41 A unique cause of low Hb concentrations in pregnant women is the physiological expansion of plasma volume across gestation. To account for the hemodynamic changes in anemia determination, Hb cutoffs used to define anemia across pregnancy are trimester specific and are adjusted downward. However, as found in the general population, the majority of anemia in pregnant women is believed to be attributable to ID.42 This is supported by our study and the studies by others in minority populations demonstrating that approximately one-third to one-half of the cases with anemia were associated with ID defined by low SF.15,40,43 Although ID cutoffs are not adjusted for plasma volume expansion and remain static over gestation, the significant correlations observed between Hb and serum iron status indicators in our studies in pregnant adolescents,15,40 as well as the similar pattern of increases in both ID and anemia across gestation,4 provide further validation of the close relationship between ID and anemia in pregnancy.
There was no evidence for a negative impact of maternal ppBMI and GWG on neonatal iron status. Consistent with findings in adult pregnant women,12 neonates born to obese adolescents had higher Hb than those born to normal-weight mothers. This observation may reflect higher red blood cell mass in infants born to mothers with high ppBMI. Similar to the findings in nondiabetic pregnant women,12 there was no significant relationship between maternal BMI and cord SF in pregnant adolescents. It is unclear why we, and others,12 did not observe an inverse relationship between maternal BMI and cord serum iron as reported previously.5 More studies are needed to clarify the impact of maternal weight on neonatal iron status in women with healthy pregnancies.
Major strengths of this study included (1) the large sample size, (2) concurrent measurement of inflammatory cytokines (IL-6 and leptin) and hepcidin at multiple time points across gestation, (3) controlling for key confounding variables including dietary iron intake and delivery mode, and (4) the use of a comprehensive panel of iron status indicators to assess iron status in the mother and her neonate. Literature on the association between obesity and hepcidin during pregnancy is scarce, and even less is known about whether maternal obesity and inflammation negatively impacts neonatal iron status. The sample size of the current analysis is the largest among all studies to date on the topic of adiposity and iron status during pregnancy and allowed for a comparison of iron status across all prepregnancy weight and GWG categories. Additional measurements of IL-6 and leptin further strengthened the biological plausibility of the obesity-iron link and addressed the paucity of literature on the determinants of iron status in pregnant populations with a high prevalence of obesity and excessive GWG.
This study also has limitations. First, prepregnancy weight was self-reported and may have led to misclassification of ppBMI and may have biased the difference between weight groups toward null. Due to the large number of unplanned pregnancies and unavailability of measured weight at medical visits around the time of conception, prepregnancy weight is seldom available in the prenatal care setting44 and is frequently estimated by self-reported weight and weight at the first prenatal visit. It has been reported that ppBMI categorization based on self-reported weight is comparable to measured weight for the majority of women (>73%),45 and our prior research in 1120 pregnant adolescents in Baltimore showed that self-reported prepregnancy weight was plausible in more than 90% of the adolescents based on their first measured weight in the first trimester combined with expected rates of weight gain.46 Thus, although we recognize that the self-reported weight may not be accurate, it is reasonable to assume that such misclassification would probably be small, and if it had occurred, it would have reduced our ability to find meaningful associations. Another limitation of this study is the small number of adolescents in the class 2 and class 3 obesity categories. The small sample size in these groups undermined our ability to conduct subgroup analyses of obesity, inflammation, and iron status. Future studies in severely obese populations are needed to elucidate the possible threshold effects of inflammation on hepcidin and to examine the impact of severe maternal obesity on neonatal iron status.
In this large cohort of healthy pregnant adolescents, prepregnancy obesity had no negative impact on maternal and neonatal iron status. Maternal IL-6 increased across pregnancy and was positively associated with hepcidin only at delivery. Our results suggest that low-grade inflammation during pregnancy does not override the suppressive effect of iron depletion on hepcidin, supporting the notion that hepcidin expression is driven by the strength of each individual regulator under conditions of opposing stimuli.
Acknowledgments
The authors thank Tera Kent for laboratory support and Jay Barry and Francoise Vermeylen for statistical advice. The authors are grateful to the health project coordinators (Allison McIntyre, Hannah Stillings, Sarah Caveglia, and Lauren Cowen) for their assistance in study recruitment, the Highland Hospital staff, the University of Rochester Medical School Midwifery Group for their clinical assistance, and all the study volunteers for their participation.
Footnotes
Authors’ Note: KOO designed the research. KOO, EKP, EMC, and RG conducted the research and coordinated sample and data collection. MW performed hepcidin analysis. KOO and CC conceived the idea of the study, undertook the statistical analysis, and wrote the paper. All authors reviewed the manuscript and approved its final content. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Mark Westerman is an officer, stockholder, and developer of the hepcidin assay described herein. He holds issued U.S. patents on methods and compositions used to develop the assay.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the USDA National Institute of Food and Agriculture, award numbers 2005-35200-15218 and 2009-35200-05171. Additional support was provided by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number T32DK007158.
Supplemental Material: The online data and table are available at http://rs.sagepub.com/supplemental
References
- 1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 2. Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines. Washington, DC: National Academy Press; 2009. [PubMed] [Google Scholar]
- 3. American College of Obstetricians and Gynecologists. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol. 2013;121(1):213–217. [DOI] [PubMed] [Google Scholar]
- 4. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr. 2011;93(6):1312–1320. [DOI] [PubMed] [Google Scholar]
- 5. Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link? J Perinatol. 2013;33(3):177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7):2461–2463. [DOI] [PubMed] [Google Scholar]
- 7. Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung B, Matak P, McKie AT, Sharp P. Leptin increases the expression of the iron regulatory hormone hepcidin in HuH7 human hepatoma cells. J Nutr. 2007;137(11):2366–2370. [DOI] [PubMed] [Google Scholar]
- 9. Aeberli I, Hurrell RF, Zimmermann MB. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes (Lond). 2009;33(10):1111–1117. [DOI] [PubMed] [Google Scholar]
- 10. del Giudice EM, Santoro N, Amato A, et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab. 2009;94(12):5102–5107. [DOI] [PubMed] [Google Scholar]
- 11. Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring). 2010;18(7):1449–1456. [DOI] [PubMed] [Google Scholar]
- 12. Phillips AK, Roy SC, Lundberg R, et al. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34(7):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young BE, McNanley TJ, Cooper EM, et al. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am J Clin Nutr. 2012;95(5):1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young BE, McNanley TJ, Cooper EM, et al. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with parathyroid hormone and calcitriol in pregnant adolescents. J Bone Miner Res. 2012;27(1):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee S, Guillet R, Cooper EM, et al. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr. 2014;144(10):1524–1532. [DOI] [PubMed] [Google Scholar]
- 16. National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 17. Chmitorz A, von Kries R, Rasmussen KM, Nehring I, Ensenauer R. Do trimester-specific cutoffs predict whether women ultimately stay within the Institute of Medicine/National Research Council guidelines for gestational weight gain? Findings of a retrospective cohort study. Am J Clin Nutr. 2012;95(6):1432–1437. [DOI] [PubMed] [Google Scholar]
- 18. Young MF, Griffin I, Pressman E, et al. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and nonpregnant women. J Nutr. 2010;140(12):2162–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. [DOI] [PubMed] [Google Scholar]
- 20. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. [DOI] [PubMed] [Google Scholar]
- 21. Young MF, Griffin I, Pressman E, et al. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rehu M, Punnonen K, Ostland V, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85(4):345–352. [DOI] [PubMed] [Google Scholar]
- 23. Tamura T, Goldenberg RL, Hou JR, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140(2):165–170. [DOI] [PubMed] [Google Scholar]
- 24. Cheng HL, Bryant CE, Rooney KB, et al. Iron, hepcidin and inflammatory status of young healthy overweight and obese women in Australia. PLoS One. 2013;8(7):e68675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armitage AE, Eddowes LA, Gileadi U, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118(15):4129–4139. [DOI] [PubMed] [Google Scholar]
- 27. Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, et al. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutr. 2011;93(5):975–983. [DOI] [PubMed] [Google Scholar]
- 28. Yanoff LB, Menzie CM, Denkinger B, et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond). 2007;31(9):1412–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tussing-Humphreys LM, Liang H, Nemeth E, Freels S, Braunschweig CA. Excess adiposity, inflammation, and iron-deficiency in female adolescents. J Am Diet Assoc. 2009;109(2):297–302. [DOI] [PubMed] [Google Scholar]
- 30. Derbent AU, Simavli SA, Kaygusuz I, et al. Serum hepcidin is associated with parameters of glucose metabolism in women with gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2013;26(11):1112–1115. [DOI] [PubMed] [Google Scholar]
- 31. Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117(25):e218–e225. [DOI] [PubMed] [Google Scholar]
- 32. Simavli S, Derbent AU, Uysal S, Turhan NÖ. Hepcidin, iron status, and inflammation variables among healthy pregnant women in the Turkish population. J Matern Fetal Neonatal Med. 2014;27(1):75–79. [DOI] [PubMed] [Google Scholar]
- 33. Auguet T, Quintero Y, Terra X, et al. Upregulation of lipocalin 2 in adipose tissues of severely obese women: positive relationship with proinflammatory cytokines. Obesity (Silver Spring). 2011;19(12):2295–2300. [DOI] [PubMed] [Google Scholar]
- 34. Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. [DOI] [PubMed] [Google Scholar]
- 35. Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56(10):2533–2540. [DOI] [PubMed] [Google Scholar]
- 36. Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr. 2004;28(6):410–415. [DOI] [PubMed] [Google Scholar]
- 37. Samouilidou E, Pantelias K, Petras D, et al. Serum hepcidin levels are associated with serum triglycerides and interleukin-6 concentrations in patients with end-stage renal disease. Ther Apher Dial. 2014;18(3):279–283. [DOI] [PubMed] [Google Scholar]
- 38. van Eijk LT, Kroot JJ, Tromp M, van der Hoeven JG, Swinkels DW, Pickkers P. Inflammation-induced hepcidin-25 is associated with the development of anemia in septic patients: an observational study. Crit Care. 2011;15(1):R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Briggs MM, Hopman WM, Jamieson MA. Comparing pregnancy in adolescents and adults: obstetric outcomes and prevalence of anemia. J Obstet Gynaecol Can. 2007;29(7):546–555. [DOI] [PubMed] [Google Scholar]
- 40. Iannotti LL, O’Brien KO, Chang SC, et al. Iron deficiency anemia and depleted body iron reserves are prevalent among pregnant African-American adolescents. J Nutr. 2005;135(11):2572–2577. [DOI] [PubMed] [Google Scholar]
- 41. de Benoist B, McLean E, Egli I, Cogswell M. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: WHO Press; 2008. [Google Scholar]
- 42. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201–207. [DOI] [PubMed] [Google Scholar]
- 43. Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. 2005;81(5):1218S–1222S. [DOI] [PubMed] [Google Scholar]
- 44. Holland E, Moore Simas TA, Doyle Curiale DK, Liao X, Waring ME. Self-reported pre-pregnancy weight versus weight measured at first prenatal visit: effects on categorization of pre-pregnancy body mass index. Matern Child Health J. 2013;17(10):1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mandujano A, Huston-Presley L, Waters TP, Catalano PM. Women’s reported weight: is there a discrepancy? J Matern Fetal Neonatal Med. 2012;25(8):1395–1398. [DOI] [PubMed] [Google Scholar]
- 46. Nielsen JN, O’Brien KO, Witter FR, et al. High gestational weight gain does not improve birth weight in a cohort of African American adolescents. Am J Clin Nutr. 2006;84(1):183–189. [DOI] [PubMed] [Google Scholar]