Abstract
Endometriosis is a gynecologic disease characterized by the ectopic presence of endometrial tissue on organs within the peritoneal cavity, causing debilitating abdominal pain and infertility. Current treatments alleviate moderate pain symptoms associated with the disorder but exhibit limited ability to prevent new or recurring lesion establishment and growth. Retrograde menstruation has been implicated for introducing endometrial tissue into the peritoneal cavity, but molecular mechanisms underlying attachment and invasion are not fully understood. We hypothesize that cysteine cathepsins, a group of powerful extracellular matrix proteases, facilitate endometrial tissue invasion and endometriosis lesion establishment in the peritoneal wall and inhibiting this activity would decrease endometriosis lesion implantation. To test this, we used an immunocompetent endometriosis mouse model and found that endometriotic lesions exhibited a greater than 5-fold increase in active cathepsins compared to tissue from peritoneal wall or eutopic endometrium, with cathepsins L and K specifically implicated. Human endometriosis lesions also exhibited greater cathepsin activity than adjacent peritoneum tissue, supporting the mouse results. Finally, we tested the hypothesis that inhibiting cathepsin activity could block endometriosis lesion attachment and implantation in vivo. Intraperitoneal injection of the broad cysteine cathepsin inhibitor, E-64, significantly reduced the number of attached endometriosis lesions in our murine model compared to vehicle-treated controls demonstrating that cathepsin proteases contribute to endometriosis lesion establishment, and their inhibition may provide a novel, nonhormonal therapy for endometriosis.
Keywords: cathepsin, cysteine protease, endometriosis, lesion establishment
Introduction
Endometriosis affects an estimated 5.5 million women in North America alone.1 Symptoms of endometriosis including dysmenorrhea, menorrhagia, and infertility likely result from the ectopic implantation of endometrial tissues, causing lesion formation on the peritoneal wall, uterus, and ovaries among other sites.2,3 Although current treatments can alleviate moderate pain symptoms, their ability to prevent lesion formation is inconsistent and varies among patients.4 Retrograde menstruation has been highly implicated in disease pathogenesis, since it enables endometrial fragments to travel upward through oviducts into the peritoneal cavity.5 However, this is unlikely the sole cause of the disorder since retrograde menstruation occurs in 90% of women,6 but only 6% to 10% of women are diagnosed with endometriosis.1 Pathogenic factors, aside from estrogen, may also contribute to disease development since lesion recurrence is prevalent even among patients after total hysterectomy and oophorectomy,7,8 highlighting the need for more effective mechanism-specific endometriosis therapies.
Ectopic endometrial cell invasion is central to endometriosis lesion establishment. Enzymes that degrade extracellular matrix (ECM) proteins such as collagen, fibronectin, and laminin that comprise peritoneal and uterine walls,9,10 may facilitate invasion. Cathepsin D has been shown to be increased in peritoneal fluid of patients with endometriosis,11,12 but it is an aspartic protease, a different family of proteases regulated differently, though similarly named to the cysteine cathepsins. Matrix metalloproteinases (MMPs) have also been shown to be upregulated in endometriosis and lesion establishment.13,14 Yet, cysteine cathepsins are the most potent ECM proteases and can hydrolyze 2 to 6 times the proteins per unit time compared to the more commonly studied MMPs.15,16 Cysteine cathepsins are also elevated in a number of tissue invasive/destructive diseases,17–20 motivating pharmaceutical companies to develop small molecule cathepsin inhibitors,21–24 but their activity has not been demonstrated in endometriosis. Here, we investigate the contribution of cysteine cathepsin proteases to endometriotic lesion establishment using a nonsurgical, immune-competent endometriosis mouse model. This model is closer to endometriosis etiology by maintaining the immune and lesion attachment aspects of the disease.25 Our work investigates whether pharmacological inhibition of cathepsins could serve as a novel therapy to reduce endometriosis lesion establishment.
Materials and Methods
Murine Endometriosis Model
This previously established and validated25,26 model provides an immunocompetent and nonsurgical approach to study endometriosis lesion establishment. The green fluorescent protein transgenic (GFP-Tg) donor mice were treated subcutaneously with 100 mg/kg estradiol valerate (dissolved in corn oil) 1 week before being sacrificed to stimulate endometrial tissue proliferation. Uterine tissue from GFP-Tg mice was dissected, finely minced with iris scissors, and further fragmented by serial titration in 0.5 mL of sterile phosphate-buffered saline (PBS).25 Phosphate-buffered saline only (Sham) or GFP-Tg donor uterine fragments (∼35 mg) were then injected into peritoneal cavities of syngeneic mice (ages 8-19 weeks, but age-matched per experiment) to mimic lesion establishment via retrograde menstruation. To determine the contribution of cathepsin proteases to endometriotic lesion establishment, a subset of mice were administered either vehicle or the broad cathepsin inhibitor, E-64 (9 mg/kg/d, EMD Millipore, Darmstadt, Germany) by intraperitoneal (ip) injection for 9 days. At day 10 postinjection, mice were euthanized and the uteri and peritoneum removed. The GFP-positive endometriotic lesions were visible under 488 nm light (excitation wavelength peak of GFP), collected, counted, and measured. All studies were completed in compliance with protocols approved by the Institutional Animal Care and Use Committee of Emory University (Permit No: DAR-2002462-100416BN) and performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Human Endometriosis Tissues
Human tissues were obtained from patients with endometriosis (n = 3; ages 29, 33, and 40) undergoing surgery for infertility or pelvic pain as part of a previously published study.27 Peritoneal tissue excised from that same woman was also collected. None of the women had been on estrogen- or progesterone-containing medications or other forms of pituitary suppression in the previous 3 months. The stage of endometriosis in all 3 women was classified as stage III according to the Revised American Society for Reproduction Medicine classification of endometriosis, 1996.28 The protocols were approved by the Institutional Review Boards of the Emory University School of Medicine. Written informed consent was obtained from all participants prior to surgery.
Multiplex Cathepsin Zymography
Experiments were based on our previously published protocol.29 Briefly, equal protein amounts from tissues were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) containing 0.2% gelatin. Enzymes were renatured, then incubated in assay buffer at pH 4 which is preferable for cathepsins L and V activity,29–31 for 18 to 24 hours in the presence or absence of E-64 or Z-FY-DMK (EMD Millipore, Darmstadt, Germany). Afterward, the gels were stained with Coomassie Blue followed by destaining. Gels were imaged with ImageQuant LAS 4000 (GE Healthcare, Pittsburgh, USA). Cleared white bands indicate cathepsin activity, and specific cathepsin activity was identified by molecular weight and quantified by densitometry.
Western Blot Analysis
Briefly, 25 μg of protein resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked with Odyssey Li-cor blocking buffer (Li-Cor, Lincoln, USA), and incubated overnight with primary antibodies: goat anti-murine cathepsin L and mouse anti-cathepsin V (R&D Systems, Minneapolis, USA); mouse anti-cathepsin K (Millipore, Darmstadt, Germany). Appropriate fluorescently tagged secondary antibodies were then used (Rockland, Pottstown, USA). Membranes were imaged on Odyssey Li-cor, and immunoreactive bands were quantified by densitometry with ImageJ software (NIH).
Statistical Analysis
Data are shown as mean ± standard error of the mean. Student t test analysis was used for comparison of 2 groups. One-way analysis of variance with Tukey posttest was used for comparison of multiple groups. Statistical significance was defined as P < .05.
Results
Mouse Endometriotic Lesions Exhibit Elevated Cathepsin Proteolytic Activity
To investigate the contribution of cysteine cathepsins to endometriotic lesion establishment, we employed a nonsurgical, immunocompetent mouse model of endometriosis. Briefly, minced uterine fragments from GFP-Tg donors were injected into the peritoneal cavity of recipient mice and allowed to implant (Figure 1A). This model simulates retrograde menses and peritoneal lesion establishment with lesions confirmed by hematoxylin and eosin histology to be nearly identical to those observed in the human counterpart. These lesions are also capable of forming cysts containing blood cells and enveloped by dense epithelial tissues and endometrial stroma.25 After 10 days, GFP-positive lesions, neighboring peritoneum tissue where no lesion implanted, and peritoneum tissue (Peri) of mice receiving injections of PBS only (Sham) were collected. This tissue was homogenized and prepared for multiplex cathepsin zymography to quantify the amounts of active, mature cathepsins to test the hypothesis that cathepsins were elevated during endometriotic lesion implantation and invasion. Cleared white bands on the dark background indicate active cathepsins. Cathepsin activity was significantly elevated in endometriotic lesions for the 100 and 20 kDa cathepsin signatures compared to the peritoneum of injected (Peri) or sham injected (Sham) mice by more than 5-fold (n = 8; *P < .05; Figure 1B). Peritoneal tissue was used for comparison since excising the lesions for testing may have contained some of the peritoneal wall tissue to which the lesions were attached.
Figure 1.
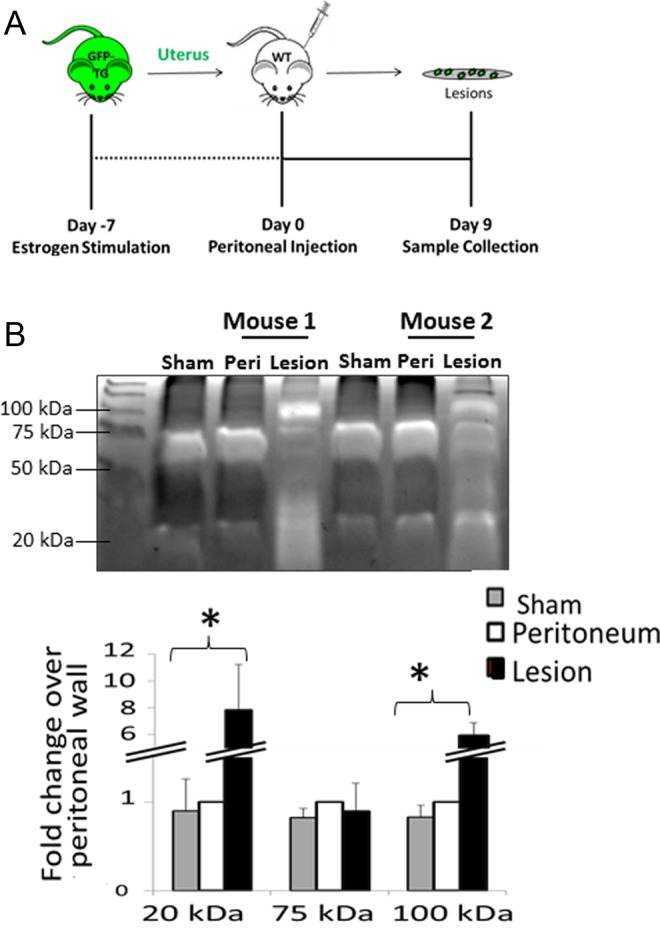
Mouse endometriotic lesions exhibit elevated cathepsin proteolytic activity. Uterine tissues from green fluorescent protein transgenic (GFP-Tg) mice were minced into solution and injected into the peritoneal cavity of recipient mice. Lesions were allowed to implant over 9 days (A). On day 10, the peritoneum and attached endometriosis lesions were collected and analyzed by multiplex cathepsin zymography (B). Endometriotic lesions exhibited a greater than 5-fold increase in cathepsin activity (20 and 100 kD) when compared to peritoneal samples (C, n = 8; *P < .05).
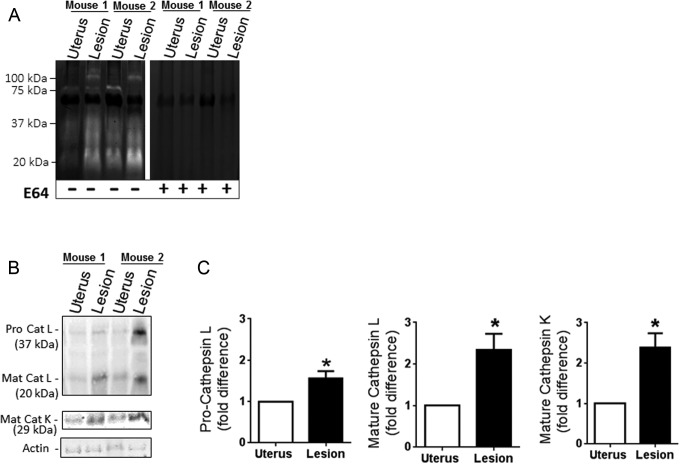
Active cathepsin levels in attached endometriotic lesions were also compared to that of uterine tissue since the endometrial tissue injected into recipient mice was derived from uteri of GFP-mice. Attached lesions exhibited greater cathepsin activity when compared to animal-matched uterine tissue that included tissue from both endometrium and myometrium layers (Figure 2A). To confirm the activity was due to cysteine cathepsins, the broad cathepsin small molecule inhibitor E-64 was incubated with zymograms overnight during the substrate degradation phase of the assay. Failure of white bands to appear confirmed that signals were due to active cysteine cathepsins in the uterus and lesion samples (Figure 2A). Since zymography only indicates the active form of the enzymes, we used Western blots to quantify the amount of protein expression of cathepsins L and K in the uterus and attached lesions (Figure 2B). Both the pro- and mature forms of the cathepsin L were detected with a 1.5-fold increase in procathepsin L (37 kDa) and a 2-fold increase in mature cathepsin L (25 kDa) in lesions compared to uterine tissue (n = 6, P < .05; Figure 2C). Statistically significant elevations in mature cathepsin K were also detected in lesions when compared to the uterine tissue and quantified with densitometry (n = 6, P < .05; Figure 2C).
Figure 2.
E-64 blocks all cathepsin proteolytic activity in murine endometriotic lesions. Incubation with the broad cathepsin inhibitor, E-64, completely abolished cathepsin activity detected by zymography identifying the active bands as cysteine cathepsins (A). Cathepsin protein levels in lesions were compared to eutopic uterine tissue by Western blot (B, n = 6). Representative immunoblots are shown. Cathepsins L and K were significantly increased in endometriotic lesions as compared to eutopic uterine controls (C; n = 6; *P < .05).
Cathepsin L is highly active among the other cathepsin bands induced in attached endometriotic lesions but is not responsible for all of the activity.
Cathepsin L and its human ortholog cathepsin V show increased activity by zymography when incubated at pH 4,29–31 which was used here to show the highest cathepsin signatures. However, in our previous examinations by multiplex cathepsin zymography of other tissues for active cathepsins, including arteries, lungs, muscle, bone, cervix, and breast,17,29 these cathepsin proteolytic profiles were different. To confirm the identity of these bands and whether their signal was due to cathepsin L, we repeated cathepsin zymography but incubated the gel in the presence or absence of the cathepsin L inhibitor Z-FY-DMK (5 µmol/L) during the overnight degradation step. Z-FY-DMK reduced the intensity of many of the active cathepsin bands, leaving only faint bands of activity at ∼40 to 50 kDa (Figure 3); this was determined previously to be where cathepsin K appears in multiplex cathepsin zymography,29,31 supporting the cathepsin K Western blot data (Figure 2B).
Figure 3.
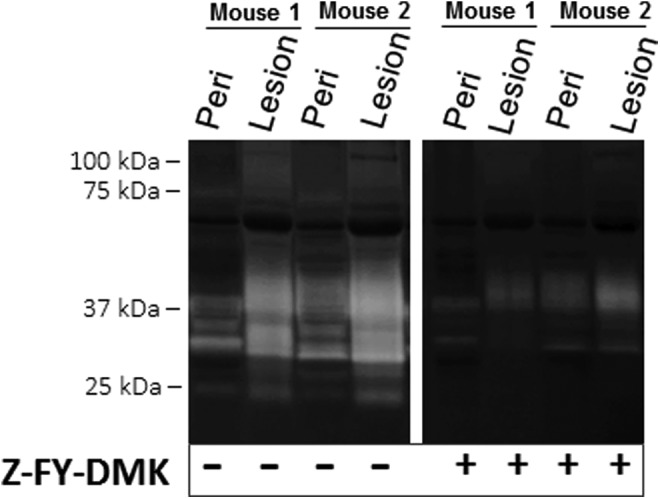
Z-FY-DMK cathepsin L inhibitor does not inhibit all cathepsin activity of murine endometriotic lesions. Incubation with Z-FY-DMK, a selective inhibitor of cathepsin L, inhibited many of the cathepsin active bands but did not block all active cathepsin bands. This suggested that the residually active band was not cathepsin L or susceptible to inhibition by Z-FY-DMK. Representative zymogram is shown from 2 matched mice tissue samples of 6 tested (n = 6).
Human Endometriotic Lesions also Contain Active Cathepsin L
If cathepsin L was elevated in our immunocompetent, nonsurgical mouse model of endometriosis, the next question was whether it would also be active in human endometriotic lesions. Similar to murine lesions, cathepsin L activity was found in human endometriotic lesions and could be abrogated by incubation with the cathepsin L inhibitor, Z-FY-DMK, which confirmed the identity as cathepsin L/V (Figure 4A). Particular bands of activity at the 20 and 37 kDa sizes were reduced, indicative of cathepsins L and V, respectively,29 and the protein identity and amounts were confirmed by Western blotting (Figure 4B). It is important to clarify that human cathepsin V is the ortholog of mouse cathepsin L, sharing greater than 80% sequence homology, and there is no murine ortholog for human cathepsin L.32
Figure 4.
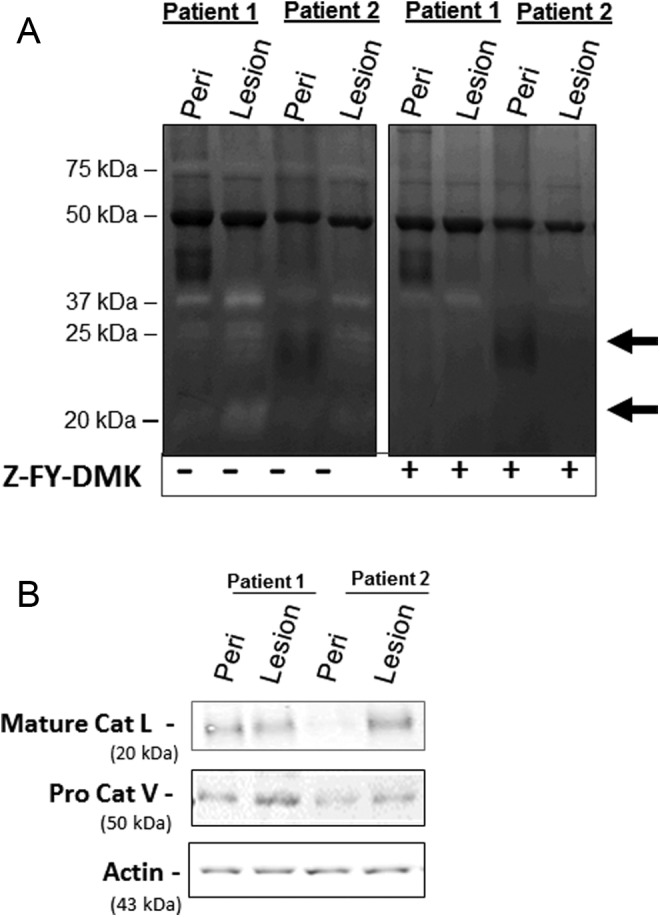
Human endometriotic lesions also contain active cathepsin L. A, Lesions from patients with endometriosis were tested for cathepsin L and V activity and patient-matched peritoneal tissue samples (n = 3, with 2 representative patients shown) were run to confirm that lesion activity was not due to peritoneal tissue contamination. Incubation with the cathepsin L specific inhibitor, Z-FY-DMK, blocked cathepsin L signals, confirming the cathepsin L bands in the zymogram (indicated by arrows). B, Cathepsin L and V protein expression in the human endometriotic lesion tissue was also confirmed by Western blot.
In Vivo, Broad Inhibition of Cysteine Cathepsins With E-64 Reduces Endometriotic Lesion Establishment in Mice
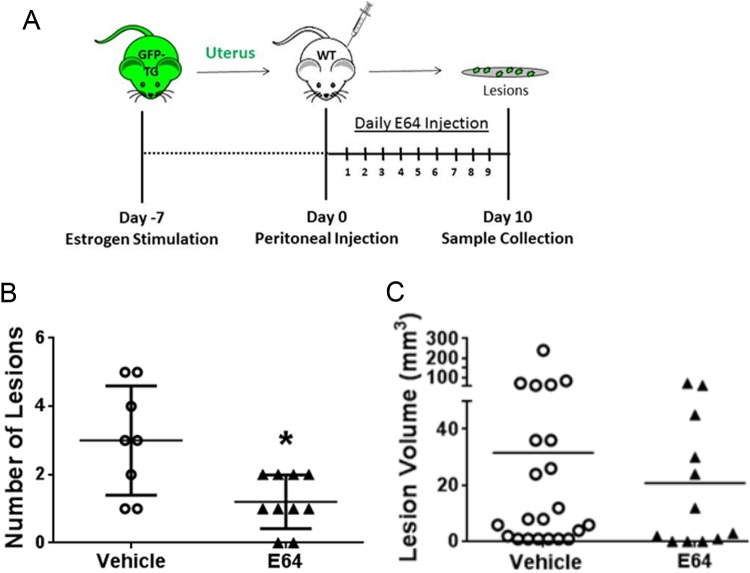
Since the proteolytic profile of mouse lesions exhibited strong similarities to the cathepsin signature of human patients, we tested the hypothesis that inhibiting all cysteine cathepsin activity could block endometriosis lesion attachment and implantation in vivo. E-64 was selected over Z-FY-DMK since Z-FY-DMK left residual, noncathepsin L activity in the zymograms (Figures 3 and 4), whereas E-64 showed complete inhibition (Figure 2A). Recipient mice were administered 9 mg/kg of the broad cathepsin inhibitor, E-64 daily via ip injection after implantation of the minced GFP-uterine fragments (Figure 5A). After 10 days, recipient mice were sacrificed, and lesions were collected, counted, and measured for size to assess lesion establishment. E-64 significantly reduced the number of implanted lesions in treated mice (n = 10) compared to vehicle control (n = 8, P < .01; Figure 5B), such that 2 of the mice even had complete prevention of lesion establishment by E-64 as they did not have any attached lesions at the end of the experiment. There also appeared to be a reduction in the volume of lesions with E-64 treatment, but this was not statistically significant (Figure 5C).
Figure 5.
Inhibiting cysteine cathepsins reduces endometriotic lesion establishment. Endometrial fragments from female green fluorescent protein transgenic (GFP Tg) mice were injected into the peritoneum of wild-type (WT) mice and allowed to form lesions for 10 days. Mice were administered vehicle or the broad cathepsin inhibitor, E-64 at 9 mg/kg/d via intraperitoneal injection. Lesions were then counted and measured on day 10 (A). Mice administered E-64 exhibited significant reductions in lesion number (B, n = 8, *P < .01) but not size (C, n = 8, P = .058) when compared to untreated controls. Two of the mice treated with E-64 did not have any implanted lesions.
Discussion
This work demonstrates the novel role of cysteine cathepsin proteases in endometriosis lesion establishment. Cysteine cathepsin expression and activity were significantly increased in endometriosis lesions that implanted and attached to the peritoneal wall when compared to either eutopic uterine or peritoneal tissues. Incubating the zymograms with the cathepsin L inhibitor Z-FY-DMK inhibited most of the cathepsin signal, implicating cathepsin L, but E-64, the broad cathepsin inhibitor, blocked all of this activity confirming the activity of other cathepsins in the attached endometriotic lesions. Further, endometriosis lesions excised from female patients also exhibited cathepsin L activity, confirming that cathepsins L and V are active in human endometriotic lesions as well. Then finally to test the efficacy of cathepsin inhibition as a therapy for endometriosis, E-64 peritoneal injections significantly reduced the numbers of attached lesions in our immunocompetent, nonsurgical mouse model. These data suggest a significant role for cysteine cathepsins in the invasion and implantation of endometrial tissue introduced into the peritoneal cavity and, taken together, suggest that targeted pharmaceutical inhibition of these proteases may effectively reduce initial endometriotic lesion establishment.
The etiological difference underlying the propensity of ectopic tissues to invade and establish endometriotic lesions in one woman over another has not been elucidated. We have observed in other studies that new cathepsin proteolytic profiles emerged following heterotypic cell–cell contact when compared to one cell type alone,33,34 and it may be the case here that when the endometriotic lesions contact the peritoneal wall, that new cathepsin activity is induced to facilitate attachment and early invasion. Our data also suggest that cathepsin activity may be important in this initial establishment of lesions, and one of these patient specific differences distinguishing women at risk for endometriosis could be inherent cathepsin activity. To validate this, it will be an important next step to compare cathepsin activity of peritoneal tissue from women with and without endometriosis and also cathepsin activity from eutopic endometrial tissue from women with and without endometriosis. Indeed, we have previously shown that there are wide ranges of variation of cathepsin expression between different individuals by profiling monocyte-derived macrophages,31 suggesting differences in tissue destructive disease prognosis.
A previously disrupted peritoneum is not required for endometriosis lesion formation,35 so it may be that the ectopic endometrial cells’ ability to proteolytically invade through peritoneal wall basement membrane may be an essential step of lesion establishment, and active cysteine cathepsins produced by that tissue could mediate this. The inflammatory mediators released by ectopic endometrial tissues and/or activated macrophages during lesion establishment36 may also modulate cathepsin expression and activity and will be important to understand for proper dosing of cathepsin inhibitors. One limitation of this study when interpreting ectopic endometrial cell attachment is that the myometrium was not separated from the endometrium of the mouse uteri prior to injection. This was an unavoidable limitation in order to obtain adequate viable endometrial tissue for producing reproducible results in terms of lesion development. Therefore the lesion versus eutopic tissue comparisons may contain some ratio of myometrial cells as well. This was not a concern for the human tissue since eutopic biopsies as well as the lesions were predominantly endometrial tissue.
Z-FY-DMK, the cathepsin L inhibitor used here, was shown in other studies to almost completely inhibit invasion by fibroblasts cocultured with melanoma cells,37 however it was not the only active cathepsin in the endometriotic lesion tissue. E-64, the broad cathepsin inhibitor, is not safe for systemic use in humans as there are many homeostatic cathepsin functions that, when inhibited, cause unpleasant side effects. However, a number of pharmaceutical inhibitors of cysteine cathepsins have been designed and advanced to human clinical trials but have failed due to side effects.38–39 Systemic administration of these inhibitors may be detrimental, but repurposing them for targeted gynecologic or peritoneal delivery to mitigate endometriosis may revive their utility. The identification of cysteine cathepsin proteases as contributors to endometriosis lesion establishment would highlight them as potential therapeutic targets for patients with endometriosis.
Footnotes
Declaration of Conflicting Interests: The authors(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Emory University FIRST Fellowship (K12GM000680, NIH/NIGMS) awarded to Kristi Porter. Additional funds provided by NIH Award Number DP2OD007433 and the Eunice Kennedy Shriver NICHD as part of the Cooperative Research Partnerships to Promote Workforce Diversity in the Reproductive Sciences (UO1 HD66439).
References
- 1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258. [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- 4. Soares SR, Martinez-Varea A, Hidalgo-Mora JJ, Pellicer A. Pharmacologic therapies in endometriosis: A systematic review. Fertil Steril. 2012;98(3):529–555. [DOI] [PubMed] [Google Scholar]
- 5. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110. 143. [PMC free article] [PubMed] [Google Scholar]
- 6. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–154. [PubMed] [Google Scholar]
- 7. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Repetitive conservative surgery for recurrence of endometriosis. Obstet Gynecol. 1991;77(3):421–424. [PubMed] [Google Scholar]
- 8. Namnoum AB, Hickman TN, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898–902. [DOI] [PubMed] [Google Scholar]
- 9. Witz CA, Montoya-Rodriguez IA, Cho S, Centonze VE, Bonewald LF, Schenken RS. Composition of the extracellular matrix of the peritoneum. J Soc Gynecol Investig. 2001;8(5):299–304. [DOI] [PubMed] [Google Scholar]
- 10. Sillem M, Hahn U, Coddington CC, III, Gordon K, Runnebaum B, Hodgen GD. Ectopic growth of endometrium depends on its structural integrity and proteolytic activity in the cynomolgus monkey (Macaca fascicularis) model of endometriosis. Fertil Steril. 1996;66(3):468–473. [DOI] [PubMed] [Google Scholar]
- 11. Bergqvist A, Ferno M, Mattson S. A comparison of cathepsin D levels in endometriotic tissue and in uterine endometrium. Fertil Steril. 1996;65(6):1130–1134. [PubMed] [Google Scholar]
- 12. Suzumori N, Ozaki Y, Ogasawara M, Suzumori K. Increased concentrations of cathepsin D in peritoneal fluid from women with endometriosis. Mol Hum Reprod. 2001;7(5):459–462. [DOI] [PubMed] [Google Scholar]
- 13. Chung HW, Lee JY, Moon HS, et al. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78(4):787–795. [DOI] [PubMed] [Google Scholar]
- 14. Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99(12):2851–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Bromme D. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279(35):36761–36770. [DOI] [PubMed] [Google Scholar]
- 16. Garnero P, Borel O, Byrjalsen I, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273(48):32347–32352. [DOI] [PubMed] [Google Scholar]
- 17. Chen B, Platt MO. Multiplex zymography captures stage-specific activity profiles of cathepsins K, L, and S in human breast, lung, and cervical cancer. J Transl Med. 2011;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Sukhova GK, Yang JT, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184(2):302–311. [DOI] [PubMed] [Google Scholar]
- 19. Cheng XW, Shi G-P, Kuzuya M, Sasaki T, Okumura K, Murohara T. Role for cysteine protease cathepsins in heart disease: Focus on biology and mechanisms with clinical implication. Circulation. 2012;125:1551–1562. [DOI] [PubMed] [Google Scholar]
- 20. Mohamed MM, Sloane BF. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. [DOI] [PubMed] [Google Scholar]
- 21. Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des. 2007;13:387–403. [DOI] [PubMed] [Google Scholar]
- 22. Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: Its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol. 2011;7:447–456. [DOI] [PubMed] [Google Scholar]
- 23. Desmarais S, Masse F, Percival MD. Pharmacological inhibitors to identify roles of cathepsin K in cell-based studies: A comparison of available tools. Biol Chem. 2009;390:941–948. [DOI] [PubMed] [Google Scholar]
- 24. Bromme D, Lecaille F. Cathepsin K inhibitors for osteoporosis and potential off-target effects. Expert Opin Investig Drugs. 2009;18:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wieser F, Wu J, Shen Z, Taylor RN, Sidell N. Retinoic acid suppresses growth of lesions, inhibits peritoneal cytokine secretion, and promotes macrophage differentiation in an immunocompetent mouse model of endometriosis. Fertil Steril. 2012;97(6):1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirata T, Osuga Y, Yoshino O, et al. Development of an experimental model of endometriosis using mice that ubiquitously express green fluorescent protein. Hum Reprod. 2005;20(8):2092–2096. [DOI] [PubMed] [Google Scholar]
- 27. Pierzchalski K, Taylor RN, Nezhat C, et al. Retinoic acid biosynthesis is impaired in human and murine endometriosis. Biol Reprod. 2014;91(4):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Revised american society for reproductive medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 29. Wilder CL, Park KY, Keegan PM, Platt MO. Manipulating substrate and pH in zymography protocols selectively distinguishes cathepsins K, L, S, and V activity in cells and tissues. Arch Biochem Biophys. 2011;516(1):52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li WA, Barry ZT, Cohen JD, et al. Detection of femtomole quantities of mature cathepsin K with zymography. Anal Biochem. 2010;401(1):91–98. [DOI] [PubMed] [Google Scholar]
- 31. Park KY, Li WA, Platt MO. Patient specific proteolytic activity of monocyte-derived macrophages and osteoclasts predicted with temporal kinase activation states during differentiation. Integr Biol (Camb). 2012;4(12):1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bromme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38(8):2377–2385. [DOI] [PubMed] [Google Scholar]
- 33. Keegan PM, Surapaneni S, Platt MO. Sickle cell disease activates peripheral blood mononuclear cells to induce cathepsins K and V activity in endothelial cells. Anemia. 2012;2012:201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keegan PM, Wilder CL, Platt MO. Tumor necrosis factor alpha stimulates cathepsin K and V activity via juxtacrine monocyte-endothelial cell signaling and JNK activation. Mol Cell Biochem. 2012;367(1-2):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nair AS, Nair HB, Lucidi RS, et al. Modeling the early endometriotic lesion: Mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril. 2008;90(4 suppl):1487–1495. [DOI] [PubMed] [Google Scholar]
- 36. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. [DOI] [PubMed] [Google Scholar]
- 37. Yin M, Soikkeli J, Jahkola T, Virolainen S, Saksela O, Holtta E. TGF-beta signaling, activated stromal fibroblasts, and cysteine cathepsins B and L drive the invasive growth of human melanoma cells. Am J Pathol. 2012;181(6):2202–2216. [DOI] [PubMed] [Google Scholar]
- 38. Wijkmans J, Gossen J. Inhibitors of cathepsin K: a patent review (2004 - 2010). Expert Opin Ther Pat. 2011;21(10):1611–1629. [DOI] [PubMed] [Google Scholar]
- 39. Lee-Dutra A, Wiener DK, Sun S. Cathepsin S inhibitors: 2004-2010. Expert Opin Ther Pat. 2011;21(3):311–337. [DOI] [PubMed] [Google Scholar]