Abstract
Objective:
Fenretinide is a synthetic retinoid analogue that promotes apoptosis but has decreased toxicity when compared to other retinoids. We have previously shown that retinoic acid (RA) production in endometriotic tissue is decreased, resulting in reduced estrogen metabolism and apoptotic resistance. We hypothesize fenretinide may induce apoptosis in endometriotic cells and tissues, thereby reducing disease burden.
Materials and Methods:
Primary endometriotic stromal cells were collected, isolated, cultured, and treated with fenretinide in doses from 0 to 20 µmol/L. Cell count, viability, and immunoblots were performed to examine apoptosis. Quantitative reverse transcription-polymerase chain reaction from endometriotic cells treated with fenretinide was used to examine expression of genes involved in RA signaling including stimulated by RA 6 (STRA6), cellular RA binding protein 2 (CRABP2), and fatty acid binding protein 5 (FABP5). Endometriotic tissue was xenografted subcutaneously into the flanks of mice which were treated with fenretinide for 2 weeks, after which the mice were killed and lesion volumes calculated. Statistical analysis was performed using t test and analysis of variance.
Results:
Treatment with fenretinide significantly decreased total cell count (doses 5-20 µL) and viability (doses 10-20 µmol/L). Fenretinide increased protein levels of the apoptotic marker poly (ADP ribose) polymerase (starting at 10 µmol/L) and decreased proliferation marker proliferating cell nuclear antigen (10 µmol/L, starting at 8-day treatment). Examination of genes involved in retinoid uptake and action showed that treatment induced STRA6 expression while expression of CRABP2 and FABP5 remained unchanged. Fenretinide also significantly decreased the endometriotic lesion xenograft volume.
Conclusions:
Fenretinide increases STRA6 expression thereby potentially reversing the pathological loss of retinoid availability. Treatment with this compound induces apoptosis. In vivo treatments decrease lesion volume. Targeting the RA signaling pathway may be a promising novel treatment for women with endometriosis.
Keywords: endometriosis, fenretinide, apoptosis, retinol, retinoid, STRA6, retinoic acid
Introduction
Endometriosis is an estrogen-dependent disease characterized by the growth of endometrial stroma and glands outside of the uterus.1 Women afflicted with this condition often present with chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility.1,2 Retrograde menstruation is often cited as the cause of this disease; however retrograde menstruation occurs in nearly all women yet not all women are afflicted with this condition. Therefore, it has been suggested that women with endometriosis are likely to have underlying molecular abnormalities allowing for the continued growth of the endometrial tissues outside the uterus.3
Since endometriosis is a heterogeneous disorder, therapies may be beneficial to some patients but not others. Therefore, there is a continued need to study novel pathways involved in disease initiation, maintenance, and progression in order to develop better therapies.1,4–6 We have recently shown that retinoid uptake, metabolism, and action are deficient in endometriotic cells compared to normal endometrial cells.7,8 We believe that this decreased expression in genes regulating retinoid uptake and action affects differentiation in endometriotic cells. We hypothesize that targeting this pathway may lead to novel treatments for this disorder.
Retinoic acid (RA) has been established as a potent modulator of gene transcription by directing control over cell growth, differentiation, and apoptosis.9 Because endometriosis is characterized by a resistance to apoptosis, we believe that perturbations in the RA signaling pathway are crucial to the establishment and progression of the disease.7,10,11 Our previous work indicated that RA production in endometriotic tissue is reduced relative to healthy endometrium, resulting in blunted estrogen metabolism.7,8 We recently characterized the expression of the genes responsible for retinoid uptake, synthesis, and degradation in both normal and endometriotic tissues and found a decreased expression of the genes involved in RA synthesis and trafficking, indicating that this signaling pathway plays a central role in the pathogenesis of endometriosis.
Fenretinide is a synthetic retinoid analogue with decreased toxicity compared with other retinoids. It is a differentiation-inducing agent and promotes apoptosis in a variety of tissues and cell types.12 The mechanism by which fenretinide inhibits growth and induces apoptosis is not well understood and may be independent of binding to nuclear retinoid receptors.12 Very preliminary studies done in leiomyoma cells have shown that it may block growth and induces apoptosis.13 Fenretinide has also been shown to reduce aromatase activity in vitro.14,15 Because of its ability to reduce aromatase, as well as induce apoptosis, we believe it will be efficacious in the treatment of endometriosis. We therefore hypothesize that treatment with fenretinide will inhibit the growth of human endometriotic tissues.
Material and Methods
Ethics Statement and Tissue Acquisition
Human tissue acquisition was approved by the Northwestern Institutional Review Board for human research. All the animal experiments were approved by Northwestern University Animal Care and Use Committee. Written consent was obtained from women undergoing surgery for endometriosis. Endometriosis (OSIS) tissue was obtained from cyst walls of ovarian endometriomas (n = 10). Because we are only able to obtain excess pathological tissues, all of our experiments are conducted using endometriomas.7,8,16 All the patients were premenopausal and were not on any hormonal therapy before the surgery. The average age of the patients was 38 ± 6 years.
Isolation and Culture of Primary Stromal Cells
Stromal cells were isolated from these endometriomas by using a protocol described by Ryan et al with a few modifications.17,18 Briefly, the tissues were minced and digested with pronase, deoxyribonuclease, and collagenase (Sigma) at 37°C for 40 minutes. A second round of digestion with hyaluronidase (Sigma, St. Louis, USA) was performed for another 20 minutes. Filtration through 70- and 20-µm sieves was used to separate stromal cells from epithelial cells. The cells were then resuspended in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12 1:1; GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) with 100 µg/mL streptomycin and 100 IU/mL penicillin.19 A humidified atmosphere at 5% CO2 and 37°C was used to grow the cells. All the cells were passaged once before being used for experiments. Media were changed every 48 hours. For all in vitro experiments, light exposure was minimized during treatment with fenretinide.
Cell Count Assay
At 60% to 80% confluency, OSIS cells were trypsinized and counted. Equal numbers of live cells were seeded in plastic cell culture plates. The cells were cultured overnight at 37°C and 5% CO2 in full media. The day after trypsinization, these cells were serum starved overnight to regulate cell cycle. After 14 to 16 hours of serum starvation, OSIS cells were placed in full media and treated with different concentrations of fenretinide (from 5 to 20 mol/L) while minimizing exposure to light. After 6 days of treatment with fenretinide, the cells were counted using a Bio-Rad TC20 Automated Cell Counter (Bio Rad, Cat # 1450102).20 Treatment length of 6 days was chosen based on preliminary time course experiments (data not shown). Results represent the mean ± standard error of the mean (SEM) for 6 independent experiments using tissues from different subjects.
Cell Viability Assay
At 60% to 80% confluency, OSIS cells were trypsinized and equal number of live cells were seeded in 96-well plates. The cells were initially cultured overnight in full medium then serum starved. After 14 to 16 hours of serum starvation, OSIS cells were placed in full media and treated with different concentrations of fenretinide (from 5 to 20 µmol/L) for 6 days while minimizing exposure to light. CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega) cell viability reagent was used to estimate cell viability and cytotoxicity according to manufacturer’s direction. The cytotoxicity of the cells was measured by adding MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] reagent to the cells, according to the manufacturer’s instructions. Absorbance was measured at 490 nm using the KC4 3.4 software. Background readings were done using wells containing media but no cells. Results represent the mean ± SEM for tissues from 6 different subjects.
RNA Extraction and Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
Cells were treated with 2 to 10 µmol/L of fenretinide for 6 days. Total RNA was isolated using TRIzol reagent (Sigma) following the manufacturer’s protocol. One microgram of RNA was used to generate complementary DNA (cDNA) using q-script cDNA SuperMix (Quanta Biosciences, Gaithersburg, Maryland). Real-time quantitative polymerase chain reaction (PCR) was performed using ABI 7900 Sequence Detection and the ABI Power SYBR Green gene expression systems (Applied Biosystems, Foster City, California) to quantify messenger RNA (mRNA) levels for stimulated by RA 6 (STRA6), cellular retinol binding protein 1 (CRBP1), cellular retinoic acid binding protein 2 (CRABP2), fatty acid binding protein 5 (FABP5), and 18S. 18S was used for normalization and is the housekeeping gene we have used in multiple studies.7,8 All PCR were run for 40 cycles (95°C for 15 seconds, 60°C for 1 minute) after 10 minutes of incubation at 95°C. The fold change in expression of each gene was calculated using the change in cycle threshold value method (ΔΔ Ct). Commercially available primers were used for all genes quantified (QIAGEN, Valencia, California). Results represent the mean ± sem for tissues from 6 different subjects.
Western Blot
When the cells were at 60% to 80% confluency, OSIS cells were trypsinized and seeded in Techno Plastic Products (TTP) tissue culture dishes (sterilized 22.1 cm, DNA/RNA, DNase/RNase, and pyrogen free). As soon as the desired incubation time with fenretinide was over, the cells were processed with radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors (Sigma) to extract proteins. Cells were washed with phosphate-buffered saline, collected, and homogenized in 120 µL RIPA buffer (1% Nonidet P-40 [NP-40], 0.5% sodium deoxycholate, 50 nmol/L Tris pH 7.6, 0.1% sodium dodecyl sulfate, and 150 nmol/L NaCl). The lysates were centrifuged and equal amounts of protein were resolved on NuPAGE Novex 4-12% bis-Tris Gels (Life Technologies). Transfer and membrane blocking were performed as described.21 Incubation with primary antibodies was performed at 4°C in 2.5% nonfat milk overnight, after which the membranes were washed and incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies. For proliferating cell nuclear antigen (PCNA), experiments were done using fenretinide 10 µmol/L. Westerns with poly (ADP-ribose) polymerases (PARP) and cleaved PARP were done at a 6-day time point. Data are representative of experimental replicates from 4 different patients, and results were quantified using Image J software.
Xenograft Mouse Model
A xenograft mouse model was adapted from the model previously described by Bruner-Tran et al.22–24 A total of 30 aged 8- to 9-week-old female CD1 athymic nude mice were purchased from Charles River Laboratories. The mice were maintained under pathogen free conditions in accordance with Northwestern University’s Institutional Animal Care and Use Committee. The mice were given standard food pellets and water and were allowed to acclimatize to their new environment for up to10 days. The mice were then ovariectomized to be able to better regulate estrogen and progesterone levels. An estradiol pellet (1.7 mg/pellet, 60-day release; Innovative Research of America) was implanted subcutaneously. The mice were then put in recovery cages for 10 days. Fresh OSIS tissue was acquired, weighed, cut into 1 mm × 1 mm fragments, reweighed, and divided into equal number of parts. The tissue fragments were cultured overnight at 37°C and 5% CO2 in 1000 µL phenol-red-free DMEM/F12 + 2% fetal bovine serum (sFBS) + 1% penicillin/streptomycin (P/S) with 10 nmol/L estradiol. The tissue fragments were then injected subcutaneously in each flank. A progesterone pellet (0.001 mg/pellet, 45-day release time) was also implanted at this time. Mice were weighed before, after, and periodically throughout the treatment. The size of the implanted tissue was measured with calipers throughout the course of treatment. Tissue volume was calculated after the mice were killed using the following formula: volume = 0.5 × length × width2.24 Fenretinide was freshly prepared every 3 days, kept at 4°C and protected from light.25,26 We injected mice (n = 16) with 120 mg/kg of fenretinide with intraperitoneal injections 5 times per week for 2 weeks. This dose was chosen based on other publications using this compound, and in line with what has been used clinically.27–33 Control mice (n = 14) were simultaneously treated with 5% ethanol in 0.9% NaCl solution containing 1.65 mg/mL bovine serum albumin.25 At the end of the experiment, the mice were euthanized and the endometriotic tissue lesions were resected. This tissue was embedded in paraffin blocks.
Immunohistochemistry Staining
Endometriotic tissue lesions were fixed in formalin, paraffin embedded, and 5 µm tissue sections were cut. After deparaffinization, heat-induced antigen retrieval was performed in a 10 mmol/L sodium citrate buffer (pH 6.0) with 0.05% Tween (Sigma) for 20 minutes in a pressure cooker. The slides were allowed to cool for 30 minutes at room temperature and were then washed in Tris-Buffered Saline - Tween 20 (TBS-T) for 5 minutes. The Dako EnVision HRP IHC kit was used (Dako North America, Inc, Carpinteria, California). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide and then washed. For Ki67 immunolabelling, nonspecific binding sites were blocked with protein block, and the tissue sections were then incubated with Ki67 primary antibody (Dako) overnight at 4°C in a humidified chamber. Antimouse secondary antibody was then applied to the tissue sections for 1 hour at room temperature and then washed. For cleaved caspase 3, nonspecific proteins were blocked using 5% normal donkey serum, incubated with cleaved caspase 3 (Cell Signaling Technology, Danvers, MA, USA) overnight, then the protocol provided by ABC kit (Vector Laboratories Inc, Burlingame, California) was followed. 3,3′-Diaminobenzidin (DAB) solution was used to develop color, and Mayer’s hematoxylin was used to counterstain the sections. The 28% ammonium hydroxide was used as a bluing reagent. The sections were dehydrated with 95% ethanol, 100% ethanol, and xylene, and then mounted onto cover slips using Cytoseal XYL mounting media (Richard-Allan Scientific). The slides were visualized using Zeiss upright AXIO Tissue FAXS version 3.5.5120.120 scope (TissueGnostics GmbH, Vienna, Austria). Data are representative of experimental replicates from at least 4 patients.
Statistical Analysis
Western Blot analysis and statistical analyses were performed with SPSS version 19 (IBM Corporation). A normal distribution of data was noted, so parametric testing was done. The average cell count and viability results were statistically analyzed using a one-way analysis of variance. For the volume experiments, statistical analysis was performed using a t test. A P value <.05 was considered significant for all the tests. All the values are plotted as the mean, with error bars indicating standard error of the mean.
Results
Fenretinide Decreases Cells Count and Cell Viability
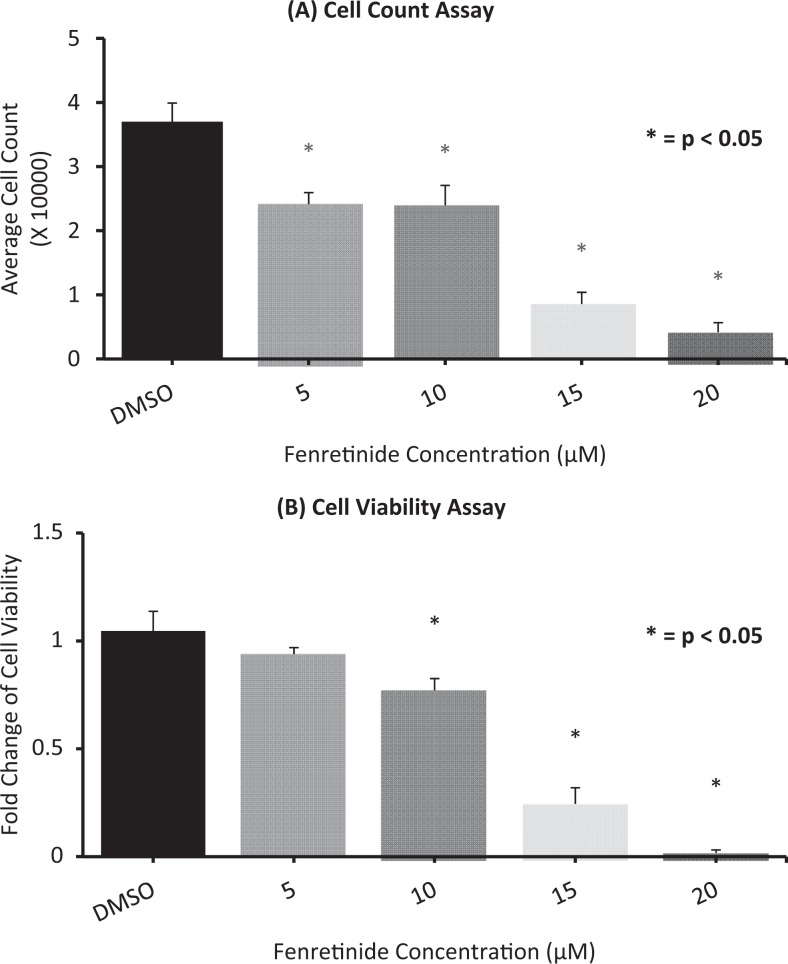
To test the effect of fenretinide on OSIS cell counts and viability, these cells were treated with fenretinide in a dose-dependent manner. As seen in Figure 1, fenretinide caused a significant decrease in the total cell count as well as the viability at 10 µmol/L concentrations over a 6-day treatment. Treatment for 6 days was chosen based on preliminary time course experiments (data not shown).
Figure 1.
Fenretinide decreases (A) cell count and (B) cell viability: Endometriotic cells were treated with vehicle (dimethyl sulfoxide [DMSO]) and increasing concentration of fenretinide (5, 10, 15, 20 µmol/L) for 6 days. Cell count was done using Bio-rad cell counter, and the viability was measured using 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. Data are presented as (A) total average cell count and (B) fold change in viability compared to control samples. Results represent the mean ± standard error of the mean (sem) from 6 independent experiments using tissues from different subjects.
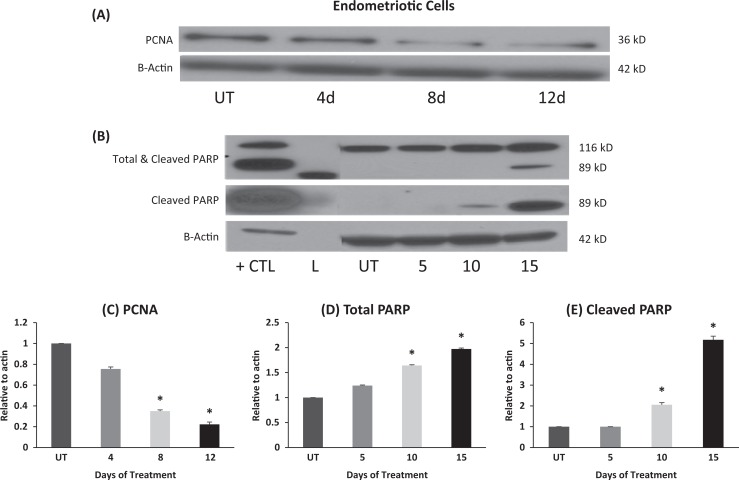
Fenretinide Decreases PCNA While Increasing the Cleaved PARP Protein Levels
We also tested the effects of fenretinide on PCNA and PARP over a course of up to12 days. As shown in Figure 2, we found that PCNA decreased in the OSIS cells treated with fenretinide suggesting a decrease in proliferation with treatment. We observed that treatment with fenretinide increased the cleaved PARP protein levels in a dose-dependent manner, while decreasing the total PARP levels, suggesting cell damage and death.
Figure 2.
Fenretinide decreases proliferating cell nuclear antigen (PCNA) and increases cleaved poly (ADP-ribose) polymerases (PARP) protein levels: A, OSIS cells were treated with vehicle (dimethyl sulfoxide [DMSO]) and fenretinide for 12 days. B, OSIS cells were treated and increasing concentration of fenretinide (vehicle, 5, 10, 15, 20 µmol/L). Western blot was performed to check PCNA, PARP (total and cleaved), and actin protein levels. (L = ladder, UT = DMSO control, CTL= tumor necrosis factor α [TNF-α], apoptosis control). Data are representative of experimental replicates from 4 patients (*p < 0.05).
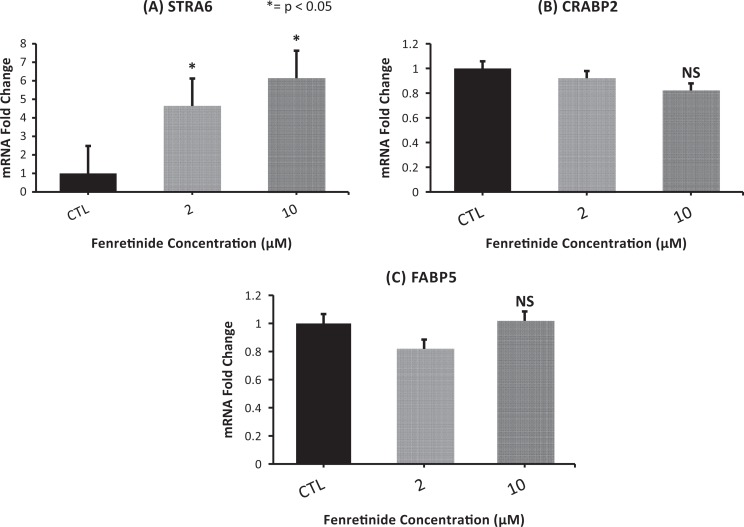
Fenretinide Treatment Causes an Increase in STRA6 Expression
To evaluate the mechanism of action of fenretinide, genes involved in retinoid uptake and action were assessed. Cells were treated with increasing doses of fenretinide, and RNA was isolated after 6 days. As shown in Figure 3, we found that the genes involved in retinoid transport into the nucleus, CRABP2, and FABP5 were unchanged with treatment. We found that STRA6 mRNA, the main retinol-binding protein receptor in cells, was significantly higher in cells treated with fenretinide when compared to the control cells (Figure 3).8 In addition, cellular retinol binding protein 1 (CRBP1) expression increased, although this was not statistically significant (Supplemental Figure 2).
Figure 3.
Fenretinide treatment increases STRA6 expression: stimulated by retinoic acid 6 (STRA6) messenger RNA (mRNA) fold expressions increase significantly when the cells were treated with fenretinide for 6 days. CRBP1 was also induced, nearing significance (P = .05). There was no change in cellular retinoic acid binding protein 2 (CRABP2) or fatty acid binding protein 5 (FABP5) expression. Results represent the mean ± standard error of the mean (sem) from 6 independent experiments using tissues from different subjects. NS indicates not significant.
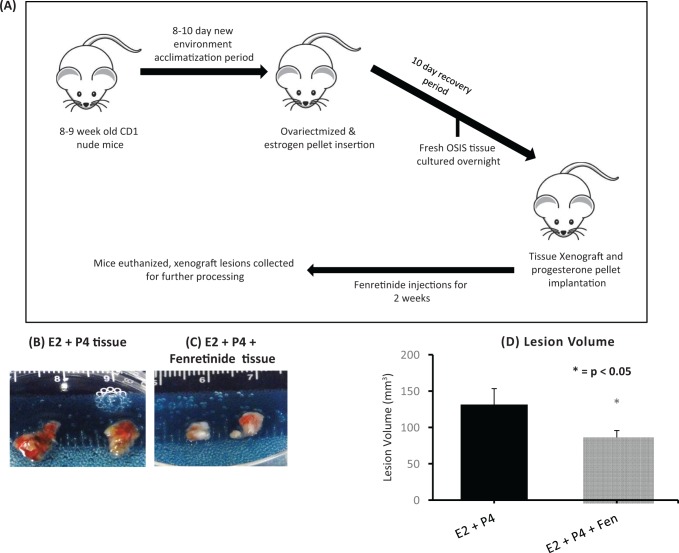
Fenretinide Treatment Causes a Decrease in Tissue Volume
To test the effects of fenretinide on human endometriotic tissues in an in vivo model, tissue fragments from ovarian endometriomas were injected subcutaneously into nude ovariectomized mice, as described by Bruner-Tran et al.22–24 As seen in Figure 5, fenretinide caused a significant decrease in the lesion volumes after 2 weeks of treatment compared to control. There was also a decrease in lesion weights after treatment with fenretinide as seen in supplemental Figure 1. In addition, the weight of the mice was stable throughout, suggesting that fenretinide was overall well tolerated (Supplemental Figure 1). Two-week treatment time period was chosen based on our previous work using this compound.31
Figure 5.
Fenretinide treatment decrease xenograft lesion volume. Compared to control (A), treatment with fenretinide (B) decreased the xenograft lesion. C, Xenograft lesion volume decreased significantly after the mice were subjected to fenretinide treatment when compared to the control group.
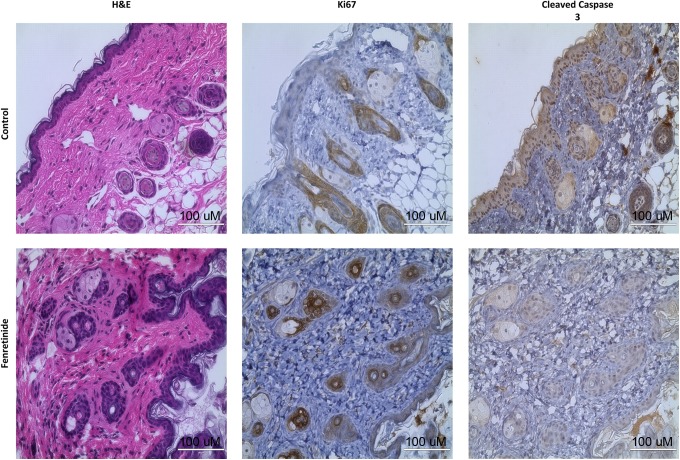
Fenretinide Treatment Causes a Decrease in Ki67 but an Increase in Cleaved Caspase 3 Staining
After the xenografts were harvested from the mice, the tissue was obtained and subjected to processing and embedding. Sections were cut and slides were stained for classic proliferation and apoptosis markers. As shown in Figure 4, fenretinide caused a decrease in the proliferation marker Ki67 and increased the apoptosis marker cleaved caspase 3, suggesting that treatment with this compound decreased disease burden.
Figure 4.
Immunohistochemistry results: hematoxylin and eosin (H&E), Ki67, cleaved caspase 3 immunohistochemistry staining of the control and fenretinide treatment xenografts at the time of harvest. The H&E sections show the presence of endometrial stroma and glands, indicating that xenografting did not alter this tissue. Treatment with fenretinide showed a decrease in Ki67 staining and an increase in cleaved caspase 3 staining when compared to controls, indicating a decrease in proliferation and an increase in apoptosis. Note: cells with large amounts of cytoplasm are adipose (xenografts were placed subcutaneously). Data are representative of experimental replicates from 4 different patient xenografts in each treatment group.
Discussion
In this article, we have shown using both in vitro and in vivo studies that fenretinide may act to inhibit the growth of human endometriotic tissues. In addition, we have shown that fenretinide increases the expression of STRA6, and we believe that this increase in retinol uptake may contribute to the apoptotic actions of this compound. We have also shown that treatment with fenretinide increases apoptosis and decreases cell proliferation.
It is well known that retinoids and their derivatives, both natural and synthetic, have a role in the regulation of cell growth, differentiation, and apoptosis.8,9 Our laboratory has previously shown that STRA6 is the principal regulator of retinol uptake in the endometrium and that the decreased expression of this gene is found in endometriosis. We have also previously shown that this decrease in STRA6 expression in endometriosis, and subsequent decrease in retinol uptake, leads to decreased 17-beta-hydroxylase mRNA expression.8 Ultimately, this causes an increase in local estradiol, the main mitogen in the maintenance and progression of endometriosis.34 Our laboratory has also demonstrated that endometrial and endometriotic cell fate can be influenced by the CRABP2:FABP5 ratio.8 We had initially hypothesized that the increase in apoptosis and decrease in cellular proliferation that was noted would be secondary to a change in the expression of CRABP2 or FABP5. However, as shown in Figure 3, treatment with this compound increased STRA6 expression but did not alter expression of either CRABP2 or FABP5. We now believe that this compound may increase retinol uptake into endometriotic cells. We believe that this increase in retinol uptake may ultimately lead to an increase in apoptotic activity in these pathogenic tissues. It has been shown that STRA6 may function as a cytokine receptor which activates JAK/STAT signaling in cells, and we believe that this may ultimately lead to increase in apoptosis.35 We hope to study this further in the future. Another group has also found that simvastatin induces expression of STRA6 and may contribute the apoptosis in endometriosis, although their study used human endometrial xenografts and not endometrotic tissue.36
We found that treatment with fenretinide decreased the size of endometriotic lesions in the mouse model. Other studies have also demonstrated the clinical efficacy of fenretinide. In a phase III trial done in 3000 premenopausal women with early stage cancer, administration of this drug resulted in a 35% reduction in contralateral breast cancer and ipsilateral reappearance.37,38 It was also shown to reduce specific serum markers associated with breast cancer recurrence.39,40 In another study, a complex of fenretinide and human serum albumin showed a high level of antitumor activity through the reduction of tumor mass using a lung cancer xenograft model.41 In addition, fenretinide is being investigated as a treatment for neuroblastoma as an agent for oral cancer chemoprevention as well as a treatment for age-related macular degeneration.28,29,42,43
A major drawback with the use of retinoids clinically has been their poor side effect profile.44 However, pilot studies with fenretinide have shown that it is well tolerated.45 The most common adverse effects with use of this compound were diminished dark adaptation (19%) and dermatologic issues (18.6%). Both of these events were noted to decrease with time. Symptoms were found to resolve spontaneously and did not require early discontinuation of treatment or a reduction of dose. Other side effects included gastrointestinal symptoms (13%) which tended to decrease with time and disorders of the ocular surface (10.9%). The rate of ocular surface issues was noted to be stable over time. Ocular surface issues and gastrointestinal issues prompted treatment discontinuation in a small number of patients (48 out of 1432).45 In our study, all the mice seemed to tolerate treatment with minimal side effects (Supplemental Figure 1).
Another issue with the use of retinoids in women of reproductive age is their teratogenicity, which may result in fetal malformations.46 In the in vivo portion of this study, mice were treated with fenretinide in the presence of both estrogen and progesterone. We feel that any studies done in reproductive aged women using this compound would need to be performed while women were on oral contraceptive pills or another reliable form of birth control. As our findings indicate, even in presence of estrogen and progesterone, tissue volume in the mice treated with fenretinide did decrease (Figure 5).
In summary, we have found that fenretinide decreases proliferation and increases apoptosis in human endometriotic lesions. Studies using this compound in women have found that it is overall well tolerated. Since current treatments for endometriosis are less than ideal, our study suggests that further research on the potential use of fenretinide in women with this disease is warranted.
Supplementary Material
Acknowledgements
This work was partially presented at the American Society for Reproductive Medicine 2014 meeting in Boston, MA.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially funded by:K12HD050121, Friends of Prentice (to MEP) R37HD038691 (to SEB).
Supplemental Material: The online [appendices/data supplements/etc] are available at http://rs.sagepub.com/supplemental.
References
- 1. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84(1):16–21. [DOI] [PubMed] [Google Scholar]
- 4. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 5. Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann N Y Acad Sci. 2002;955:252–264; discussion 293-255, 396-406. [DOI] [PubMed] [Google Scholar]
- 6. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pavone ME, Dyson M, Reirstad S, et al. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26(8):2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95(11):300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umesono K, Giguere V, Glass CK, Rosenfeld MG, Evans RM. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988;336(6196):262–265. [DOI] [PubMed] [Google Scholar]
- 10. Jones S, Meng L, Parsons DW, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33(1):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nasu K, Yuge A, Tsuno A, Nishida M, Narahara H. Involvement of resistance to apoptosis in the pathogenesis of endometriosis. Histol Histopathol. 2009;24(9):1181–1192. [DOI] [PubMed] [Google Scholar]
- 12. Formelli F, Barua AB, Olson JA. Bioactivities of N-(4-hydroxyphenyl) retinamide and retinoyl beta-glucuronide. Faseb J. 1996;10(9):1014–1024. [DOI] [PubMed] [Google Scholar]
- 13. Broaddus RR, Xie S, Hsu CJ, Wang J, Zhang S, Zou C. The chemopreventive agents 4-HPR and DFMO inhibit growth and induce apoptosis in uterine leiomyomas. Am J Obstet Gynecol. 2004;190(3):686–692. [DOI] [PubMed] [Google Scholar]
- 14. Ciolino HP, Dai Z, Nair V. Retinol inhibits aromatase activity and expression in vitro. J Nutr Biochem. 2011;22(6):522–526. [DOI] [PubMed] [Google Scholar]
- 15. Ciolino HP, Wang TT, Sathyamoorthy N. Inhibition of aromatase activity and expression in MCF-7 cells by the chemopreventive retinoid N-(4-hydroxy-phenyl)-retinamide. Br J Cancer. 2000;83(3):333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis. Fertil Steril. 2012;98(6):1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78(3):642–649. [DOI] [PubMed] [Google Scholar]
- 18. Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82(2):600–606. [DOI] [PubMed] [Google Scholar]
- 19. Dyson MT, Roqueiro D, Monsivais D, et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bio-rad. Bio-Rad TC20 Automated Cell Counter Description. Cell Counter Description. Web site http://www.bio-rad.com/en-us/product/tc20-automated-cell-counter?tab=Description.
- 21. Dyson MT, Jones JK, Kowalewski MP, et al. Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in MA-10 mouse leydig tumor cells. Biol Reprod. 2008;78(2):267–277. [DOI] [PubMed] [Google Scholar]
- 22. Bruner KL, Eisenberg E, Gorstein F, Osteen KG. Progesterone and transforming growth factor-beta coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis. Steroids. 1999;64(9):648–653. [DOI] [PubMed] [Google Scholar]
- 23. Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99(12):2851–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eaton JL, Unno K, Caraveo M, Lu Z, Kim JJ. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab. 2013;98(12):e1871–e1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Formelli F, Cleris L. Therapeutic effects of the combination of fenretinide and all-trans-retinoic acid and of the two retinoids with cisplatin in a human ovarian carcinoma xenograft and in a cisplatin-resistant sub-line. Eur J Cancer. 2000;36(18):2411–2419. [DOI] [PubMed] [Google Scholar]
- 26. Carrera S, Cuadrado-Castano S, Samuel J, et al. Stra6, a retinoic acid-responsive gene, participates in p53-induced apoptosis after DNA damage. Cell Death Differ. 2013;20(7):910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Decensi A, Robertson C, Guerrieri-Gonzaga A, et al. Randomized double-blind 2× 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27(23):3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villablanca JG, London WB, Naranjo A, et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: a report from the Children’s Oncology Group. Clin Cancer Res. 2011;17(21):6858–6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maurer BJ, Kang MH, Villablanca JG, et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: a report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr Blood Cancer. 2013;60(11):1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ratko TA, Detrisac CJ, Dinger NM, Thomas CF, Kelloff GJ, Moon RC. Chemopreventive efficacy of combined retinoid and tamoxifen treatment following surgical excision of a primary mammary cancer in female rats. Cancer Res. 1989;49(16):4472–4476. [PubMed] [Google Scholar]
- 31. Mittal N, Malpani S, Dyson M, et al. Fenretinide: a novel treatment for endometrial cancer. PLoS One. 2014;9(10):e110410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gopal AK, Pagel JM, Hedin N, Press OW. Fenretinide enhances rituximab-induced cytotoxicity against B-cell lymphoma xenografts through a caspase-dependent mechanism. Blood. 2004;103(9):3516–3520. [DOI] [PubMed] [Google Scholar]
- 33. Mukherjee N, Reuland SN, Lu Y, et al. Combining a BCL2 inhibitor with the retinoid derivative fenretinide targets melanoma cells Including melanoma initiating cells. J Invest Dermatol. 2015;135(3):842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng YH, Yin P, Xue Q, Yilmaz B, Dawson MI, Bulun SE. Retinoic acid (RA) regulates 17beta-hydroxysteroid dehydrogenase type 2 expression in endometrium: interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J Clin Endocrinol Metab. 2008;93(5):1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berry DC, O’Byrne SM, Vreeland AC, Blaner WS, Noy N. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol Cell Biol. 2012;32(15):3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sokalska A, Anderson M, Villanueva J, et al. Effects of simvastatin on retinoic acid system in primary human endometrial stromal cells and in a chimeric model of human endometriosis. J Clin Endocrinol Metab. 2013;98(3):e463–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veronesi U, De Palo G, Marubini E, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91(21):1847–1856. [DOI] [PubMed] [Google Scholar]
- 38. Veronesi U, Mariani L, Decensi A, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17(7):1065–1071. [DOI] [PubMed] [Google Scholar]
- 39. Torrisi R, Parodi S, Fontana V, et al. Effect of fenretinide on plasma IGF-I and IGFBP-3 in early breast cancer patients. Int J Cancer. 1998;76(6):787–790. [DOI] [PubMed] [Google Scholar]
- 40. Koay DC, Zerillo C, Narayan M, Harris LN, DiGiovanna MP. Anti-tumor effects of retinoids combined with trastuzumab or tamoxifen in breast cancer cells: induction of apoptosis by retinoid/trastuzumab combinations. Breast Cancer Res. 2010;12(4):r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Durante S, Orienti I, Teti G, et al. Anti-tumor activity of fenretinide complexed with human serum albumin in lung cancer xenograft mouse model. Oncotarget. 2014;5(13):4811–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mata NL, Lichter JB, Vogel R, Han Y, Bui TV, Singerman LJ. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33(3):498–507. [DOI] [PubMed] [Google Scholar]
- 43. Holpuch AS, Phelps MP, Desai K-GH, et al. Evaluation of a mucoadhesive fenretinide patch for local intraoral delivery: a strategy to reintroduce fenretinide for oral cancer chemoprevention. Carcinogenesis. 2012;33(5):1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Costa A, Malone W, Perloff M, et al. Tolerability of the synthetic retinoid fenretinide® (HPR). Eur J Canc Clin Oncol. 1989;25(5):805–808. [DOI] [PubMed] [Google Scholar]
- 45. Camerini T, Mariani L, De Palo G, et al. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19(6):1664–1670. [DOI] [PubMed] [Google Scholar]
- 46. Soprano DR, Soprano KJ. Retinoids as teratogens. Annu Rev Nutr. 1995;15(1):111–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.