Abstract
The objective of this study was to examine B-cell CLL/lymphoma 6 (BCL6) expression in human eutopic endometrium across the menstrual cycle in women with and without endometriosis and to establish a cutoff for future studies. This design was a series of case–control studies in tertiary University teaching hospitals. We examined BCL6 expression by messenger RNA and immunohistochemically in prospectively collected samples in both the proliferative (P) and the secretory phases. BCL6 is minimally increased in the mid-secretory phase of the menstrual cycle compared to the P phase in normal patients. BCL6 protein expression was significantly higher in the secretory phase of patients with endometriosis (n = 29) versus fertile controls without endometriosis at laparoscopy (n = 20; P < .0001). Normal fertile controls (n = 28) recruited for endometrial biopsy also had low levels of secretory phase BCL6 expression compared to women with unexplained infertility (UI; n = 119). A receiving–operator characteristic analysis of these data revealed an area under the curve of 94% (95% confidence interval 85%-100%; P < .0001) with an HSCORE cutoff of 1.4 to differentiate cases with and without endometriosis. Using this cutoff value, BCL6 was positive in 88% of cases with UI. Laparoscopic examination of a subset of 65 patients confirmed abnormalities in 98% of cases; 61 (93.8%) were found to have endometriosis, 3 (4.6%) with hydrosalpinx, and 1 (1.5%) with a normal pelvis. These data suggest that BCL6 is a promising candidate as a single diagnostic biomarker for detection of endometriosis in women with otherwise UI and may be associated with endometrial dysfunction, including progesterone resistance.
Keywords: BCL6, endometriosis, endometrium, biomarker, infertility
Introduction
The human endometrium is a dynamic, hormone-responsive tissue that undergoes repetitive, cyclic proliferation, differentiation, apoptosis, tissue breakdown, and repair to support its major function of regulating embryo implantation. These dynamic changes are orchestrated, directly and indirectly, by the sex steroids estrogen and progesterone and mediated by paracrine factors including classical immune system cytokines and chemokines.1
According to the American Society for Reproductive Medicine, unexplained infertility (UI) is estimated to represent 5% to 10% of couples with infertility.2 Unexplained causes account for at least 40% of recurrent pregnancy loss.3 Endometriosis is the predominant finding in UI, when diagnostic laparoscopy is performed.4–6 While laparoscopy is an important part of the workup for women with subfertility, this intervention is invasive, expensive, and not readily available to all women. Therefore, a diagnostic test for endometriosis would be a valuable tool for this population of women with otherwise UI.
Endometriosis is a common, inflammatory condition7 and a leading cause of pain and infertility, affecting an estimated 176 million women worldwide.8,9 Large differences in reproductive hormonal cycles between human and animal models (other than old-world primates), reproductive tract anatomy, and mechanisms governing endometrial function at embryo implantation have impeded progress in understanding the effects of endometriosis on human fertility. Further, the lack of validated biomarkers to screen for or diagnose endometriosis or to monitor recurrence of this disease accounts in part for the long delay in diagnosis.10
Endometriosis is associated with biological changes in the eutopic endometrium, which includes increased endometrial proliferation and inflammation, decreased apoptosis, altered cellular immunity, and diminished responses to progesterone also known as progesterone resistance.11 The tumor suppressor protein p53 is significantly decreased in endometriosis.12 Expression of p53 is specifically inhibited by B-cell CLL/lymphoma 6 (BCL6), a nuclear gene repressor associated with cellular proliferation.13,14 BCL6 is upregulated by Signal transducer and activator of transcription 3 (STAT3)15 which we recently reported as phosphorylated (pSTAT3) in eutopic endometrium of women with endometriosis compared to those without this disease.16 Like pSTAT3,17,18 BCL6 is overexpressed in different types of cancer,19–22 while p53 is decreased.23 These relationships may account for the proliferative and inflammatory phenotype of endometriosis. Further, as a gene repressor, BCL6 may account for progesterone resistance through decrease in known progesterone-mediated factors, including the Indian Hedgehog pathway involving COUP transcription factor 2 (COUP-TFII).24,25
There is little information available on BCL6 expression in endometrium of humans. The objectives of this study were to investigate BCL6 expression in human eutopic endometrium across the menstrual cycle in women with and without proven endometriosis and to evaluate the potential for BCL6 protein immunostaining as an endometriosis biomarker by describing the differences between populations, to establish an immunostaining cutoff to best define normal versus abnormal, and to apply the test to a large group of women with UI before undergoing laparoscopy as a prevalidation of BCL6 testing.
Materials and Methods
Study Design and Settings
This is a case–control study derived from prospectively obtained samples at the University of North Carolina (UNC) at Chapel Hill and from Greenville Health System (GHS), Greenville, South Carolina, between January 2002 and December 2013. Additional fertile controls who underwent laparoscopic tubal ligation were prospectively obtained from the Institute for Maternal and Child Research, Santiago, Chile, were studied.
Participants
The study was approved by the institutional review committees at both UNC–Chapel Hill and GHS. Participants were given written consent for collection and use of their tissues. Three different populations were used in this study: (1) endometrium from paid volunteers who served as normal fertile controls (endometrial tissue from controls); (2) endometrium obtained from women with infertility and or pain who underwent laparoscopy (women with and without endometriosis and BCL6 expression in eutopic endometrium); and (3) endometrium obtained in urinary luteinizing hormone (LH)-timed, mid-secretory (MS) phase of the menstrual cycle from women with UI (endometrial sample of women with UI).
Endometrial tissue from controls
Endometrial samples for normal controls were derived from prospective studies at the UNC at Chapel Hill described elsewhere.26 Briefly, women were recruited as paid volunteers to undergo endometrial biopsy in the proliferative (P) phase or in LH-timed sampling in the early secretory (ES), mid-secretory (MS), and late secretory (LS) phases. None had pain or infertility or signs or symptoms of endometriosis. Endometrial samples were obtained with Pipelle endometrial curette (Cooper Surgical, Trumbull, CT) across the menstrual cycle phase determined by urinary LH surge monitoring (Ovuquick; Quidel, San Diego, California). Samples were classified as P, ES (ES—LH surge + 1 to 5 days), MS (MS—LH surge + 6 to 10 days), or LS phases (LS—LH surge +11 to 14 days). Those with body mass index (BMI) ≥ 30, who took medications known to affect reproductive hormones during the previous 3 months, and those who had known anatomic or functional reproductive tract abnormalities were excluded. Samples were included only if endometrial dating agreed with the cycle day of the biopsy (±2 days).
A second set of fertile controls included women with prior fertility undergoing diagnostic laparoscopy for tubal ligation (n = 18) or fibroids or uterine prolapse (n = 2). Laparoscopic inspection showed no endometriosis, and each patient had a luteal phase endometrial biopsy at the time of surgery. None had received hormone therapy for at least 3 months.
Women with endometriosis
Endometrium was obtained by Pipelle endometrial biopsy from women who underwent laparoscopy for pelvic pain and/or infertility. The biopsy was obtained prior to or at the time of surgery. Endometrial samples were obtained from both P and secretory phases of the menstrual cycle, depending on where that patient was at the time of surgery. Secretory phase samples were assigned as ES, MS, or LS based on the date of the last menstrual cycle and by histological dating according to the Noyes criteria.27 Endometriosis was visually confirmed by laparoscopy according and stage assigned based on the Revised American Society for Reproductive Medicine criteria.28 Those who refused endometrial biopsy were excluded.
Endometrial samples of women with UI
A separate set of MS endometrial samples was prospectively obtained as part of an infertility workup in women with UI (n = 119). For the purposes of these studies, UI diagnosis required only that the patient had normal ovulatory menstrual cycles (25-35 days), no history of pelvic infection, and a partner with normal sperm count and motility according to the World Health Organization.29 Fallopian tube patency was determined by hysterosalpingography or at the time of a laparoscopy by chromopertubation. Exclusion criteria included age ≥40, history of pelvic infection or pelvic inflammatory disease, polycystic ovary syndrome, or the presence of fibroid tumors.
Variables/Data Sources/Measurement
Expression of BCL6, either by reverse transcriptase polymerase chain reaction (RT-PCR) or by immunohistochemistry, using the 2−ΔCt method and the HSCORE, respectively, were the outcomes analyzed. Details of the measurement are described in the RNA isolation and quantification and immunohistochemistry sections. Women with endometriosis, with UI, and controls were the groups analyzed and compared. Parity was a potential confounder, and separate statistical analysis was used to verify the effect of parity on BCL6 expression.
Cell Separation
Endometrial samples obtained from normal controls during the secretory phase of the menstrual cycle were washed with Opti-mem media supplemented with fetal bovine serum (FBS) and antibiotics (10 000 IU/mL penicillin, 10 000 IU/mL streptomycin; Life Technologies, Grand Island, New York). Tissue was recovered via centrifugation and incubated with collagenase-containing medium (phenol red-free Dulbecco Modified Eagle Medium/F12, 0.5% collagenase I, 0.02% DNase, and 5% FBS). Cell types were separated as described previously.30
RNA Isolation and Quantification
Total RNA from endometrial frozen tissue was isolated using RNAqueous-4 PCR kit (Ambios, Austin, Texas), quantification was performed using RiboGreen (Invitrogen, Carlsbad, California, and complementary DNA was synthesized as described previously.31 Reverse transcription conditions were 25°C for 5 minutes, 42°C for 15 minutes, and 95°C for 5 minutes. Quantitative real-time RT-PCR was performed on total RNA using primer-probe sets specific for BCL6 (Gene Expression Assays; Applied Biosystems (Foster City, CA)—assay HS00153368), and expression was normalized with the constitutively expressed gene, peptidylprolyl isomerase A (PPIA; Hs99999904 ml; Applied Biosystems). PPIA was chosen after a validation of 12 housekeeping genes in endometrium as described before.32 Real-time PCR employed Brilliant II QPCR Master Mix (Stratagene) in a total reaction volume of 20 µL and was performed on a Stratagene MX3000P machine (Stratagene, La Jolla, CA) using 40 two-step cycles (95°C for 25 seconds, followed by 60°C for 1 minute). The RT-PCR data were analyzed using the 2−ΔCt method, as described.33
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks were sectioned at 4 µm. Slides were stained with hematoxylin–eosin, and consecutive sections stained with ready-to-use antibodies against BCL6 (clone LN22; Leica Microsystems, Buffalo grove, Illinois). Immunohistochemistry was performed on an automated system by a certified Pathology Laboratory at GHS (Pathology Associates, GHS, Greenville, South Carolina) using the Bond immunostainer platform (Leica Microsystems). Following exposure to the horseradish peroxidase-conjugated streptavidin substrate, positive immunoreactivity (red precipitate) was detected using the Vectastain Elite DAB kit (Vector Laboratories, Burlingame, CA). Negative control sections included P phase endometrium from normal fertile individuals, and positive controls included lymph node sections. The semiquantitative assessment of expression was made using the HSCORE (0-4), calculated using the following equation: HSCORE = ∑ Pi (i + 1)/100, where i = intensity of staining with a value of 1, 2, or 3, (weak, moderate, or strong, respectively) and Pi is the percentage of stained epithelial cells for each intensity, varying from 0% to 100%. The use of HSCORE has been previously as a semiquantitative assay for immunohistochemical staining.34
The use of an automated system for immunostaining reduced a potential bias in immunohistochemical analysis, read by a gynecologic pathologist (D.P.S). Certified Board gynecologists with experience in endometriosis diagnosis were responsible for the laparoscopies, reducing detection bias.
Study Size, Quantitative Variables, and Ethical Approval
The institutional review boards at the UNC and GHS approved all protocols for collection and use of human samples. Written consent was obtained from each patient prior to enrollment. Sample size for BCL6 immunohistochemical analysis was calculated according to the literature35 using the following formula: n = [(zα + zβ)2 . 2 . s2]/d2, where alpha error (zα) as 0.02, power (zβ) of 0.9, an estimated standard deviation (s) of BCL6 of 0.2, and a difference (d) between means of both groups (endometriosis vs control) of 0.5 points in an HSCORE scale that ranges from 0 to 4. The variance (s2) was obtained from a pilot study with 15 cases of normal endometrium. These figures yielded a minimum of 4 cases in each group because the standard deviation of BCL6 was low, that is, 0.2. Sample size for receiving–operating characteristics (ROC) curve was calculated as described,36 considering a zα as 0.05, power (zβ) of 0.9, an expected accuracy of 95%, and a ratio of 2:1 of patients with endometriosis and without endometriosis. These figures yielded a sample size of 20 and 10 patients with and without endometriosis, respectively. Gaussian distribution was verified using the D’Agostino and Pearson omnibus normality test. The Student t test was used to compare means. If normality of the data were not confirmed, the Mann-Whitney U or Kruskal-Wallis nonparametric tests were used to compare medians. Dunn correction for multiple comparisons was used as a post hoc test in Kruskal-Wallis test. Statistical analysis was performed using GraphPad Prism version 6 for Macintosh (GraphPad software, Inc, San Diego, California). Analysis of covariance (ANCOVA) was used for adjusting HSCORE between groups using an online calculator (http://vassarstats.net/ancova2L.html), since parity in controls and cases (endometriosis) was significantly different. The ANCOVA online calculator was used for statistical analysis.
Results
Participants and Descriptive Data
Demographic data of the studied populations are shown in Tables 1 and 2. Among the variables, BMI and parity were higher in the fertile control groups compared to those with endometriosis at laparoscopy (Table 1; P < .001 and P < .0001, respectively). The fertile control group had a higher parity than the UI group (Table 2; P < .001).
Table 1.
Demographics of Patients Who Underwent Laparoscopy.
| Characteristics | No Endometriosis (n = 20) | Endometriosis Cases (n = 29) | P Value |
|---|---|---|---|
| Age, mean (SD) | 34.2 (6.6) | 32.4 (4.5) | .27a |
| Body mass index, mean (SD) | 31.6 (4.4) | 23.3 (4.8) | <.001a |
| Parity | <.0001b | ||
| Median (range) | 2.4 (2-5) | 0 (0-2) | |
| n/total (% parous) | 20 (100%) | 6/29 (20.7%) | |
| Endometriosis stage, n (%)c | |||
| I/II | 21 (72) | ||
| III/IV | 8 (28) | ||
Abbreviation: SD, standard deviation.
a Student t test.
b Mann-Whitney U test.
c Pathology report was obtained from 24 of 29 cases.
Table 2.
Demographics of Unexplained Infertility (UI) and Fertile Controls for BCL6 Testing.
| Characteristic | Control Group (n = 28) | UI Group (n = 119) | P Value |
|---|---|---|---|
| Age, mean (SD) | 32.77 (2.6) | 33.23 (4.2) | .59a |
| Body mass index, mean (SD) | 25.6 (4.7) | 25.3 (5.5) | .08a |
| Parity | <.0001b | ||
| Median (range) | 2 (1-3) | 0 (0-3) | |
| n/total (% parous) | 28/28 (100%) | 22/119 (18.4%) | |
| Race, n (%) | .3c | ||
| Caucasian | 23 (82.1%) | 106 (89%) | |
| African American | 2 (7.4%) | 5 (4.2%) | |
| Other | 3 (11.1%) | 8 (6.7%) | |
Abbreviation: SD, standard deviation.
a Student’s t test.
b Mann-Whitney U test.
c Chi-square for trend.
Expression of BCL6 in Endometrial Biopsies From Controls
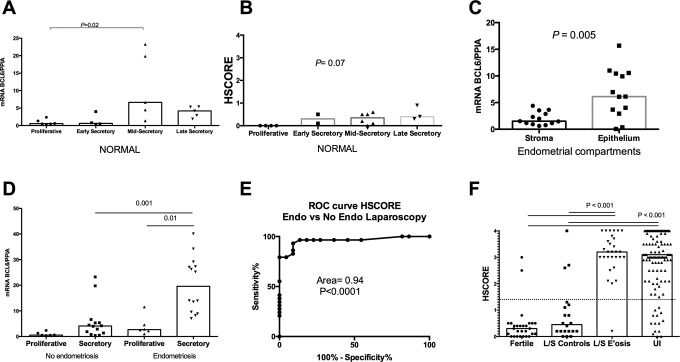
Whole endometrial messenger RNA (mRNA) analysis using quantitative RT-PCR for BCL6 over the menstrual cycle revealed that BCL6 is minimally increased in the MS phase compared to the P phase (P = .02, Kruskal-Wallis with Dunn post hoc test; Figure 1A). Immunohistochemical analyses of BCL6 expression in normal eutopic endometrium across the menstrual cycle (Figures 1B and 2A-D) were similar to those found with RT-PCR. No expression was noted in the P phase, and staining across the secretory phases was similar, maintaining low levels of expression across the ES, MS, and LS phases, with none achieving statistical significance. The differential expression of BCL6 predominantly in endometrial epithelium was demonstrated by RT-PCR in enzymatically separated endometrial glands and stroma from the secretory phase (P = .005, Mann-Whitney U; Figure 1C).
Figure 1.
B-cell CLL/lymphoma 6 (BCL6) expression at different states. A, Quantitative RT polymerase chain reaction (RT-PCR) levels of BCL6 messenger RNA (mRNA) in whole normal endometrium evaluated each cycle phase, determined by cycle day and urine luteinizing hormone (LH) detection. Mid-secretory levels of BCL6 were significantly higher compared to proliferative phase (P = .02, Kruskal-Wallis with Dunn post hoc test). B, HSCORE values based on immunohistochemical expression of BCL6 across the menstrual cycle in normal women. No statistical difference was found among groups (Kruskal-Wallis). C, Quantitative RT-PCR levels of BCL6 mRNA in separated stromal and epithelium cells from whole endometrial tissue. A higher level of expression was found in the epithelial compartment (P = .0005, Mann-Whitney U test). D, Quantitative RT-PCR levels of BCL6 mRNA obtained from proliferative and secretory phase in women with and without endometriosis. Significant difference was found between secretory phases of both groups and between proliferative and secretory groups of endometriosis (P = .001 and 001, respectively—Kruskal-Wallis with Dunn post hoc test). E, Receiving–operator characteristic (ROC) analysis comparing laparoscopically proven endometriosis to fertile women without endometriosis at the time of laparoscopy. The dotted line represents the ROC-determined HSCORE cutoff of 1.4. F, Immunohistochemical expression (HSCORE) of BCL6 between fertile controls (Fertile) and fertile women without endometriosis at laparoscopy (L/S Controls) compared to those with endometriosis at laparoscopy (L/S E’osis; P < .0001, Mann-Whitney). A further comparison was made between immunohistochemical expression (HSCORE) of BCL6 between women with unexplained infertility (UI) and both fertile control groups (P < .0001, Mann-Whitney U test). All boxes in each graph represent the median levels of mRNA of expression or HSCORE.
Figure 2.
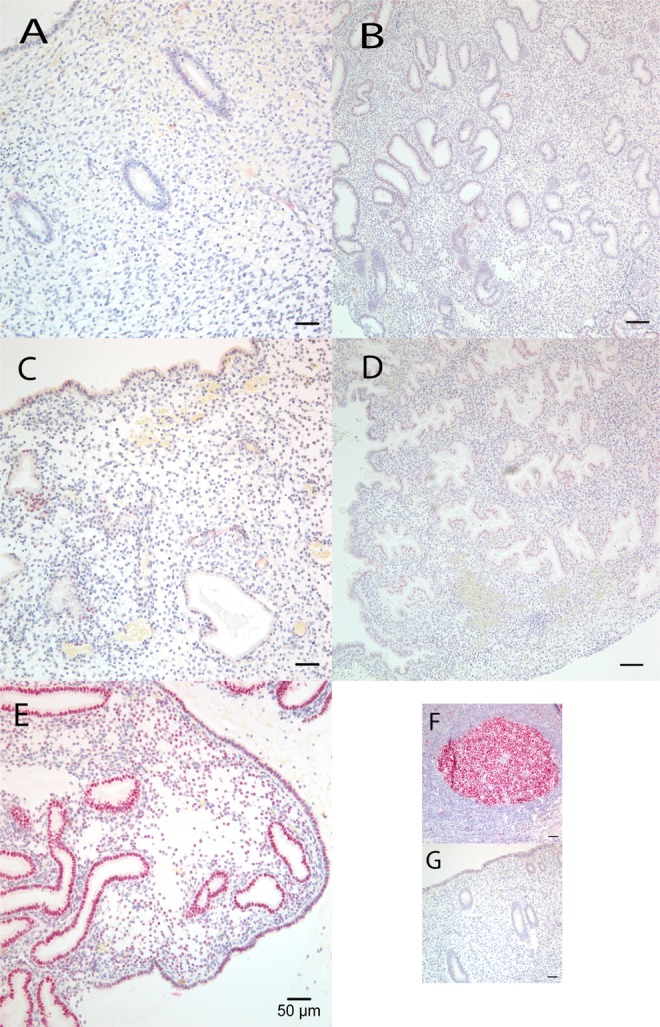
Representative B-Cell CLL/Lymphoma 6 (BCL6) protein immunostaining expression in endometrial paraffin sections over the menstrual cycle. (A) normal proliferative, (B) normal early secretory, (C) normal mid-secretory, (D) normal late secretory, and (E) endometriosis—mid-secretory. Positive (F) and negative controls (G).
Expression of BCL6 in Endometrial Biopsies From Fertile Controls and Infertile Women With Endometriosis
Significantly higher BLC6 mRNA levels were seen in the secretory phase of infertile women with endometriosis (P = .001, Kruskal-Wallis with Dunn post hoc test; Figure 1D). Secretory phase BCL6 was also higher in the P phase in women with endometriosis (P = .01, Kruskal-Wallis with Dunn post hoc test; Figure 1D). In the 2 fertile control groups, average BCL6 expression was low in both the groups. Women with proven fertility but no laparoscopy had elevated BCL6 levels in 2 (7%) cases. In women with prior fertility and laparoscopy showing no endometriosis, 3 (13.6%) of 22 had high levels of BCL6 expression (Figure 1F).
Based on these results, comparison of BCL6 was standardized to the MS phase in women undergoing laparoscopy. BCL6 immunohistochemical expression was significantly higher in patients with endometriosis at laparoscopy (n = 29), compared to women without endometriosis (n = 20; P < .0001, Mann-Whitney test; Figure 1F). Receiving–operator characteristic analysis revealed that the area under the curve was 96% (95% confidence interval [CI] 0.908-1.01; P < .0001) and an HSCORE cutoff of 1.4 (Figure 1E—dotted line).
Women With UI
Using this ROC-defined HSCORE cutoff, we determined BCL6 expression in 119 women with UI. All samples were analyzed by immunohistochemistry for BCL6 expression in urinary LH-timed, MS phase biopsies of eutopic endometrium. BCL6 was significantly overexpressed in cases of UI compared to controls (P < .0001—Mann-Whitney U test; Figure 1F). Based on the preestablished HSCORE cutoff, 105 of 119 with UI tested positive for BCL6 (88%; 95%CI 81%-92%). Of the 105 patients, 65 underwent subsequent laparoscopy with findings of endometriosis in 61 (93.8%), hydrosalpinx in 3 (4.6%), and a normal appearing pelvis in 1. In the cases with hydrosalpinx, tubal patency had not been established prior to surgery, rather was determined during surgery by chromopertubation. We compared these cases with UI to normal, fertile volunteers with LH-timed, MS phase in which 2 (7.1%) of 28 tested positive for BCL6. In another set of laparoscopically studied fertile controls, 3 (13.9%) of 22 were positive for BCL6 staining (both P < .001).
Other Analyses
Analysis of covariance was performed to identify whether parity between groups had influence on BCL6 expression in the comparison between infertile women with and without endometriosis, and in the prospective cohort of fertile women and women with UI. Levene test of equality of error variances was 0.4 and 0.5, respectively. After running ANCOVA, parity was adjusted, and the P value the between groups was <.0001 in both analyses, confirming the significant difference, despite the parity difference between the groups.
Discussion
This study provides the first detailed examination of BCL6 expression in the human endometrium throughout the menstrual cycle, in women with and without endometriosis. BCL6 protein expression has a nuclear localization, and both mRNA and protein expressions are increased in endometrial epithelium of infertile women with endometriosis in the secretory phase.
It is known that endometriosis is an inflammatory condition7,37 and that inflammatory cytokines are associated with signs of progesterone resistance.25 Inflammatory cytokines, such as interleukin 6 (IL-6), are elevated in peritoneum and in plasma of women with endometriosis.38,39 We previously showed that IL-6 increases pSTAT3 (activated) in the endometrium of women with endometriosis,16 and other authors demonstrated that STAT3 upregulates BCL6.15 Therefore, overexpression of BCL6 in eutopic endometrium of women with endometriosis is both logical and consistent with these known changes in eutopic endometrium.
With the identification of BCL6 as an endometrial protein expressed in the secretory phase, we hypothesized that an endometrial biopsy-based test for endometriosis may be feasible. To address this question, we first compared secretory endometrium, from laparoscopically proven cases of endometriosis to endometrium from fertile women without disease at laparoscopy. After establishing that the area under the curve exceeded 50%,36 we proceeded to find an HSCORE cutoff with the highest likelihood ratio. Next, we applied the test to a group of prospectively obtained, MS endometrial samples from women with UI.
We demonstrate that BCL6 positivity was more frequent in the UI group compared to those with proven normal fertility (Figure 1F). Of the 65 cases who had laparoscopy, 61 (94%) had endometriosis, 3 (4.6%) had unsuspected hydrosalpinx, and 1 was found to be normal. While the incidence of endometriosis predicted by BCL6 expression in women with UI was high, other laparoscopic studies have suggested a very high incidence in this population.4–6 Although the population that underwent laparoscopy due to clinician practice may be enriched for endometriosis, it is important to note the historical trend of increasing prevalence of endometriosis in this population due to improved laparoscopic equipment and a better appreciation of subtle forms of endometriosis.40 Based on the findings presented here, we predict further increases in the reported prevalence of endometriosis as better diagnostic biomarkers become available. Women with proven fertility also occasionally tested about the ROC defined cutoff value of 1.4 (Figure 1F). This may indicate other inflammatory conditions such as adenomyosis not appreciated at the time of laparoscopy.
Strengths of our study include a sufficient sample size and appropriate statistical analysis of normal BCL6 expression through the normal menstrual cycle. All endometrial samples were obtained prior to laparoscopy in women with and without this disorder. Based on power analysis and the differential staining for BCL6 in women with endometriosis, we had 4 times the number of samples needed to have a 95% chance of detecting, as significant at the 1% level, an increase in the primary outcome from 0.4 in the control group to 3.1 HSCORE in the endometriosis group. Based on ROC analysis, a defined HSCORE cutoff for BCL6 discriminates between women with and without this disorder.
Potential limitations include the case–control study design, which prevents us from properly calculating positive and negative predictive value. However, these data provide an HSCORE cutoff for future clinical studies. It is reassuring that only 7% of the nonlaparoscoped fertile controls tested positive, since this is similar to the published prevalence in the general population of 5% to 8%.40 However, even fertile controls without endometriosis proven by laparoscopy occasionally tested positive for BCL6, even without visible abnormalities, suggesting other inflammatory abnormalities might be present (ie, adenomyosis).
The diagnosis of endometriosis by visual inspection, and not solely by pathology, is a potential bias. Minimal or mild endometriosis is often treated by laser ablation or cautery, with no specimen available for pathological confirmation. Nevertheless, most of our cases (24 of 29) had pathologically confirmed endometriosis. It is possible that patients selected for surgery were more likely to have endometriosis. Nevertheless, these data confirm that BCL6-positive cases were associated with pathologic findings. These data also suggest that endometriosis is not the only diagnosis associated with BCL6 positivity. Like integrin testing,41 BCL6 may have a more global application for diseases that cause inflammation, rather than just indicating endometriosis.
It is too soon to extrapolate these data to all women with endometriosis. Future studies will need to be performed on women without pain, or women with endometriosis but without infertility, to determine whether BCL6 is diagnostic for endometriosis in this group of women as well. Since endometriosis can exist in infertile women without symptoms, a biomarker for this population would be of value. In addition, ongoing studies in animal models are being performed to determine whether BCL6 is a direct result of endometriosis lesion establishment and the inflammatory responses generated by this disease.
Conclusion
Based on both mRNA and protein analysis, we have strong evidence that BCL6 has low expression in women without endometriosis. In contrast, it is highly overexpressed in women with endometriosis during the secretory phase of the menstrual cycle. The significant difference in BCL6 protein expression between women with and without endometriosis testing yields an area under the curve of 94%. A cutoff of 1.4 in HSCORE provides an likelihood ratio of 15.4 and 0.04 for positive and negative results, respectively. These results, in conjunction with clinical signs and symptoms, including infertility, will need to be applied in prospective trials to determine the diagnostic accuracy of BCL6 as a biomarker for endometriosis. Considering the difficulty in diagnosis of endometriosis or prospectively predicting cases of diminished endometrial receptivity, a reliable marker for endometriosis and/or reduced endometrial receptivity would result in a paradigm shift for both diagnosis and treatment of infertile women.
Acknowledgments
We acknowledge the excellent technical assistance of Gail Grossman (UNC) and Angela Houwing (GHS). We thank Janetta Phillips for helping coordinate sample collection and maintaining data on human subjects.
Authors’ Note: Bruce Lessey and Emily Evans-Hoeker contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) via R01 HD067721 (S.Y. and B.L.), R01 HD057873 (J.J.) and U54 HD 40093 (A.F.) and an American Cancer Society Research Grant RSG-12-084-01-TBG (J.J.).
References
- 1. Large MJ, Demayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Optimal evaluation of the infertile female. Fertil Steril. 2006;86(5 suppl 1):S264–S267. [DOI] [PubMed] [Google Scholar]
- 3. Kutteh WH. Novel strategies for the management of recurrent pregnancy loss. Semin Reprod Med. 2015;33(3):161–168. [DOI] [PubMed] [Google Scholar]
- 4. Nakagawa K, Ohgi S, Horikawa T, Kojima R, Ito M, Saito H. Laparoscopy should be strongly considered for women with unexplained infertility. J Obstet Gynaecol Res. 2007;33(5):665–670. [DOI] [PubMed] [Google Scholar]
- 5. Tsuji I, Ami K, Miyazaki A, Hujinami N, Hoshiai H. Benefit of diagnostic laparoscopy for patients with unexplained infertility and normal hysterosalpingography findings. Tohoku J Exp Med. 2009;219(1):39–42. [DOI] [PubMed] [Google Scholar]
- 6. Bonneau C, Chanelles O, Sifer C, Poncelet C. Use of laparoscopy in unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 2012;163(1):57–61. [DOI] [PubMed] [Google Scholar]
- 7. McKinnon BD, Bertschi D, Bersinger NA, Mueller MD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. 2015;26(1):1–10. [DOI] [PubMed] [Google Scholar]
- 8. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holoch KJ, Lessey BA. Endometriosis and infertility. Clin Obstet Gynecol. 2010;53(2):429–438. [DOI] [PubMed] [Google Scholar]
- 10. Stratton P. The tangled web of reasons for the delay in diagnosis of endometriosis in women with chronic pelvic pain: will the suffering end? Fertil Steril. 2006;86(5):1302–1304; discussion 17. [DOI] [PubMed] [Google Scholar]
- 11. Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28(1):5–16. [DOI] [PubMed] [Google Scholar]
- 12. Allavena G, Carrarelli P, Del Bello B, Luisi S, Petraglia F, Maellaro E. Autophagy is upregulated in ovarian endometriosis: a possible interplay with p53 and heme oxygenase-1. Fertil Steril. 2015;103(5):1244–1251. e1. [DOI] [PubMed] [Google Scholar]
- 13. Phan RTand Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432(7017):635–639. [DOI] [PubMed] [Google Scholar]
- 14. Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13(2):199–212. [DOI] [PubMed] [Google Scholar]
- 15. Arguni E, Arima M, Tsuruoka N, Sakamoto A, Hatano M, Tokuhisa T. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol. 2006;18(7):1079–1089. [DOI] [PubMed] [Google Scholar]
- 16. Kim BG, Yoo JY, Kim TH, et al. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30(5):1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu L, Guo L, Liang Y, Liu X, Jiang L, Wang L. Curcumin suppresses stem-like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncol Rep. 2015;34(6):3311–3317. [DOI] [PubMed] [Google Scholar]
- 18. Carro MS, Lim WK, Alvarez MJ, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ye BH, Lista F, Lo Coco F, et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262(5134):747–750. [DOI] [PubMed] [Google Scholar]
- 20. Lossos IS, Jones CD, Zehnder JL, Levy R. A polymorphism in the BCL-6 gene is associated with follicle center lymphoma. Leuk lymphoma. 2001;42(6):1343–1350. [DOI] [PubMed] [Google Scholar]
- 21. Ci W, Polo JM, Cerchietti L, et al. The BCL6 transcriptional program features repression of multiple oncogenes in primary B cells and is deregulated in DLBCL. Blood. 2009;113(22):5536–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peh SC, Gan GG, Lee LK, Eow GI. Clinical relevance of CD10, BCL-6 and multiple myeloma-1 expression in diffuse large B-cell lymphomas in Malaysia. Pathol Int. 2008;58(9):572–579. [DOI] [PubMed] [Google Scholar]
- 23. Yao D, Cai GH, Chen J, Ling R, Wu SX, Li YP. Prognostic value of p53 alterations in human osteosarcoma: a meta analysis. Int J Clin Exp Pathol. 2014;7(10):6725–6733. [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Large MJ, Creighton CJ, et al. COUP-TFII regulates human endometrial stromal genes involved in inflammation. Mol Endocrinol. 2013;27(12):2041–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin SC, Li YH, Wu MH, et al. Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2014;99(3):E427–E437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franasiak JM, Holoch KJ, Yuan L, Schammel DP, Young SL, Lessey BA. Prospective assessment of midsecretory endometrial leukemia inhibitor factor expression versus alphanubeta3 testing in women with unexplained infertility. Fertil Steril. 2014;101(6):1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1(1):3–25. [DOI] [PubMed] [Google Scholar]
- 28. The Revised American Society for Reproductive Medicine Classification of endometriosis: 1996. Fertil Steril. 1997;67(5):822–829. [DOI] [PubMed] [Google Scholar]
- 29. Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. [DOI] [PubMed] [Google Scholar]
- 30. Arnold JT, Kaufman DG, Seppala M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16(5):836–845. [DOI] [PubMed] [Google Scholar]
- 31. Plante BJ, Lessey BA, Taylor RN, et al. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod Sci. 2012;19(7):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roemer KL, Young SL, Savaris RF. Characterization of GAB1 expression over the menstrual cycle in women with and without polycystic ovarian syndrome provides a new insight into its pathophysiology. J Clin Endocrinol Metab. 2014;99(11):E2162–E2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 34. Budwit-Novotny DA, McCarty KS, Cox EB, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419–5425. [PubMed] [Google Scholar]
- 35. Jekel JF, Elmore JG, Katz DL. Jekel’s Epidemiology, Biostatistics, Preventive Medicine, and Public Health. 1st ed Philadelphia, PA: W.B. Saunders; 1996, p.xiii, 405 p. [Google Scholar]
- 36. Obuchowski NA, Lieber ML, Wians FH., Jr ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem. 2004;50(7):1118–1125. [DOI] [PubMed] [Google Scholar]
- 37. Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62(3):139–147. [DOI] [PubMed] [Google Scholar]
- 38. Buyalos RP, Funari VA, Azziz R, Watson JM, Martinez-Maza O. Elevated interleukin-6 levels in peritoneal fluid of patients with pelvic pathology. Fertil Steril. 1992;58(2):302–306. [PubMed] [Google Scholar]
- 39. Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohi J, Simon C. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril. 1998;70(3):425–431. [DOI] [PubMed] [Google Scholar]
- 40. D’Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21(2):243–254. [DOI] [PubMed] [Google Scholar]
- 41. Meyer WR, Castelbaum AJ, Somkuti S, et al. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod. 1997;12(7):1393–1398. [DOI] [PubMed] [Google Scholar]