Abstract
Background:
Several studies suggest that resistance to progesterone may contribute to the pathophysiology of endometriosis. Progesterone mediates its biological activity via the 2 progesterone receptor (PR) isoforms (PR-A and PR-B). Effects of progesterone are determined by the PR-A:PR-B ratio such that a PR-B-dominant state promotes progesterone signaling, whereas a PR-A-dominant state decreases progesterone responsiveness. Our objective was to compare the abundance and cellular localization of the PR isoforms in endometrium and endometriotic lesions from women with and without peritoneal and ovarian endometriosis.
Methods:
This in vitro study was conducted in a tertiary care facility. Reproductive-age women with surgically diagnosed endometriosis (n = 18) and asymptomatic control individuals (n = 20) were prospectively recruited at the late proliferative and the early secretory phases. At laparoscopy, samples of eutopic endometrium, peritoneal and ovarian endometriosis, and disease-free peritoneum were obtained for subsequent immunohistochemical and immunoblot analysis of PR-B and total PR localization and PR-A and PR-B abundance, respectively.
Results:
The PR-A and PR-B were detected in eutopic endometrium and in peritoneal and ovarian endometriosis but not in disease-free peritoneum from patients with and without endometriosis. In peritoneal endometriosis, PR-A was the predominant isoform detected, whereas both receptors were detected in ovarian endometriosis and eutopic endometrium. In eutopic endometrium, levels of PR-A were significantly elevated in women with endometriosis compared with women without disease, regardless of menstrual phase. The PR-A levels were significantly elevated in ovarian endometriosis compared with peritoneal endometriosis.
Conclusions:
Endometriotic lesions and eutopic endometrium from women with endometriosis are uniform in a PR-A-dominant state. The data suggest that menstrual efflux of a PR-A-dominant endometrial tissue into the peritoneal cavity may play a role in the pathophysiology of endometriosis.
Keywords: endometrium, endometriosis, progesterone receptors
Introduction
Endometriosis is the presence of glandular and stromal endometrial implants at an extrauterine (ectopic) site—usually in the peritoneal cavity. It is generally considered that the endometrial tissue enters the peritoneal cavity mainly by retrograde menstruation via the oviducts.1 A key unanswered question regarding the etiology of endometriosis is why it affects 5% to 10% of women even though retrograde menstruation occurs in most women.2 One explanation is that endometriosis arises due to intrinsic changes in endometrial cells causing them to have a predisposition to form ectopic implants when they are shed into the peritoneum.
The ovarian steroid hormones, estrogen and progesterone, have profound effects on the pathophysiology of endometriosis. Multiple studies have shown that estrogens promote, whereas progesterone inhibits, endometriosis disease severity and progression.3 Thus, the etiology and progression of endometriosis could be affected by aberrant estrogen or progesterone signaling in endometrial cells such that cells reaching the peritoneum have increased risk of forming ectopic endometriotic implants. In support of this hypothesis, several studies suggest that endometriotic lesions derive from eutopic endometrial tissue with decreased progesterone responsiveness.4 This study tested the hypothesis that decreased progesterone responsiveness associated with endometriosis disease is due to aberrant expression of progesterone receptors (PRs) in eutopic and ectopic endometrial cells.
Progesterone actions in endometrial cells are mediated primarily by the nuclear PR isoforms, PR-A and PR-B,5 that function as ligand-activated transcription factors.6 Progesterone target cells coexpress PR-A and PR-B in varying relative amounts depending on tissue type and pathologic condition. Consequently, progesterone responsiveness is determined by the net effects of PR-A and PR-B. In most cell types, PR-B has strong ligand-induced transcriptional activity, whereas PR-A on its own has minimal transcriptional activity and, depending on its amount relative to PR-B, represses the transcriptional activity of PR-B.7 Thus, PR-A and PR-B are thought to comprise an opposing system for target cell control of progesterone responsiveness whereby responsiveness to progesterone is inversely related to the PR-A:PR-B ratio. Indeed, the pathophysiology of some progesterone-related disorders, including endometriosis, is thought to be due, in part, to abnormal progesterone signaling secondary to aberrant relative expression of PR-A and PR-B.8
Previous studies of PR isoform expression in endometriosis have produced conflicting results. Studies using assays that did not discriminate between PR-A and PR-B reported lower PR levels in ectopic compared with eutopic endometrium,9 with the exception of 1 study reporting no difference.10 Attia et al4 measured PR-A and PR-B by immunoblotting and reported that peritoneal endometriotic tissue did not express PR-B and had reduced levels of PR-A compared with eutopic endometrium. In contrast, messenger RNA (mRNA) analysis of ovarian endometriotic tissue showed dominant expression of PR-B,11 whereas studies of PR gene structure in endometriosis showed hypermethylation of the PR-B promoter consistent with decreased PR-B expression.12 Studies of PR-A and PR-B levels in eutopic endometrium in women with and without endometriosis produced similar conflicting outcomes.13 Thus, the current body of published data regarding the levels of PR-A and PR-B in eutopic and endometriotic tissue is equivocal. In the present study, we sought to resolve this uncertainty by measuring the extent of expression and cellular localization of PR-A and PR-B in peritoneal and ovarian endometriosis and eutopic endometrium from women with and without endometriosis using highly specific and sensitive immunoblot and immunohistochemical assays.
Materials and Methods
Patient Recruitment, Tissue Procurement, and Processing
The study was approved by the institutional review board (IRB) for human research and was conducted between January 2011 and April 2013 (IRB protocol number is 12-10-28). Inclusion criteria were women with regular predictable menstrual cycles lasting 25 to 35 days and no hormonal treatments to modulate fertility and/or menstrual cycles for at least 3 months prior to surgery. After informed consent, biopsies of endometrium, peritoneal endometriosis, ovarian endometriosis (ie, ovarian endometriomata), and adjacent disease-free peritoneum were obtained from women undergoing operative laparoscopy for a variety of endometriosis-associated symptoms (n = 18). Biopsies of endometrium and disease-free peritoneum were also obtained from women undergoing laparoscopic tubal ligation (n = 15) and diagnostic laparoscopy for unexplained infertility (n = 5). No visible endometriosis was detected in these women at the time of laparoscopy. Synchronous sampling of endometrium and peritoneum was performed in all patients. Sampling of endometriomas was performed in 9 of the 18 patients with endometriosis. Tissue samples were stratified according to the stage of the menstrual cycle at the time of collection based on the menstrual history and confirmed by endometrial histology. All tissue samples were collected during the late proliferative or early secretory phase of the menstrual cycle. Every patient was represented by only 1 sample per tissue type. Tissues were washed several times in ice-cold phosphate-buffered saline to remove blood. A portion of each specimen was formalin fixed for histological examination, and the remaining tissue was frozen in liquid nitrogen and stored at −80°C. Histopathologic analysis of all endometrium specimens was used to confirm the stage of the menstrual cycle at the time of procurement.
Protein Extraction and PR Immunoblot Analysis
Protein extraction and immunoblot assays were performed as previously described.14 Briefly, total cell lysate was prepared from each tissue specimen by homogenization on ice in radioimmunoprecipitation assay extraction buffer (25 mmol/L Tris-HCl, pH 7.6); 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS; Sigma, St Louis, Missouri), supplemented with protease and phosphatase inhibitors (0.5 mmol/L phenylmethylsulfonyl fluoride, 86 µmol/L leupeptin, 77 µg/mL aprotinin, 1.4 µmol/L pepstatin A, and 100 µg/mL bacitracin; Roche, Indianapolis, Indiana) and then centrifuged at 14 000g for 10 minutes at 4°C. The supernatant (ie, tissue lysate) was then collected and assayed for protein content by the bicinchoninic acid method (Thermo Scientific, Rockford, Illinois). Lysate containing ∼100 µg of protein was diluted in gel loading buffer (40% glycerol, 1 mol/L Tris-HCl, 2.5% β-mercaptoethanol, 8% SDS, and 0.01% bromophenol blue), heated for 5 minutes at 100°C, and subjected to denaturing (in 10% SDS) polyacrylamide gel electrophoresis using the Novex tris-glycine system (Life Technologies, Grand Island, New York) and electrotransferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, California). For immunoblotting, the PVDF membrane was blocked with 5% nonfat milk powder in Tris-buffered saline (TBS: 20 mmol/L Tris, 500 mmol/L NaCl, pH 7.5) containing 0.05% Tween-20 (TTBS) for 1 hour at room temperature and then incubated overnight at 4°C with primary antibodies in TTBS containing 5% nonfat milk powder. The primary antibodies were PgR1294 (Dako North America, Carpinteria California, Cat # M3568 1:1000 dilution) that detects PR-A and PR-B with equal affinity and sc-32233 (Santa Cruz Biotechnology, Santa Cruz, California, Cat # 1:50 000 dilution) that detects glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The membrane was then rinsed 3 times at room temperature in TTBS and incubated with horseradish peroxidase (HRP)-conjugated antimouse immunoglobulin G (IgG) HRP antibody (Cell Signaling, Danvers, Massachusetts) for 1 hour at room temperature. Immunoreactivity was then detected using HyGLO Chemiluminescent HRP antibody detection reagent (Denville Scientific, Metuchen, New Jersey) and visualized on a digital imager (FluoroChem E imager; ProteinSimple, Santa Clara, California). Intensity of immunoreactive bands was quantified by digital densitometry using the NIH Image J software (NIH, Bethesda, Maryland) using exposures in which the target bands were in the linear range for digital densitometry.
Protein was also extracted from the immortalized human myometrial cell line, hTERT-HMA/B, in which expression of PR-A and PR-B can be independently controlled by exposure to the following specific inducers: doxycycline (DOX) for PR-A and GenoStat ligand (GSL) for PR-B. Cells were cultured in the presence of DOX or GSL and DOX for 24 hours and then subjected to immunoblotting using the PgR1294, C1A2, and GAPDH antibodies as previously described.14
Progesterone Receptor Immunohistochemistry
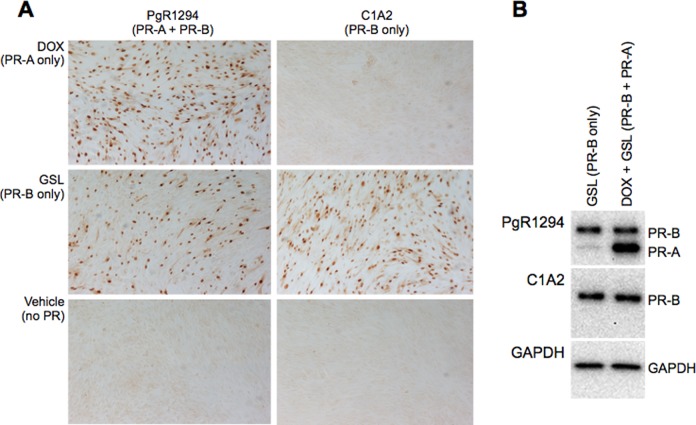
Formalin-fixed tissues were embedded in paraffin, and sections (5 µm) were cut and mounted on glass slides. For immunohistochemistry (IHC), sections were deparaffinized in xylene and rehydrated in graded ethanols and subjected to antigen retrieval/unmasking by heating at 95°C in 10 mmol/L citric acid, 0.05% Tween 20, pH 6.0 for 20 minutes. After cooling to room temperature, the sections were rinsed in TTBS and treated with 3% H2O2 at room temperature for 5 minutes to inhibit endogenous peroxidase activity. Sections were then covered with primary antibody: either PgR1294, which detects PR-A and PR-B, or C1A2 (Cell Signaling Technology, Inc, Boston, Massachusetts), which detects only PR-B (each diluted 1:250 in 1% BSA in TBS) or nonimmune IgG of the same species and isotype as the primary antibody (Santa Cruz Inc) at room temperature for 1 hour and then washed in TTBS. Bound primary antibodies were then detected using a peroxidase/3,3′-diaminobenzidine-based visualization kit (Universal LSAB Kit; Dako North America) and processed for light microscopy. Immunostaining intensity and cellular localization were analyzed and scored under light microscopy. Staining intensity was scored on a scale of 0 to 3 (with 0 = no staining, 1 = faint staining, 2 = moderate staining, and 3 = strong staining) by 2 independent observers blinded to sample identity according to the method described by Mote et al.15 To confirm that PgR1294 and C1A2 produce comparable staining for PR-B, IHC (Figure 1A) and immunoblot (Figure 1B) analyses were also performed on fixed cells and total cell lysate from an immortalized human myometrial cell line that was genetically modified to express PR-A and/or PR-B in response to independent inducers as previously described.14 The data show essentially identical PgR1294 and C1A2 immunoreactive staining for PR-B (Figure 1).
Figure 1.
Comparison of immunostaining and verification of antibody specificity for PgR1294 (detects PR-A and PR-B with equal affinity) and C1A2 (detects only PR-B) using the hTERT-HMA/B human myometrial cell line that expresses PR-A only in response to doxycycline (DOX) and PR-B only in response to GenoStat ligand (GSL). Immunohistochemistry (IHC) was performed on formalin-fixed cells and immunoblot analysis was performed on total lysate. PR indicates progesterone receptor.
Statistical Analyses
Immunoblot data were analyzed by Student t test (equal variance). Differences were considered statistically significant if 2-sided P values were <.05. With a sample size of 15 women in each group, this study would have an 80% power to detect approximately 2 times difference in mean PR-A relative optical density between both groups (women with and without endometriosis) and 2.5 difference in mean PR-B relative optical density. This calculation assumed 0.8 mean PR-A relative optical density with standard deviation of 0.7 and 0.7 mean PR-B relative optical density with standard deviation of 1 among disease-free women. It also assumes equal standard deviations for PR-A and PR-B in both groups of women. For the overall comparisons, we had a larger sample size (18 women with endometriosis and 20 women without endometriosis), yielding approximately 90% power to detect these differences.
Results
Patient Characteristics
The mean (±SD) age of the endometriosis group (n = 18) was 35.5 ± 3.5 years compared with 34.9 ± 2.5 years for the control group (n = 20; P = .08). The body mass index (BMI) of the endometriosis group was 25.5 ± 2.5 kg/m2 and the BMI for the control group was 26.6 ± 3.4 kg/m2 (P = .79). All patients were caucasian.
Abundance and Cellular Localization of PR-A and PR-B in Eutopic Endometrium
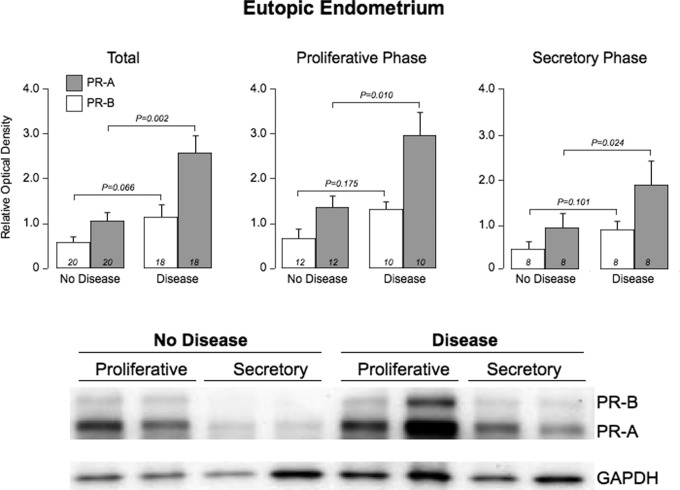
Robust levels of PR-A and PR-B were detected by immunoblotting in endometrium from patients with (n = 18) and without (n = 20) endometriosis. The mean level of PR-A (relative to GAPDH) was significantly higher in endometrium from patients with endometriosis compared with patients without disease (P = .002, Figure 2). In contrast, the mean level of PR-B was not different in endometrium from the 2 patient groups. This relationship between endometriosis and PR-A was maintained upon stratifying patients in both groups according to the phase of the menstrual cycle (P = .01 for the proliferative phase and P = .02 for the secretory phase). Neither PR-A nor PR-B isoforms were detected in the disease-free peritoneum from both groups (data not shown).
Figure 2.
Expression of progesterone receptor (PR) isoforms in eutopic endometrium of women with and without endometriosis. The upper panel shows the mean (±standard error [SE]) relative abundance of both isoforms in patients with and without the disease; the lower panel shows the corresponding western plots.
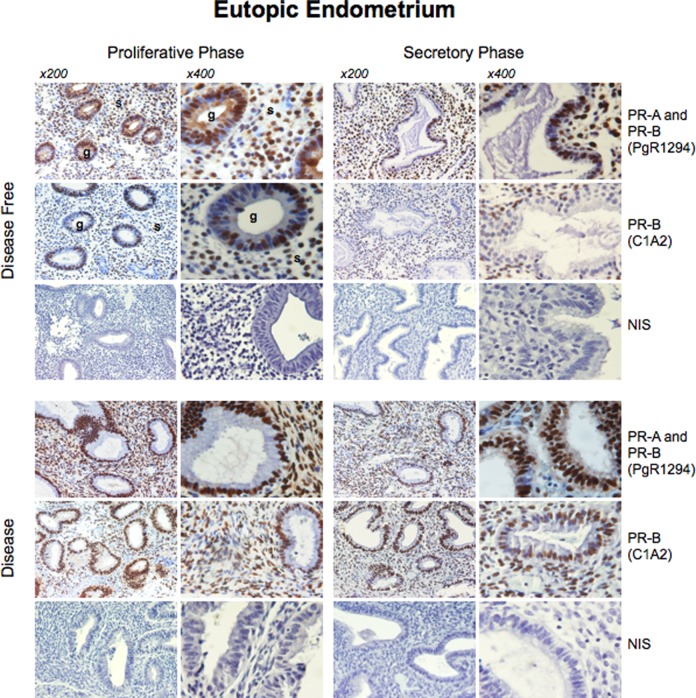
Immunoreactive staining with PgR1294 (detects PR-A and PR-B) was limited to the nucleus of glandular and stromal cells in late proliferative and early secretory phase endometrium (Figure 3). Immunoreactive staining with the C1A2 antibody (detects only PR-B) exhibited the identical pattern of cellular and subcellular localization as that of PgR1294. No PR immunostaining was detected with either antibody in the disease-free peritoneum from both groups (data not shown). The intensity of total PR staining (ie, using the PgR1294 antibody) was significantly higher in patients with endometriosis compared with endometrium from patients without disease (Table 1), whereas intensity of staining for PR-B (ie, using the C1A2 antibody) was similar in both groups. Using the PgR1294 antibody, the intensity of staining was significantly higher in the late proliferative phase at the glandular (P = .0006) and the stromal levels (P = .0007) in patients with endometriosis versus controls (Table 1). Similarly, the intensity of staining was significantly higher in the early secretory phase at the glandular (P = .0013) and the stromal levels (P = .0001) in patients with endometriosis versus controls (Table 1). Using the C1A2 antibody, there was no difference in the intensity of staining of the PR-B isoform between patients and controls at the glandular and the stromal level irrespective of the phase of the menstrual cycle. This difference appeared consistent with increased expression of PR-A in the endometrium of women with endometriosis.
Figure 3.
Immunohistochemical localization of PR-A and PR-B isoforms in eutopic endometrium of women with and without endometriosis. The upper panel shows the immunohistochemistry staining patterns of patients without endometriosis during the proliferative and the secretory phases. The lower panel shows immunohistochemistry staining patterns of patients with endometriosis during the proliferative and the secretory phases. NIS: staining with nonimmune immunoglobulin G (IGG). PR indicates progesterone receptor.
Table 1.
Analysis of PR Isoform Staining Intensity in the Glandular and the Stromal Components of the Eutopic Endometrium in Patients With and Without Endometriosis.
| Disease: Proliferative (n = 5) | No Disease: Proliferative (n = 5) | P Value | Disease: Secretory (n = 5) | No Disease: Secretory (n = 5) | P Value | |
|---|---|---|---|---|---|---|
| PgR1294 (PR-A and PR-B) | ||||||
| Glandular | 2.75 .70.23 | 2.00 .00.20 | .0006 | 2.5 .50.23 | 2.00 .00.00 | .0013 |
| Stromal | 2.5 .50.1 | 1.75 .70.3 | .0007 | 2.5 .50.1 | 2.00 .00.00 | .0001 |
| C1A2 (PR-B) | ||||||
| Glandular | 2.2 .20.4 | 2.00 .00.5 | .50 | 1.5 .50.00 | 1.75 .70.5 | .296 |
| Stromal | 2.00 .00.2 | 1.75 .70.5 | .329 | 1.75 .70.2 | 2.00 .00.5 | .329 |
Abbreviation: PR, progesterone receptor.
PR-A and PR-B in Peritoneal and Ovarian Endometriosis
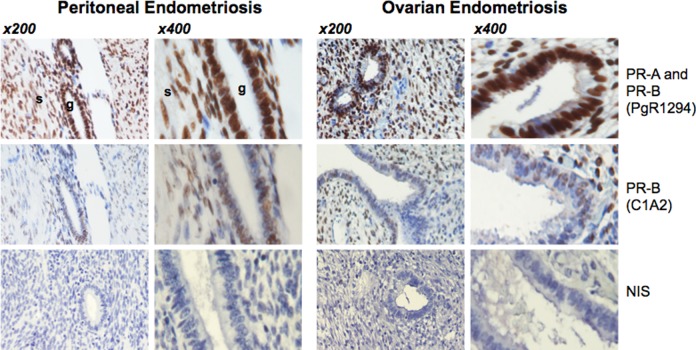
PgR1294 and C1A2 staining was localized to the nuclei of glandular and stromal cells in peritoneal endometriotic implants and ovarian endometriosis (Figure 4). Both PR isoforms were detected by immunoblotting in peritoneal and ovarian endometriotic tissue, albeit at variable levels (Figure 5). The mean levels of PR-A and PR-B (based on band intensity relative to GAPDH) were significantly higher in eutopic endometrium compared to levels in peritoneal endometriotic implants. In contrast, PR-A and PR-B levels were not different in eutopic endometrium compared with levels in ovarian endometriomata. The PR-A levels were significantly higher in ovarian endometriomata compared with peritoneal endometriosis. Mean PR-B levels were also higher in ovarian endometriomata compared with peritoneal endometriosis but the difference did not achieve statistical significance.
Figure 4.
Immunohistochemical localization of progesterone receptor (PR) isoforms in peritoneal and ovarian endometriosis. PgR1294 and C1A2 staining in peritoneal implants and ovarian endometrioma.
Figure 5.
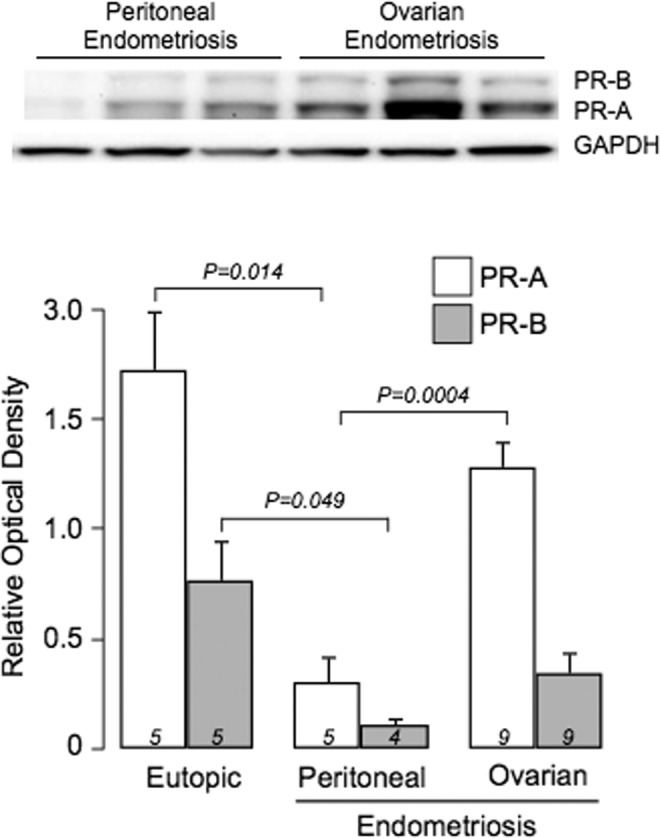
Abundance of PR-A and PR-B in peritoneal implants and ovarian endometrioma assessed by immunoblotting. Upper panel: representative PgR1294 immunoblots of total lysate from peritoneal implants and ovarian endometrioma. Lower panel: mean (±standard error [SE]) levels of PR-A and PR-B (assessed by digital densitometry of PgR1294 bands) in each tissue type. PR indicates progesterone receptor.
Discussion
Progesterone plays a key role in the pathophysiology of endometriosis. Detailed analyses of the endometrial cell transcriptome by Burney et al16 demonstrated that eutopic endometrium in women with endometriosis has decreased progesterone responsiveness (based on the level of expression of progesterone responsive genes) and defective transition from proliferative to secretory phase. This implies 2 possible mechanisms with respect to the role of progesterone responsiveness in endometriosis: (1) a causal relationship whereby disruption of endometrial cell progesterone responsiveness increases the likelihood of the tissue forming endometriotic implants when it is shed from the uterus into the peritoneum and/or (2) the existence of endometriosis causes the eutopic endometrium to exhibit reduced progesterone responsiveness. Our objective was not to solve this conundrum but insisted to determine whether decreased progesterone responsiveness is due to changes in endometrial cells PR isoform expression. To this end, we determine whether the pattern and abundance and localization of PR-A and PR-B in the endometrium in women with endometriosis are different to that of women without disease, and if so, whether it is consistent with decreased progesterone responsiveness.
A common problem with previous studies addressing the same question is that the various PR assays employed did not discriminate between PR-A and PR-B.9 Only more recent studies by Attia et al4 and Igarashi et al17 examined PR-A and PR-B protein levels in endometrium and endometrial implants. Igarashi et al17 reported that the PR-B/PR-A ratio assessed by immunoblotting of tissue obtained during the proliferative phase was less in the endometrium of women with endometriosis compared with endometrium from normal women. However, it is unclear from that study whether the decrease was due to decreased levels of PR-B or increased levels of PR-A; only the ratio was reported and immunoblot images were not shown. Nonetheless, our data showing increased PR-A relative to PR-B in endometrium from women with endometriosis are consistent with those of Igarashi et al. In contrast, Attia et al,4 also using immunoblotting, reported that PR-B was not detectable and that PR-A was markedly decreased in peritoneal endometriotic implants compared with eutopic endometrium. However, their assay had low sensitivity and required enrichment by PR immunoprecipitation (IP) to achieve detectable PR levels. Interestingly, even after IP, Attia et al did not detect PR-B in endometriotic tissue and only barely detected PR-A, suggesting that overall PR expression is decreased in endometriotic implants compared with eutopic endometrium. Misao et al,11 on the other hand, reported that the PR-B/total PR mRNA ratio was higher in eutopic endometrium compared with ectopic endometriotic lesions, suggesting the PR-B is indeed expressed by eutopic and ectopic endometrium. Clearly, the existing literature contains highly disparate outcomes. Our goal was to resolve this issue.
A major strength of our study is that PR antibodies, with high specificity for PR-B (C1A2) and PR-A/PR-B (PgR1294) in optimized IHC and immunoblot assays, were used to determine the cellular localization and relative abundance, respectively, of the PR isoforms in endometrium from women with and without endometriosis and in peritoneal and ovarian endometriotic implants. Our data show that aberrant PR-A overexpression occurs in eutopic and ectopic endometrial cells in women with endometriosis. We found markedly increased abundance of PR-A relative to PR-B (ie, a significant increase in the PR-A:PR-B ratio) in endometrium of women with endometriosis compared with endometrium from women without disease. Levels of PR-B and the localization pattern of PR isoforms in eutopic endometrium were not affected. Ovarian endometriosis has increased PR-A dominance compared with peritoneal endometriosis and had higher levels of PR-A and PR-B. This difference could be explained by variable disease load. Ovarian endometriomata is usually localized to the cyst wall and can occupy as much as 90% of the cyst wall surface area,18 whereas peritoneal lesions may be less pure, comprising a higher proportion of nonendometrial cells. Interestingly, the elevated PR-A:PR-B ratio in ovarian endometriosis correlates with a more aggressive phenotype compared to peritoneal disease.19 In most cell types, PR-A inhibits the transcriptional activity of PR-B,7 and overexpression of PR-A in breast tumors is associated with aggressive growth and increased expression of genes associated with invasion and poor prognosis even in the absence of ligand.20 Although the mechanistic link between increased PR-A and tumor growth and invasion is unclear, it is possible that similar effects occur in PR-A-dominant endometrial tissue that translocates to the peritoneum instead of being shed from the body. Thus, our data suggest that overexpression of PR-A by endometrial cells (at least in the late proliferative and the early secretory phases) is a factor in the pathophysiology of endometriosis.
An alternate hypothesis is that the altered peritoneal environment containing endometriotic implants somehow augments PR-A expression in eutopic endometrium. Although a mechanism for this effect remains unknown, it may involve proinflammatory cytokines. Inflammation is considered to play a key role in the etiology and progression of endometriosis, with tissue-level inflammation being a distinct characteristic of the ectopic implantation site.21 We previously found that in myometrial cells, expression of PR-A but not PR-B is specifically increased by proinflammatory stimuli.22 Thus, it is possible that endometriosis creates an inflammatory milieu that augments PR-A expression in ectopic and eutopic endometrium. Under normal circumstances, progesterone, presumably acting mainly via PR-B, is required for the appropriate transition of the endometrium from the proliferative to the secretory phenotype.23 It also may prevent—likely via PR-B-mediated anti-inflammatory action—the formation of endometriotic implants. Attenuation and/or disruption of this effect by increased PR-A, together with PR-A-mediated stimulation of invasion and growth, may not only interfere with the phenotypic ontogeny of the eutopic endometrium during the menstrual cycle but also promote the development and growth of ectopic lesions. Thus, the etiology of endometriosis may involve a positive-feedback loop whereby inflammation increases endometrial cell PR-A, which then inhibits the anti-inflammatory activity of PR-B, which in turn increases the risk of forming endometriotic implants causing more inflammation.
In conclusion, our finding of concordant PR-A dominance in endometriosis and in eutopic endometrium of women with endometriosis supports the hypothesis that the etiology of endometriosis involves altered progesterone signaling in eutopic endometrial cells, although further studies are needed to establish the causal relationship between increased PR-A expression and the development and progression of endometriosis. Nonetheless, the recently reported properties of PR-A, including its proinflammatory, proinvasive, and antiapoptotic actions, suggest that overabundance of this PR isoform (relative to PR-B) contributes to the development and progression of endometriosis. Our findings also suggest that development of selective PR modulators, especially compounds that specifically inhibit the transrepressive activity of PR-A, could be a promising strategy to treat endometriosis.24
Summary
Endometriotic lesions and eutopic endometrium from women with endometriosis are uniform in a PR-A-dominant state. Specifically, levels of PR-A were significantly elevated in the eutopic endometrium of women with endometriosis compared with women without disease, regardless of menstrual phase. In addition, PR-A levels were significantly elevated in ovarian endometriomas compared with peritoneal endometriosis. Given the association of PR-A overexpression in certain cancers with aggressive growth, invasion, and poor prognosis, similar effects may occur in endometriosis. Further research is necessary to determine the specific role of PR-A in mediating progesterone actions in endometriosis and to evaluate the use of selective PR modulators as a potential treatment strategy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by NIH grant #HD069819 (SM) and a grant from the Lerner Fund (JHL).
References
- 1. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 2. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258. [DOI] [PubMed] [Google Scholar]
- 3. Kaunitz AM. Injectable depot medroxyprogesterone acetate contraception: an update for U.S. clinicians. Int J Fertil Womens Med. 1998;43(2):73–83. [PubMed] [Google Scholar]
- 4. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 5. Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84(8):2963–2971. [DOI] [PubMed] [Google Scholar]
- 6. Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5518. [DOI] [PubMed] [Google Scholar]
- 7. Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20(9):3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87(10):4782–4791. [DOI] [PubMed] [Google Scholar]
- 9. Lyndrup J, Thorpe S, Glenthoj A, Obel E, Sele V. Altered progesterone/estrogen receptor ratios in endometriosis. A comparative study of steroid receptors and morphology in endometriosis and endometrium. Acta Obstet Gynecol Scand. 1987;66(7):625–629. [DOI] [PubMed] [Google Scholar]
- 10. Nisolle M, Casanas-Roux F, Wyns C, de Menten Y, Mathieu PE, Donnez J. Immunohistochemical analysis of estrogen and progesterone receptors in endometrium and peritoneal endometriosis: a new quantitative method. Fertil Steril. 1994;62(4):751–759. [DOI] [PubMed] [Google Scholar]
- 11. Misao R, Iwagaki S, Fujimoto J, Sun W, Tamaya T. Dominant expression of progesterone receptor form B mRNA in ovarian endometriosis. Hormone Res. 1999;52(1):30–34. [DOI] [PubMed] [Google Scholar]
- 12. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111. [DOI] [PubMed] [Google Scholar]
- 13. Prentice A, Randall BJ, Weddell A, et al. Ovarian steroid receptor expression in endometriosis and in two potential parent epithelia: endometrium and peritoneal mesothelium. Hum Reprod. 1992;7(9):1318–1325. [DOI] [PubMed] [Google Scholar]
- 14. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97(5):E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mote PA, Johnston JF, Manninen T, Tuohimaa P, Clarke CL. Detection of progesterone receptor forms A and B by immunohistochemical analysis. J Clin Pathol. 2001;54(8):624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 17. Igarashi TM, Bruner-Tran KL, Yeaman GR, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84(1):67–74. [DOI] [PubMed] [Google Scholar]
- 18. Muzii L, Bianchi A, Bellati F, et al. Histologic analysis of endometriomas: what the surgeon needs to know. Fertil Steril. 2007;87(2):362–366. [DOI] [PubMed] [Google Scholar]
- 19. Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab. 1999;84(8):2963–2971. [DOI] [PubMed] [Google Scholar]
- 20. McGowan EM, Clarke CL. Effect of overexpression of progesterone receptor A on endogenous progestin-sensitive endpoints in breast cancer cells. Mol Endocrinol. 1999;13(10):1657–1671. [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez-Ramos R, Donnez J, Defrere S, et al. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Human Reprod. 2007;13(7):503–509. [DOI] [PubMed] [Google Scholar]
- 22. Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab. 2004;89(2):1010–1013. [DOI] [PubMed] [Google Scholar]
- 23. Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83(3):529–537. [DOI] [PubMed] [Google Scholar]
- 24. Jeng CJ, Chuang L, Shen J. A comparison of progestogens or oral contraceptives and gonadotropin-releasing hormone agonists for the treatment of endometriosis: a systematic review. Expert Opinion Pharmacother. 2014;15(6):767–773. [DOI] [PubMed] [Google Scholar]