Abstract
Objective:
Angiotensin-converting enzyme (ACE) and angiotensin-converting enzyme 2 (ACE2) are key enzymes of the renin–angiotensin system. We investigated developmental changes in renal ACE and ACE2 gene expression and activity in both male and female sheep.
Methods:
Three groups of sheep (fetus, newborn, and adult) were used. Renal ACE and ACE2 activities, messenger RNA (mRNA), and protein expression were studied.
Results:
Renal ACE and ACE2 activities increased at 1 year in males, while there were no changes throughout development in females. Renal ACE and ACE2 mRNA and protein showed no sex differences but increased by 1 year of age.
Conclusion:
There are sex-related differences in the development of renal-converting enzyme activities that may have functional implications in terms of the regulation of blood pressure and renal function in men and women. The difference in the patterns of gene expression and enzyme activity indicates that changes in gene expression may not accurately reflect changes in activity.
Keywords: angiotensin-converting enzyme, angiotensin-converting enzyme 2, renin–angiotensin system, kidney, sex
Introduction
The renin–angiotensin system (RAS) was initially viewed as an endocrine system with circulating angiotensin II (Ang II) as its functional effector hormone. Systemically, the juxtaglomerular cells of the kidney release active renin into the circulation.1 In the blood, renin cleaves liver-derived angiotensinogen to form inactive angiotensin I (Ang I). An angiotensin-converting enzyme (ACE), which is a sessile zinc-containing metalloproteinase, further removes 2 c-terminal amino acids of Ang I thereby generating Ang II. Actions of Ang II are imparted by its binding to specific receptors, type 1 and type 2 (AT1 and AT2).2 Most of the known effects of Ang II, such as vasoconstriction, aldosterone release, and cell proliferation, are medicated by the AT1 receptor.2–5 Ang II may also interact with AT2 receptors, which generally oppose the actions of the Ang II-AT1 axis.6
More than a decade ago, a new arm of the RAS, the angiotensin-converting enzyme 2 (ACE2)–angiotensin-(1-7)/Mas receptor axis, was identified. Unlike ACE, ACE2 does not convert Ang I to Ang II, and the enzyme is resistant to ACE inhibitors.7 The ACE2 exhibits much greater catalytic activity for Ang II to form Ang-(1-7) rather than converting Ang I to Ang-(1-9).8 The ANG-(1-7), the main product of ANG II degradation by ACE2, has opposite properties to that of ANG II. The ANG-(1-7) promotes natriuresis, antiproliferation, antihypertrophy, and in some circumstances vasodilation.9,10
Together with ACE, ACE2 is abundantly expressed in the kidney.11,12 Recent evidence has indicated that intrarenal RAS activity, governed in part by the balance of ACE and ACE2, may be involved in organogenesis and development of adult disease including hypertension and renal disease.13–16 It has been shown that ACE activity is important in the development of high blood pressure.17,18 Locally, ACE2 mediated production of Ang-(1-7) may counteract the effects of locally generated Ang II by ACE in the proximal tubule.19 This may help protect against the development of high blood pressure and progressive kidney injury.19,20
Striking sex differences have been documented in the prevalence and/or progression of hypertension and renal disease. For example, compared to girls and young women, boys and young men developed higher serum ACE activity and higher blood pressure.21,22 Also, kidneys from males appear more susceptible to renal disease in both human and animal studies than do kidneys from females.23–27 The mechanisms contributing to these sex differences are unclear.
From the above, it is clear that ACE and ACE2 are 2 key components of RAS, which help regulate multiple physiological functions in both males and females. However, the development of renal ACE and ACE2 activities is not well defined. Therefore, the present study was designed to determine renal ACE and ACE2 levels and messenger RNA (mRNA) expression prior to birth, in the newborn period, and in the first year of life in both male and female sheep. We chose to examine these questions in sheep because they are a major animal model used in studying the development of renal function and the RAS28–30 and to study both males and females to determine whether sex-related differences in the developmental patterns of enzyme activities exist.
Materials and Methods
Animal and Experimental Protocols
All study protocols were approved by the institutional Animal Care and Use Committee of Wake Forest University School of Medicine. Three groups of date-mated sheep (5 animals of each gender) were studied (fetus at 135 days of gestation age, newborn at 5-7 days, and adult at 1 year). Sheep (mixed breeds) were anesthetized and nephrectomy was performed, and the kidney cortex was dissected on ice and frozen at −80°C for further study.
Renal ACE and ACE2 Activities
Renal cortical membranes were prepared by homogenization in assay buffer (10 mmol/L HEPES [4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid], 125 mmol/L NaCl, 10 μmol/L ZnCl2, pH 7.4) followed by centrifugation at 28 000g for 10 minutes at 4°C. The supernatant was removed, the pellet was resuspended, and incubated overnight at 4°C in 0.5% Triton-Assay buffer to solubilize the membranes. The sample was centrifuged at 28 000g for 5 minutes at 4°C. The supernatant was saved for further study. Protein concentration was determined using the bovine serum albumin method according to the manufacturer’s instructions (Pierce, Rockford, Illinois).
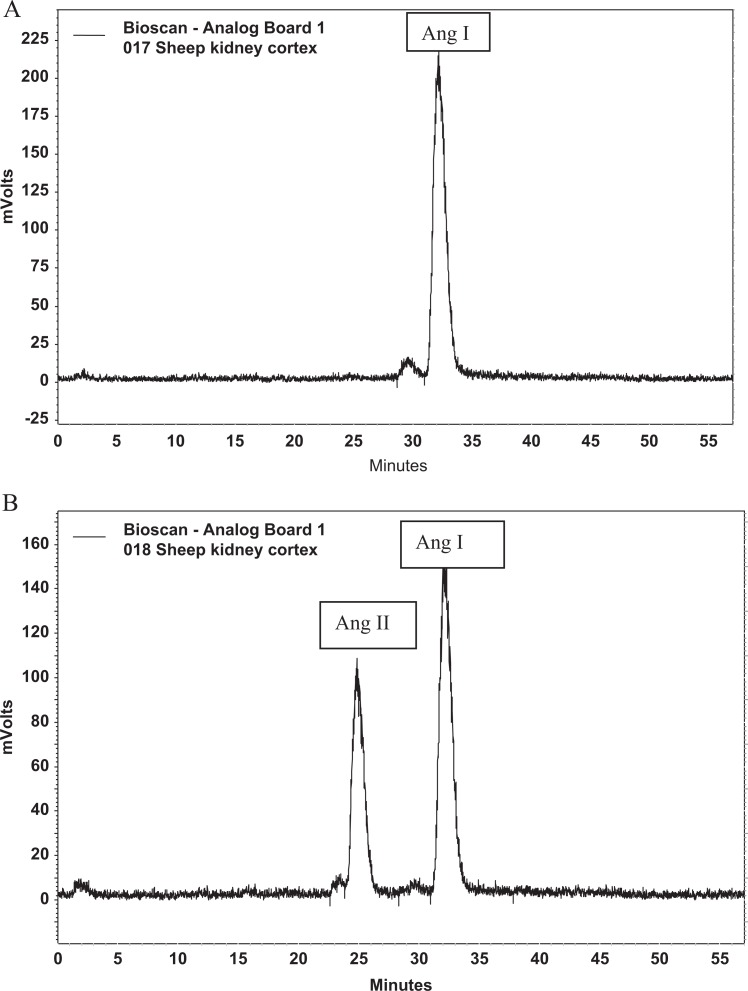
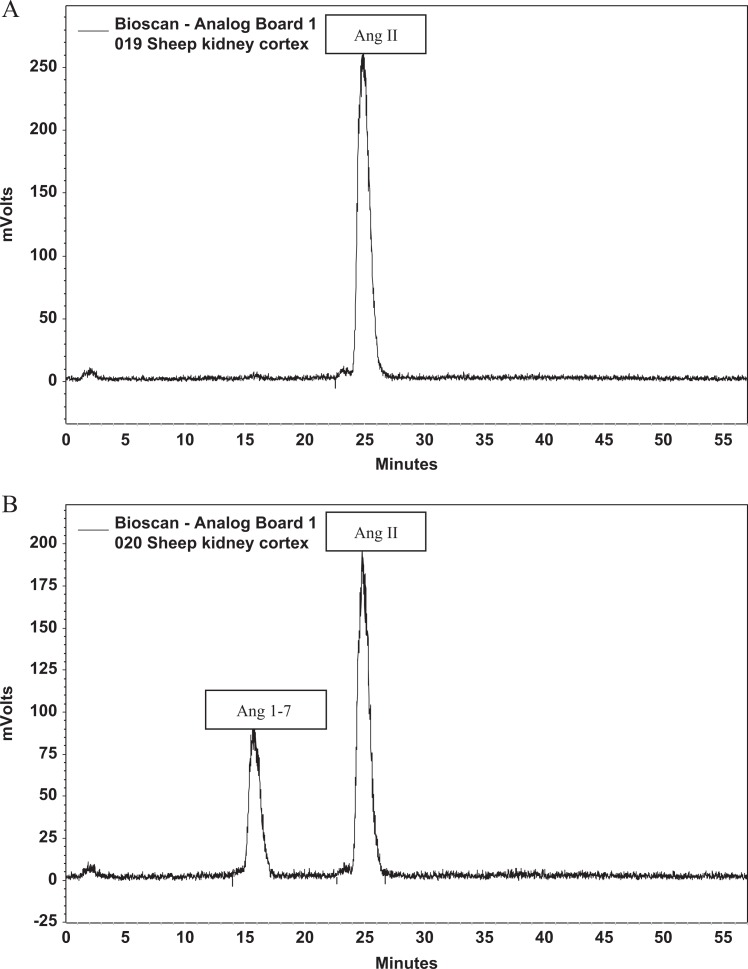
Solubilized membranes of 4 μg were then incubated at 37°C for 30 minutes with either 125I-Ang I or 125I-Ang II in the presence or absence of Lisinopril (at 10 μmol/L final concentration) to inhibit ACE activity or C-16 (an ACE2 inhibitor; Millennium Pharmaceuticals, Baltimore, Maryland, at 10 μmol/L final concentration) to inhibit ACE2 activity. The general inhibitor cocktail, which was used in all assays included amastatin (2 μmol/L), bestatin (10 μmol/L), chymostatin (10 μmol/L), benzyl succinate (10 μmol/L), para-chloro-mercuribenzoic acid (0.5 mmol/L), and SCH39370 (10 μmol/L). The reaction was stopped by addition of 1.0% phosphoric acid, centrifuged at 16 000g, and the supernatant was stored at −20°C. Samples were filtered before separation by reverse-phase high-performance liquid chromatography (HPLC), and the 125I products were monitored by a Bioscan flow-through γ detector as described.31 Products were identified by comparison of retention times to 125I standard peptides. Peptides were iodinated by the chloramine T method and purified by HPLC (specific activity of 2200 Ci/mmol).32 The activities of ACE (Figure 1) and ACE2 (Figure 2) were then quantified by calculating the area under the curve for each product and converted to fmol of product/mg protein/min of incubation.
Figure 1.
Representative chromatograms of ACE activity. A, The figure shows the quantity of angiotensin I (Ang I) after a control membrane sample incubated with Ang I with ACE activity blocked with lisinopril. B, The figure shows the quantity of Ang I and angiotensin II (Ang II) after a control membrane sample incubated with Ang I without blockade of ACE. ACE indicates angiotensin-converting enzyme.
Figure 2.
Representative chromatograms of ACE2 activity. A, The figure shows the quantity of angiotensin II (Ang II) after a control membrane sample incubated with Ang II with ACE2 activity blocked with C16. B, The figure shows the quantity of Ang II and angiotensin 1-7 (Ang-1-7) after a control membrane sample incubated with Ang I without blockade of ACE2. ACE2 indicates angiotensin-converting enzyme 2.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction
The relative abundance of ACE1 and ACE2 mRNA transcripts in kidney cortex was measured by quantitative real-time reverse transcription polymerase chain reaction (PCR) using TaqMan PCR (Applied Biosystems, Foster City, CA). Total RNA was isolated from kidney cortex tissue using the TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, California). RNA from kidney cortex was quantified by measuring absorbance at 260 nm, and 1 μg of total RNA was reverse transcribed in a 20-μL reaction mixture using an ABI High Capacity complementary DNA (cDNA) Archive Kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, California). The reaction contained 1× reverse transcription (RT) buffer, 100 μmol/L of each deoxynucleoside triphosphate, 1× random primer, and 100 U of reverse transcriptase. The reaction was carried out at 25°C for 10 minutes then at 37°C for 2 hours. Control reactions were those in which the RT enzyme or the target RNA was omitted from the reaction.
TaqMan PCR was performed on the cDNA samples using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). For each gene tested (see Table 1), PCR was carried out in multiplex mode, with every 20 μL reaction containing 2 μL of cDNA reaction, 1× TaqMan universal PCR master mix, 250 nmol/L of a gene-specific primer, 250 nmol/L of FAM (6-carboxy-fluorescein)-labeled fluorogenic TaqMan probe, and 2.5 U of TaqMan enzymes. The thermal cycling conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute. An increase in fluorescence was obtained at the annealing and extension step at 60°C.
Table 1.
Primers for ACE and ACE2 mRNA.
| Gene | Primers | Sequence |
|---|---|---|
| ACE1 | Forward | CCGCCTGGGACTTCTTCA |
| Reverse | ACTGAGGTGCACTGCTTGATC | |
| Probe | ACGGCAAGGACTTTAG | |
| ACE2 | Forward | ACCTCACTATTTGAAAGCACTTGGT |
| Reverse | GCTTGCTTGAGCAGGAAGTTTATTT | |
| Probe | TCTGGCACCCGATTTT |
Abbreviations: ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; mRNA, messenger RNA.
The relative level of expression of each gene in the samples was determined using the relative 2ΔΔCt expression method as previously described according to the manufacturer’s instructions (Applied Biosystems. Relative Quantitation of Gene Expression, User Bulletin #2, ABI PRISM 7700 Sequence Detection System. 2001:11-5). After the linear range of amplification (threshold cycle, Ct) was determined for the genes of interest, it was normalized against an endogenous glyceraldehyde 3-phosphate dehydrogenase control and then against a control sample (aliquots of this sample from a naive sheep were used in each assay as the calibrator). The value of the relative level of expression for the gene of interest represents 2 independent reactions performed in triplicate.
Western Blot for Enzyme Proteins
Frozen kidney cortex was fractured in liquid nitrogen and then homogenized at 4°C with 2 mL homogenization buffer (0.1 mmol/L EDTA, 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Triton X, 0.1% sodium dodecyl sulfate, 1% Na deoxycholate, and 0.5 mmol/L dithiotreitol) and 1:1000 protein inhibitor. Samples were then centrifuged at 2000 rpm for 10 minutes. The supernatant was then centrifuged at 45 000 rpm for 60 minutes. The supernatant was kept for further study. Protein concentration was determined using the BCA method (Pierce).
Protein of 25 μg was electrophoresed and transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 6% nonfat dry milk in 0.1% Tween in Tris-buffered saline (TBST) for 60 minutes at room temperature before incubation with an ACE antibody (Abcam, Massachusetts) at a 1:150 dilution at 4°C overnight. Immunoblots were then resolved with Amersham ECL plus Western blotting detection reagents, as described by the manufacturer, and exposed to AmershamHyperfilm. Membranes were then stripped and again blocked with 6% nonfat dry milk in 0.1% TBST for 60 minutes prior to incubation with ACE2 antibody (Abcam) at a 1:2000 dilution at 4°C overnight. Immunoblots were then resolved with Amersham ECL plus Western blotting detection reagents, as before, and exposed to AmershamHyperfilm ECL (Amersham, Piscataway, New Jersey). β-Actin was detected as internal control using standard protocols.
Immunohistochemical Determination for ACE and ACE2
Sections (5 μm) of kidney cortex of 1-year-old sheep were cut from 4% paraformaldehyde-fixed, paraffin-embedded tissue blocks and mounted on polylysin-coated slides. The antigen was retrieved by heating the sections in a 10-mmol/L citrate buffer (90°C, 30 minutes). For ACE immunostaining, sections were incubated at 4°C overnight with the mouse monoclonal anti-ACE antibody (Abcam) at a 1:1000 dilution in 0.5 mol/L TBST. The secondary antibody was coupled to alexafluor488 antimouse immunoglobulin G (IgG; Invitrogen Corp) used at a 1:200 dilution. For ACE2 immunostaining, sections were incubated at 4°C overnight with the rabbit polyclonal anti-ACE2 antibody (Abcam) at a 1:1000 dilution in 0.5 mol/L TBST. The secondary antibody was coupled to alexafluor 546 antirabbit IgG (Invitrogen Corp) used at a 1:200 dilution. Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole at mounting medium (H-1200; VECTOR Lab Inc, Burlingame, CA). Sections were kept in the dark until the fluorescence was studied using a microscope (LEICA DM 4000; LEICA Microsystem, Germany). Control slides were incubated with the appropriate IgG but without the primary antibody.
Statistics
All of the measurements were expressed as the means ± standard error of means. Two-way analysis of variance with Bonferroni posttest was used for developmental data analysis. A P value <.05 was used as statistical significance.
Result
Immunohistochemical Determination for ACE and ACE2
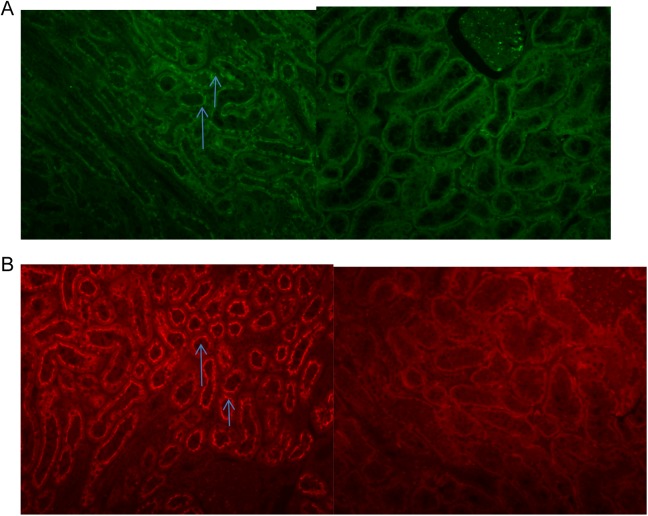
The locations of both ACE and ACE2 in the renal cortex are shown in Figure 3. Both ACE (Figure 3, panel A, red) and ACE2 (Figure 3, panel B, green) were primarily localized to the brush border of proximal tubules in the renal cortex.
Figure 3.
Fluorescent stain of ACE and ACE2 in the renal cortex. A, The figure shows the fluorescent ACE staining in the renal cortex was primarily localized to the brush border of proximal tubules (left: bright green shows ACE. Right, negative control. Magnification: 1:20). B, The figure shows the fluorescent ACE2 staining in the renal cortex was primarily localized to the brush border of proximal tubules (left, bright red shows ACE2. Right, negative control. Magnification: 1:20). ACE indicates angiotensin-converting enzyme.
Developmental Change in Renal ACE and ACE2 Activities
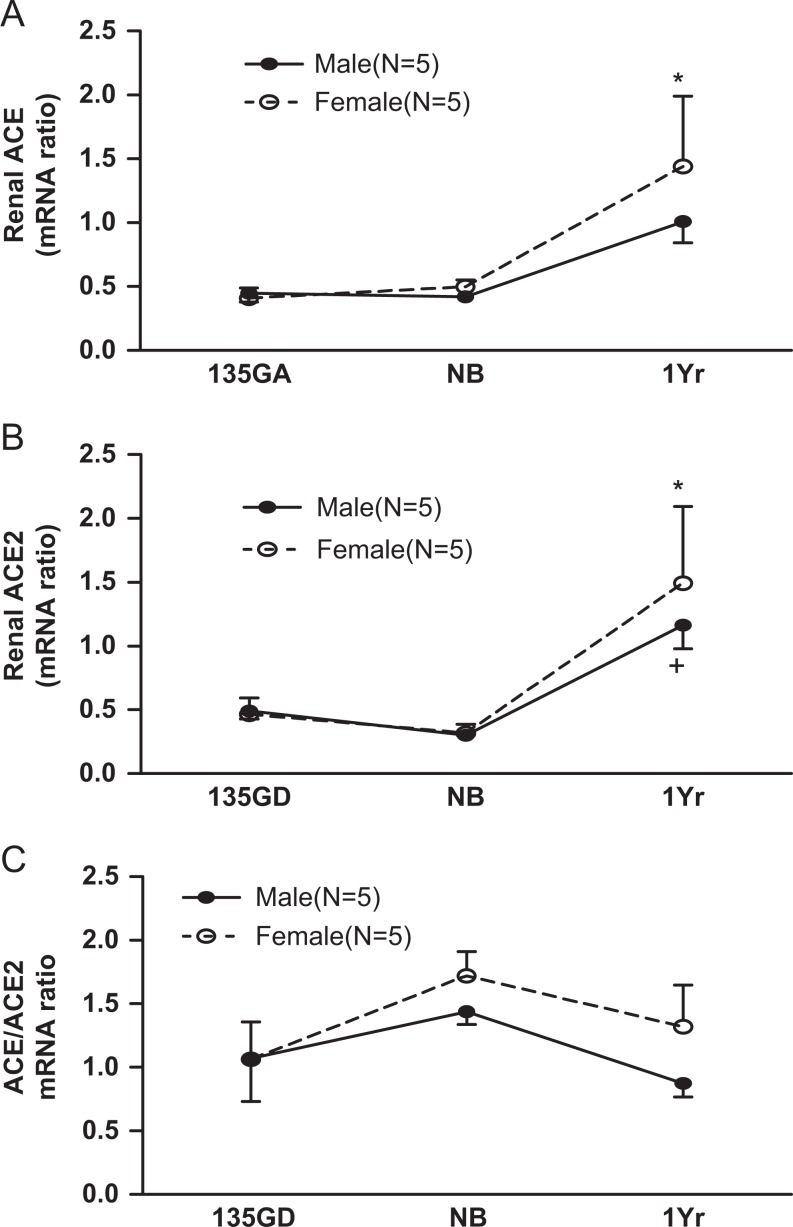
Figure 4 shows the developmental changes in renal ACE activity, ACE2 activity, and their ratios. There were age (F = 3.642, P = .0409) and sex (F = 9.090, P = .0058) effects in ovine renal cortical ACE activity. In males, renal ACE activity (Figure 4, Panel A) increased approximately 50% at 1 year compared with levels in the fetus (late gestation, P < .01) and newborns (P < .001). In contrast, renal ACE activity in females had no change throughout development. Males had higher renal ACE activity compared with that in females at 1 year of age (P < .001).
Figure 4.
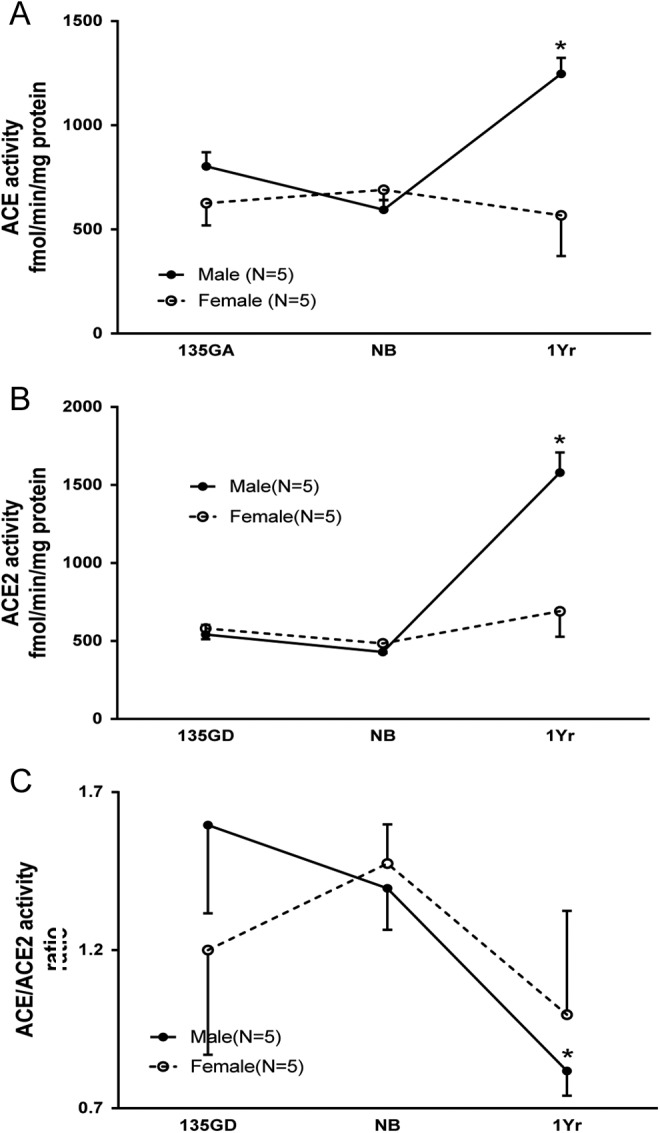
The developmental changes in renal ACE activity, ACE2 activity, and their ratio. A. Renal ACE activity during development. Renal ACE activity increased at 1 year of age (1 year; *P < .01 vs 135 days of gestation [135 GA], P < .001 vs newborns [NBs]) in males. Males had higher renal ACE activity compared to that in females at 1 year of age (+P < .001). B, Renal ACE2 activity during development. Renal ACE2 activity increased at 1 year of age (*P < .001 vs 135 GA and NBs) in males. Males had higher renal ACE2 activity compared to that in females at 1 year of age (+P < .001). C, ACE/ACE2 activity ratio during development. The ACE/ACE2 ratio decreased at 1 year (*P < .05 vs NB) in males. ACE indicates angiotensin-converting enzyme.
Age (F = 27.38, P < .0001) and sex (F = 10.52, P = .0033) effects were also noted in ovine renal cortical ACE2 activity. In males, renal ACE2 activity (Figure 3, Panel B) increased about 300% at 1 year compared with the late gestation (P < .001) and newborn periods (P < .001). In contrast, renal ACE2 in females had no change throughout development. Males had higher renal ACE2 activity compared with that in females at 1 year of age (P < .001).
The ACE/ACE2 activity ratio (Figure 4, Panel C) showed age (F = 3.399, P = .0495) but not sex (F = 0.062, P = .804) effects. The ACE/ACE2 ratio decreased at 1 year in males (P < .05) compared with newborns but did not change during development in females.
Developmental Changes in Renal ACE mRNA and ACE2 mRNA Expression
Renal cortical ACE and ACE2 mRNA expression and the ACE/ACE2 mRNA ratio are shown in Figure 5. There was an age (F = 7.473, P = .0029) but not a sex (F = 0.656, P = .42) effect in sheep renal ACE mRNA during development. Renal cortical ACE mRNA expression (Figure 4, Panel A) increased at 1 year in males compared with the newborns and fetuses.
Figure 5.
The developmental changes in renal cortical ACE mRNA, ACE2 mRNA, and their ratio. A, Renal cortical ACE mRNA expression. Renal ACE mRNA increased at 1 year of age in female (*P < .05 vs newborns [NBs] and 135 GA) but not in males. B, Renal cortical ACE2 mRNA expression during development. Renal ACE2 mRNA expression was significantly increased at 1 year of age in both males (P < .05 vs NBs) and females (P < .05 vs NBs and 135 GA). C, The ACE/ACE2 mRNA expression ratio during development. There were no effects of age or sex and no interactions. ACE indicates angiotensin-converting enzyme; mRNA, messenger RNA; 135 GA, 135 days of gestation.
There was an age (F = 9.00, P = .001) but not a sex (F = 0.25, P = .62) effect in the developmental change in sheep renal ACE2 mRNA levels. Renal ACE2 mRNA expression (Figure 5, Panel B) was significantly increased at 1 year in both males and females compared with newborns. The ACE/ACE2 mRNA expression ratio (Figure 5, Panel C) had neither age (F = 2.81, P = .08) nor sex (F = 1.58, P = .23) effects throughout development.
Developmental Change in Renal ACE and ACE2 Protein Expression
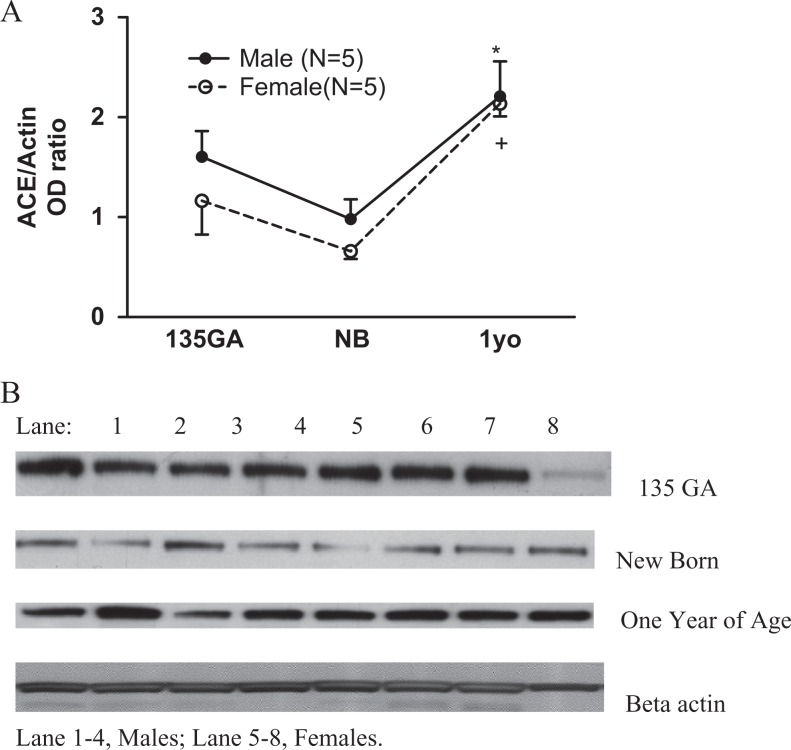
Renal cortical ACE and ACE2 protein expression are shown in Figures 6 and 7. There was an age (F = 12.25, P = .0003) but not a sex (F = 1.63, P = .21) effect in the developmental change in sheep renal ACE protein expression. Renal ACE protein expression increased at 1 year in both males and females compared with newborns (Figure 6, panel A).
Figure 6.
The developmental change in renal cortical ACE protein. Panel A: renal cortical ACE protein expression. Renal ACE protein expression increased at 1 year old in both males (*P < .01 vs newborns [NBs]) and females (*P < .01 vs NB). Panel B: Western blot of renal cortical ACE. ACE indicates angiotensin-converting enzyme.
Figure 7.
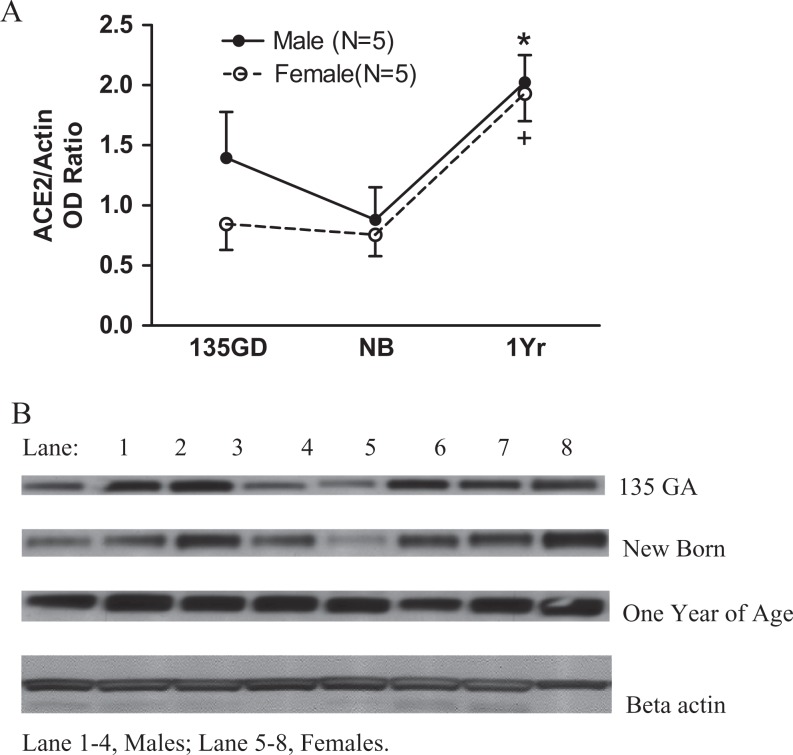
The developmental change in renal cortical ACE2 protein. Panel A: renal cortical ACE2 protein expression. Renal ACE2 protein expression increased at 1 year old in both males (*P < .01 vs NB) and females (*P < .05 vs NB and 135 days of gestation [135 GA]). Panel B: Western blot of renal cortical ACE2. ACE2 indicates angiotensin-converting enzyme 2
The developmental change in sheep renal ACE2 protein expression had an age (F = 9.04, P = .0014) but not a sex (F = 1.33, P = .26) effect. Renal ACE2 protein expression increased at 1 year in both males (compared with newborns) and females (compared with both the newborns and fetuses; Figure 7, panel A).
Discussion
The purpose of this study was to define renal ACE and ACE2 levels and mRNA expression prior to birth, in the newborn period and in the first year of life and to determine whether sex influences any changes observed. We studied sheep, which are a widely used animal model for studying renal physiology during development. We chose the renal cortex because it is a primary target for Ang II and Ang-(1-7), which is locally produced by ACE and ACE2, respectively.33 We found the developmental pattern of ACE and ACE2 activities was different in males and females with males having an increase in both activities at 1 year of age while females showed no increase. Thus, there are sex differences in the development of renal cortical ACE and ACE2 activities after birth. We also found that the pattern of change in ACE and ACE2 activities in females differed from the pattern of change in mRNA and protein expression. This difference was not seen in males. This suggests there are some posttranslational modifications of the enzyme occurring as development progresses in the females that are lacking in the males.
At present, the mechanisms underlying the sex differences in renal cortical ACE and ACE2 development are unknown. Our study shows that the sex difference in development of renal ACE and ACE2 activities in sheep does not appear until postpuberty. This is consistent with human studies in which, compared to girls, boys have the same serum ACE activity before puberty but their blood serum ACE activity starts to increase and becomes significantly higher after puberty.21 These findings suggest that sex hormones may be involved in this age-related change and some studies indicate that estrogen and testosterone may modulate ACE activity in other species.34–37 It seems that ACE activity is likely decreased by estrogen and increased by androgens.38
But the regulation of ACE2 is more complex and the effect of estrogen on ACE2 is different depending upon the physiological or pathophysiological status of the subject. For example, in normal mice and normotensive Lewis rats, females had lower renal ACE2 activity compared to males. This was similar to what we found in adult sheep. This difference depended on the presence of ovaries and ovarian hormones.39,40 On the other hand, ovariectomy attenuated renal ACE2 activity in a rat model of progressive renal disease, and this effect was prevented by estrogen treatment.41 These findings suggest that renal ACE2 is differentially regulated by estrogen under normal and pathological conditions and that the enzyme may play different physiological roles in normal and disease states.
The pattern of changes in ACE and ACE2 activities in females differed from the pattern of changes in mRNA and protein expression in our study. The different patterns of change were not seen in males. This suggests there were some posttranslational modifications of the enzyme occurring in the females that were lacking in the males. The lack of parallelism in mRNA expression, protein expression, and protein activity of ACE2 was also noted previously in both renal cortex and lung in rats.42,43 Thus, our data and these reports all suggest that ACE and ACE2 activity measurements are needed to fully understand the effects of interventions on the enzymes rather than measurements of only gene or protein expression, especially when making comparisons between males and females.
The mechanisms producing the discrepancy among ACE and ACE2 mRNA, protein expression, and enzyme activity in female sheep are still unclear but might be related to the effect of estrogen in posttranscriptional and/or posttranslational regulation. A posttranslational effect of estrogen has been reported in a recent study in which a novel mechanism of estrogen-mediated promotion of inflammatory responses, which involves posttranslational modification of signal transducer and activation of transcription-1 protein, was noted.44 Whether or not such mechanisms are operative in controlling ACE and ACE2 expression is not known at present.
Recently several studies have provided another potential mechanism to explain this phenomenon.36,45–47 For example, it was noted that xanthenone, a small drug-like compound (molecular weight <500), may enhance ACE2 activity which can cause considerable reductions in blood pressure without changing ACE2 mRNA or protein expression.47 Other small molecules have similar effects on ACE2 activity.36,46 This opens the possibility that endogenous small molecules could serve as sex-specific posttranslational regulators of ACE and ACE2 activities and would be consistent with our observations.
Little is known about the physiological consequences of the sex difference we noted in the development of renal cortical ACE. The increased ACE in adult males that is absent in females may contribute to differences in blood pressure in males and females as both animal and human studies have shown that males have higher ACE levels and blood pressure compared to females.6,17,18,21,22,40,48,49 These reports are all consistent with the idea that ACE acts to increase blood pressure and that some sex-related differences in blood pressure may be associated with differences in ACE activity in males and females.
Compelling evidence suggests that ACE2-Ang-(1-7)–Mas receptor axis may oppose actions of the ACE-Ang II-AT1 receptor pathway.50 We found that, besides higher renal ACE activity, 1-year-old male sheep also had higher ACE2 activity compared to females. The same phenomenon has been reported in both mice and rats.39,40 The physiological impact of this sex difference is not clear. Some evidence suggests a link between changes in blood pressure and changes in ACE2 in adults. For example, compared to normotensive Wistar Kyoto rats, an increase in renal ACE2 was paralleled by increases in renal ACE before the onset of hypertension in spontaneously hypertensive rats (SHRs).16 Then, with, the onset of hypertension1 renal ACE2 declined significantly16 and became lower in the hypertensive adult male SHR.16,51 It was hypothesized that the increased renal ACE2 might reflect the compensatory role of ACE2 before the onset of hypertension.16 Thus, the developmental increase in ACE2 activity in male sheep may play a role in modulating the effect of increased ACE activity before the development of age-related hypertension in males.52
Besides earlier development of hypertension, studies also show that, compared to females, males appear more susceptible to renal disease23–27,53 and show greater deleterious effects earlier than do females in response to reductions in nephron number.54 The emerging evidence suggests that the interaction of ACE and ACE2 maybe involved in the development of renal disease,13 with intrarenal ACE playing a determining role.55,56 For example, increased renal ACE is associated with age-related renal fibrosis in male SD rats,57,58 and renal fibrosis is well known as one of the major mechanisms in the progression of renal disease.59–62 We demonstrated that renal ACE activity was significantly increased and higher in male sheep than in females at 1 year. The higher local ACE activity may be responsible, at least partially, for the sex susceptibility to renal disease in males.23–27
On the other hand, increased renal ACE2 expression is considered as a renoprotective mechanism.63,64 Spontaneously hypertensive rats treated with ACE2 activators have significantly attenuated interstitial renal fibrosis,45 and ACE2 overexpression in diabetic mice ameliorated their glomerular injury.65 Changes in ACE/ACE2 ratio have a significant impact on renal injury,45,63–66 with increased ACE/ACE2 ratio possibly contributing to hypertensive and diabetic nephropathy in rats67,68 and age-dependent glomerulosclerosis in male mice.63 Interestingly, increased ACE2 expression and consequently lower ACE/ACE2 ratios were seen in the kidneys of diabetic mice before development of diabetic nephropathy. This suggested that the increased ACE2 and decreased ACE/ACE2 ratio represented an early protective response by attenuating Ang II accumulation and increasing Ang-(1-7) formation.20 If this is the case, then a higher ACE/ACE2 ratio67,68 seen in pathological conditions might indicate loss of renal protective mechanisms which may foster kidney damage. We noted that in male sheep at 1 year, renal ACE activity increased less (about a 30% increase compared to late gestation and newborn) than did renal ACE2 activity (about 300% increase compared to late gestation and newborn); consequently the renal ACE/ACE2 ratio decreased. Therefore, the greater increase in renal ACE2 activity compared to the increase in ACE activity during development might indicate an early protective response to delay the onset of renal injury in male sheep.
In conclusion, the ontogeny of renal cortical ACE and ACE2 enzymatic activity is developmentally regulated in a sex-specific manner in sheep. These sex differences may be influenced by sex hormones and could be related to sex differences in renal function or the ability to respond to endogenous and/or exogenous insults. Gene or protein expression alone may not accurately reflect the effects of interventions on both renal ACE and ACE2 activities. The physiological consequences and mechanisms involved in the sex-specific ontogeny of renal ACE and ACE2 activities merit further investigation.
Footnotes
Authors’ Note: Jianli Bi and Yixin Su contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH grants RO1 HD017644 and PO1 HD047584.
References
- 1. Givertz MM. Manipulation of the renin–angiotensin system. Circulation. 2001;104(5):E14–E18. [DOI] [PubMed] [Google Scholar]
- 2. Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45(2):205–251. [PubMed] [Google Scholar]
- 3. Peach MJ. Molecular actions of angiotensin. Biochem Pharmacol. 1981;30(20):2745–2751. [DOI] [PubMed] [Google Scholar]
- 4. Csikos T, Gallinat S, Unger T. Extrarenal aspects of angiotensin II function. Eur J Endocrinol. 1997;136(4):349–358. [DOI] [PubMed] [Google Scholar]
- 5. Bottari SP, De GM, Steckelings UM, Levens NR. Angiotensin II receptor subtypes: characterization, signalling mechanisms, and possible physiological implications. Front Neuroendocrinol. 1993;14(2):123–171. [DOI] [PubMed] [Google Scholar]
- 6. Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor: the AT2 receptor comes of age. Hypertension. 2005;45(5):840–844. [DOI] [PubMed] [Google Scholar]
- 7. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. [DOI] [PubMed] [Google Scholar]
- 8. Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277(17):14838–14843. [DOI] [PubMed] [Google Scholar]
- 9. Santos RA, Haibara AS, Campagnole-Santos MJ, et al. Characterization of a new selective antagonist for angiotensin-(1-7), D-pro7-angiotensin-(1-7). Hypertension. 2003;41(3 pt 2):737–743. [DOI] [PubMed] [Google Scholar]
- 10. Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289(6):H2281–H2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27(3 pt 2):523–528. [DOI] [PubMed] [Google Scholar]
- 12. Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regul Pept. 2000;91(1-3):45–62. [DOI] [PubMed] [Google Scholar]
- 13. Wakahara S, Konoshita T, Mizuno S, et al. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology. 2007;148(5):2453–2457. [DOI] [PubMed] [Google Scholar]
- 14. Forhead AJ, Gillespie CE, Fowden AL. Role of cortisol in the ontogenic control of pulmonary and renal angiotensin-converting enzyme in fetal sheep near term. J Physiol. 2000;526(pt 2):409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song R, Preston G, Yosypiv IV. Ontogeny of angiotensin-converting enzyme 2. Pediatr Res. 2012;71(1):13–19. [DOI] [PubMed] [Google Scholar]
- 16. Tikellis C, Cooper ME, Bialkowski K, et al. Developmental expression of ACE2 in the SHR kidney: a role in hypertension? Kidney Int. 2006;70(1):34–41. [DOI] [PubMed] [Google Scholar]
- 17. Baumbach GL, Chillon JM. Effects of angiotensin-converting enzyme inhibitors on cerebral vascular structure in chronic hypertension. J Hypertens Suppl. 2000;18(1):S7–S11. [PubMed] [Google Scholar]
- 18. Salvetti A, Pedrinelli R, Arzilli F, et al. Angiotensin-converting enzyme inhibitors in hypertension: a review. Int J Clin Pharmacol Res. 1985;5(6):429–438. [PubMed] [Google Scholar]
- 19. Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int. 2006;69(12):2212–2218. [DOI] [PubMed] [Google Scholar]
- 20. Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension. 2004;43(5):1120–1125. [DOI] [PubMed] [Google Scholar]
- 21. Landazuri P, Granobles C, Loango N. Gender differences in serum angiotensin-converting enzyme activity and blood pressure in children: an observational study. Arq Bras Cardiol. 2008;91(6):352–357. [DOI] [PubMed] [Google Scholar]
- 22. Zapater P, Novalbos J, Gallego-Sandin S, Hernandez FT, bad-Santos F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J Cardiovasc Pharmacol. 2004;43(5):737–744. [DOI] [PubMed] [Google Scholar]
- 23. Gandolfo MT, Verzola D, Salvatore F, et al. Gender and the progression of chronic renal diseases: does apoptosis make the difference? Minerva Urol Nefrol. 2004;56(1):1–14. [PubMed] [Google Scholar]
- 24. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11(2):319–329. [DOI] [PubMed] [Google Scholar]
- 25. Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5(suppl A):S3–S10. [DOI] [PubMed] [Google Scholar]
- 26. Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25(4):515–533. [DOI] [PubMed] [Google Scholar]
- 27. Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Adv Ren Replace Ther. 2003;10(1):3–14. [DOI] [PubMed] [Google Scholar]
- 28. Tang L, Carey LC, Bi J, et al. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R309–R317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang DH, Elijovich F. Modulation and function of extrarenal angiotensin receptors. Cell Biochem Biophys. 1999;31(1):1–17. [DOI] [PubMed] [Google Scholar]
- 30. Chen K, Carey LC, Liu J, Valego NK, Tatter SB, Rose JC. The effect of hypothalamo-pituitary disconnection on the renin–angiotensin system in the late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1279–R1287. [DOI] [PubMed] [Google Scholar]
- 31. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. [DOI] [PubMed] [Google Scholar]
- 32. Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1---7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol. 2000;279(5):F841–F850. [DOI] [PubMed] [Google Scholar]
- 33. Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53(2):404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Freshour JR, Chase SE, Vikstrom KL. Gender differences in cardiac ACE expression are normalized in androgen-deprived male mice. Am J Physiol Heart Circ Physiol. 2002;283(5):H1997–H2003. [DOI] [PubMed] [Google Scholar]
- 35. Brosnihan KB, Weddle D, Anthony MS, Heise C, Li P, Ferrario CM. Effects of chronic hormone replacement on the renin–angiotensin system in cynomolgus monkeys. J Hypertens. 1997;15(7):719–726. [DOI] [PubMed] [Google Scholar]
- 36. Kulemina LV, Ostrov DA. Prediction of off-target effects on angiotensin-converting enzyme 2. J Biomol Screen. 2011;16(8):878–885. [DOI] [PubMed] [Google Scholar]
- 37. Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension. 1999;33(1 pt 2):323–328. [DOI] [PubMed] [Google Scholar]
- 38. Komukai K, Mochizuki S, Yoshimura M. Gender and the renin–angiotensin–aldosterone system. Fundam Clin Pharmacol. 2010;24(6):687–698. [DOI] [PubMed] [Google Scholar]
- 39. Liu J, Ji H, Zheng W, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17beta-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 2010;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295(1):H10–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol. 2008;93(5):648–657. [DOI] [PubMed] [Google Scholar]
- 42. Wysocki J, Ye M, Soler MJ, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132–2139. [DOI] [PubMed] [Google Scholar]
- 43. Wosten-van Asperen RM, Lutter R, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–627. [DOI] [PubMed] [Google Scholar]
- 44. Dai R, Phillips RA, Karpuzoglu E, Khan D, Ahmed SA. Estrogen regulates transcription factors STAT-1 and NF-kappaB to promote inducible nitric oxide synthase and inflammatory responses. J Immunol. 2009;183(11):6998–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hernandez Prada JA, Ferreira AJ, et al. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51(5):1312–1317. [DOI] [PubMed] [Google Scholar]
- 46. Qi Y, Zhang J, Cole-Jeffrey CT, et al. Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension. 2013;62(4):746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lloyd LJ, Foster T, Rhodes P, Rhind SM, Gardner DS. Protein-energy malnutrition during early gestation in sheep blunts fetal renal vascular and nephron development and compromises adult renal function. J Physiol. 2012;590(2):377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74(5):953–965. [PubMed] [Google Scholar]
- 49. Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123(5):2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandnberg ME, Smith LJ, Correa-Rotter R, Hostetter TH. The paradox of the renin–angiotensin system in chronic renal disease. Kidney Int. 1994;45(2):403–410. [DOI] [PubMed] [Google Scholar]
- 51. Zhong JC, Huang DY, Yang YM, et al. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44(6):907–912. [DOI] [PubMed] [Google Scholar]
- 52. Hilliard LM, Sampson AK, Brown RD, Denton KM. The “his and hers” of the renin–angiotensin system. Curr Hypertens Rep. 2013;15(1):71–79. [DOI] [PubMed] [Google Scholar]
- 53. Sandberg K. Mechanisms underlying sex differences in progressive renal disease. Gend Med. 2008;5(1):10–23. [DOI] [PubMed] [Google Scholar]
- 54. Lankadeva YR, Singh RR, Tare M, Moritz KM, Denton KM. Loss of a kidney during fetal life: long-term consequences and lessons learned. Am J Physiol Renal Physiol. 2014;306(8):F791–F800. [DOI] [PubMed] [Google Scholar]
- 55. Gilbert RE, Wu LL, Kelly DJ, et al. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol. 1999;155(2):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harris RC, Cheng HF. The intrarenal renin–angiotensin system: a paracrine system for the local control of renal function separate from the systemic axis. Exp Nephrol. 1996;4(suppl 1):2–7. [PubMed] [Google Scholar]
- 57. Schulman IH, Zhou MS, Treuer AV, Chadipiralla K, Hare JM, Raij L. Altered renal expression of angiotensin II receptors, renin receptor, and ACE-2 precede the development of renal fibrosis in aging rats. Am J Nephrol. 2010;32(3):249–261. [DOI] [PubMed] [Google Scholar]
- 58. Vio CP, Jeanneret VA. Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int Suppl. 2003;(86):S57–S63. [DOI] [PubMed] [Google Scholar]
- 59. Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77(6):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lafayette RA, Mayer G, Park SK, Meyer TW. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992;90(3):766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. [DOI] [PubMed] [Google Scholar]
- 62. Rose e M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17(11):3067–3075. [DOI] [PubMed] [Google Scholar]
- 63. Oudit GY, Herzenberg AM, Kassiri Z, et al. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168(6):1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50(4):596–599. [DOI] [PubMed] [Google Scholar]
- 65. Liu CX, Hu Q, Wang Y, et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17(1-2):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1-7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55(1):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172(5):1174–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tikellis C, Johnston CI, Forbes JM, et al. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41(3):392–397. [DOI] [PubMed] [Google Scholar]