Abstract
Objective:
The aim of this study was to evaluate whether ubiquitin-specific peptidase 26 (USP26) gene variations were associated with nonobstructive azoospermia (NOA).
Methods:
Seven hundred and seventy-six patients diagnosed with NOA and 709 proven fertile men were included in this study. Genetic variations of infertility-related genes, including USP26, were identified by selected exonic sequencing. The effects of USP26 mutations on androgen receptor (AR) binding, ubiquitination, and transcriptional activity were detected by immunoprecipitation and luciferase assay in Hela and TM4 cells.
Results:
Six novel missense mutations and 1 novel synonymous mutation of USP26 unique to the patients with NOA were identified. Of these missense mutations, USP26 R344W remarkably reduced the binding affinity and deubiquitinating activity of USP26 to AR, thus eliminated the inhibitory effect of USP26 on transcriptional activity of AR in Hela and TM4 cells.
Conclusion:
A novel USP26 variant p.R344W is associated with NOA probably through affecting AR function.
Keywords: nonobstructive azoospermia, USP26, androgen receptor
Background
Nonobstructive azoospermia (NOA) is characterized by the absence of spermatozoa in the ejaculate due to testicular dysfunction, affecting 60% of azoospermia men.1,2 The etiology of NOA includes genetic defects and environmental causes.2–4 More and more genes associated with NOA have been reported through familiar case reports and mouse model studies.5–8 However, the molecular basis of NOA is still poorly understood.
Ubiquitin-specific peptidase 26 (USP26) is an X-linked gene, encoding a deubiquitinating enzyme (DUB).9 In mouse testis, USP26 is highly expressed in spermatogonia, preleptotene spermatocyte, round spermatids, and at the blood–testis barrier.10 In human, USP26 is also expressed in Leydig cells and Sertoli cells and colocalizes with androgen receptor (AR).11 Ubiquitin-specific peptidase 26 has been reported to act as a regulator of AR hormone-induced action involved in spermatogenesis and steroid production in in vitro studies.12 It was reported that USP26 contained 3 important nuclear receptor interaction motifs and could bind to AR through these motifs to modulate AR ubiquitination and transcriptional activity.12 Prior mutation screening has demonstrated that several USP26 gene variations were associated with male infertility, yet few functional studies have been performed.13–16 No causative mutation in USP26 has been confirmed in infertile men.
Given the findings above, we speculate that genetic variants of USP26 that affect AR function confer high susceptibility to NOA. In this study, selected exonic sequencing was performed to identify mutations of USP26 among 766 patients with NOA and 709 controls. In addition, bioinformatic analysis combined with functional studies was conducted to systematically assess the effects of these mutations on USP26 function.
Methods
Participants
A total of 1880 patients with azoospermia were recruited for this study from the Center of Reproductive Medicine, Tongji Medical College, Huazhong University of Science and Technology, from January 2007 to October 2011.6–8 Among them, 776 Han Chinese patients fulfilled the following criteria for NOA diagnosis: (1) no sperm detected in the pellets of semen samples on 3 different occasions; (2) no obstruction, inflammation, or injury of the reproductive system or pelvic cavity; and (3) no karyotypic abnormality or Y chromosome microdeletion.6–8 A total of 709 fertile Han Chinese men from the Center of Physical Examination, Peking University Shenzhen Hospital, were recruited as controls who had fathered at least 1 child without assisted reproductive techniques, such as in vitro fertilization, intracytoplasmic sperm injection, or intracytoplasmic morphologically selected sperm injection.6–8 After a panel resequencing study and quality control steps, 776 patients aged 24 to 46 years (average of 30.6 years) and 709 fertile men aged 29 to 51 years (average of 35.6 years) were available for further analysis.6–8 This study was approved by the ethics committee of Peking University Shenzhen Hospital and Tongji Medical College in accordance with the Declaration of Helsinki. Informed, written consents were obtained from all participants.
Sequencing and Mutational Analysis of USP26 Gene
The selected exonic sequencing and data analysis were performed in Beijing Genomics Institute at Shenzhen as described in detail previously.6–8 Briefly, genomic DNA was extracted using the AllPrep DNA/RNA Mini Kit (Qiagen, Germantown, Maryland). The exon capture was performed using NimbleGen custom array (Roche NimbleGen, Madison, Wisconsin). The sequencing (paired-end 90-base pair reads) was performed on an Illumina Hiseq 2000 platform (Illumina, San Diego, California) using recommended protocols from the manufacturer. After removing the low-quality bases and adaptor sequences, the sequencing reads were aligned against the human reference genome (NCBI build 37.1, hg19) using the SOAPaligner software (2.21).6
Variations within USP26 were further validated by polymerase chain reaction (PCR) and Sanger sequencing (Invitrogen, Shanghai, China) with the following 4 sets of primers: AAAACATGGCTGCCCTATTCCTAC (F1), GTCCCACTTCCTTTTGCTATCTCA (R1); TCATGCATCATGAACACCACTG (F2), CCTGACCACAAGCTTTACAAGC (R2); ACAACAAGGGTATAGTGACG (F3), TTTGGGGAAGGTTGATGG (R3); TGAGTGAGGATGGAGAAATTACAG (F4), TGGTTTTCACATATTTCTTTCGTT (R4).
Plasmid Construction
Human USP26 complementary DNA (cDNA) obtained from GeneCopoeia (Guangzhou, China) was subcloned into pcDNA3.1-HA and pEGFP-C1 using primers “ATGGCTGCCCTATTCCT” and “TTCCTTCTGAAGGGTC, so that USP26 protein overexpressed could be detected by HA tag antibody or green fluorescent protein (GFP) antibody.” Six missense mutants were obtained by site-directed mutagenesis, as described previously.6,7 Human AR was cloned into pcDNA3.1 expression vector. Human ubiquitin cDNA was synthesized at Sangon Biotech (Shanghai, China) and then inserted into pcDNA3.1-HA.
Cell Culture and Transfection
Hela and TM4 cells (ATCC, Manassas, Virginia) were cultured in Dulbecco modified Eagle medium (Gibco BRL, Gaithersburg, Maryland) supplemented with 10% fetal bovine serum (Gibco). All transfections were performed transiently using Lipofectamine 2000 (Invitrogen, Carlsbad, California) according to the manufacturer’s guidelines.
Luciferase Assay
Hela and TM4 cells were transfected with pcDNA3.1-AR, pMMTV-Luc (a firefly luciferase reporter gene under the control of mouse mammary tumor virus long terminal repeat), pRL-TK (Renilla luciferase gene driven by HSV-thymidine kinase promoter), and HA-USP26 (wild-type USP26 fused with HA tag) or mutant expression vectors. Cells were treated with 10 nmol/L testosterone 6 hours after transfection. Twenty-four hours later, cells were harvested and the expression of firefly and Renilla luciferase was assessed by Dual-Luciferase Reporter Assay System (E1910; Promega, Madison, Wisconsin), according to the manufacturer’s protocols, with a Modulus single-tube multimode reader (Turner Biosystems Inc., Sunnyvale, CA, USA). The Renilla luciferase activity was normalized to that of firefly luciferase. After normalization for transfection efficiency, induction factors were calculated as the ratio of the average of the luciferase luminescence value for the testosterone-stimulated samples versus the nonstimulated (ethanol vehicle treated) samples.6,7
Immunoprecipitation
For coimmunoprecipitation, EGFP-USP26 and AR were cotransfected into Hela cells. Thirty-six hours after transfection, cells were treated with 10 μg/mL MG132 (Sigma-Aldrich, St Louis, Missouri) and 10 nmol/L testosterone overnight.12 Then, cells were lysed in radioimmunoprecipitation assay buffer (with phenylmethanesulfonyl fluoride and proteases inhibitor cocktail; Beyotime, Shanghai, China) and centrifugated at 12 000 rpm for 30 minutes. Supernatants were incubated under gentle rotation with Dynabeads (Life Technologies AS, Oslo, Norway) prebound with anti-GFP antibody (sc-9996; Santa Cruz Biotechnology, TX, USA) or anti-AR antibody (ab74272; Abcam, Cambridge, UK) for 1 hour. Normal immunoglobulins G (mouse sc-2025, rabbit sc-2027; Santa Cruz Biotechnology) were used as controls. Beads were washed 3 times with lysis buffer and then boiled with sodium dodecyl sulfate (SDS) loading buffer for SDS polyacrylamide gel electrophoresis (SDS-PAGE).
For detection of AR ubiquitination, EGFP-USP26, AR, and ubiquitin-HA expression vectors were transfected into Hela cells. Thirty-six hours after transfection, cells were incubated with 10 μg/mL MG132 overnight and then treated with 10nmol/L testosterone for another 2 hours.12 Cells were lysed and immunoprecipitated with anti-AR antibody as described before; the immunoprecipitated AR was detected with anti-HA antibody (Sigma-Aldrich). All experiments were repeated at least 3 times.
Western Blot
Protein samples were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. Then, membranes were blocked with 5% nonfat milk in TBST buffer (20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.1% Tween 20) and incubated with anti-GFP antibody or anti-AR antibody at 4°C overnight. After washing 3 times with TBST buffer, membranes were incubated with horseradish peroxidase-labeled secondary antibody (Abgent, San Diego, California) at room temperature for 1 hour. The protein bands were visualized by chemiluminescence using enhanced chemiluminescence (ECL) reagents (WBKLS0100; Millipore Corporation, Billerica, Massachusetts).
Statistical Analysis
Data are shown as the mean ± standard error of the mean (SEM) of values obtained in at least 3 independent experiments. The statistical significance of the differences between 2 groups was determined by Student’s t test using SPSS 17.0 statistical software. Statistical significance was set at P < .05.
Results
Identification of USP26 Mutations in Patients With NOA
Massively parallel sequencing had been performed to screen variations in 654 infertility-related genes potentially associated with NOA,6–8 including USP26. As shown in Table 1, 8 missense mutations and 2 synonymous mutations were identified in USP26. Of these 8 missense mutations, except for c.957T>C and c.779 C>T, 6 other missense mutations were not observed in 709 fertile men. Clinical data about patients with NOA harboring these mutations are listed in Table 2. Patients (W173, W1575) carrying R344W mutation had normal hormone levels, except follicle-stimulating hormone level was slightly higher in 1 patient (W173). Spermatogenesis was arrested at spermatocytes and no sperm could be detected in the testis of 1 patient (W173) harboring USP26 R344W mutation, as revealed by histological analysis (Figure 1).
Table 1.
Mutations of USP26 Identified in Patients With NOA and Controls.
| No | Genomic Location | Nucleotide Variants | Amino Acid Changes | Patients (n = 776) | Fertile Men (n = 709) | Allele Frequency in ExAC |
|---|---|---|---|---|---|---|
| Missense mutations | ||||||
| 1 | ChrX:133026284 | c.1989 C>T | p.R646Q | 1 | 0 | 0 |
| 2 | ChrX:133026314 | c.1959T>C | p.N636S | 1 | 0 | |
| 3 | ChrX:133027144 | c.1129 C>T | p.M359I | 1 | 0 | |
| 4 | ChrX:133027191 | c.1082 G>A | p.R344W | 2 | 0 | 0.0007588 |
| 5 | ChrX:133027316 | c.957T>C | p.N302S | 0 | 1 | |
| 6 | ChrX:133027494 | c.779 C>T | p.A243T | 0 | 2 | 0.001057 (rs185719820) |
| 7 | ChrX:133027908 | c.365 C>T | p.E105K | 1 | 0 | 0.0006045 |
| 8 | ChrX:133028096 | c.177 A>G | p.F42S | 1 | 0 | 0.000151 |
| Synonymous mutations | ||||||
| 9 | ChrX:133027965 | c.308 A>G | None | 1 | 0 | |
| 10 | ChrX:133028109 | c.170 G>A | None | 0 | 1 | |
Abbreviations: NOA, nonobstructive azoospermia; USP26, ubiquitin-specific peptidase 26; ExAC, the exome aggregation consortium.
Table 2.
Clinical Information of Patients With NOA Having Missense Mutations in USP26.
| Nucleotide Changes | Sample IDs | Age | Testicular Volume, mL | FSH (1.5-12.5), mIU/mL | LH (1.7-8.6), mIU/mL | T (2.5-8.0), ng/mL |
|---|---|---|---|---|---|---|
| c.177 A>G | W492 | 28 | 10 | 20.42 | 9.31 | 2.87 |
| c.365 C>T | W659 | 34 | 5 | 14.80 | 3.80 | 2.10 |
| c.1082 G>A | W173 | 32 | 12 | 15.10 | 6.20 | 3.80 |
| c.1082 G>A | W1575 | 31 | 15 | 11.90 | 2.90 | NA |
| c.1129 C>T | W168 | 27 | 8 | 18.20 | 3.70 | NA |
| c.1959T>C | W385 | 29 | 5 | 23.14 | 10.38 | 3.17 |
| c.1989 C>T | W718 | 26 | 12 | 17.59 | 10.55 | 3.44 |
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; NA, not available; NOA, nonobstructive azoospermia; T, testosterone; USP26, ubiquitin-specific peptidase 26.
Figure 1.
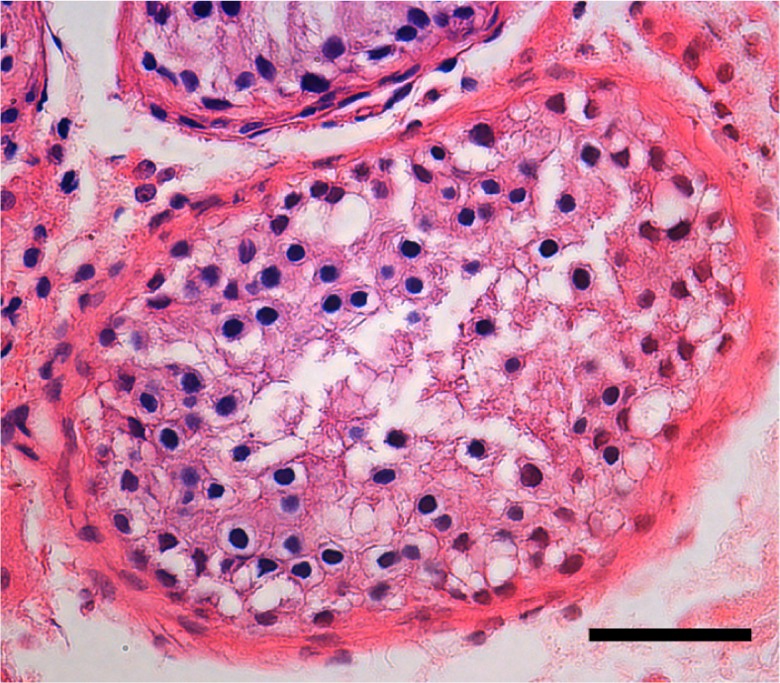
Morphology of testes from 1 patient harboring ubiquitin-specific peptidase 26 (USP26) R344W mutation by hematoxylin and eosin staining. The spermatogenesis was arrested at spermatocytes. Bar: 50 μm.
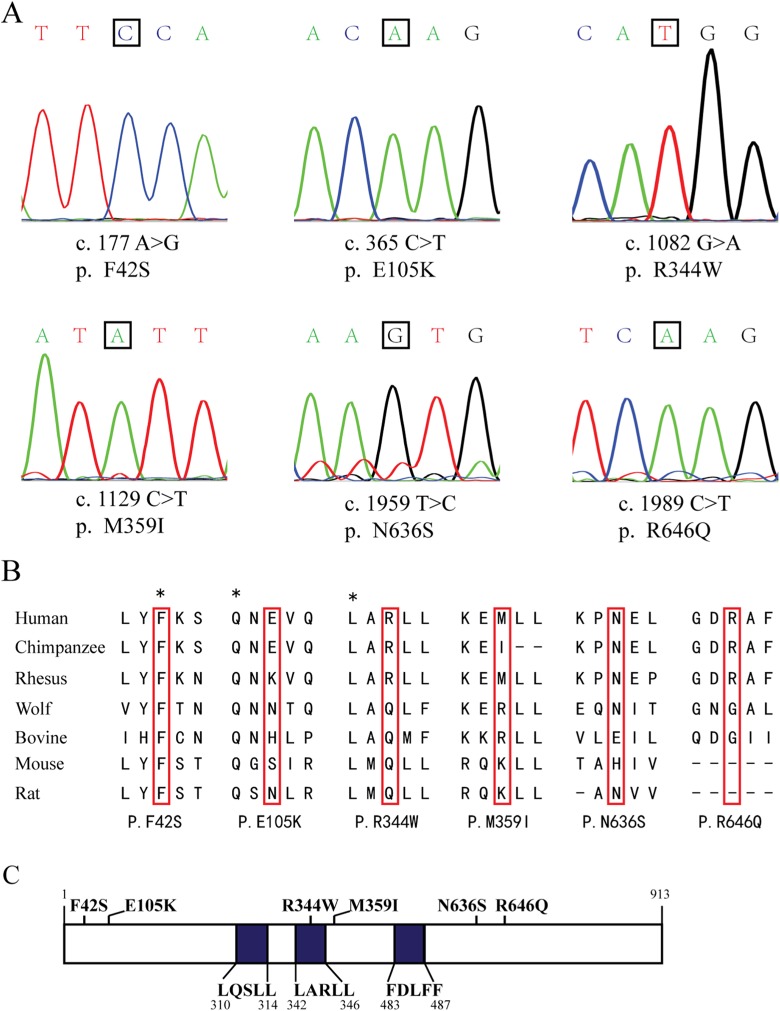
These 6 missense mutations were further validated in corresponding individuals using PCR and Sanger sequencing (Figure 2A). The evolutionary conservation analysis by multiple sequence alignments of USP26 protein and its homologues showed that amino acids at 42 and 344 were evolutionarily conserved, whereas amino acids affected by other 4 mutations were hardly conserved (Figure 2B). Finally, the impact of the 6 missense mutations on USP26 protein was assessed by Polymorphism Phenotyping v2 (PolyPhen-2)17 and Sorting Intolerant From Tolerant (SIFT).18 When USP26 R344W mutation occurred, an alkaline amino acid located at the binding motif was substituted into an aromatic one occupying larger volume (Figure 2C). The results indicated that p.E105K, p.M359I, p.N636S, and p.R646Q mutations were tolerable, but p.F42S and p.R344W mutations were probably damaging (Table 3).
Figure 2.
Missense mutations of ubiquitin-specific peptidase 26 (USP26) identified in patients with nonobstructive azoospermia (NOA). A, Six missense mutations were validated by polymerase chain reaction (PCR) and Sanger sequencing. Locations of the mutations were marked with black box. B, The evolutionary conservation was shown for each amino acid altered by these mutations through multiple protein sequence alignments. C, Six missense mutations were labeled in the schematic diagram of USP26 protein, with the binding motifs of USP26 protein to AR highlighted in blue.
Table 3.
Effects of USP26 Missense Mutations on the Protein Function Predicted by PolyPhen-2 and SIFT.
| No. | Nucleotide Changes | Amino Acid Changes | PolyPhen-2 | SIFT |
|---|---|---|---|---|
| 1 | c.177 A>G | p.F42S | Probably damaging | Damaging |
| 2 | c.365 C>T | p.E105K | Benign | Tolerated |
| 3 | c.1082 G>A | p.R344W | Probably damaging | Damaging |
| 4 | c.1129 C>T | p.M359I | Possibly damaging | Tolerated |
| 5 | c.1959T>C | p.N636S | Benign | Tolerated |
| 6 | c.1989 C>T | p.R646Q | Possibly damaging | Tolerated |
Abbreviations: USP26, ubiquitin-specific peptidase 26; PolyPhen-2, polymorphism phenotyping v2; SIFT, sorting intolerant from tolerant.
Ubiquitin-Specific Peptidase 26 R344W Severely Diminished the Interaction Between USP26 and AR
Ubiquitin-specific peptidase 26 was reported to interact with AR, modulate AR ubiquitination, and consequently regulate transcriptional activity of AR.12 To further explore the impact of these mutations on USP26 function, coimmunoprecipitation was first performed to assess the binding ability of USP26 mutants to AR. In Figure 3, GFP antibody was used to immunoprecipitate AR, and the coimmunoprecipitated AR was detected with AR antibody. The results showed that all other 5 EGFP-USP26 mutants, including F42S, E105K, M359I, N636S, and R646Q, could coimmunoprecipitate AR as well as wild-type EGFP-USP26, but EGFP-USP26 R344W could not. This was further demonstrated by the reverse experiment in which AR antibody was used to pull down EGFP-USP26. These results demonstrated that USP26 R344W mutation disrupted the interaction between USP26 and AR.
Figure 3.
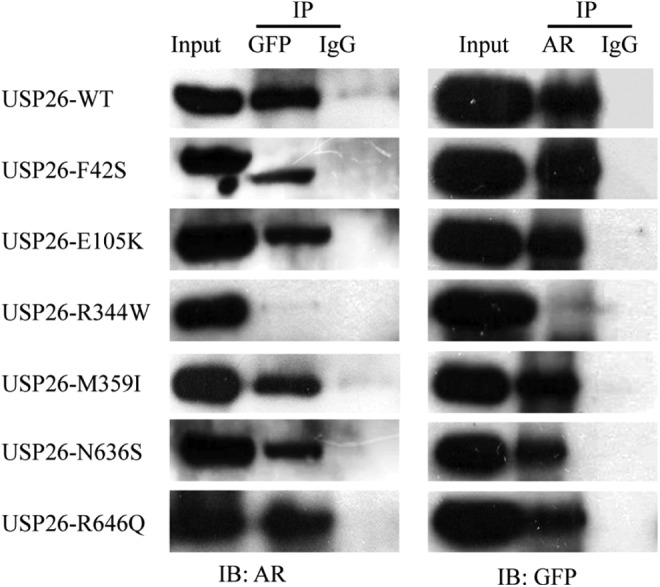
Ubiquitin-specific peptidase 26 (USP26) R344W disrupted the interaction between USP26 and androgen receptor (AR). Hela cells were cotransfected with AR and wild-type or mutant EGFP-USP26 expression vectors, as indicated. On the left panel, immunoprecipitation was performed with green fluorescent protein (GFP) antibody, and the coimmunoprecipitated AR was detected with AR antibody. On the right panel, immunoprecipitation was performed with AR antibody, and the coimmunoprecipitated USP26 was detected with GFP antibody. Immunoglobulin G (IgG) was used as control. The results showed that except R344W, all other 6 USP26 constructs could bind to AR. Abbreviation: EGFP, enhanced green fluorescent protein.
Ubiquitin-Specific Peptidase 26 R344W Reduced the Deubiquitinating Activity of USP26 Toward AR
Then, to test whether these mutations affected deubiquitinating activity of USP26, ubiquitination profiles of AR were investigated by immunoprecipitation. HA antibody was used to detect ubiquitinated AR. As shown in Figure 4, the polyubiquitinated AR bands were clearly visualized when EGFP-USP26 R344W was overexpressed in Hela cells. Compared with wild-type EGFP-USP26, the deubiquitinating activity of EGFP-USP26 R344W was significantly decreased, however, no significant difference was observed in other 5 mutants. These results showed that USP26 R344W mutation significantly reduced the deubiquitinating activity of USP26 toward AR.
Figure 4.
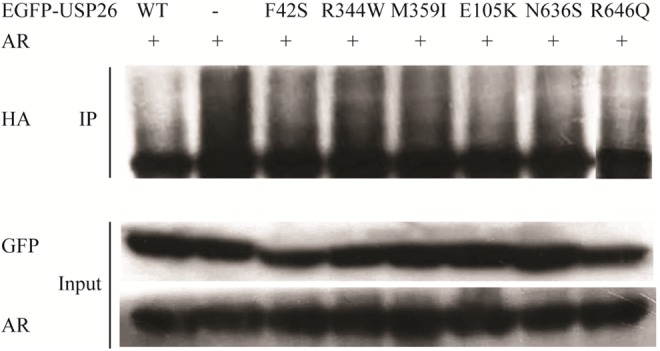
Ubiquitin-specific peptidase 26 (USP26) R344W reduced deubiquitinating activity of USP26 to androgen receptor (AR). Hela cells were transfected with AR, ubiquitin-HA, wild-type or mutant EGFP-USP26 expression vectors, as indicated. Androgen receptor ubiquitination was assessed with HA antibody. Compared with wild-type USP26 and other mutants, the deubiquitinating activity of USP26 R344W was significantly reduced. Abbreviation: EGFP, enhanced green fluorescent protein.
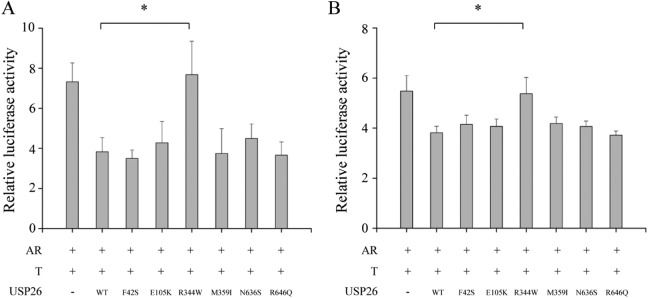
Ubiquitin-Specific Peptidase 26 R344W Failed to Repress the Transcriptional Activity of AR
Finally, in order to analyze whether these mutations eliminated the regulatory effect of USP26 on AR function, luciferase activity driven by AR-responsive MMTV promoter was measured. As shown in Figure 5, both in Hela and TM4 cells, wild-type USP26-HA repressed the transcriptional activity of AR, compared with that in the absence of USP26-HA. The inhibitory effect on AR activity of USP26 R344W was diminished, while no change was observed in other 5 mutants. This functional assay revealed that USP26 R344W mutation eliminated the inhibitory effect of USP26 on AR activity.
Figure 5.
Ubiquitin-specific peptidase 26 (USP26) R344W eliminated the inhibitory effect of USP26 on androgen receptor (AR) function. The AR expression vector, testosterone-inducible pMMTV-Luc (a firefly luciferase reporter gene under the control of mouse mammary tumor virus long terminal repeat), and pRL-TK (Renilla luciferase gene driven by HSV-thymidine kinase promoter) vector were transfected with Hela cells (A) and TM4 (B) cells with either wild-type or mutant HA-USP26 expression vectors, as indicated. Luciferase assay was performed to evaluate the transcriptional activity of AR. Induction factors were calculated as the ratios of the average luciferase value of the testosterone-stimulated samples versus ethanol vehicle-treated samples. Compared with wild-type USP26 and other 5 mutants, USP26 R344W failed to repress AR activity. T bars represented the standard error of the mean (SEM). Bars marked with asterisks showed significant differences (P < .05).
Discussion
Spermatogenesis is a complex yet delicate process, in which spermatogonia must go through mitosis, meiosis, and spermiogenesis to become elongated spermatid. Cumulative evidence shows that genetic defects are main causes of abnormal spermatogenesis, one etiology of NOA.2–8 Thus, massively parallel sequencing was performed to identify genetic abnormalities in a large cohort of patients with NOA and controls, and lots of genetic variations have been found in male infertility-related genes, including USP26.8 Ubiquitin-specific peptidase 26 gene is located on X chromosome, which limits it as a single allele in an individual.9 Ubiquitin-specific peptidase 26 protein belongs to a large family of DUBs, which play an important role in diverse cellular activities and protein turnover during spermatogenesis.19 In addition, USP26 was initially reported as a testis-specific gene in both human and mouse.9 These features above make it as an excellent candidate gene to study male infertility.
About 20 variations in USP26 have been reported to be associated with male infertility.20 Among these, several frequent mutations, including c.363_364insACA, c.494T>C, and c.1423C>T cluster halotype, were reported to be significantly associated with azoospermia and severe oligozoospermia.21–23 However, results from other investigators are conflicting,24,25 probably due to different ethnic origins and number of patient samples, as well as different infertility definition of investigators. In this study, both patients and controls were from the same ethnic origin and selected after strict quality control as described before. Six novel missense mutations in USP26 were identified among 766 Han Chinese patients with NOA, which were absent in 709 controls.
Androgen receptor signaling pathway is essential for the maintenance of spermatogenesis. Posttranslational modifications, including phosphorylation, acetylation, and ubiquitination, could influence AR signaling at all levels.12 Ubiquitination modifications of AR, which is specifically regulated by ubiquitin ligases and DUBs, function as molecular switches regulating transcription of target genes. Transformed mouse 3T3 cell double minute 2 (Mdm2) was reported to mediate multiple monoubiquitination of AR, which triggered transcriptional activation within hours of hormone stimulation, and polyubiquitination of AR, which was to be degraded after a further delay.26 Ring finger protein 6 mediated K6/K27 mixed ubiquitination of AR, and this kind of modification was proposed not to degrade AR but serve as a scaffold recruiting cofactors.27 But, very little is known about DUBs that deubiquitinated AR. USP26 is one DUB that has been recognized as a regulator of AR signaling pathway through regulating AR ubiquitination.12
Ubiquitin-specific peptidase 26 was reported to physically interact with AR through 3 important nuclear receptor interaction motifs, deubiquitinate polyubiquitinated AR, and stimulate AR signaling in HEK293 and LnCap cells.12 However, our results showed that USP26 repressed AR signaling in Hela and TM4 cells, which was consistent with results reported in HepG2 cells.12 Discrepancies of AR cofactor function in different cell lines were observed previously, yet no functional explanation has been given.28 As reviewed by Aoyagi and Archer, the differences in timing of USP26 deubiquitination activity could be caused by variations in the composition or dynamics of assembly of multicomponent AR transcriptional complexes in the examined cell lines.12,29
Ubiquitin-specific peptidase 26 R344W mutation was detected in 2 patients with NOA in this study (Table 1). Bioinformatic analysis indicated that this mutation was damaging. When USP26 R344W mutation occurred, an alkaline amino acid was substituted into an aromatic one occupying larger volume, which would probably influence the structure of USP26. In addition, the mutation site is located in the binding motif between USP26 and AR, raising the possibility of deleterious effect of USP26 R344W mutation. Functional assays proved that the interaction of USP26 with AR was severely diminished, which resulted in that polyubiquitination of AR could not be removed. In Hela cells, polyubiquitinated AR probably was not to be degraded but act as a scaffold to recruit coactivators.27 Consequently, compared with wild-type USP26, USP26 R344W failed to repress AR activity, which was probably the reason why spermatogenesis was impaired in patients with NOA carrying USP26 R344W mutation.
Five other USP26 mutations neither affected AR binding, ubiquitination, or AR activity. However, it can’t rule out the possibility that they are associated with NOA. One possible explanation is the insensitivity of pMMTV-Luc promoter to those mutations not so devastating.6 It also should be noted that these functional assays were performed in vitro and might not sufficiently reflect functions of USP26 in vivo. Thus, further studies are still needed to elucidate the effect of these mutations on the subtle changes in the structure and other unknown functions of USP26.
In summary, our study evaluated the association between USP26 variations and NOA in a large cohort of Han Chinese patients and controls. These results showed that USP26 R344W mutation was associated with NOA through affecting transcriptional activity of AR. This study advances our understanding of the molecular basis of NOA. Further investigations focusing on the transcriptional network of AR regulated by USP26 during spermatogenesis will be of great importance to elucidate the etiology of abnormal spermatogenesis such as found in NOA.
Acknowledgments
The authors thank the patients and the family members for their cooperation during the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (31471344, 81501311), China Postdoctoral Science Foundation (grant 2015M572344), Guangdong Natural Science Foundation of China (2015A030310029), and Shenzhen Foundation of Science and Technology (JCYJ20150403110829616).
References
- 1. Jarvi K, Lo K, Fischer A, et al. CUA guideline: the workup of azoospermic males. Can Urol Assoc J. 2010;4(3):163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wosnitzer M, Goldstein M, Hardy MP. Review of azoospermia. Spermatogenesis. 2014;4:e28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferlin A, Raicu F, Gatta V, et al. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14(6):734–745. [DOI] [PubMed] [Google Scholar]
- 5. Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11(6):415–425. [DOI] [PubMed] [Google Scholar]
- 6. Mou L, Xie N, Yang L, et al. A novel mutation of DAX-1 associated with secretory azoospermia. PLoS One. 2015;10(7):e0133997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mou L, Zhang Q, Diao R, Cai Z, Gui Y. A functional variant in the UBE2B gene promoter is associated with idiopathic azoospermia. Reprod Biol Endocrinol. 2015;13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Huang Y, Li H, et al. Excess of rare variants in genes that are key epigenetic regulators of spermatogenesis in the patients with non-obstructive azoospermia. Sci Rep. 2015;5:8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27(4):422–426. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Tian H, Huo YW, et al. The expression of Usp26 gene in mouse testis and brain. Asian J Androl. 2009;11(4):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wosnitzer MS, Mielnik A, Dabaja A, et al. Ubiquitin specific protease 26 (USP26) expression analysis in human testicular and extragonadal tissues indicates diverse action of USP26 in cell differentiation and tumorigenesis. PLoS One. 2014;9(6):e98638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dirac AM, Bernards R. The deubiquitinating enzyme USP26 is a regulator of androgen receptor signaling. Mol Cancer Res. 2010;8(6):844–854. [DOI] [PubMed] [Google Scholar]
- 13. Stouffs K, Lissens W, Tournaye H, Van Steirteghem A, Liebaers I. Possible role of USP26 in patients with severely impaired spermatogenesis. Eur J Hum Genet. 2005;13(3):336–340. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Qiu SD, Li SB, et al. Novel mutations in ubiquitin-specific protease 26 gene might cause spermatogenesis impairment and male infertility. Asian J Androl. 2007;9(6):809–814. [DOI] [PubMed] [Google Scholar]
- 15. Ribarski I, Lehavi O, Yogev L, et al. USP26 gene variations in fertile and infertile men. Hum Reprod. 2009;24(2):477–484. [DOI] [PubMed] [Google Scholar]
- 16. Asadpor U, Totonchi M, Sabbaghian M, et al. Ubiquitin-specific protease (USP26) gene alterations associated with male infertility and recurrent pregnancy loss (RPL) in Iranian infertile patients. J Assist Reprod Genet. 2013;30(7):923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11(5):863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suresh B, Lee J, Hong SH, Kim KS, Ramakrishna S. The role of deubiquitinating enzymes in spermatogenesis. Cell Mol Life Sci. 2015;72(24):4711–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stouffs K, Tournaye H, Liebaers I, Lissens W. Male infertility and the involvement of the X chromosome. Hum Reprod Update. 2009;15(6):623–637. [DOI] [PubMed] [Google Scholar]
- 21. Paduch DA, Mielnik A, Schlegel PN. Novel mutations in testis-specific ubiquitin protease 26 gene may cause male infertility and hypogonadism. Reprod Biomed Online. 2005;10(6):747–754. [DOI] [PubMed] [Google Scholar]
- 22. Lee IW, Kuan LC, Lin CH, et al. Association of USP26 haplotypes in men in Taiwan, China with severe spermatogenic defect. Asian J Androl. 2008;10(6):896–904. [DOI] [PubMed] [Google Scholar]
- 23. Xia JD, Chen J, Han YF, et al. Association of 370-371insACA, 494T>C, and 1423C>T haplotype in ubiquitin-specific protease 26 gene and male infertility: a meta-analysis. Asian J Androl. 2014;16(5):720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ravel C, El Houate B, Chantot S, et al. Haplotypes, mutations and male fertility: the story of the testis-specific ubiquitin protease USP26. Mol Hum Reprod. 2006;12(10):643–646. [DOI] [PubMed] [Google Scholar]
- 25. Zhang W, Liu T, Mi YJ, et al. Evidence from enzymatic and meta-analyses does not support a direct association between USP26 gene variants and male infertility. Andrology. 2015;3(2):271–279. [DOI] [PubMed] [Google Scholar]
- 26. Gaughan L, Logan IR, Neal DE, Robson CN. Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 2005;33(1):13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu K, Shimelis H, Linn DE, et al. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer Cell. 2009;15(4):270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotaja N, Aittomaki S, Silvennoinen O, Palvimo JJ, Janne OA. ARIP3 (androgen receptor-interacting protein 3) and other PIAS (protein inhibitor of activated STAT) proteins differ in their ability to modulate steroid receptor-dependent transcriptional activation. Mol Endocrinol. 2000;14(12):1986–2000. [DOI] [PubMed] [Google Scholar]
- 29. Aoyagi S, Archer TK. Dynamics of coactivator recruitment and chromatin modifications during nuclear receptor mediated transcription. Mol Cell Endocrinol. 2008;280(1-2):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]