Abstract
We have recently shown that platelets play important roles in development of endometriosis and proposed that endometriotic lesions are essentially wounds that undergo repeated tissue injury and repair (ReTIAR). Further investigation indicated that endometriotic lesions, stimulated by platelet-derived transforming growth factor β1 (TGF-β1), activate the TGF-β1/Smad3 signaling pathway and undergo epithelial–mesenchymal transition (EMT) and fibroblast-to-myofibroblast transdifferentiation (FMT), resulting in increased cellular contractility and collagen production and increased smooth muscle metaplasia (SMM), leading to fibrosis. Using serially dissected endometriotic tissue samples from baboons with induced endometriosis, we tested the hypothesis of progressive EMT, FMT, SMM, and fibrosis through TGF-β1/Smad activation using immunohistochemistry and immunoflurescence staining analyses. We found that platelets are aggregated in endometriotic lesions, and vimentin expression was increased in the epithelial compartment of the lesions as they progressively developed. We also found that the number of smooth muscle cells (SMCs) appeared to increase with time as lesions progressed and was concomitant with the increased vimentin-positive glandular epithelial cells in the lesions. As lesion development progressed, TGF-β1 and phosphorylated-Smad3 staining was elevated and the number of α-smooth muscle actin-positive myofibroblasts and highly differentiated SMCs increased in the stromal compartment, which correlated with the increasing extent of fibrosis. These results, taken together, provide support for the notion that ReTIAR occurs in the endometriotic lesions, resulting in EMT and FMT, leading to SMM and ultimately fibrosis as lesions progress. Consequently, our data also provide corroborative evidence that platelets drive the EMT and FMT in endometriotic lesions over time, promoting SMM and resulting ultimately in fibrosis in the endometriotic lesions. These findings cast a new light on the natural history of endometriosis which so far has been elusive.
Keywords: baboon, endometriosis, epithelial–mesenchymal transition, fibroblast-to-myofibroblast transdifferentiation, fibrosis, Smad3, smooth muscle metaplasia, TGF-β1
Introduction
Endometriosis, characterized by the presence and growth of endometrial-like tissues outside the uterine cavity, is an estrogen-dependent disorder and a major contributor to pelvic pain and subfertility affecting 6% to 10% of reproductive-age women.1 Despite exponential growth in the number of publications on endometriosis in the last 4 decades,2 our understanding of its pathophysiology is still fragmentary.1 The lack of a clear understanding of the pathophysiology of endometriosis has been a seemingly insurmountable barrier to the development of effective, targeted therapy or preventative measures for this debilitating disease, especially in the development of nonhormonal drugs.2
One apparent hurdle to the elucidation of the pathophysiology of endometriosis is the lack of understanding of the natural history of endometriosis development. By the time a patient seeks medical attention due to overt endometriosis-related symptoms, the endometriotic lesions would have already been in her system for some time. In fact, given the well-documented diagnostic delay of about 4 to 12 years from the onset of first endometriosis-related symptoms to the definitive diagnosis,3–5 the endometriotic lesions at the time of surgery or diagnosis may have already evolved and developed into something that could be quite different from the initiating lesions. Naturally, ethical as well as logistic constraints preclude any serial laparoscopic examination of the developing lesions.
While serial observation of the developing endometriotic lesions in humans is not feasible, the baboon model of endometriosis is quite suitable for serial laparoscopic evaluation on the development of endometriosis.6–8 Like human females, female baboons also menstruate and can develop spontaneous and induced endometriosis.9 As such, the baboon model may well be the best animal model of endometriosis which is a close approximation to the human condition and can be used for the elucidation of endometriosis pathophysiology.10–12 To further understand the underlying causes of endometriotic lesion development, it is important to characterize the fundamental features of lesion development during the entire natural history of endometriosis. For these purposes, the baboon serves as an invaluable model for serial studies.
We have recently shown that platelets play important roles in the development of endometriosis13–15 and proposed that endometriotic lesions are essentially wounds that undergo repeated tissue injury and repair (ReTIAR).13,16 Further investigation indicated that, as the result of this ReTIAR, the endometriotic lesions, stimulated by platelet-derived transforming growth factor β1 (TGF-β1), activate the TGF-β1/Smad3 signaling pathway and undergo epithelial–mesenchymal transition (EMT) and fibroblast-to-myofibroblast transdifferentiation (FMT), resulting in increased cellular contractility and collagen production, leading ultimately to fibrosis.45 Prolonged exposure to activated platelets also leads to increased expression of α-smooth muscle actin (α-SMA) as well as markers of differentiated smooth muscle cells (SMCs) in endometriotic stromal cells, which may be responsible for what is termed smooth muscle metaplasia (SMM) that is universally seen in endometriotic lesions.17–20
In light of these findings, we hypothesized that during the progression of endometriosis, we should see indications of the activation of the TGF-β1/Smad3 signaling pathway, progressive EMT, and FMT as evidenced by increased vimentin expression but decreased E-cadherin expression in the epithelial component of the endometriotic lesions. In addition, we should see increased expression of α-SMA, a marker for myofibroblasts and SMCs,21,22 in the stromal component of the lesions. Concomitant with the increased α-SMA expression and other markers of SMCs, we should see progressive increase in SMM as well as fibrotic tissue content in the endometriotic lesions. This serial immunohistochemistry (IHC), immunofluorescence, and histochemistry study was undertaken to test this hypothesis.
Materials and Methods
Animals and Induction of Experimental Endometriosis
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois, Chicago, and Michigan State University. Endometriosis was experimentally induced in female baboons (Papio anubis) by intraperitoneal inoculation with menstrual endometrium on 2 consecutive menstrual cycles, as described previously.11,23 Animals had documented regular menstrual cycles and had not undergone any previous surgeries. Menstrual endometrium was harvested on days 1 to 2 of menses using a Unimar Pipelle (Cooper Surgical Inc, Shelton, Connecticut) immediately prior to laparoscopy. Under laparoscopic guidance, approximately 1 g of menstrual tissue and fluid was deposited from the Pipelle at 4 sites: the pouch of Douglas, the uterine fundus, the cul de sac, and the ovaries. At subsequent menses, the animals underwent a second laparoscopy and endometrial reseeding at the same ectopic sites. The progression of disease was monitored in each animal by laparoscopic evaluation at 3 (n = 6), 6 to 8 (n = 7), 10 to 12 (n = 3), and 15 (n = 6) months after inoculation during the window of uterine receptivity (days 9-11 postovulation in the baboon). After laparoscopic entry, a complete systemic survey of the abdomen and pelvic cavity was performed, and the number, color, and position of each visible lesion were digitally documented.24 The presence of peritoneal fluid, extent of adhesions, level of surface vasculature, scar tissue, and corpora lutea was noted. Following each laparoscopy, a laparotomy was performed, and ectopic lesions were harvested to evaluate progression of fibrosis during endometriotic lesion development.
Immunohistochemistry
Tissue samples were fixed with 10% formalin (w/v) and paraffin embedded. Serial 4-μm sections were obtained from each block, with the first resultant slide being stained with hematoxylin and eosin to confirm pathologic diagnosis, and the subsequent slides for IHC analysis for TGF-β1 (1:50; Abcam, Cambridge, United Kingdom), phosphorylated Smad3 (p-Smad3; 1:50, Bioworld, St Louis Park, Minnesota), E-cadherin (1:400; Cell Signaling Technology, Boston, Massachusetts), α-SMA (1:100, Abcam), desmin (1:100, Abcam), and smooth muscle, myosin heavy chain (SM-MHC; 1:100, Abcam). Routine deparaffinization and rehydration procedures were performed. For antigen retrieval, the slides were heated at 98°C in a citrate buffer (pH6.0) for a total of 30 minutes and cooled to room temperature. The slides were then incubated with the primary antibodies overnight at 4°C. After the slides were rinsed, the horse raddish peroxidae-labeled secondary antibody Detection Reagent (Sunpoly-HII; BioSun Technology Co, Ltd, Shanghai, China) was added and incubated at room temperature for 30 minutes. The bound antibody complexes were stained for 3 to 5 minutes or until appropriate for microscopic examination with diaminobenzidine and then counterstained with hematoxylin (30 seconds) and mounted. Images were obtained with the microscope (Olympus BX53; Olympus, Tokyo, Japan) fitted with a digital camera (Olympus DP73; Olympus). Three to five randomly selected images at 400× magnification of each sample were taken to get a mean optional density value by Image Pro-Plus 6.0 (version 6.0.0.206; Media Cybernetics, Inc, Bethesda, Maryland).
Masson Trichrome Staining
Masson trichrome staining was used for the detection of collagen fibers in tissues. Tissue sections were deparaffinized in xylene and rehydrated in a graded alcohol series and then were immersed in Bouin solution at 37°C for 2 hours. Bouin solution was made with 75 mL of saturated picric acid, 25 mL of 10% formalin (w/v) solution, and 5 mL of acetic acid. Tissue sections were stained using the Masson Trichrome Staining kit (Baso, Wuhan, China) following the manufacturer’s instructions. The areas of the collagen fiber layer stained in blue in proportion to the entire field of the ectopic implants were calculated by the Image Pro-Plus 6.0 (Media Cybernetics, Inc).
Immunofluorescence
Sections were heated for antigen retrieval in the citrate buffer (pH 8.0), and they were incubated with 5% goat serum for blocking nonspecific binding sites. The slides were then incubated overnight at 4°C with primary antibodies, including rabbit anti-human α-SMA (1:100; Abcam), rabbit anti-human CD42b (1:100; Abcam), and mouse anti-human vimentin (1:250; Abcam) antibodies. The slides were incubated with 1:500 AlexaFluor 647-conjugated goat anti-rabbit secondary antibody and 1:500 AlexaFluor 488-conjugated goat anti-mouse secondary antibody (Cell Signaling Technology) for 1 hour at 37°C. Counterstaining of nuclei with DAPI (Beyotime, Shanghai, China) was also performed. Fluorescent slides were examined under a confocal laser scanning microscope (Leica TCS SP5 Confocal Microscope, Solms, Germany) at room temperature and then exported as a TIFF-format digital file. Image-Pro Plus 6.0 was used to process the images (brightness and contrast) and to construct merged images.
Statistical Analysis
The comparison of distributions of continuous variables between or among 2 or more groups was made using the Wilcoxon and Kruskal test, respectively. Pearson correlation coefficient was used when evaluating correlations between the staining levels of 2 markers (both square-root transformed to improve normality, unless stated otherwise). Multivariate linear regression analyses were used to determine whether the time since endometriosis induction was associated with the immunostaining levels or the extent of fibrosis. Since in normal endometrium no fibrotic tissue was found, the percentage of fibrotic content was treated as 0.
A hierarchical cluster analysis was carried out with scaled data and the Euclidean distance as the similarity metric, with the average linkage being the clustering method. The resulting dendrogram was represented as a heat map. A multidimensional scaling analysis was performed to discriminate all baboons used in this study. P values of less than .05 were considered statistically significant. All computations were made with R 3.2.2 (www.r-project.org).25
Results
Evidence Consistent With the Activation of the TGF-β1/Smad3 Signaling Pathway
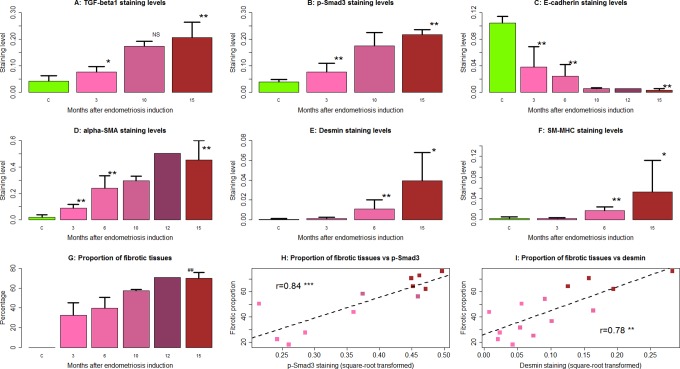
We first performed an IHC analysis of TGF-β1 and p-Smad3 in ectopic and control endometrial samples. We found that TGF-β1 staining was seen mostly in cytoplasm and membranes, while p-Smad3 staining was seen in nuclei as well as cytoplasm in both endometriotic epithelial and stromal cells. The nuclei staining of p-Smad3 appeared to be more prominent as the lesions aged (Figure 1). The immunostaining levels of TGF-β1 and p-Smad3 were progressively elevated as endometriosis progressed (Figures 1 and 2A and B), consistent with the notion that the TGF-β1/Smad3 signaling pathway is activated during the progression of endometriosis. The linear regression analyses indicated that staining levels of both TGF-β1 and Smad3 were positively associated with the time at which the tissue samples were harvested (P = .0002, R 2 = .77 and P = 3.3 × 10−5, R 2 = .83, respectively; Figure 2A and B). The staining levels of TGF-β1 were highly correlated with that of p-Smad3 (r = .90, P = 6.7 × 10−7, both square root transformed to increase normality).
Figure 1.
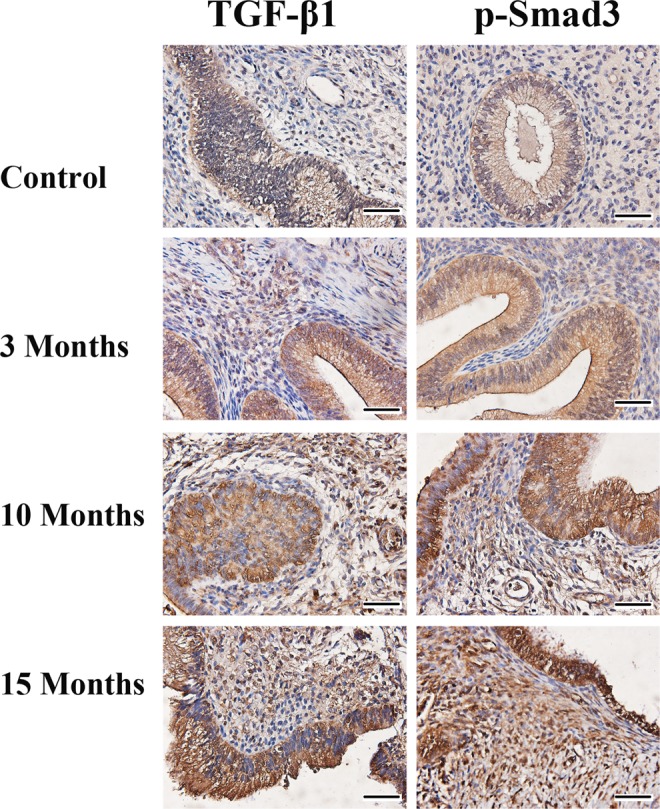
Representative photomicrographs of serial immunohistochemistry analysis of transforming growth factor β1 (TGF-β1) and p-Smad3 in control endometrium and in endometriotic tissue samples harvested at different time points. Magnification: ×400. Scale bar = 50 μm.
Figure 2.
Immunostaining levels of transforming growth factor β1 (TGF-β1) (A), p-Smad3 (B), E-cadherin (C), α-smooth muscle actin (α-SMA; D), desmin (E), smooth muscle, myosin heavy chain (SM-MHC; F), and the percentage of fibrotic content (G) in endometriotic tissue samples harvested from baboons at different time points after the induction of endometriosis. Except E-cadherin, in which staining was quantified in the epithelial component, all staining was quantified in the stromal component. Scatter plot shows the relationship between the extent of fibrosis via Masson trichrome staining and p-Smad3 (H) and desmin (I) immunoreactivity levels in endometriotic lesions. The numbers shown in (H)-(I) are Pearson correlation coefficients, and each dot represents 1 data point, with its color corresponding to the time point at which the tissue sample was taken, as shown in (A)-(G). *P < .05 **; ## P < .01; ***P < 0.001. NS indicates not statistically significant (ie, P > .05). For (A) to (G), the comparison was made with reference to the control endometrium by group-wise Wilcoxon rank-sum test. In (G), the ## refers to the significance level when compared to the proportion at 3 months. Data are represented in mean ± standard deviation (SD).
Immunofluorescence Evidence for EMT and FMT in the Progression of Endometriosis
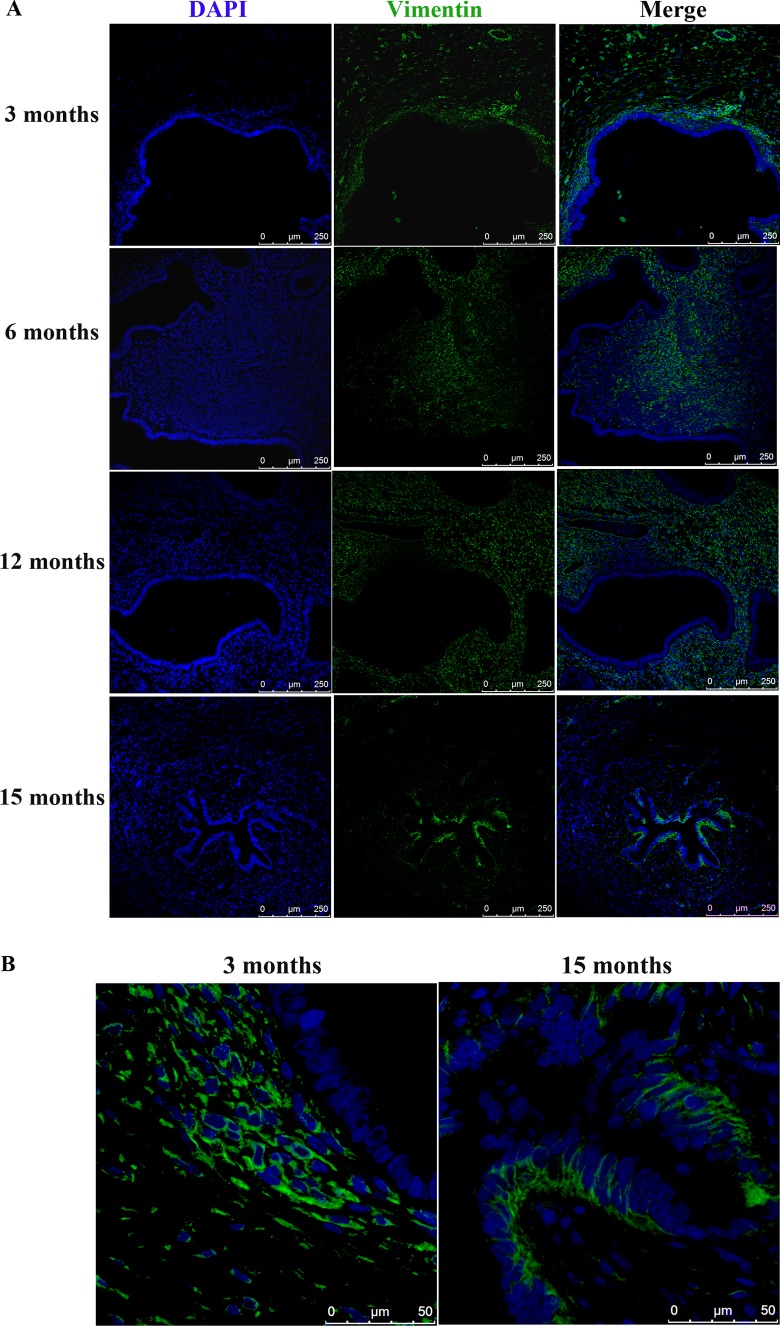
Since one consequence of EMT is the acquirement of mesenchymal markers in epithelial cells, we first examined the expression of vimentin, a mesenchymal marker, in the epithelial compartment in the ectopic endometrium using baboon lesion samples harvested at 3, 6, 12, and 15 months after the induction of endometriosis (n = 2-3 for each sampling point). We found that the glandular epithelial cells did not express vimentin until about 12 months after induction (Figure 3). In 15-month-old lesions, the vimentin-positive epithelial cells were conspicuous, especially compared to lesion samples obtained at 3 months (Figure 3B).
Figure 3.
(A) Increased vimentin expression in epithelial cells of endometriotic lesions as endometriosis progresses at 3, 6, 12, and 15 months following the induction of endometriosis in baboons. Magnification: ×200. Scale bar = 250 μm. (B) Representative photomicrographs of vimentin expression in epithelial cells of endometriotic lesions harvested at 3 and 15 months after induction. Magnification: ×1000. Scale bar = 50 μm.
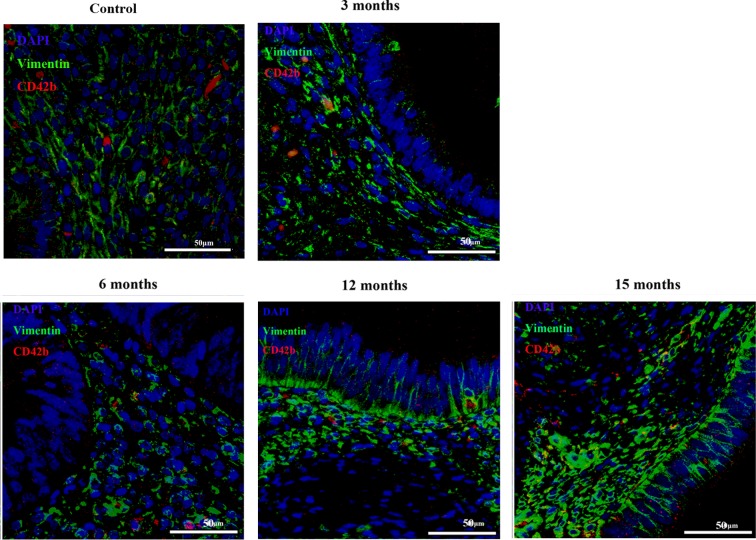
Consistent with aggregated platelets in endometriotic lesions as shown previously,13 we found CD42b-positive platelets scattered in endometriotic lesion samples from all time points (Figure 4). In particular, platelets appeared to be aggregated within vimentin-positive stromal cells in lesions from earlier time points but aggregated near glandular epithelial cells that were differentiated into mesenchymal stromal cells as the disease progressed (Figure 4). This difference seemed to correspond with the apparent absence of EMT in the former and the evidence of EMT in the latter (Figure 4).
Figure 4.
Localization of vimentin staining and CD42b-positive platelets in endometriotic lesions at different time points during lesion development. CD42b (in red) is a marker for platelets, and vimentin (in green) is a marker for stromal cells, which is also expressed in endometriotic glandular epithelial cells during the process of epithelial–mesenchymal transition. Magnification: ×1000. Scale bar = 50 μm.
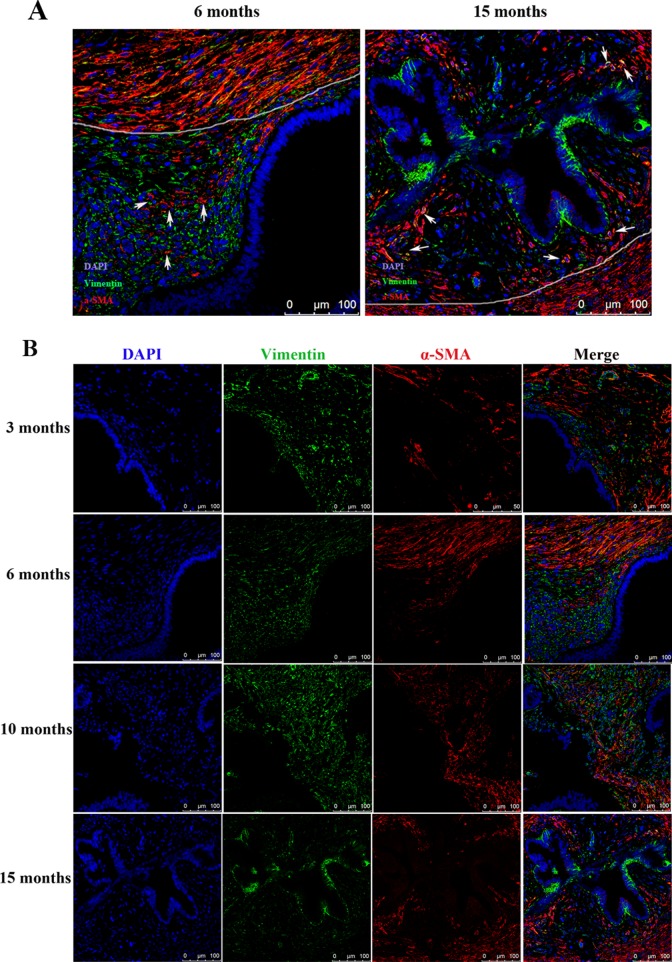
α-Smooth muscle actin is a well-recognized marker for myofibroblasts and also for SMCs.21,22 We found that some α-SMA-positive SMCs were distributed sporadically in the stroma near the glandular epithelium (which are called intrastromal SMCs or ISMC26), while others were grouped together as smooth muscle tissue around the lesions, termed surrounding SMCs or SSMC (Figure 5A and B). Their distinction was so conspicuous that a clear demarcation line can be made to separate the 2 types of cells (Figure 5). Remarkably, the number of ISMCs appeared to increase with time as lesions progressed, concomitant with the increased vimentin-positive glandular epithelial cells (Figure 5).
Figure 5.
(A) Localization of α-smooth muscle actin (α-SMA) and vimentin in younger (6 months) and older (15 months) endometriotic lesions. The white line was drawn to demarcate areas with different features. The areas with extensive α-SMA-positive myofibroblasts or smooth muscle cells within the stromal component contain intrastromal smooth muscle cells or ISMCs as described by Barcena de Arellano et al.26 The white arrows point to ISMCs. Magnification: ×400. Scale bar = 100 μm. (B) Serial immunofluorescent staining of vimentin and α-SMA showing progressive epithelial–mesenchymal tranision (EMT), as shown by increased vimentin-positive epithelial cells, and fibroblast-to-myofibroblast transdifferentiation (FMT), as shown by the increased acquirement of α-SMA positivity in the stromal component, in endometriotic lesions as endometriosis progresses. Magnification: ×400. Scale bar = 100 μm.
Immunohistochemistry Evidence Consistent With EMT and FMT
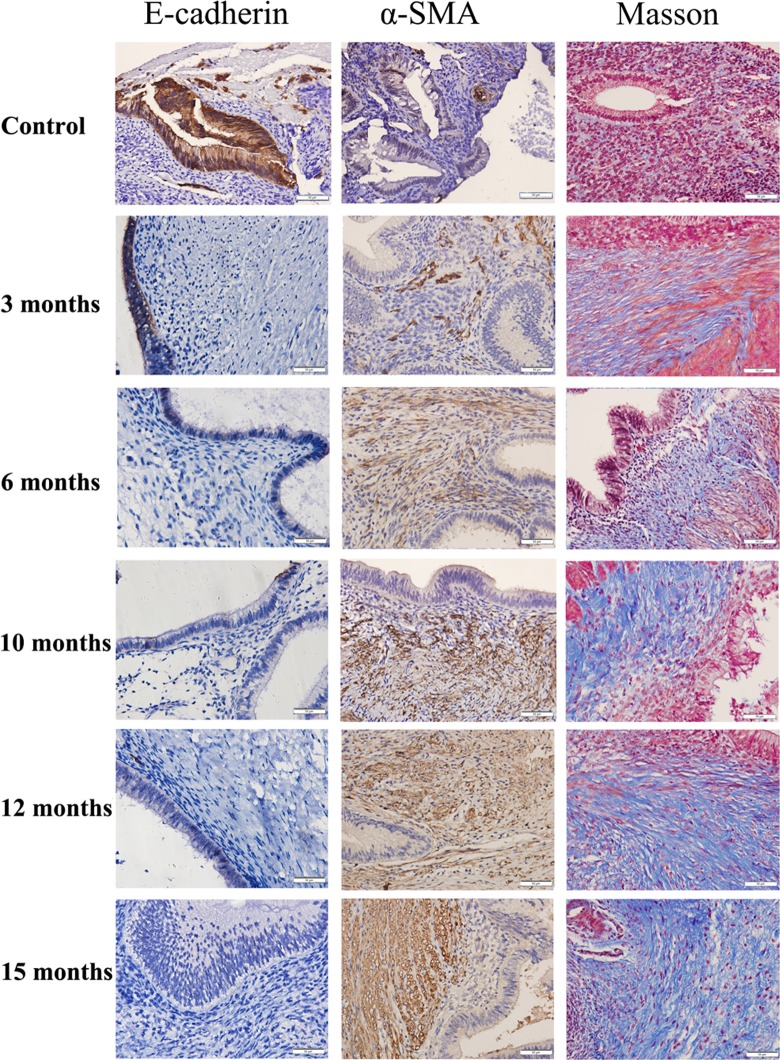
We first performed an IHC analysis of E-cadherin and α-SMA on ectopic and control endometrial samples. We found that E-cadherin staining was seen mostly in cytoplasm and membranes in endometriotic epithelial cells, but its immunoreactivity levels were decreased as endometriosis progressed (Figures 2C and 6), suggesting that a progressive EMT occurred as the disease progressed. Concomitantly, the immunoreactivity against α-SMA was seen mostly in cytoplasm in endometriotic stromal cells and also elevated as endometriosis progressed (Figures 2D and 6), consistent with the notion of progressive FMT in the development of endometriosis.
Figure 6.
Representative photomicrographs of serial immunohistochemistry analysis of E-cadherin and α-smooth muscle actin (α-SMA), along with Masson trichrome staining in control endometrium and in endometriotic lesions of different ages. Magnification: ×400. Scale bar = 50 μm.
In fact, α-SMA staining levels in lesions and control endometrium correlated negatively with that of E-cadherin (r = −.89, P < .001). For both markers, the staining levels correlated significantly with the time since induction (r = −.82, P = 4.8 × 10−6, and r = .85, P = 1.1 × 10−6, respectively). While E-cadherin staining correlated negatively with both TGF-β1 and p-Smad3 staining levels (r = −.86, P = 1.1 × 10−5, and r = −.92, P = 1.6 × 10−7, respectively), α-SMA correlated positively with them (r = .89, P = 1.5 × 10−6, and r = .94, P = 2.4 × 10−8, respectively). These results are consistent with the notion that as endometriosis progresses, progressive EMT and FMT, likely driven by the TGF-β1/Smad3 signaling pathway, occurred in the lesions.
Immunohistochemistry Evidence Consistent With Progressive SMM and Fibrosis
We also performed Masson trichrome staining to quantify the extent of fibrosis in endometriotic lesions and IHC analysis of desmin and SM-MHC, 2 markers of SMCs (Figures 2E and F and 7). Both markers correlated positively with the TGF-β1 and p-Smad3 staining levels (all rs > .84, all P values <.0002). We found that both desmin and SM-MHC staining levels, which were positive correlated (r = .95, P = 3.4 × 10−10), increased progressively as endometriotic lesions evolved with time. In fact, multiple linear regression analysis indicated that both desmin and SM-MHC staining levels were positively correlated with the time since induction (r = .76, P = .0015 and r = .70, P = .0053, respectively). Starting from 6 months after induction, the staining levels of both desmin and SM-MHC were significantly higher than that in control endometrium (Figures 2E and F and 7), indicating the gradual and progressive accumulation of SMM in ectopic endometrium. Consistent with this observation, the extent of fibrosis was also significantly and progressively increased in endometriotic lesions as endometriosis progressed, as shown with Masson trichrome staining (Figures 2G and 6). The extent of fibrosis in ectopic endometrium correlated positively with the time since the induction (r = .84, P = 1.5 × 10−6).
Figure 7.
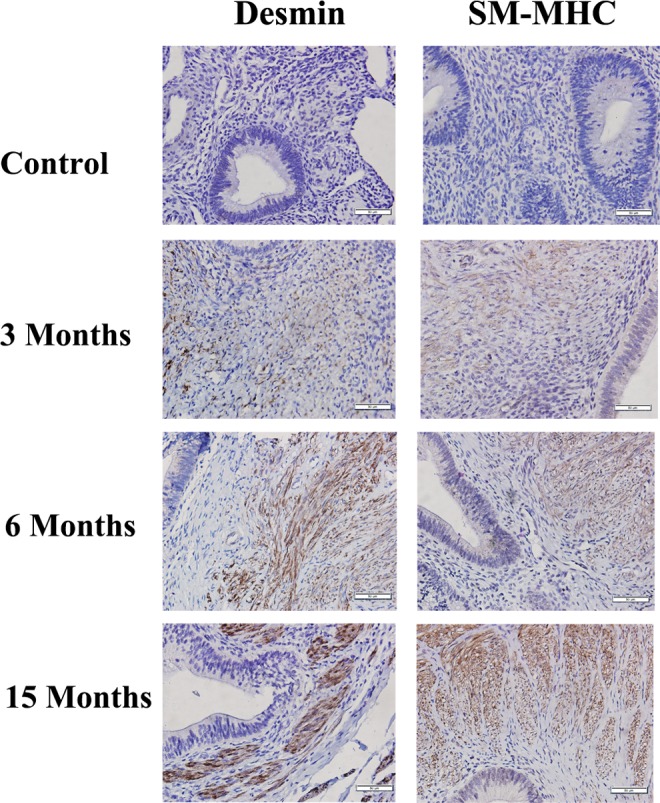
Representative photomicrographs of serial immunohistochemistry analysis of desmin and smooth muscle, myosin heavy chain (SM-MHC) in control endometrium and in endometriotic tissue samples harvested at different time points. Magnification: ×400. Scale bar = 50 μm.
The extent of fibrosis in endometriotic lesions was found to be correlated positively with the staining levels of TGF-β1, p-Smad3, desmin, SM-MHC, and α-SMA (all rs > .68, P < .0011) but negatively with that of E-cadherin staining levels (r = −.66, P < .01; Figure 2H to I) in endometriotic lesions. These data are consistent with progressive EMT, FMT, and SMM in endometriotic lesions, concomitant with increased extent of fibrosis.
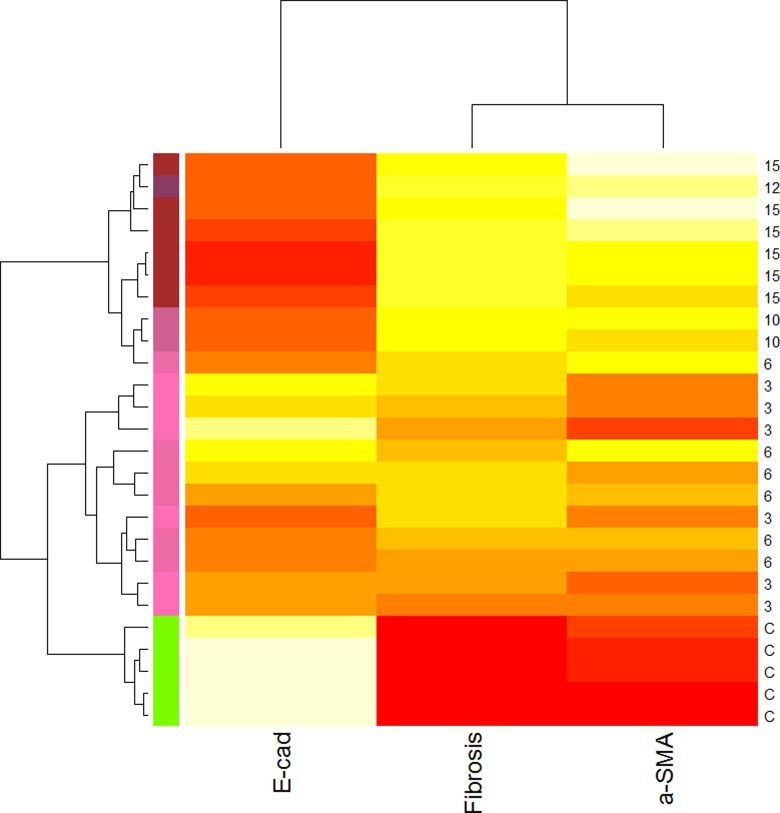
Hierarchical Cluster Analysis of Select EMT/FMT/Fibrosis Markers
To gain more insights into the possible molecular mechanisms underlying the time-dependent changes as endometriosis progresses, we performed a hierarchical cluster analysis of data on E-cadherin (a marker of EMT), α-SMA (result of the FMT), and the extent of fibrosis, since these 3 measurements represent distinct but possibly overlapping events in the development of endometriosis. In addition, these data were chosen also because they were complete for all baboon tissue samples. The results are presented as a heat map (Figure 8). As shown in Figure 8, all tissue samples could be grouped roughly into 3 clusters: cluster 1 is featured by a high E-cadherin immunoreactivity but low or no staining of α-SMA and the absence of fibrosis. This cluster includes exclusively all control endometrial tissue samples from baboons without endometriosis. Cluster 2 is the opposite and is characterized by low E-cadherin immunoreactivity but elevated staining of α-SMA and the higher extent of fibrosis. This cluster includes all ectopic endometrial tissue samples that were harvested 10 months after induction. Cluster 3 includes all ectopic endometrial tissue samples that were harvested at 3 and 6 months after induction and is featured by intermediate levels of E-cadherin and α-SMA staining and the presence of some fibrosis (Figure 8).
Figure 8.
Hierarchical clustering heat map of select immunohistochemistry (E-cadherin and α-smooth muscle actin [α-SMA]) and histochemistry (extent of fibrosis) measurements and baboon tissue samples. The heat map was organized by clustering both tissue samples (by rows) and immunohistochemistry and histochemistry measurements (by columns). The red color represents the minimal values, while the yellow color represents the maximal values. The designation on the right panel represents either the type of tissues (C as control endometrium) or the age of the endometriotic lesions, where C indicates a normal endometrial tissue sample and the number represents the ectopic endometrial tissue samples harvested from baboons at the indicated number of months following endometriosis induction. The designation at the bottom represents the names of the immunohistochemistry and histochemistry measurements. E-cad indicates E-cadherin; a-SMA: α-smooth muscle actin; fibrosis: the extent of fibrosis as measured by the Masson trichrome staining.
From the heat map, it is apparent that, through the use of 2 IHC markers and one histochemistry measurement, we could distinguish tissue samples that are free of endometriosis (normal endometrium), in the early stage of endometriosis development, and the later stage of endometriosis. Consequently, we used these measurements to carry out a multidimensional scaling analysis. The results shown in Figure 9 demonstrate that the use of these measurements can group these tissues to correlate with the type of tissue (normal vs. lesions) as well as the developmental stages of the lesions (Figure 9).
Figure 9.
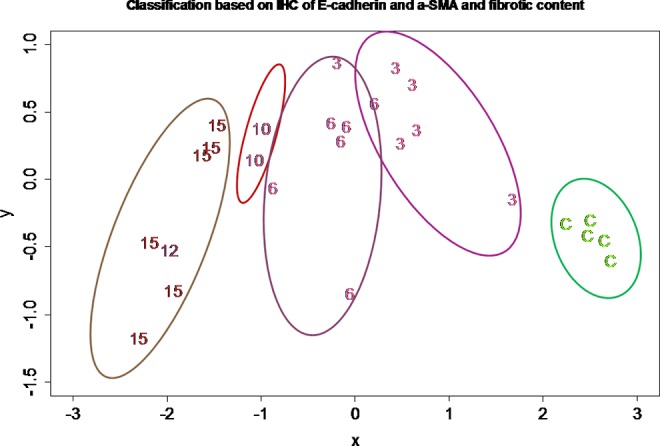
Results of multidimensional scaling analysis using the E-cadherin and α-smooth muscle actin (α-SMA) immunostaining levels and the extent of fibrosis. All tissues are represented by either the type of tissues (C as control endometrium) or the ectopic endometrial tissue samples from the indicated number of months following endometriosis induction, which are the same as used in Figure 8.
Discussion
We have shown in this study that in endometriotic lesions harvested from baboons with induced endometriosis, platelets are aggregated in the stromal compartment and TGF-β1 and p-Smad3 expression increases progressively as lesions develop. The expression of vimentin is increased in the epithelial compartment of the lesions as the lesions progressively develop. We also have shown that the number of ISMCs appears to be increased as lesions progressed with time, concomitant with the increased vimentin-positive glandular epithelial cells in the lesions. As the lesion progresses, the number of α-SMA-positive myofibroblasts and highly differentiated SMCs is increased in the stromal compartment, which correlates with the increasing extent of fibrosis. These results, taken together, corroborate with the notion that ReTIAR occurs in the endometriotic lesions, resulting in TGF-β1/Smad3-driven EMT and FMT, leading to SMM and ultimately fibrosis as lesions progress.
The EMT is a highly conserved cellular process that allows polarized and generally immotile epithelial cells to convert to motile mesenchymal cells and occurs during embryonic development, in cancer, and as a physiological response to injury.27 It allows the transformed cells to enhance their migratory and invasive capabilities. The FMT is a key event in physiological and pathological tissue repair and fibrosis.28 Through the evaluation of serially harvested endometriotic tissue samples from baboons, our data provide evidence that corroborates with our in vitro results that platelet-derived TGF-β1 drives fibrogenesis through EMT and FMT in endometriotic epithelial and stromal cells, respectively, and prolonged exposure to activated platelets induce expression of highly specific marker of differentiated SMCs.45 These data also are consistent with the finding that older ovarian cysts, having experienced more bleeding episodes, contain chocolate fluid that is higher in viscosity, density, and iron content and have a higher fibrotic content than younger ones.16 In other words, irrespective location or subtype, all endometriotic lesions share the same commonality as being the wounds that undergo ReTIAR, resulting eventually in fibrosis. Viewed through this perspective, it is perhaps not difficult to understand the natural history of endometriotic lesions and appreciate why endometriosis is challenging to treat.
The SMM is frequently found in peritoneal,17–20 deep,19,29,30 ovarian,17,20,31,32 extragenital,33,34 and pleuropulmonary endometriosis35 as well as in adenomyosis.36 The possibility of stromal cells differentiating into SMC, as happened in physiological SMM in the junctional zone of the uterus,37,38 has been entertained20,30 but nonetheless dismissed due to insufficient evidence.20 The prevailing view appears to be that they originate either from the metaplasia of the “second Müllerian system” or, more likely, from the basal layer of the endometrium with stem cell features that were regurgitated through the fallopian tubes because of dysperistalsis.20,39,40 Nonetheless, there are no data that confirm or refute any of these hypotheses.26 This study provides corroborative evidence that, as endometriotic lesions develop and are thus consistently in contact with activated platelets, there is an increasing SMM in lesions, concomitant with increased fibrotic contents. Thus, platelets appear to be a driver in SMM and fibrogenesis during the development of endometriosis.
Due to the methods we used, the results presented here cannot, in and by themselves, provide a direct proof that platelet-derived TGF-β1 drives fibrogenesis through EMT and FMT in endometriotic epithelial and stromal cells, respectively, leading ultimately to fibrosis. However, given the cohesive and biologically plausible hypothesis and the serial observation of this study and in light of our data indicating that, as the result of this ReTIAR, platelet-derived TGF-β1 induces the activation of the TGF-β1/Smad3 signaling pathway that modulates EMT and FMT in endometriotic epithelial and stromal cells, respectively,45 our data, coupled with the in vitro human and in vivo mouse data, lend strong support for the hypothesis we postulated. It should also be noted that adenomyosis seems to have a similar developmental process41,42 because both endometriosis and adenomyosis share the commonality of experiencing ReTIAR in ectopic endometrium.
Over time endometriosis is a progressive and dynamic disease in spontaneous and induced endometriosis.6,24 But repeated laparocopy may itself promote the development of endometriosis.43 In view of this, while our serial analysis of baboon endometriotic lesions corroborate with our human in vitro and mouse in vivo data,45 caution should be exercised since repeated laparoscopy may accelerate the development of endometriosis.
One important implication of our results is the biological justification for a histological staging of endometriotic lesions, since it is well known that the currently widely used rAFS/rASRM staging system does not correlate well with the severity of symptoms, progression, or prognosis.44 Both hierarchical cluster analysis and multidimensional scaling analysis indicate that, through the use of select IHC and histochemistry measurements, we can nearly perfectly identify not only changes in lesion tissues compared to the normal endometrium but also lesions at different stages of development based simply on merely 3 markers of the lesions, one on EMT, one on FMT, and the other on the extent of fibrosis. Given the inherent variation in IHC and histochemistry analyses, these data are of importance and indicate that, once the molecular mechanisms underlying the development of endometriotic lesions are elucidated, just a few select IHC and histochemistry measurements could “date” the age of lesions. In other words, endometriotic lesions, in and by themselves, may have a built-in record to evaluate their progressive age. Thus, in conjunction with finding that similar developmental process appears to occur also in adenomyosis,41,42 our data suggest that a histological classification of endometriosis progression could be within reach. In fact, this has been validated in principle in ovarian endometriomas using cyst fluid.16 Given the increased extent of fibrosis concomitant with decreased PR-B expression in ectopic endometrium,41 it is likely that such a classification system might have potential in predicting response to medical treatment or even recurrence. The investigation into the relationship between endometriosis progression and the severity of pain or subfertility and the recurrence risk is currently underway.
In summary, our data provide corroborative evidence that, through the activation of the TGF-β1/Smad3 signaling pathway, platelets drive the EMT and FMT in endometriotic lesions over time, promoting SMM and resulting ultimately in fibrosis in endometriotic lesions during the progression of disease development.
Footnotes
Author Contribution: Qi Zhang and Mark Olson contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by grants 81270676 (SWG), 81471434 (SWG), and 81530040 (SWG) from the National Natural Science Foundation of China, and NIH grants HD083273 and HD083453 (ATF).
References
- 1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 2. Guo SW. An overview of the current status of clinical trials on endometriosis: issues and concerns. Fertil Steril. 2014;101(1):183–190. e4. [DOI] [PubMed] [Google Scholar]
- 3. Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod. 1996;11(4):878–880. [DOI] [PubMed] [Google Scholar]
- 4. Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238–243. [DOI] [PubMed] [Google Scholar]
- 5. Arruda MS, Petta CA, Abrao MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756–759. [DOI] [PubMed] [Google Scholar]
- 6. D’Hooghe TM, Bambra CS, Raeymaekers BM, Koninckx PR. Serial laparoscopies over 30 months show that endometriosis in captive baboons (Papio anubis, Papio cynocephalus) is a progressive disease. Fertil Steril. 1996;65(3):645–649. [PubMed] [Google Scholar]
- 7. Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod. 2013;88(2):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JJ, Taylor HS, Lu Z, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13(5):323–332. [DOI] [PubMed] [Google Scholar]
- 9. D’Hooghe TM, Bambra CS, De Jonge I, Lauweryns JM, Koninckx PR. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75(2):98–101. [DOI] [PubMed] [Google Scholar]
- 10. D’Hooghe TM. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil Steril. 1997;68(4):613–625. [DOI] [PubMed] [Google Scholar]
- 11. Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;4(suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D’Hooghe TM, Kyama CM, Chai D, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16(2):152–161. [DOI] [PubMed] [Google Scholar]
- 13. Ding D, Liu X, Duan J, Guo SW. Platelets are an unindicted culprit in the development of endometriosis: clinical and experimental evidence. Hum Reprod. 2015;30(4):812–832. [DOI] [PubMed] [Google Scholar]
- 14. Guo SW, Ding D, Geng JG, Wang L, Liu X. P-selectin as a potential therapeutic target for endometriosis. Fertil Steril. 2015;103(4):990–1000. e8. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Q, Ding D, Liu X, Guo SW. Activated platelets induce estrogen receptor beta expression in endometriotic stromal cells. Gynecol Obstet Invest. 2015;80(3):187–192. [DOI] [PubMed] [Google Scholar]
- 16. Guo SW, Ding D, Shen M, Liu X. Dating endometriotic ovarian cysts based on the content of cyst fluid and its potential clinical implications. Reprod Sci. 2015;22(7):873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoga T, Matsumoto T, Takeuchi H, et al. Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis. Pathol Int. 2003;53(6):371–375. [DOI] [PubMed] [Google Scholar]
- 18. Khare VK, Martin DC, Eltorky M. A comparative study of ovarian and pelvic wall-infiltrating endometriosis. J Am Assoc Gynecol Laparosc. 1996;3(2):235–239. [DOI] [PubMed] [Google Scholar]
- 19. Matsuzaki S, Darcha C. Involvement of the Wnt/beta-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLoS One. 2013;8(10):e76808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mechsner S, Bartley J, Loddenkemper C, Salomon DS, Starzinski-Powitz A, Ebert AD. Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts. Fertil Steril. 2005;83(suppl 1):1220–1231. [DOI] [PubMed] [Google Scholar]
- 21. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasegawa T, Hasegawa F, Hirose T, Sano T, Matsuno Y. Expression of smooth muscle markers in so called malignant fibrous histiocytomas. J Clin Pathol. 2003;56(9):666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fazleabas AT. A baboon model for inducing endometriosis. Methods Mol Med. 2006;121:95–99. [DOI] [PubMed] [Google Scholar]
- 24. Harirchian P, Gashaw I, Lipskind ST, et al. Lesion kinetics in a non-human primate model of endometriosis. Hum Reprod. 2012;27(8):2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 26. Barcena de Arellano ML, Gericke J, Reichelt U, et al. Immunohistochemical characterization of endometriosis-associated smooth muscle cells in human peritoneal endometriotic lesions. Hum Reprod. 2011;26(10):2721–2730. [DOI] [PubMed] [Google Scholar]
- 27. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. [DOI] [PubMed] [Google Scholar]
- 28. Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13(1):7–12. [DOI] [PubMed] [Google Scholar]
- 29. Bonte H, Chapron C, Vieira M, et al. Histologic appearance of endometriosis infiltrating uterosacral ligaments in women with painful symptoms. J Am Assoc Gynecol Laparosc. 2002;9(4):519–524. [DOI] [PubMed] [Google Scholar]
- 30. van Kaam KJ, Schouten JP, Nap AW, Dunselman GA, Groothuis PG. Fibromuscular differentiation in deeply infiltrating endometriosis is a reaction of resident fibroblasts to the presence of ectopic endometrium. Hum Reprod. 2008;23(12):2692–2700. [DOI] [PubMed] [Google Scholar]
- 31. Doss BJ, Wanek SM, Jacques SM, Qureshi F, Ramirez NC, Lawrence WD. Ovarian smooth muscle metaplasia: an uncommon and possibly underrecognized entity. Int J Gynecol Pathol. 1999;18(1):58–62. [DOI] [PubMed] [Google Scholar]
- 32. Fukunaga M. Smooth muscle metaplasia in ovarian endometriosis. Histopathology. 2000;36(4):348–352. [DOI] [PubMed] [Google Scholar]
- 33. Haga T, Kumasaka T, Kurihara M, Kataoka H, Miura M. Immunohistochemical analysis of thoracic endometriosis. Pathol Int. 2013;63(9):429–434. [DOI] [PubMed] [Google Scholar]
- 34. Mechsner S, Bartley J, Infanger M, Loddenkemper C, Herbel J, Ebert AD. Clinical management and immunohistochemical analysis of umbilical endometriosis. Arch Gynecol Obstet. 2009;280(2):235–242. [DOI] [PubMed] [Google Scholar]
- 35. Flieder DB, Moran CA, Travis WD, Koss MN, Mark EJ. Pleuro-pulmonary endometriosis and pulmonary ectopic deciduosis: a clinicopathologic and immunohistochemical study of 10 cases with emphasis on diagnostic pitfalls. Hum Pathol. 1998;29(12):1495–1503. [DOI] [PubMed] [Google Scholar]
- 36. Mechsner S, Grum B, Gericke C, Loddenkemper C, Dudenhausen JW, Ebert AD. Possible roles of oxytocin receptor and vasopressin-1alpha receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil Steril. 2010;94(7):2541–2546. [DOI] [PubMed] [Google Scholar]
- 37. Fujii S, Konishi I, Mori T. Smooth muscle differentiation at endometrio-myometrial junction. An ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1989;414(2):105–112. [DOI] [PubMed] [Google Scholar]
- 38. Konishi I, Fujii S, Okamura H, Mori T. Development of smooth muscle in the human fetal uterus: an ultrastructural study. J Anat. 1984;139(pt 2):239–252. [PMC free article] [PubMed] [Google Scholar]
- 39. Leyendecker G, Herbertz M, Kunz G, Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod. 2002;17(10):2725–2736. [DOI] [PubMed] [Google Scholar]
- 40. Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280(4):529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen M, Liu X, Zhang H, Guo SW. Transforming growth factor β1 signaling coincides with -mediated epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in drive the development of adenomyosis in mice. Hum Reprod. 2016;31(2):355–369. [DOI] [PubMed] [Google Scholar]
- 42. Liu X, Shen S, Qi Q, Zhang H, Guo S-W. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiationin the development of adenomyosis. Hum Reprod. 2016;31(4):734–749. [DOI] [PubMed] [Google Scholar]
- 43. D’Hooghe TM, Bambra CS, Raeymaekers BM, Koninckx PR. Development of spontaneous endometriosis in baboons. Obstet Gynecol. 1996;88(3):462–466. [DOI] [PubMed] [Google Scholar]
- 44. Koninckx PR, Ussia A, Adamyan L, Wattiez A. An endometriosis classification, designed to be validated. Gynecol Surg. 2011;8(1):1–6. [Google Scholar]
- 45. Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol, 2016;428:1–16. [DOI] [PubMed] [Google Scholar]