Abstract
The uterine vasculature undergoes marked changes during pregnancy in order to provide the necessary increase in blood flow to support growth and nutrition of the uterus, placenta, and developing fetus. Pregnancy-associated uterine vascular transformations are orchestrated by a complex array of endocrine and cellular mechanisms to bring about structural modifications at the maternal–fetal interface, which collectively lead to development of the uteroplacental circulation. Understanding intrinsic uterine vascular remodeling in pregnancy is essential for understanding the physiologic and pathophysiologic regulation of maternal uterine blood flow. Aberrations of uterine vascular remodeling are potentially involved in the etiology of several pregnancy disorders, for example, preeclampsia, fetal growth restriction, and preterm labor; therefore, it is essential for subspecialist clinicians and investigators interested in reproductive physiology to fully understand the establishment of uteroplacental circulation. The foundational literature in this area is extensive; thus, a succinct review is likely to be a useful resource. Herein, we present and discuss a historical perspective on uterine vascular anatomy, maternal vascular growth associated with decidualization, trophoblast invasion, intervillous circulation, aberrations in uterine vascular modeling, and the clinical implications of improper development of the uteroplacental circulation.
Keywords: placenta, pregnancy complications, preeclampsia
Introduction
During gestation, the uterine vasculature undergoes numerous physiologic modifications in order to bring about the necessary quantitative increase in blood flow to support the development of the uterus, placenta, and fetus. Modifications to the intrinsic, within the uterine tissues, and extrinsic, within the mesometrium, vasculature are orchestrated by endocrine and paracrine mediators, decidualization, and trophoblast invasion. Our primary focus is on the intrinsic vascular changes. In the current context, the term “remodeling” refers to the intrinsic vascular alternations which occur during pregnancy which are largely, but not completely, reversed following delivery. It is important to note that, in the cardiovascular literature, the term remodeling is used to describe permanent cellular and biochemical alterations in the vessels, including inward eutrophic and hypertrophic remodeling, contributing to elevated systemic vascular resistance in hypertension and its consequences.1–3 Transformation of the maternal uterine vasculature is critical for a successful pregnancy and forms the fundamental basis for understanding the increase in the uteroplacental blood flow during gestation. Unsuccessful uterine vascular remodeling is believed to result in pregnancy complications as gestation progresses, for example, preeclampsia, fetal growth restriction, and preterm birth.4–8 Thus, the importance of a thorough understanding of the large volume of literature on this topic within the clinical and translational research settings cannot be stressed enough. As there are already excellent reviews published addressing the most recent advancements in this field, we present a succinct review of the seminal literature in this area which was essential for shaping our current knowledge of the uteroplacental circulation. Many of the seminal works were conducted in nonhuman primates, which provided a more continuous understanding of the development of uteroplacental circulation and guided the research on human uteroplacental circulation. It is important to note that there are many differences between human and nonhuman primate pregnancy, including but not limited to length of gestation, placental architecture, and trophoblast invasiveness; therefore, we have provided references to the original nonhuman primate work, as well as comparative reviews but will henceforth focus on the development of the uteroplacental circulation as it occurs in the human.9–17 In the current review, uterine vascular anatomy, maternal vascular growth including both decidualization and trophoblast invasion-associated changes, intervillous circulation, aberrations in uterine vascular remodeling, and clinical implications of improper uteroplacental vascular development are discussed.
Uterine Vascular and Early Implantation Anatomy
We first present a brief overview of the uterine vascular anatomy to provide a structural orientation for the discussion of the vascular remodeling in pregnancy. The uteroplacental vascular anatomy has been diagrammatically shown in Figure 1.
Figure 1.
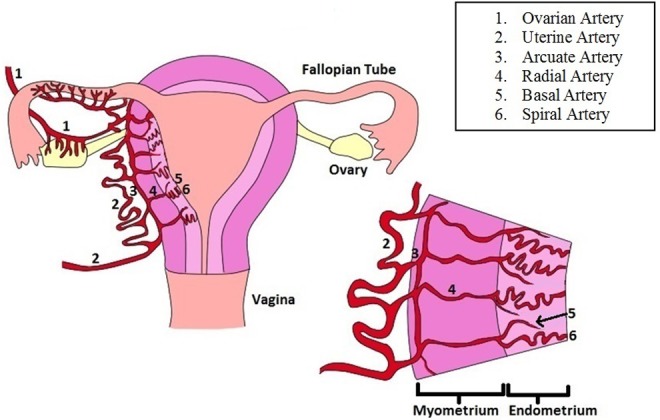
Uterine vascular anatomy: The uterine artery is the major vessel which provides blood to the uterus. At the surface of the uterus, the uterine artery branches into the arcuate artery. The radial artery, an offshoot of the arcuate artery, travels through the myometrium before giving off 2 branches at the myoendometrial junction: the basal artery and the spiral artery. Both the basal and spiral arteries transverse the endometrium and branch into capillary beds, but only the spiral artery reaches the luminal surface of the endometrium. The basal artery concludes at the basal surface of the endometrium.
The uterus is supplied by 1 uterine artery on each side and uterine branches of the ovarian arteries, which traverse along the utero-ovarian ligaments and anastomose with the terminal branch of the uterine arteries at the cornu.18 The uterine artery has branches, called arcuate arteries, which are within the myometrium, but near the surface of the uterus. A branch of the arcuate artery, known as the radial artery, transverses through the uterine myometrial wall radially.18,19 Within the myometrium near the endometrial junction, the radial artery gives off at least 2 branches, the basal artery and the spiral artery. More than 1 basal artery may be present, but there is only 1 spiral artery. Despite its name, the spiral artery does not spiral around its own axis but instead follows a tortuous course which gives the impression of spiraling in some histological sections.11 The basal artery and spiral artery grow toward the uterine cavity branching into arterioles and capillaries.18,19 Following the menstrual cycle or the delivery of the placenta at birth, the basal artery gives rise to a new spiral artery.18,19
The human embryo undergoes interstitial implantation causing the underlying endometrial glands and opposing surface epithelium to be compressed with the growth of the conceptus.20 The maternal sex steroids, estrogen, and progesterone, as well as myriad growth factors and cytokines, prime the endometrial stromal tissue for implantation during the luteal or secretory phase of the menstrual cycle. Sex steroids are also involved in facilitating endometrial gland differentiation, which is essential for implantation and the nutritional support of the growing conceptus prior to the establishment of maternal perfusion.21,22 Following implantation, stromal tissue further differentiates into what is known as the decidua.20 During implantation, about two-thirds of the thickness of the decidua is incorporated into the placenta through the formation of the basal plate and maternal septa, which, in combination with fetal tissues, produces an organ which is both maternal and fetal in origin.
Pregnancy-Associated Changes in Uterine Vasculature
Both the decidual and myometrial portion of the spiral arteries and the distal part of the radial arteries are subject to the physiologic transformations of pregnancy. Following transformation, the arteries are known as the uteroplacental vessels. Alterations in the anatomy and physiology of maternal vasculature are best described as 2 distinct phenomena: (1) uterine vascular growth with decidualization and (2) uterine vascular remodeling secondary to trophoblast invasion.
Maternal Vascular Growth With Decidualization-Associated Changes
By the end of 5 weeks of gestation, spiraling, that is tortuosity, of the uterine vessels is more pronounced as a result of axial longitudinal growth of the vessel in excess to the increase in endometrial thickness. The terminal stretch of the vessel is appreciably increased due to hormones, primarily estrogen, and growth factors, primarily vascular endothelial (VE) growth factor and fibroblast growth factor, mediated modifications in the vascular wall.23,24 The increase in vessel wall diameter and thinning of the vessel wall is regarded as a prerequisite to trophoblast invasion.25 By the end of week 10, the endometrium becomes greatly thinned due to the growth of the conceptus. As a result, the course of the spiral arteries becomes oblique, that is diagonal, from the previous radial direction. The tortuosity of the uterine vessels increases due to further lengthwise growth and their accommodation within a relatively narrower, that is thinner uterine wall. As gestation progresses through the second trimester, spiraling is further intensified through continued longitudinal vessel growth, and by 18 to 24 weeks, the uterus has undergone marked enlargement with an increase in the thickness of the uterine wall. The straightening or “paying out” of the uterine arteries because of an increase in uterine wall thickness results in reduction of the previous apparent coils and allows the arteries to assume an undulating course. The growth and lengthwise expansion of the vessels, as well as increases in diameter and reduction in the degree of tortuosity, continues as gestation advances further.
Vascular Invasion by Trophoblasts
Human trophoblasts invade the maternal tissue beginning at 6 to 8 weeks after implantation with invasion generally completed by 19 to 20 weeks of gestation.25 Trophoblast invasion is categorized, based on location and course of invasion, as vascular or interstitial.
In vascular invasion, the endovascular trophoblast cells invade the maternal blood vessels at the terminal portion of the arteriole. The migration of trophoblast cells then occurs along the inner walls of the arterioles in sheets with deposition of fibrinoid material on the luminal surface. Once the trophoblasts reach the spiral arteries, an accumulation of cells occurs, almost to the point of occlusion of the lumen, forming the spiral arteriole plug. Maternal blood flow is drastically reduced in these plugged arteries such that nutritional support of the embryo is largely dependent upon the uterine glands for the first 10 weeks of gestation. Minimal blood flow early in pregnancy and the resulting hypoxic environment helps direct trophoblast proliferation and differentiation and alters endothelial and vascular smooth muscle cell response to external stimuli, which may be important for trophoblast-mediated vascular remodeling.26,27 Over time, the integrity of the trophoblast plug diminishes, and by 10 to 12 weeks, the plug has loosened sufficiently to allow continuous maternal blood flow into the intervillous space.28,29 Trophoblast migration continues beyond the spiral plugs into the deeper myometrial segment of the arteries, that is the terminal portion of the radial artery.30,31 As illustrated in Figure 2, uterine vascular remodeling in pregnancy significantly alters the structural and cellular composition of the spiral artery. Trophoblasts use cell surface proteins including VE-cadherin, platelet endothelial cell adhesion molecule 1 (PECAM-1), compliment protein C1q, and various chemokine receptors to migrate across the endothelial cell layer before inserting into the interendothelial junction. The dynamics of trophoblast–endothelium interactions are essential for successful vascular remodeling. Evidence suggests that endothelial cells are active participants in the recruitment of trophoblasts to the spiral arteries through the release of chemokines such as interleukin 8 and growth-regulated oncogene alpha (GRO-α).32 As trophoblasts migrate across the endothelium, endothelial cells undergo apoptosis, leaving trophoblasts to replace them as the lining of the lumen within the spiral arteries.30,33,34 Interestingly, evidence suggests that as trophoblasts replace the resident endothelial cells, they themselves adopt an endothelial-like phenotype producing an apparent re-endothelialization of the vessels.35 Trophoblast invasion results in disorganization and removal of the tunica media, that is muscularis layer, and disruption of the internal elastic lamina.36 Some remnants of the internal elastic lamina remain, but largely, there is replacement of the tunica muscularis layer by trophoblast and fibrinoid layers, and thus following remodeling, the spiral arteries lack the ability to constrict in any significant manner.30
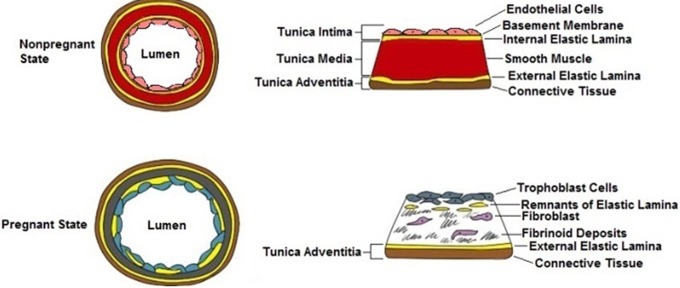
Figure 2.
Spiral artery remodeling in pregnancy: in the nonpregnant state, the spiral artery can be partitioned into 3 distinct layers: tunica intima, tunica media, and tunica adventitia. The vessel contains endothelial cells, shown in pink, and a smooth muscle layer, shown in red. In the pregnant state, the spiral artery retains only one of the original layers, the tunica adventitia. Endothelial cells are replaced by trophoblasts, shown in blue, and the layer of smooth muscle cells is also eliminated and supplanted by fibroblast cells and fibrinoid deposits which are shown in gray.
Trophoblast invasion occurs by disruption of the internal lamina and removal of vascular smooth muscle cells. With only the outer adventitial connective tissue layer remaining, the uterine arteries demonstrate enhanced stretch and decreased contractile capabilities.37,38 The loss of vascular smooth muscle cells is critical, as evidence suggests that blood flow is controlled primarily by muscular arteries proximal to the myoendometrial junction.19,39 Loss of contractility increases blood flow and reduces the force of pulsatility as blood enters the intervillous space. In addition, circumferential growth and stretching of the uterus contribute to the increase in diameter of the remodeled arteries. The resultant uteroplacental vessels have a mean diameter of 500 microns; a significant increase compared to the 200-micron diameter commonly seen in the nonpregnant uterine spiral arteries.37 In contrast, the basal arteries, which do not participate in the intervillous circulation, do not show decidualization-associated structural changes to the same degree as the spiral arteries. The basal vessels are not subject to trophoblast invasion and have an approximate diameter of 120 microns at term gestation.37
Insufficient vascular invasion is a nearly certain harbinger of complications later in pregnancy though the mechanisms involved remain to be fully understood. Zhou et al have demonstrated low levels of integrin, cadherin, and immunoglobulin superfamily members on the surface of trophoblast cells from preeclamptic placentas, suggesting that failure to assume a vascular adhesion phenotype negatively affects spiral artery remodeling.35,40 Other groups have shown the importance of the immune system in healthy pregnancy. Stimulation of immune cells, in particular decidual natural killer (NK) cells, not only moderates the immune response to foreign fetal antigens and produces a “tolerant” maternal–fetal interface but also regulates trophoblast invasion and vascular growth through the release of promigratory chemokines, matrix metalloproteinases, and angiogenic factors.41–44 Indeed, loss of decidual NK cells is known to lead to poor vascular remodeling.45 Additionally, the placenta plays an active role in moderating the maternal immune response at the maternal–fetal interface through the production of various proteins such as placental protein 13 (PP13). The PP13 immunostaining strongly coincides with decidual necrosis zones that include T cells, neutrophils, and macrophages in the first trimester. There is a relationship between low PP13 in maternal serum, loss in immune populated necrosis zones, and severe preeclampsia and HELLP syndrome.46
Interstitial Trophoblast Invasion
In addition to spiral artery invasion, trophoblasts also invade the decidual tissue between the blood vessels. The invasion by the extravillous trophoblast is known as interstitial invasion. Interstitial invasion begins at the anchoring villi with columns of cells proliferating and separating from the deeper most attachment of the villi. Within the decidua, extravillous trophoblasts tend to cluster around spiral arteries, whereas those in the junctional zone are more dispersed. Extravillous trophoblasts begin invading the decidua as early as 5 weeks of gestation and can be observed in the myometrial portion of the arteries by 14 weeks of gestation.25
Extravillous trophoblasts invading the decidua can differentiate into 2 subpopulations: those that invade the spiral arteries and participate in remodeling in the vessel lumen, similar to endovascular trophoblasts, and those that do not.37 Therefore, the trophoblast invasion of the vessel wall occurs at more than 1 site, and a single spiral artery may have multiple openings into the same intervillous space.37 Adequate extravillous invasion is essential for the spiral arteries to achieve maximum remodeling and vasodilation partly because extravillous trophoblasts appear to be the primary cells responsible for smooth muscle cell remodeling. Extravillous trophoblasts may arrive at the spiral artery and initiate disorganization and degradation of vascular smooth muscle cells prior to the arrival of endovascular trophoblasts. It is hypothesized that loss of smooth muscle cells prior to endovascular remodeling facilitates endothelial cell replacement by trophoblasts.26 Extravillous trophoblasts also promote further invasion by producing a favorable uterine environment through the release of metalloproteinase and disintegrin family members, which are associated with the activation of cell surface receptors for cytokines and growth factors.47
Gross Anatomy of Placental Development
Normal placental development in humans occurs circumferentially, resulting in a discoid structure.48,49 The process of trophoblast invasion occurs continuously, and along the periphery of the placenta, into the second trimester. During placental development, new maternal uterine blood vessels continue to be incorporated as uteroplacental vessels providing circulation to the developing areas of the placenta. The mature human placenta demonstrates the deepest trophoblast invasion of spiral arteries at its center, with the depth gradually decreasing toward the periphery of the placenta.48,49 The variation in trophoblast invasion in different areas of the placenta occurs because trophoblast invasion goes on for a longer period of time at the earliest invasion site, that is at the center, and for a shorter period at the periphery, which is also the “newest” part of the placenta. This phenomenon has implications for collecting placental bed tissue biopsies. In order to compare biopsies and results between studies, the location of the biopsy, in reference to placental diameter, must be recorded. There is currently no standardized method for recording the biopsy location in translational research investigations. This can make comparing placental biopsy results between different studies difficult.31,50 It is likely that tissue samples taken from the center of the placenta and at specified intervals from the center with the placental diameter information will provide the most reproducible data.
Intervillous Circulation
Following vascular remodeling, the uteroplacental vessels form the afferent channels of the maternal intervillous blood flow to the placenta, the decidual veins being the efferent channels. There is a general agreement among placental biologists that the arterial and venous openings are irregularly distributed on the basal decidual plate, but that most arterial openings are present in close proximity to the decidual projections and the septa.51 According to Boyd52 and Brosens et al,51 there are approximately 100 to 120 maternal arteries that open into the intervillous space with many spiral arteries having multiple openings. Studies indicate that intervillous blood flow enters in spurts and is generally segmental in the units perfused by 1 or 2 maternal arteries and drained by the corresponding vein or veins on the basal plate; however, dispersion between different intervillous spaces is not completely restricted.52
Although the human placenta is not classified as cotyledonary, as in ruminants, the human placenta has been suggested to have “functional fetal cotyledons” which are illustrated in Figure 3. Ramsey et al defined functional cotyledons as distinct anatomic units of fetal stem vessels perfusing a segment of the fetal-placental/villous tissue associated with its intervillous space(s). At term, an average placenta contains 38 to 40 functional cotyledons. Such abundance is believed to confer an evolutionary advantage as a normal placenta can lose functionality of up to one-third of the functional cotyledons without producing a significant impact on placental function. On the maternal side of these functional cotyledons, the units of the placenta are divided by the decidual projections known as septae, though the borders of the maternal and fetal side are generally not paired complementarily in humans as is seen in true cotyledonary placentation. These septae are made primarily of the maternal tissue.51 Anatomic studies of these septa indicate that they do not form complete partitions of placenta, and that they do not necessarily traverse vertically into the fetal tissue of the placenta; many septae transverse radially and horizontally. Thus, intervillous spaces may intercommunicate within the placenta in a cavernous manner.
Figure 3.
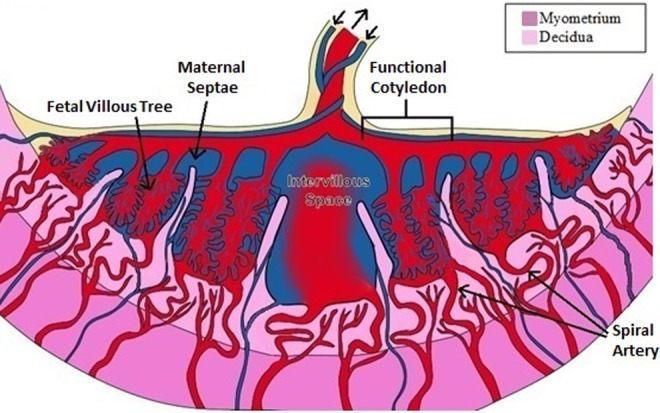
The intervillous circulation: Following pregnancy-associated vascular remodeling, the spiral arteries are known as the uteroplacental vessels. The uteroplacental vessels provide blood to the intervillous space, where the maternal blood is free to interact with the fetal villous tree. Although the term “cotyledon” has sometimes been used to refer to the incomplete partitions of the placenta, the human placenta is not truly cotyledonary and thus the term “functional cotyledon” is more appropriate.
The Aberrations of Vascular Remodeling
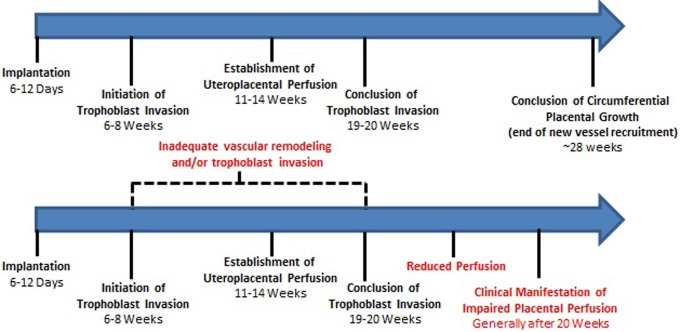
A major difference between normal and aberrant vascular remodeling during pregnancy is the depth of the trophoblast invasion. The depth of trophoblast invasion has been described as shallow in preeclampsia, with or without preexisting hypertension, and also in fetal growth restriction, with and without hypertension, and in preterm births.4,6,23,37 Brosens et al conducted a review of their material to define whether the extent of vascular remodeling of the spiral arteries could be related to the incidence of preeclampsia. Based on their seminal observations, they described that trophoblast invasion of the spiral arteries was deficient in patients with preeclampsia.37 Furthermore, as described by Brosens et al, the extent of vascular modeling in the arteries of the placental bed is less deep in all areas of the placenta in patients with preeclampsia when compared with normal pregnancies (Figure 4).27,37
Figure 4.
Key events in the establishment of the uteroplacental circulation in normal and complicated pregnancies: In normal pregnancy (top), proper trophoblast invasion and vascular remodeling lay the foundation for a successful pregnancy. In pregnancies where these processes are interrupted or impaired (bottom) complications such as preeclampsia can occur.
Deficient trophoblastic invasion of the spiral arteries and the resultant reduction in vessel remodeling prevents the arteries from achieving maximum dilation and distensibility. Although normal pregnancy is characterized by wide diameter vessels with a mean diameter of 500 microns, preeclamptic patients typically demonstrate arteries with a mean diameter of 200 microns. Preeclamptic vessels remain similar in size and cellular composition to nonpregnant spiral arteries, which also have a mean diameter of 200 microns.37 As shown in Figure 5, these relatively narrow uteroplacental vessels are restrictive and thus are unable to provide the necessary increase in blood supply to the growing conceptus. The most important aspect of this aberration, in our opinion, is that the vasocontractile elements remain intact in preeclampsia in the myometrial segment of the artery. As a result, these vessels remain responsive to vasomotor stimuli, as also noted by Brosens et al.30 This heightened vasomotor activity increases uterine vascular impedance and thus becomes problematic, as fetal nutritional and oxygen demands increase with gestational age and are not met due to a limited increase in uteroplacental blood flow. Importantly, even in instances in which trophoblast invasion is sufficiently deep, improper vascular remodeling may still occur. Alterations in the expression or signaling pathways of angiogenic factors, matrix metalloproteinases, and growth factors can also contribute to insufficient vascular remodeling.53–55
Figure 5.
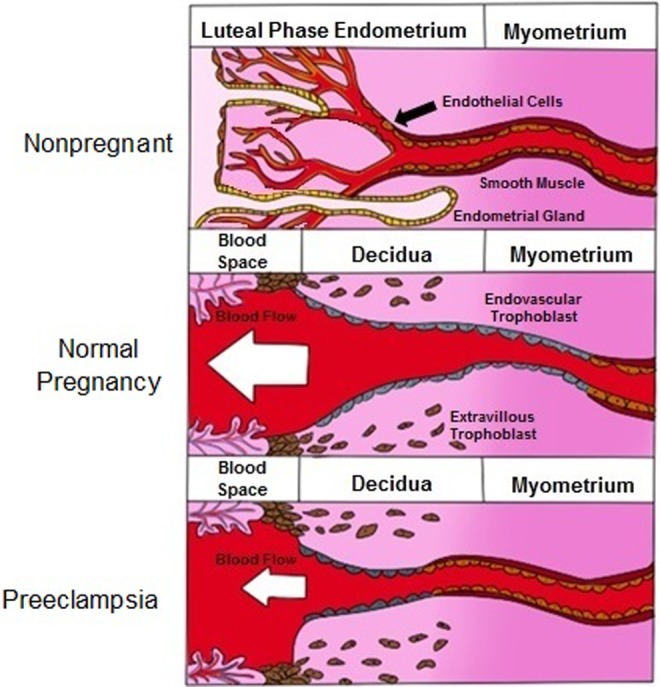
Comparison of spiral artery remodeling in the nonpregnant, normal pregnant, and preeclamptic pregnant state: Normal, nonpregnant endometrium contains capillary beds and glands, which provide nourishment to the uterine endometrium. During pregnancy, these capillary beds and endometrial glands are destroyed, and the spiral arteries dilate and open directly into the intervillous space; thus, providing low-pressure, high-volume blood flow. In preeclampsia, some remodeling allows for a small degree of spiral artery dilation, but the volume of blood being delivered to the placenta is considerably less than in normal pregnancy.
Pathologic Arterial Lesions in Preeclampsia
Zeek and Assali were the first to describe uterine vascular lesions of the spiral arteries. The lesions contained lipid-laden cells, or lipophages, within the vascular wall of the vessel along with fibrinoid necrosis and mononuclear perivascular infiltrate.56–58 Zeek and Assali called this lesion acute atherosis, which is not to be confused with similar terms including “atheroma,” “atheromatous,” and “atherosclerosis,” which describe a different vascular pathology (Figure 6).30,59
Figure 6.
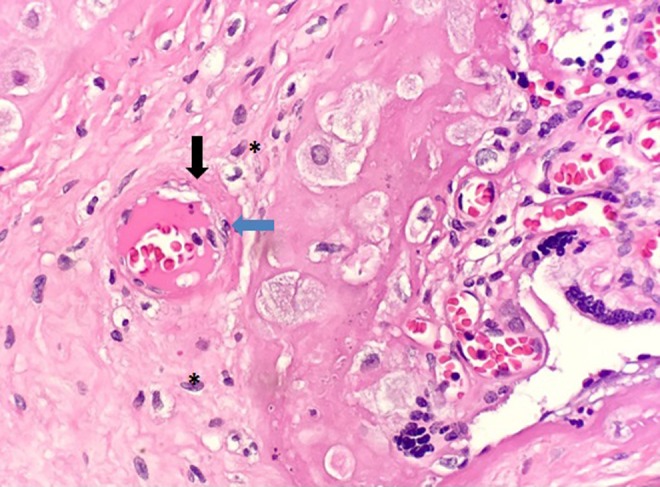
Maternal spiral arteries which fail to undergo remodeling may demonstrate a vascular lesion known as acute atherosis. Lipid laden cells (blue arrow) and thickened, fibrinoid deposits in the vessel wall (black arrow) are characteristics of this lesion. Potential lipid containing macrophages (denoted by asterisk) are also apparent in this section.
Although the incidence of acute atherosis in preeclampsia may vary greatly, anywhere from 5% to 40%, it is essential to understand that acute atherosis occurs only in the spiral arteries that lack trophoblast invasion.30,60,61 Lipid-containing cells have been identified as macrophages, which may also exhibit intracellular inclusions of cellular debris similar to foam cells described in atherosclerosis.62,63 Boyd and Hamilton have described lipid accumulation in the senescent trophoblasts in the walls of the uteroplacental arteries, which is a feature of normal pregnancy and must be distinguished from the acute atherosis as described earlier in a vessel that lacks trophoblast invasion.30,52 In contrast to preeclampsia alone, essential hypertension with superimposed preeclampsia demonstrates vascular medial hypertrophy in addition to the findings of lipid-laden macrophages.5 Necrotic lesions are more pronounced in essential hypertension with superimposed preeclampsia than in preeclampsia without preexisting hypertension.5
The occurrence of acute atherosis in placental vessels has been shown to correlate with severity of placental pathology, and fetal clinical outcomes suggesting that this vasculopathy occurs as a direct result of the preeclamptic environment.64 Currently, it is unknown whether acute atherosis occurs as a result of an underlying maternal vascular abnormality that predisposes women to preeclampsia or if preeclampsia itself is responsible for the formation of lesions.65
Pathologic Arterial Lesions in Intrauterine Growth Restriction and Preterm Birth
Acute atherosis, once proposed as a vasculopathy unique to preeclampsia, has also been described in intrauterine growth restriction (IUGR) and preterm birth. Sheppard and Bonnar described plaques within the spiral arteries of normotensive women who gave birth to infants affected by IUGR.66 These plaques, consisting of mononuclear, lipid-laden cells, fibrin, and smooth muscle cells, were in some cases large enough to occlude the entire vessel lumen. The plaques were seen in spiral arteries, which failed to undergo normal physiologic transformations, for example, trophoblast-mediated remodeling.67 Sheppard and Bonnar later confirmed maternal vascular lesions in both decidual and myometrial portions of the spiral arteries, in nearly all pregnancies with IUGR but not in pregnancies free from IGUR.68 Comparable observations have been made in cases complicated by preterm birth. Arias et al reported that, in a cohort of 235 women with preterm premature rupture of membranes, 54.9% demonstrated maternal uterine vascular lesions with significant narrowing of the spiral arteries.69 Atherosis in conjunction with failed remodeling of spiral arteries has also been described in vessels from women with spontaneous, idiopathic preterm birth.6,8,70,71 At this time, the question remains if the maternal uterine vascular lesion observed in preeclampsia, IUGR, and preterm birth is the same lesion or if they simply share a similar histologic appearance.
Conclusion
The uteroplacental circulation provides the necessary nutritional resources to the uterus, placenta, and fetus during gestation; thus, proper development of the uteroplacental circulation is critical for a successful pregnancy. Decidualization-associated modifications in uterine vasculature provide a platform for the development of the placenta, particularly through spiral artery growth. The invasion by endovascular and extravillous trophoblasts causes profound changes to the spiral arteries, including loss of vascular smooth muscle and endothelial cells, as well as an increase in luminal diameter. The transformed uteroplacental vessels serve as low-resistance channels in which maternal blood can travel with reduced force from the maternal circulation into the intervillous space. When uterine vasculature is not properly modified to accommodate the needs of pregnancy, complications such as preeclampsia, fetal growth restriction, and preterm birth can occur. Aberrations observed in these complications, including shallow trophoblast invasion, failed remodeling of spiral arteries, and uterine vascular lesions, appear similar despite the differences between the disease progression, which suggests the presence of a fundamental connection between these complications. This provides an exciting avenue for new research related to the clinical implications of uteroplacental development.
Acknowledgments
This article is dedicated in honor of Dr Chester B. Martin, Jr. Chair of the Department of Obstetrics and Gynecology at the UW-Madison School of Medicine 1985–1992. The authors would like to thank Ms Susanna Zheng and Rakesh Sarda for their assistance in preparing the figures for this publication.
Authors’ Note: This work was partial fulfillment of Kenna Degner’s PhD for the Endocrinology and Reproductive Physiology Training Program of the UW Graduate School.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding provided by P01HD38843 and R01HL117341 and Department of Obstetrics and Gynecology, UWSMPH, Madison, WI, USA. Funding was also received by Dinesh Shah (U01HDO87261) and Kenna Organ (5T32HD041921).
References
- 1. Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension.2001;38(3 pt 2):581–587. [DOI] [PubMed] [Google Scholar]
- 2. Mulvany MJ. Vascular remodelling of resistance vessels: can we define this? Cardiovasc Res. 1999;41(1):9–13. [DOI] [PubMed] [Google Scholar]
- 3. Renna NF, de Las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens. 2013;2013:808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dixon HG, Robertson WB. A study of the vessels of the placental bed in normotensive and hypertensive women. J Obstet Gynaecol Br Emp. 1958;65(5):803–809. [DOI] [PubMed] [Google Scholar]
- 6. Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189(4):1063–1069. [DOI] [PubMed] [Google Scholar]
- 7. Page E. The relation between hydatid moles, relative ischemia of the gravid uterus, and the placental origin of eclampsia. Am J Obstet Gynecol. 1939;37(6):291–293. [Google Scholar]
- 8. Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter AM, Enders AC, Pijnenborg R. The role of invasive trophoblast in implantation and placentation of primates. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Markee E. Menstruation in intraocular endometrial transplants in the rhesus monkey. Contrib Embryol. 1940;28(177):221–308. [Google Scholar]
- 12. Martin CB, Jr, Ramsey EM, Donner MW. The fetal placental circulation in rhesus monkeys demonstrated by radioangiography. Am J Obstet Gynecol. 1966;95(7):943–947. [DOI] [PubMed] [Google Scholar]
- 13. Ramsey E. Venous drainage of the placenta of the rhesus monkey (Macaca mulatta). Contrib Embryol. 1954;35(151):151–173. [Google Scholar]
- 14. Ramsey EM. The vascular pattern of the endometrium of the pregnant rhesus monkey (Macaca mulatta). Contrib Embryol. 1949;33(213-221):113–148. [PubMed] [Google Scholar]
- 15. Ramsey EM, Corner GW, Donner MW, Stran HM. Radioangiographic studies of circulation in the maternal placenta of the rhesus monkey: preliminary report. Proc Natl Acad Sci U S A. 1960;46(7):1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramsey EM, Houston ML, Harris JW. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol. 1976;124(6):647–652. [DOI] [PubMed] [Google Scholar]
- 17. Ramsey EM, Martin CB, Jr, Donner MW. Fetal and maternal placental circulations. Simultaneous visualization in monkeys by radiography. Am J Obstet Gynecol. 1967;98(3):419–423. [DOI] [PubMed] [Google Scholar]
- 18. Burbank F. Hemodynamic changes in the uterus and its blood vessels in pregnancy: Fibroids, Menstruation, Childbirth, and Evolution: The Fascinating Story of Uterine Blood Vessels. Tucscon, AZ: Wheatmark; 2009:177–184. [Google Scholar]
- 19. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guzeloglu-Kayisli O, Basar M, Arici A. Basic aspects of implantation. Reprod Biomed Online. 2007;15(6):728–739. [DOI] [PubMed] [Google Scholar]
- 21. Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87(6):2954–2959. [DOI] [PubMed] [Google Scholar]
- 22. Filant J, Spencer TE. Uterine glands: biological roles in conceptus implantation, uterine receptivity and decidualization. Int J Develop Biol. 2014;58(2-4):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. [DOI] [PubMed] [Google Scholar]
- 24. Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod. 2001;64(4):1033–1040. [DOI] [PubMed] [Google Scholar]
- 25. Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1(1):3–19. [DOI] [PubMed] [Google Scholar]
- 26. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9-10):939–958. [DOI] [PubMed] [Google Scholar]
- 27. Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31(6):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merce LT, Barco MJ, Alcazar JL, Sabatel R, Troyano J. Intervillous and uteroplacental circulation in normal early pregnancy and early pregnancy loss assessed by 3-dimensional power Doppler angiography. Am J Obstet Gynecol. 2009;200(3):315 e311–e318. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Zhao S. Placental blood circulation In: Granger DN, Granger J, eds. Vascular Biology of the Placenta. San Rafael, CA: Morgan & Claypool Life Sciences; 2010:Chap 2. [PubMed] [Google Scholar]
- 30. Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187(5):1416–1423. [DOI] [PubMed] [Google Scholar]
- 31. De Wolf F, Brosens I, Robertson WB. Ultrastructure of uteroplacental arteries. Contrib Gynecol Obstet. 1982;9:86–99. [PubMed] [Google Scholar]
- 32. Aldo PB, Krikun G, Visintin I, Lockwood C, Romero R, Mor G. A novel three-dimensional in vitro system to study trophoblast–endothelium cell interactions. Am J Reprod Immunol. 2007;58(2):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bulla R, Agostinis C, Bossi F, et al. Decidual endothelial cells express surface-bound C1q as a molecular bridge between endovascular trophoblast and decidual endothelium. Mol Immunol. 2008;45(9):2629–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bulla R, Villa A, Bossi F, et al. VE-cadherin is a critical molecule for trophoblast–endothelial cell interaction in decidual spiral arteries. Exp Cell Res. 2005;303(1):101–113. [DOI] [PubMed] [Google Scholar]
- 35. Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99(9):2152–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marais WD. Human decidual spiral arteries. Ultrastructure of the intima in normal vessels. J Obstet Gynaecol Br Commonw. 1968;75(5):552–567. [DOI] [PubMed] [Google Scholar]
- 37. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 38. Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. 2001;158(5):1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenfeld CR, DeSpain K, Word RA, Liu XT. Differential sensitivity to angiotensin II and norepinephrine in human uterine arteries. J Clin Endocrinol Metab. 2012;97(1):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou Y, Fisher SJ, Janatpour M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. a strategy for successful endovascular invasion? J Clin Invest. 1997;99(9):2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–1074. [DOI] [PubMed] [Google Scholar]
- 42. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107(20):9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lash GE, Otun HA, Innes BA, et al. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod. 2010;25(5):1137–1145. [DOI] [PubMed] [Google Scholar]
- 44. Moffett A, Hiby SE, Sharkey AM. The role of the maternal immune system in the regulation of human birthweight. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yagel S. The developmental role of natural killer cells at the fetal–maternal interface. Am J Obstet Gynecol. 2009;201(4):344–350. [DOI] [PubMed] [Google Scholar]
- 46. Than NG. PP13, decidual zones of necrosis, and spiral artery remodeling – preeclampsia revisited? Reprod Sci. 2012;19(1):14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pollheimer J, Fock V, Knofler M. Review: the ADAM metalloproteinases – novel regulators of trophoblast invasion? Placenta. 2014;35(suppl):S57–S63. [DOI] [PubMed] [Google Scholar]
- 48. Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4(4):397–413. [DOI] [PubMed] [Google Scholar]
- 49. Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta. 1981;2(4):303–316. [DOI] [PubMed] [Google Scholar]
- 50. Robertson WB, Khong TY, Brosens I, De Wolf F, Sheppard BL, Bonnar J. The placental bed biopsy: review from three European centers. Am J Obstet Gynecol. 1986;155(2):401–412. [DOI] [PubMed] [Google Scholar]
- 51. Brosens I, Dixon HG. The anatomy of the maternal side of the placenta. J Obstet Gynaecol Br Commonw. 1966;73(3):357–363. [DOI] [PubMed] [Google Scholar]
- 52. Boyd J, Hamilton W. The Human Placenta. Cambridge, MA: Heffer & Sons, Ltd; 1970. [Google Scholar]
- 53. Goldman-Wohl D, Yagel S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol. 2002;187(1-2):233–238. [DOI] [PubMed] [Google Scholar]
- 54. Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54(2-3):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu JY, Pang ZJ, Yu YH. Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol. 2012;5(3-4):e137–e143. [PMC free article] [PubMed] [Google Scholar]
- 56. Dixon HG, Robertson WB. Vascular changes in the placental bed. Pathol Microbiol (Basel). 1961;24:622–629. [DOI] [PubMed] [Google Scholar]
- 57. Shanklin DR, Sibai BM. Ultrastructural aspects of preeclampsia. I. Placental bed and uterine boundary vessels. Am J Obstet Gynecol. 1989;161(3):735–741. [DOI] [PubMed] [Google Scholar]
- 58. Zeek PM, Assali NS. Vascular changes in the decidua associated with eclamptogenic toxemia of pregnancy. Am J Clin Pathol. 1950;20(12):1099–1109. [DOI] [PubMed] [Google Scholar]
- 59. Rogers BB, Bloom SL, Leveno KJ. Atherosis revisited: current concepts on the pathophysiology of implantation site disorders. Obstet Gynecol Surv. 1999;54(3):189–195. [DOI] [PubMed] [Google Scholar]
- 60. Kim JY, Kim YM. Acute atherosis of the uterine spiral arteries: clinicopathologic implications. J Pathol Trans Med. 2015;49(6):462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim YM, Chaemsaithong P, Romero R, et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015;28(17):2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol. 1975;123(2):164–174. [DOI] [PubMed] [Google Scholar]
- 63. Khong TY, Mott C. Immunohistologic demonstration of endothelial disruption in acute atherosis in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1993;51(3):193–197. [DOI] [PubMed] [Google Scholar]
- 64. Bird IM, Zheng J, Cale JM, Magness RR. Pregnancy induces an increase in angiotensin II type-1 receptor expression in uterine but not systemic artery endothelium. Endocrinology. 1997;138(1):490–498. [DOI] [PubMed] [Google Scholar]
- 65. Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–1034. [DOI] [PubMed] [Google Scholar]
- 66. Sheppard BL, Bonnar J. The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. Br J Obstet Gynaecol. 1976;83(12):948–959. [DOI] [PubMed] [Google Scholar]
- 67. Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046–1054. [DOI] [PubMed] [Google Scholar]
- 68. Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol. 1981;88(7):695–705. [DOI] [PubMed] [Google Scholar]
- 69. Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89(2):265–271. [DOI] [PubMed] [Google Scholar]
- 70. Defranco EA, Jacobs TS, Plunkett J, Chaudhari BP, Huettner PC, Muglia LJ. Placental pathologic aberrations in cases of familial idiopathic spontaneous preterm birth. Placenta. 2011;32(5):386–390. [DOI] [PubMed] [Google Scholar]
- 71. Kim YM, Chaiworapongsa T, Gomez R, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–1142. [DOI] [PubMed] [Google Scholar]