Abstract
Embryo implantation, which is an absolute requirement for reproduction, starts with blastocyst apposition to the uterine endometrium, followed by attachment to the endometrial surface epithelium. Recent clinical studies reported an increase in implantation and pregnancy rates among women receiving intrauterine human chorionic gonadotropin (hCG) prior to embryo transfer suggesting that, at least in some cases, female infertility is a result of inadequate secretion of hCG. In this study, we characterized the effect of hCG on trophoblast–epithelial interaction by further developing our recently described in vitro model of implantation. Here, we confirmed hCG increased attachment of trophoblast to epithelial cells, using a single-cell trophoblast–epithelial coculture system in addition to a blastocyst-like spheroid–epithelial coculture system. Furthermore, we discovered that the source and concentration was pivotal; the first preparation of hCG affected 2 molecules related to implantation, MUC16 and osteopontin, while the second preparation required additional cytokines to mimic the effects. Using this system, we can develop a comprehensive knowledge of the cellular and gene targets of hCG and other factors involved in embryo apposition and implantation and potentially increase the number of therapeutic targets for subfertile patients.
Keywords: hCG, implantation, pregnancy
Introduction
Embryo implantation, which is an absolute requirement for reproduction, starts with blastocyst apposition to the uterine endometrium, followed by attachment to the endometrial surface epithelium. In humans, the uterus becomes receptive during the mid-secretory phase of the menstrual cycle, commonly known as the window of implantation.1 Approximately half of lost pregnancies are due to implantation failure, and many of these spontaneous abortion cases are attributed to poor uterine receptivity.2
There has been renewed interest in the role of human chorionic gonadotropin (hCG) during early embryo attachment and the “implantation window” since recent clinical studies reported an increase in implantation and pregnancy rates among women receiving intrauterine hCG prior to embryo transfer.3 These findings suggest that, at least in some cases, female infertility is a result of inadequate secretion of hCG, which plays a role in setting the stage for embryo attachment and implantation; therefore, a comprehensive knowledge of the cellular and gene targets of hCG will increase the number of therapeutic targets for subfertile patients.
Several critical roles have been previously established for hCG during pregnancy, such as maintenance of progesterone secretion by the corpus luteum,4 increased angiogenesis,5 improved trophoblast invasion,6 immune tolerance,7-11 and regulation/modulation of multiple endometrial cell types.12,13 Specifically, hCG has been shown to increase secretion of several proimplantation factors including vascular endothelial growth factor,5 leukemia inhibitory factor (LIF),14,15 and trophinin16 from endometrial epithelia. The expression of LIF during early pregnancy is required for embryo attachment, although its mechanism is not well defined. The expression of trophinin in both epithelial cells and the trophoblast increases the attachment of trophoblast cells to epithelia and could explain in part the improved implantation rates of patients treated with hCG.
Mucins, including MUC117 and MUC16,18 have been shown to be a significant barrier to embryo implantation and must be downregulated in time for embryo attachment. Using an in vitro culture system, reduction in epithelial mucins with small interfering RNA increased attachment of trophoblast cells.18 Furthermore, mucins are regulated by sex hormones, ensuring embryo implantation occurs only at the optimal time.17 Once mucins are removed from the epithelial cell surface, proteins such as osteopontin (OPN) have an important role functioning as “linkers” between the epithelium and the blastocyst.19 Osteopontin binds extracellular proteins such as integrins and helps to form a firm attachment between the embryo and the endometrium.19
In this study, we characterized the effect of hCG on trophoblast–epithelial interaction and determined whether hCG could be used to improve fertility during embryo transfer. Using a modification of our recently reported in vitro model of trophoblast–epithelial interaction,20 we demonstrate that hCG increases attachment of trophoblast to epithelial cells through the regulation of MUC16 and OPN.
Materials and Methods
Cells and Reagents
Immortilized human epithelial carcinoma cells (ECC-1; American Type Culture Collection [ATCC], CRL-2923) were cultured in Roswell Park Memorial Institute medium with 10% fetal bovine serum (FBS) as recommended by ATCC, and immortalized human trophoblast Sw.71 cells constitutively expressing green fluorescent protein (GFP) 20 were cultured in Dulbecco Modified Eagle Medium/F12 with 10% FBS under 5% CO2 at 37°C. Human chorionic gonadotropin C0434 (Sigma, St Louis, Missouri) or Pregnyl (Organon Inc, West Orange, New Jersey) was used at concentrations between 2.5 and 1000 IU.
Growth Curves and Viability Assays
Cell viability was determined using the CellTiter assay (Promega, Madison, Wisconsin). Briefly, cells (5 × 103) were plated in triplicate wells in a 100-µL volume per well of a 96-well microtiter plate (BD Biosciences/Pharmingen, San Jose, California). The cells were grown to 70% confluence and incubated in reduced serum phenol red-depleted Opti-MEM medium (Invitrogen/GIBCO, Grand Island, NY) for 4 hours before treatment. The hCG treatments were added from 2.5 to 1000 IU final concentration and after 24 hours cell viability was evaluated using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega), according to the manufacturer’s instructions. Optical densities of the samples were measured at 490 nm using an automatic microplate reader (Model 550; BioRad, Hercules, California). The values of the treated cells were compared with the values generated from the untreated control and reported as percentage of viability. Each experiment was done in triplicate. Growth curves were assessed and reported using Incucyte (Essen Instruments, Ann Arbor, MI).
Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted using the RNeasy kit (Qiagen, Valencia, California). RNA concentration and purity were assessed using spectrophotometric analyses of 260/280 ratios, and only samples with values of 1.8 or higher were used for polymerase chain reaction (PCR) analysis. For quantitative analysis of messenger RNA (mRNA), 1 µg of RNA was reverse transcribed for each sample using oligo (d)T priming and SuperScript II reverse transcriptase (Invitrogen, La Jolla, California). The complimentary DNA was diluted to 1:10 in nuclease-free water and 5 µL was used in the PCR reaction. Syber green master mix (KAPA Biosystems, Woburn, Massachesetts) and gene-specific primers were added to the RT reactions and run on the CFX96, C1000 system qPCR machine (BioRad): actin: (FP) 5’-tgacaggatcgagaaggaga-3’, (RP) 5’-cgctcaggaggagcaatg-3’ and Mucin 16: (FP) 5’-gcctctaccttaacggttacaatgaa-3’, (RP) 5’-ggtaccccatggctgttgtg-3’. Values represented were normalized to β-actin and were calculated using the ΔΔCt method: ΔCt = ΔCt treated − ΔCt control; results are expressed as fold change from control (NT).
Osteopontin Protein Assay
Protein concentration was determined using a cytokine multiplex assay from BioRad 48 hours post-hCG treatment. Briefly, wells were loaded with either 50 µL of prepared standard or 50 µL of cell-free supernatant and incubated on an orbital shaker at 500 rpm for 2 hours in the dark at RT. Wells were washed 3x with BioRad wash reagent and samples were then incubated with 25 µL of biotinylated detection antibody for 30 minutes, washed, and incubated with 50 µL streptavidin-PE for 10 minutes.
After final wash, samples were resuspended in assay buffer and measured using LUMINEX 200 (LUMINEX, Austin, Texas).
Single-Cell Attachment Assay and Blastocyst-Like Spheroids
A confluent T75 flask of first-trimester trophoblast Sw.71-GFP cells was trypsinized and divided equally into 6 wells of a Costar ultra low attachment 6-well plate (Corning Incorporated, Corning, New York), and formation of spheroids was monitored until they reached a compact morphology at approximately 48 hours. Monolayers of ECC-1 cells and spheroids were either treated with 100 IU of Pregnyl hCG 24 hours or left without treatment as controls. Spheroids were then added to ECC-1 cells and monitored over 72 hours. Single Sw.71 cell attachment assays received the same treatment, but instead of forming spheroids, monolayers of Sw.71-GFP were trypsinized and cocultured with ECC-1 cells for 1 hour. Media and unattached cells were then removed and the number of GFP-positive cells was quantified after 24 hours using a Zeiss microscope (Carl Zeiss, Jena, Germany) and Volocity software (Improvision, PerkinElmer Inc, Waltham, Massachusetts).
Statistical Analysis
Differences between means were determined using independent t-test function of Graph pad in STAT statistical software (La Jolla, California). A P value of ≤.05 was considered significant and data are presented as mean ± standard error of the mean.
Results
Dose Response and Viability of Sw.71 Cells are Dependent on Source of hCG
Our first objective was to determine a safe and effective dose of hCG that may be used to evaluate the effect of hCG on trophoblast cell growth. We performed a dose–response experiment using increasing concentrations of hCG with the human trophoblast first-trimester cell line Sw.71.21 We tested concentrations of hCG ranging from 2.5 to 700 IU/mL, using both the clinical brands of hCG, Pregnyl and purified hCG from Sigma. Both types of hCG were tested to compare the quality of hCG preparations and the potential differences in their composition. Interestingly, we observed that high concentrations of hCG had a detrimental effect on trophoblast growth (>200 IU), while viability was not affected with concentrations of up to 150 to 200 IU/mL using Pregnyl. However, we did not observe any differences in cell growth and cell viability with the Sigma brand hCG, regardless of the dose tested. Figure 1 shows a representative viability curve (A) and growth curve (B) using the first-trimester trophoblast Sw.71 cell line with both hCG brands at 48 hours. Pregnyl hCG concentrations higher than 200 IU inhibit growth and this inhibitory effect is associated with decreased cell viability (Figure 1), while Sigma brand had no affect (data not shown). Although both brands are purified from human urine and are pathogen free, these results suggest that hCG can be beneficial or detrimental to trophoblast cell survival depending on the concentration and preparation; therefore, the remaining experiments were performed using a concentration between 150 and 200 IU/mL of Pregnyl hCG with all cell types.
Figure 1.
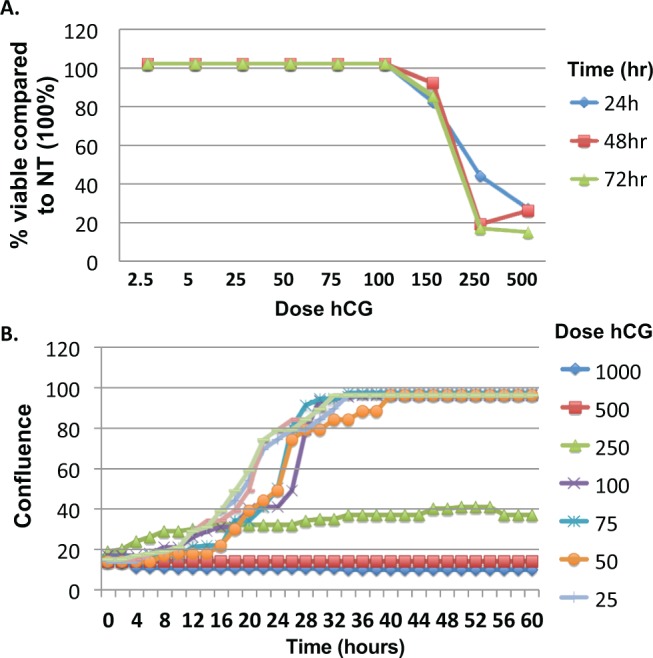
Pregnyl human chorionic gonadotropin (hCG) affects Sw.71 trophoblast cell viability at concentrations higher than 100 IU. A, Sw.71 cells were plated in triplicate and viability was determined 24, 48, and 72 hours post-hCG treatment at concentrations ranging between 2.5 and 500 IU. B, Sw.71 cell growth was further analyzed by measuring cell confluence for 60 hours post-hCG treatment at concentrations ranging between 25 and 1000 IU. Concentrations of hCG less than or equal to 100 IU did not affect viability and cells reached confluence 28 hours posttreatment, similar to cells that received no treatment.
“Pregnyl” hCG, but not Sigma hCG, Decreases MUC16 mRNA Expression in ECC-1 Cells Without Addition of Interleukin-1B
In previous studies, it was shown that hCG improved implantation rates in patients undergoing embryo transfer procedures3; therefore, the next objective was to understand how fertility was improved in these cases. We first tested the hypothesis that hCG modulates epithelial receptivity by controlling the expression of factors associated with uterine receptivity such as epithelial mucins, which must be suppressed for implantation to occur. We tested increasing concentrations of hCG, using the 2 different sources and found that there was a decrease in expression of MUC16 mRNA by the epithelial cells when treated with Pregnyl hCG (Figure 2A). This inhibitory effect was not observed when cells were treated with hCG preparation obtained from Sigma; we even observed a small increase in MUC16 mRNA at hCG concentrations of 100 IU (Figure 2B). Since previous studies16 showed that the effect of hCG was only observed in the presence of interleukin 1B (IL-1B), we supplemented the Sigma hCG with recombinant IL-1B. Interestingly, with this treatment MUC16 mRNA had a tendency to decrease with the largest change at the 750 IU dose (Figure 2C).
Figure 2.
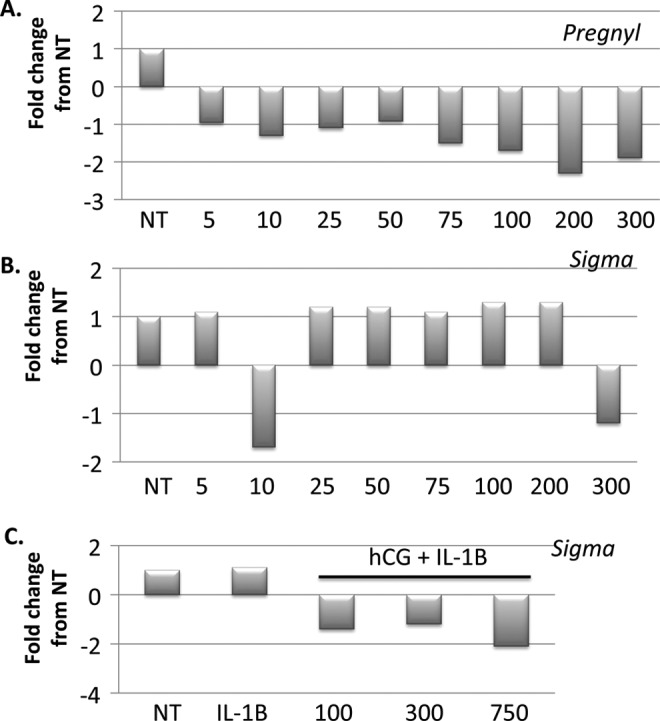
Pregnyl human chorionic gonadotropin (hCG) causes a decrease in MUC16 messenger RNA (mRNA) while Sigma hCG alone has no effect. There is a negative correlation between Pregnyl hCG concentration and MUC16 mRNA between 5 and 300 IU hCG (A). Sigma hCG does not affect MUC16 mRNA unless supplemented with interleukin 1B which results in a decrease in MUC16 concentration at 750 IU (B, C). Results are represented as fold change from nontreated control Sw.71 cells and represent 3 independent experiments.
Human Chorionic Gonadotropin Increases Secretion of OPN From ECC-1
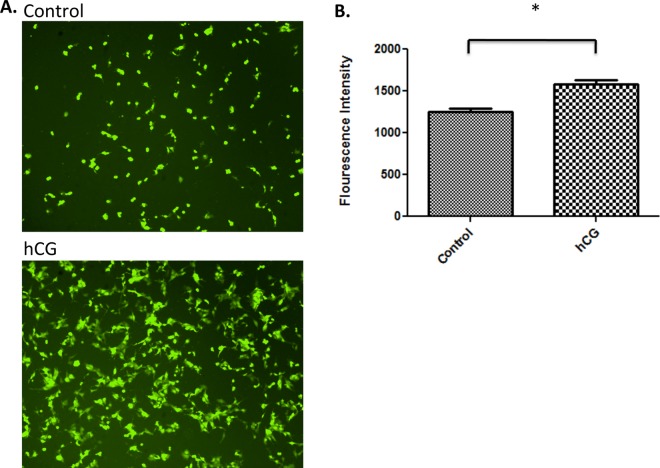
Although a decrease in mucin concentration is required for intimate contact between the early blastocyst and the uterine epithelium,17,18 the presence of adhesion and bridging molecules are also essential for the attachment of the trophoblast to the uterus. Osteopontin is a critical factor that allows strong binding of trophoblast cells to the epithelium19; therefore, we sought to determine whether hCG affected expression of this protein. Epithelial cells were treated with hCG as described earlier and the conditioned medium was collected for protein analysis. As hypothesized, OPN secretion was significantly upregulated in ECC-1 by hCG treatment (Figure 3), suggesting that increasing secretion of bridging molecules could be another mechanism with which hCG supports blastocyst implantation.
Figure 3.
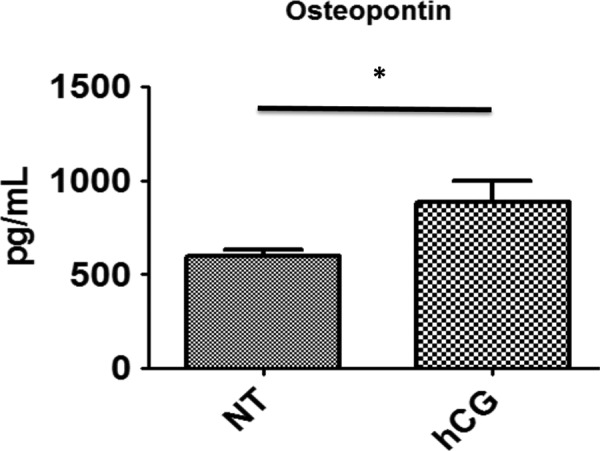
Sw.71 cells secrete higher concentrations of osteopontin (OPN) when treated with Pregnyl human chorionic gonadotropin (hCG). There were higher concentrations of OPN in Sw.71-conditioned medium following 100 IU hCG treatment compared to nontreated controls 48 hours posttreatment (n = 3, P = .038).
Human Chorionic Gonadotropin Enhances the Attachment of Trophoblast to Human Endometrial Epithelial Cells
Since we observed that hCG treatment decreased MUC16 mRNA expression and increased OPN secretion by epithelial cells, we hypothesized that these changes may enhance the ability of trophoblast cells to attach to uterine epithelial cells. Therefore, we cocultured first-trimester trophoblast Sw.71 cells that constitutively express GFP (GFP-Sw.71) with a confluent monolayer of epithelial endometrial cells ECC-1 that were previously treated with or without hCG. After 1 hour of coculture, unattached cells were removed and the number of GFP-Sw.71-positive cells attached to epithelial cells was quantified. As shown in Figure 4, there was a significant increase in the number of trophoblast cells attached to ECC-1 cells that were exposed to hCG compared to optiMEM alone (Figure 4A and B), suggesting that hCG facilitates the interaction of epithelial cells with the trophoblast.
Figure 4.
Sw.71 cells treated with 100 IU human chorionic gonadotropin (hCG) have increased attachment to endometrial epithelial cells compared to nontreated controls. Sw.71 cells constitutively expressing green fluorescent protein (GFP) were treated with 100 IU hCG for 24 hours and co-cultured with epithelial carcinoma cell 1 (ECC-1) cells for 1 hour. Once unattached cells were removed, the GFP-positive cells were quantified; one representative experiment is shown (A). Treatment with hCG increased Sw.71 attachment to the ECC-1 monolayer compared to nontreated Sw.71 controls (n = 5, P = .0006; A, B).
Human Chorionic Gonadotropin Enhances the Attachment of Blastocyst-Like Spheroids to Endometrial Epithelial Cells
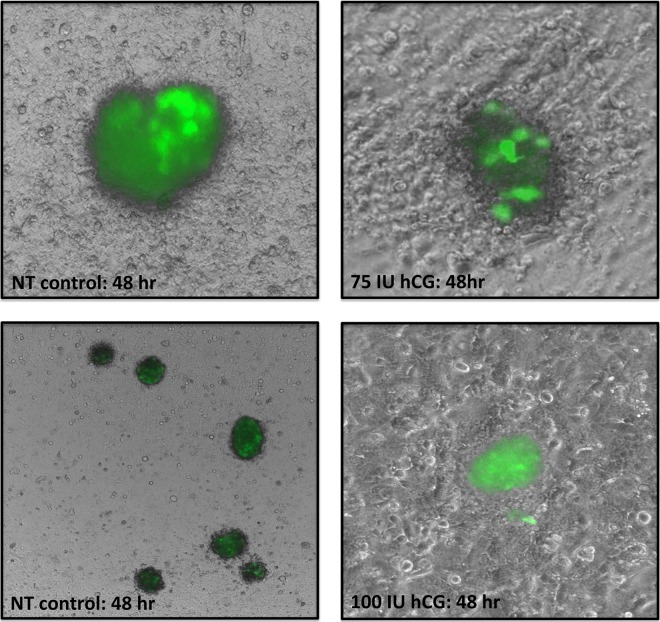
Our laboratory previously developed a model to study the interaction of 3-dimensional blastocyst-like spheroids (BLS) with uterine epithelial cells.20 In the present study, we used this system to first confirm hCG increases attachment of trophoblast cells and, second, ensure this attachment occurs when the cells are part of a 3-dimensional structure more similar to the early blastocyst. Epithelial cells were cultured until they were confluent and 20 BLS were added to each well. These spheroids were maintained on the epithelial cells for 72 hours with or without hCG and examined at 24, 48, and 72 hours. Our results showed that after 48 hours of coculture with hCG, BLS formed nest-like structures within the epithelial cells (Figure 5). After 72 hours, the medium and any floating BLS were removed and, strikingly, there were no spheroids remaining in the wells in the control groups lacking hCG, while multiple spheroids remained attached to the ECC-1 which were maintained with hCG. These findings further demonstrate that hCG changes functionally change the interaction between uterine epithelial cells and the trophoblast.
Figure 5.
Treatment with human chorionic gonadotropin (hCG) increases blastocyst-like spheroids (BLS) attachment to epithelial carcinoma cell 1 (ECC-1) monolayers. The BLS treated with hCG had more intimate contact with ECC-1 cells; ECC-1 cells form nest-like structures around hCG spheroids and there were more spheroids still attached to the monolayer after 72 hours.
Discussion
In this study, we demonstrated that hCG increased OPN secretion and downregulated mucin expression in uterine epithelial cells but the commercial source is pivotal. Confirming the relevance of the changes in the molecular targets, single-cell coculture and 3-dimensional BLS derived from trophoblast cells were used to both quantify attachment and study the cellular interaction between the “blastocysts” and the epithelia. Collectively, our data show that hCG may support trophoblast apposition and adhesion to the endometrium by regulating proteins involved in implantation and, importantly, the hCG source and dose must be carefully evaluated.
A key finding of this study is the disparity between a clinical brand of hCG and a laboratory (Sigma) brand typical in most in vitro studies. One study found that Sigma hCG did not change the expression of endometrial genes, in vitro, without the addition of the proinflammatory cytokine IL-1B16 while another in vivo study used this source affectively in mice.22 Interestingly, we found that in vitro the addition of IL-1B was indeed necessary with the Sigma brand hCG but not with the clinical brand. The Pregnyl source of hCG has been utilized in vitro,23 in vivo,10,11 and in clinical studies3 to improve implantation rates. Interestingly, in our study, we found this source of hCG resulted in cell death at much lower concentrations compared to the Sigma brand but affected expression of genes involved in implantation. These observations highlight the importance of determining dosing for clinical studies based on in vitro studies utilizing the same hormone source. Together, these findings suggest that there could be disparities not only between these 2 products but also in any different source of the hormone and should be kept in mind when comparing data from different systems or investigators and when planning clinical trials.
The 2 products used in this study were both purified from pregnant women’s urine and pathogen free. Further information about day of pregnancy and hCG isoforms contained in the preparations is not available, making speculation about the discrepancies in the products difficult. Human chorionic gonadotropin has multiple forms, including heterodimer of α and β subunits, heterodimer with nicked β form, free α and β forms, and multiple-nicked free β forms.24 All of these forms have different activities; therefore, differences in protein makeup of each preparation could be affecting their function in these experiments.
Based on the initial findings, we utilized the Pregnyl hCG in the remaining experiments to maintain consistency and because it affected target genes in our trophoblast cell line. We tested 3 approaches, in combination, to assess the effect of hCG on trophoblast–endometrial interaction. With the first approach, we determined whether hCG regulated any gene or protein targets known to be involved in implantation. It is well established that the loss of mucins during the implantation window is pivotal for pregnancy success,17,18 and interestingly, we found that these genes were downregulated by hCG at the mRNA level. Another protein, OPN, which has been found to “link” the trophoblast and the uterine epithelia via their integrins was upregulated by hCG.19 Based on these findings, it could be postulated that as the blastocyst approaches the endometrium, it produces hCG to ensure the epithelial wall is prepared for apposition; as it approaches, the local hCG concentration increases resulting in OPN release and tight regulation of barrier glycoproteins. Since these observations support the role of hCG in early embryo implantation, we next performed tests to characterize cell–cell interactions.
Two-cell adhesion tests were used to correlate the gene and protein expression with functional changes in the cells. The first cell interaction test involved addition of a single-cell suspension (trophoblast) to epithelial monolayers to quantify the adhesiveness between the cells with and without hCG. This resulted in an objective means of measuring the cellular interactions in the study since the trophoblast cells express GFP and the fluorescence can be quantified in each experimental group. We found that hCG increased the number of trophoblast cells that attached to the epithelial monolayer showing that the increase in OPN, and decrease in MUC16, correlates with increased adhesiveness.
It is known that cells behave differently in a culture plate or in a single-cell suspension as compared to a 3-dimensional multi-cell environment. A previous study showed that BLS interacted with cells uniquely based on cell type and soluble factors present,16,20 making this type of system an invaluable tool for determining whether our single-cell assays were translatable to a cellular structure more reminiscent of the embryo. We analyzed 3-dimensional spheroids layered onto epithelial monolayers with and without hCG and, indeed, the treatment with hCG increased the intimacy of the 2-cell types and maintained the cell–cell interaction over 72 hours, while nontreated BLS were easily aspirated from the wells, supporting the conclusions made from the single-cell adhesion tests.
In conclusion, we have developed a system to characterize new proteins or factors hypothesized to improve fertility by improving blastocyst apposition and attachment to the endometrium. Using this system, we tested hCG, a factor recently shown in clinical trials to improve implantation rates, and found changes in genes at the mRNA (mucin) and protein (OPN) levels that are known to contribute to embryo attachment. The attachment was further confirmed using 1- and 3-dimensional cell adhesion assays. A comprehensive knowledge of the cellular and gene targets of hCG, and other such candidates, will not only improve our understanding of the events surrounding implantation but will also increase the number of therapeutic targets for subfertile patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: Funding source is NIH/NICHD P01 HD054713-01A1 and Cooper Surgical.
References
- 1. Psychoyos A. Uterine receptivity for nidation. Ann N Y Acad Sci. 1986;476:36–42. [DOI] [PubMed] [Google Scholar]
- 2. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400–1408. [DOI] [PubMed] [Google Scholar]
- 3. Mansour R, Tawab N, Kamal O, et al. Intrauterine injection of human chorionic gonadotropin before embryo transfer significantly improves the implantation and pregnancy rates in in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril. 2011;96(6):1370–1374.e1371. [DOI] [PubMed] [Google Scholar]
- 4. Keay SD, Vatish M, Karteris E, Hillhouse EW, Randeva HS. The role of hCG in reproductive medicine. BJOG. 2004;111(11):1218–1228. [DOI] [PubMed] [Google Scholar]
- 5. Reisinger K, Baal N, McKinnon T, Munstedt K, Zygmunt M. The gonadotropins: tissue-specific angiogenic factors? Mol Cell Endocrinol. 2007;269(1-2):65–80. [DOI] [PubMed] [Google Scholar]
- 6. Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol. 2007;269(1-2):85–92. [DOI] [PubMed] [Google Scholar]
- 7. Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150(6):2882–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kosaka K, Fujiwara H, Tatsumi K, et al. Human chorionic gonadotropin (HCG) activates monocytes to produce interleukin-8 via a different pathway from luteinizing hormone/HCG receptor system. J Clin Endocrinol Metabol. 2002;87(11):5199–5208. [DOI] [PubMed] [Google Scholar]
- 9. Schumacher A, Brachwitz N, Sohr S, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182(9):5488–5497. [DOI] [PubMed] [Google Scholar]
- 10. Wan H, Versnel MA, Cheung WY, et al. Chorionic gonadotropin can enhance innate immunity by stimulating macrophage function. J Leukoc Biol. 2007;82(4):926–933. [DOI] [PubMed] [Google Scholar]
- 11. Wan H, Versnel MA, Leijten LM, et al. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J Leukoc Biol. 2008;83(4):894–901. [DOI] [PubMed] [Google Scholar]
- 12. Kornyei JL, Lei ZM, Rao CV. Human myometrial smooth muscle cells are novel targets of direct regulation by human chorionic gonadotropin. Biol Reprod. 1993;49(6):1149–1157. [DOI] [PubMed] [Google Scholar]
- 13. Sherwin JR, Sharkey AM, Cameo P, et al. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology. 2007;148(2):618–626. [DOI] [PubMed] [Google Scholar]
- 14. Perrier d'Hauterive S, Charlet-Renard C, Berndt S, et al. Human chorionic gonadotropin and growth factors at the embryonic-endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum Reprod. 2004;19(11):2633–2643. [DOI] [PubMed] [Google Scholar]
- 15. Perrier d'Hauterive S, Charlet-Renard C, Dubois M, et al. Human endometrial leukemia inhibitory factor and interleukin-6: control of secretion by transforming growth factor-beta-related members. Neuroimmunomodulation. 2005;12(3):157–163. [DOI] [PubMed] [Google Scholar]
- 16. Sugihara K, Kabir-Salmani M, Byrne J, et al. Induction of trophinin in human endometrial surface epithelia by CGbeta and IL-1beta. FEBS Lett. 2008;582(2):197–202. [DOI] [PubMed] [Google Scholar]
- 17. Meseguer M, Aplin JD, Caballero-Campo P, et al. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod. 2001;64(2):590–601. [DOI] [PubMed] [Google Scholar]
- 18. Gipson IK, Blalock T, Tisdale A, et al. MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol Reprod. 2008;78(1):134–142. [DOI] [PubMed] [Google Scholar]
- 19. Johnson GA, Burghardt RC, Bazer FW, Spencer TE. Osteopontin: roles in implantation and placentation. Biol Reprod. 2003;69(5):1458–1471. [DOI] [PubMed] [Google Scholar]
- 20. Holmberg JC, Haddad S, Wunsche V, et al. An in vitro model for the study of human implantation. Am J Reprod Immunol. 2012;67(2):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Straszewski-Chavez SL, Abrahams VM, Alvero AB, et al. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30(11):939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schumacher A, Heinze K, Witte J, et al. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol. 2013;190(6):2650–2658. [DOI] [PubMed] [Google Scholar]
- 23. Jensen F, Wallukat G, Herse F, et al. CD19+CD5+ cells as indicators of preeclampsia. Hypertension. 2012;59(4):861–868. [DOI] [PubMed] [Google Scholar]
- 24. Birken S, Kovalevskaya G, O'Connor J. Immunochemical measurement of early pregnancy isoforms of HCG: potential applications to fertility research, prenatal diagnosis, and cancer. Arch Med Res. 2001;32(6):635–643. [DOI] [PubMed] [Google Scholar]