Abstract
Endometriosis has been associated with aberrant methylation in the eutopic endometrium. Using a genome-wide methylation array, we identified differentially methylated genes in the endometrium from women with or without endometriosis. One hundred and twenty genes were significantly altered by >1.5-fold. In all, 59 genes were significantly hypermethylated and 61 genes were significantly hypomethylated. Changes in gene expression associated with the altered methylation status were validated using quantitative real-time polymerase chain reaction. A limited number of candidate genes are selectively methylated in the endometrium of women with endometriosis. Several genes not previously associated with endometriosis are aberrantly methylated and expressed. These include O-6-methylguanine-DNA methyltransferase, dual specificity phosphatase 22, cell division cycle associated 2, inhibitor of DNA binding 2, retinoblastoma binding protein 7, bone morphogenetic protein receptor, type 1B, tumor necrosis factor receptor 1B, zinc finger protein receptor 681, immunoglobulin superfamily, member 21, and tumor protein 73. Aberrant DNA methylation and gene expression of these genes may contribute to abnormal regulation of endometrial cell proliferation and function in women.
Keywords: endometriosis, methylation, epigenetic modification
Introduction
Endometriosis is a complex, estrogen-dependent disorder that affects 5% to 15% of the reproductive-age female population. It can lead to pelvic pain, dysmenorrhea, and infertility.1-3 The defining characteristic of endometriosis is the ectopic growth of endometrial stromal and glandular cells outside of the uterine cavity. The lesions typically occur in the peritoneal cavity but occur less frequently in distant sites.2,4 Common explanations for the etiology of endometriosis include Sampson's theory of retrograde menstruation, altered immunity, metastatic spread of menstrual tissue to distant sites, and contribution of bone marrow-derived stem cells.4-8 Recent studies describe the role of environmental toxins and hormone exposure as alternate contributors to the development of endometriosis.9,10
The familial predisposition of endometriosis has led to the search for genetic alterations in these women.4,11-13 Although multiple candidate genes have been postulated, a mutation that commonly leads to endometriosis has not been identified.14,15 It is clear that gene expression is commonly altered in endometriosis and in the eutopic endometrium of women with the disease. Aberrantly expressed genes include those associated with regulation of transcription, proliferation, sex steroid metabolism, apoptosis, cell cycle, and cell adhesion.14,16 Epigenetic modification is a possible mechanism by which the expression of genes necessary for the establishment of endometriosis is altered.11,12
Epigenetic modifications include heritable changes to gene expression without changes in the DNA sequence.17 Examples include DNA methylation, histone modification, and gene regulation by noncoding RNA and micro-RNAs.17 DNA methylation, a widely understood epigenetic modification, occurs at the cytosine bases adjacent to the guanine nucleotide. With the addition of a methyl group, DNA methyltransferases (DNMTs) convert cytosine into 5′-methylcytosine.11,17-19 If a certain sequence contains a large number of CpG dinucleotide repeats, this region is referred to as the CpG island. CpG islands are short (100-200 bp to several kb in size) sequences found in nearly 60% of all genes.17 DNA methylation regulates gene expression and transcription by altering the chromatin structure. If the CpG islands of the promoter region for a particular gene are methylated, the expression of that gene may be downregulated or transcriptionally silenced; in contrast, if the CpG islands in the promoter region remain unmethylated, the gene is typically more highly expressed.20,21 Activation and/or aberrant expression of a gene can contribute to the development of endometriosis, cancers, or other abnormalities.22
Recent studies have linked endometriosis to alterations in DNA methylation.11 In this study, we used a genome-wide methylation screen to identify methylated genes with the potential to increase the development and progression of endometriosis.
Materials and Methods
Tissue Sampling
Endometrial biopsies were isolated from 13 reproductive-age patients undergoing surgical treatment of endometriosis (n = 7) or endometriosis-free controls (n = 6). The samples were collected from patients who had not used hormone-based therapies for a minimum of 3 months prior to sample collection. Immediately after surgical removal, a portion of the tissue was frozen in RNA later (Qiagen, Carlsbad, California) and used for the methylation array and quantitative polymerase chain reaction (q-PCR), and the remaining tissue was formalin fixed. The diagnosis of endometriosis was confirmed histologically. The Human Investigation Committee at Yale University approved the study protocol.
Genomic DNA Extraction
Genomic DNA was isolated from the eutopic endometrial tissues using the DNeasy Blood & Tissue Kit (Qiagen) according to the manufacturer’s instructions.
Illumina Infinium HumanMethylation27 Assay
Genomic DNA of 500 ng was bisulfate converted using the Zymo Bisulfate conversion kit (Zymo Research EZ DNA Methylation Kit, Irvine, California). Methylated and nonmethylated DNA standards were used as positive and negative controls to determine the efficiency of conversion. Illumina Infinium HumanMethylation27 RevB Beadchip (Illumina, San Diego, California) was used to survey the genome-wide methylation profile. Converted DNA of 200 ng was amplified using the Illumina Infinium protocol and hybridized to the microarray for 16 hours. Hybridization was followed by a single-base extension and fluorescent amplification using Tecan Freedom Evo (Tecan, Männedorf, Switzerland). Chips were dried, scanned (Illumina iScan System), and analyzed. GenomeStudio (Illumina) was used for bioinformatics and statistical analysis.
Quantitative Real-Time PCR
To validate the methylation microarray results, total RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, California) and then purified with the RNeasy MinElute Cleanup Kit (Qiagen, Valencia, California) according to the manufacturer’s instructions. To avoid DNA contamination, total RNA was treated with RNase-Free DNase (Qiagen) before purification. Purified RNA (50 ng) was reverse transcribed using iScript complementary DNA synthesis kit (Bio-Rad Laboratories, Hercules, California). Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green (Bio-Rad Laboratories) and optimized in the MyiQ Single Color Real-Time PCR Detection System (Bio-Rad Laboratories). The specificity of the amplified transcript was confirmed by a melting curve analysis. The reactions for each gene were performed in duplicate and repeated 3 times. Expression of O-6-methylguanine-DNA methyltransferase (MGMT), dual specificity phosphatase 22 (DUSP22), cell division cycle associated 2 (CDCA2), inhibitor of DNA binding 2 (ID2), retinoblastoma binding protein 7 (RBBP7), tumor necrosis factor receptor 1B (TNFRSF1B), bone morphogenetic protein receptor, type 1B (BMPR1B), zinc finger protein receptor 681 (ZNF681), Immunoglobulin superfamily, member 21 (IGSF21), and tumor protein 73 (TP73) messenger RNA (mRNA) was quantified and standardized to a reference gene (β-actin). Primer sequences used for each gene are listed in Table 1. The relative amount of transcript generated for each primer was analyzed on the basis of the cycle threshold (Ct) value. The relative gene expression ratio was calculated using 2−ΔΔCT. A P value of .05 or less was considered significant.
Table 1.
Primer Sequences Used for Quantitative Real-Time PCR for Both the Hyper- and Hypomethylated Genes Selected From the Array.
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| MGMT | GCAATGAGAGGCAATCCTGT | TACACGTGTGTGTCGCTCAA |
| DUSP22 | GTGCTGCCAAAAAGAAAAGC | GGATCCTTACAGGCATCCAA |
| CDCA2 | TTTGAAGCACCTGCCTTTCT | CAAGATTCTCCCCCTTGTCA |
| ID2 | CGTGAGGTCCGTTAGGAAAA | ATAGTGGGATGCGAGTCCAG |
| RBBP7 | TCCCAATGATGATGCACAGT | AGGATTCTGCGGCATGTAAC |
| TNFRSF1B | GGATGAAGCCCAGTTAACCA | GCAGAGGCTTTCCACAACTC |
| BMPR1B | AAAGGTCGCTATGGGGAAGT | GCAGCAATGAAACCCAAAAT |
| ZNF681 | TGGTCTCAGCTCACTGCAAC | GGGGTCAGGAGTTCAAGACA |
| IGSF21 | AAGCGAGAGGACCTGGTGTA | GCCATGACGTTGAGGAAGAT |
| TP73 | GGCAGGTCAGCTCACATCT | GAGTGGATGTTTGTGCGTTG |
Abbreviations: MGMT, O-6-methylguanine-DNA methyltransferase; DUSP22, dual specificity phosphatase 22; CDCA2, cell division cycle associated 2; ID2, inhibitor of DNA binding 2; RBBP7, retinoblastoma binding protein 7; BMPR1B, bone morphogenetic protein receptor, type IB; TNFRSF1B, tumor necrosis factor receptor 1B; ZNF681, zinc finger protein receptor 681; IGSF21, immunoglobin superfamily, member 21; TP73, tumor protein 73.
Results
Of the 27 578 genes on the methylation array, 120 genes were significantly altered by 1.5-fold or greater. Ten genes displaying a 1.5-fold change or higher and significant methylation (P < .05) were selected for the purpose of this study. The 10 genes explored in this study were chosen due to their novel association with endometriosis; these include genes with a role in inflammatory and apoptotic pathways. Examples of hypermethylated genes include MGMT, DUSP22, CDCA2, ID2, and RBBP7. Genes with decreased methylation include BMPR1B, TNFRSF1B, ZNF681, IGSF21, and TP73. The fold change ratio in expression between controls and endometriosis as well as the statistical significance of changes in expression of MGMT, DUSP22, CDCA2, ID2, RBBP7, BMPR1B, TNFRSF1B, ZNF681, IGSF21, and TP73 are listed in Table 2.
Table 2.
Methylation Status of the Genes Selected From the Illumina Infinium HumanMethylation27 With a Fold Change of 1.5 (P < .05) or Greater When Comparing Endometriosis to Endometriosis-Free Controls.
| Methylation Status | Gene Symbol | Gene Name | Fold Change Ratio (Endometriosis/Control) | P Value | Function |
|---|---|---|---|---|---|
| Hypermethylation | MGMT | O-6-methylguanine-DNA methyltransferase | 2.220 | .0194 | DNA repair enzyme |
| DUSP22 | Dual specificity phosphatase 22 | 2.108 | 1.449e−34 | Dephosphorylate both phosphotyrosine and phosphoserine/threonine residues within 1 substrate | |
| CDCA2 | Cell division cycle associated 2 | 1.686 | .0207 | Prevent cell cycle arrest and apoptosis | |
| ID2 | Inhibitor of DNA binding 2 | 1.616 | 3.259e−06 | Negative regulator of basic helix–loop–helix transcription factors | |
| RBBP7 | Retinoblastoma binding protein 7 | 1.648 | .007 | Histone-binding protein that may target chromatin remodeling factors | |
| Hypomethylation | TNFRSF1B | Tumor necrosis factor receptor 1B | −2.375 | 1.162e−05 | Proinflammatory cytokine involved in pathogenesis of inflammatory diseases |
| BMPR1B | Bone morphogenetic protein receptor, type 1B | −2.525 | .0271 | Growth factors involved in folliculogenesis | |
| ZNF681 | Zinc finger protein receptor 681 | −1.645 | 3.209e−09 | Regulation of transcription factors | |
| IGSF21 | Immunoglobulin superfamily, member 21 | −1.723 | .0001 | Proinflammatory cytokine involved in pathogenesis of inflammatory diseases | |
| TP73 | Tumor protein 73 | −1.893 | .0008 | Cell cycle arrest and apoptosis induction |
Correlation of DNA Methylation Change and mRNA Expression by qRT-PCR
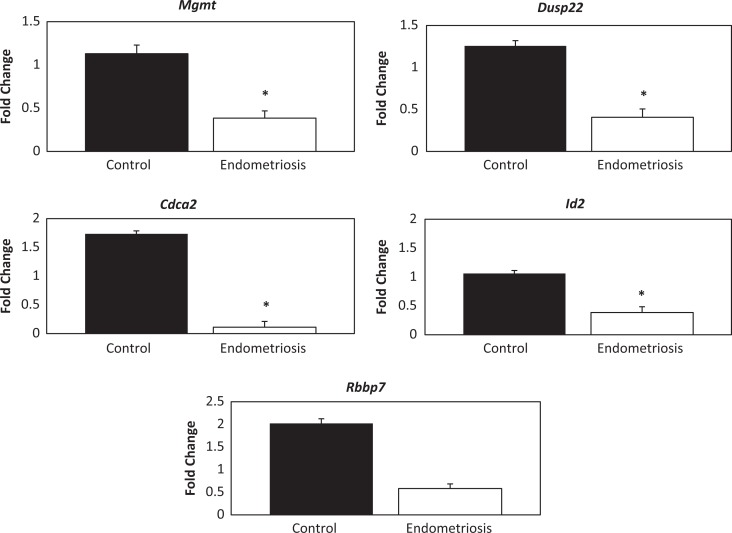
Gene expression associated with altered methylation status was validated using qRT-PCR. Hypermethylation of each gene was correlated with the level of gene expression (Figure 1). Gene expression of MGMT was significantly decreased in samples from women with endometriosis compared to controls (0.3-fold, P = .015). Expression of DUSP22 was decreased by 0.3-fold (P = .039), while expression of CDCA2 was also decreased by 0.06-fold (P = .03). Expression of ID2 was decreased by 0.4-fold (P = .004); however, RBBP7 while decreased by 0.3-fold did not reach significance (P = .069).
Figure 1.
Hypermethylated Genes: Quantitative real-time polymerase chain reaction (qRT-PCR) fold change results. * represents significance (P < .05) between the endometriosis and the control group.
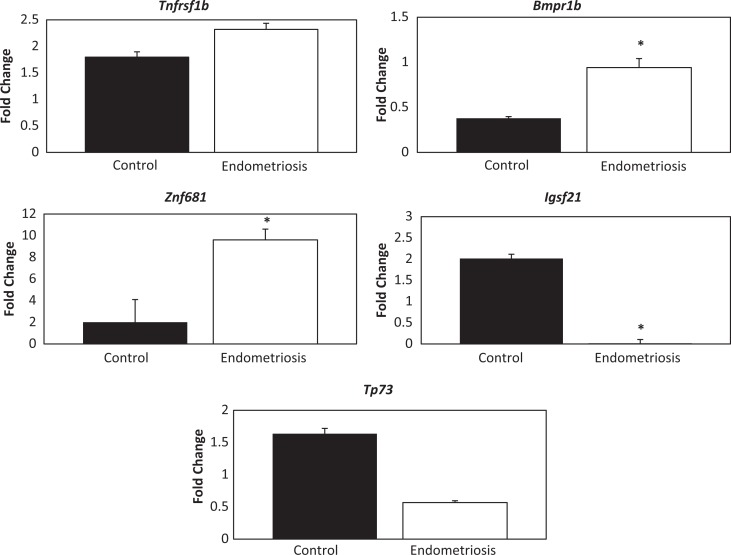
Expression was generally increased in genes noted to be hypomethylated; however, this trend was not consistently significant (Figure 2). Hypomethylation of TNFRS1B resulted in increased gene expression, specifically a 1.3-fold change, when comparing the endometriosis to the endometriosis-free controls (P > .05); BMPR1B was highly expressed in the endometrium of females with endometriosis (2.49-fold, P > .05). Expression of ZNF681 was significantly increased by a 4.8-fold change (P = .003); in contrast, expression of the hypomethylated gene IGSF21 was decreased by 0.002-fold (P = .03). Gene expression of TP73 was not significantly changed (0.346-fold; P > .05).
Figure 2.
Hypomethylated Genes: Quantitative real-time polymerase chain reaction (qRT-PCR) fold change results. * represents significance (P < .05) between the endometriosis and the control group.
Discussion
In this study, we provide a comprehensive survey on the extent of DNA methylation in the endometrium of women with endometriosis. DNA methylation is a mechanism that likely leads to altered gene expression in endometriosis and appears to be selective, limited to a relatively small number of genes. Genome-wide methylation identifies novel genes potentially involved in the development and pathogenesis of endometriosis.
Increased proliferation, invasion, and resistance to apoptosis may potentially explain the pathogenesis of endometriosis; several of these characteristic behaviors of endometriosis have been previously linked to epigenetic alterations.23,24 Sex steroid hormones regulate the proliferation of endometriosis, and the expression of steroid hormone receptors vary between normal eutopic endometrium and endometriosis.25 Expression of estrogen receptor β (ER-β) is significantly higher in endometriosis due to the hypermethylation of the CpG islands in the estrogen receptor 2 gene.25,26 Higher expression of estrogen receptor amplifies estrogenic effects and the proliferative capacity of endometrial cells.26,27 The ER-β regulates genes involved in cell cycle progression, apoptosis, and signal transduction. Ki-67 is a nuclear protein involved in cellular proliferation and highly expressed in endometriosis.24 Bcl-2 is a regulator of the programmed cell death process and inhibits apoptosis in the endometrium and the endometrial lesions.28,29 Both Ki-67 and Bcl-2 expression have been closely linked to estrogen receptor level.29 Estrogen signaling is likely epigenetically amplified in endometriosis leading to increased proliferation and resistance to apoptosis.
Previous reports have identified some of the epigenetic regulators responsible for global changes in DNA methylation in endometriosis.30 DNA methyltransferases (DNMT1, DNMT3a, and DNMT3b) are highly expressed in endometrial lesions. High levels of expression of DNMTs can lead to transcriptional activation or silencing of critical genes involved in regulating cell growth and apoptosis, respectively, thereby resulting in the survival of endometrial cells.30 Here, DNMT1 expression was increased and its methylation was altered by 1.13-fold. Expression of DNMT3a and DNMT3b was repressed and methylation altered by 1.04-fold and 1.01-fold, respectively. None of the changes in DNMT methylation met our predetermined threshold or reached statistical significance. In our study, we provide a comprehensive survey of the extent of DNA methylation in endometriosis. DNA methylation leads to altered gene expression in endometriosis; however, methylation appears to be selective, limited to a relatively small number of genes. The mechanism by which DNMTs selectively target individual genes in this disease remains to be determined.
Genome-wide methylation identifies genes potentially involved in the development and pathogenesis of endometriosis. Several genes were identified in this study that can be implicated in the pathophysiology of endometriosis. The MGMT, DUSP22, CDCA2, ID2, TNFRSF1B, ZNF681, and IGSF21 may have a role in the etiology and pathogenesis of this disease.31-40 The hypermethylation of methyltransferase (MGMT) has been reported to decrease apoptotic responses to DNA damage by TP73 and cell proliferation regulation by RBBP7, which in combination with the aberrant expression of HOXA10, increase the susceptibility for endometriosis.12,41-48 Zinc finger protein 681 contains a classical C2H2 (Kruppel) zinc finger motif and functions as an activator and repressor of transcription, regulating proliferation, differentiation, and development.38,49 The IGSF21, along with other members of the IGSF superfamily, has functions linked to endometriosis. Members of the IGSF superfamily, including intercellular adhesion molecule 1, have been shown to interfere with endometrial cell adhesion, while ALCAM, an activated leukocyte–cell adhesion molecule, has significantly decreased expression in the endometrium of women with endometriosis.39,50-52 Aberrant methylation of IGSF21 may lead to altered immune response cell signaling and cell–cell adhesion, resulting in the migration of ectopic endometrial cells and characteristic of endometriosis.
The genes discussed earlier were selected for their relatively large increase in methylation, the potential for involvement in pathways expected to be altered in endometriosis, and their novelty to the field. Numerous genes, where altered methylation in endometriosis has been previously established, were also identified in this array, although some were below our selected threshold. We have previously shown that HOXA10 expression is lower in the endometrium of women with endometriosis and is abnormally methylated.44,53,54 Here, HOXA10 expression was repressed and methylation was altered by 1.3-fold (P = .008). This level of epigenetic alteration was below our arbitrary threshold, suggesting that other genes may have alterations in methylation that regulate the development of this disease. The threshold selected is likely too restrictive to identify all genes epigenetically altered that may have a significant role in endometriosis.
We did not find significant changes in the methylation of several genes previously reported to display altered methylation in endometriosis; these include PR-B, CYP19A1 (aromatase), SF1, COX2, and ER-β. Although the differences may be due to sampling eutopic versus ectopic endometrium, Borghese et al found similar methylation profiles in the ectopic lesions and the eutopic endometrium obtained from women with disease.55 Many of the genes identified by Borghese et al as having significantly altered methylation in endometriosis were also identified in our array; these include MAFB, HOXD10, and HOXD11. Differences between our data and other published studies may be due to the ability of targeted assessment to identify small but meaningful areas of DNA methylation not identified in the array. Patient/disease characteristics, arbitrary cutoff thresholds, and location of targeted loci may have varied between studies.
Typically enhanced methylation was associated with decreased expression of a gene, although diminished methylation correlated with higher levels of gene expression. DNA methylation is thought to interfere with binding of transcriptional regulators. The assembly of a functional transcription initiation complex may be prevented by altered methylation. Alternatively, methylation that prevents the binding of a transcriptional repressor could have an opposite effect. Indeed, most of the methylated genes whose expression we examined were repressed; similarly, most of the genes with diminished methylation showed increased expression. However, in some instances, the opposite was true, suggesting that those methylated bases are sites of transcriptional repression. Future studies will explore the transcriptional regulators that bind to these sites.
Methylation was both increased and decreased at various loci in the genome. It is apparent that the changes in gene expression are context dependent and not simply the result of increased expression of a single methylating enzyme. Altered methylation in either direction implies that the mechanisms responsible may be directed by multiple pathways and vary with the type and stage of disease. More comprehensive surveys of diverse endometriosis variants will be required to categorize the extent and range of epigenetic alteration in endometriosis. After careful characterization, the extent of epigenetic regulation may be a useful marker of disease as it is detectable in eutopic endometrium.
A precise understanding of epigenetics may provide a novel approach to the treatment of endometriosis. Current therapeutic approaches are based on hormonal manipulation or the surgical removal of the endometrial implants. These treatments have limited long-term success with frequent side effects.56-59 Here, we identify epigenetic alterations in endometriosis; however, methylation and demethylation are both common, making the broad use of agents that alter methylation in a single direction impractical. The potential use of targeted therapies to correct epigenetic errors would be advantageous for the treatment of endometriosis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: NIH U54 HD052668.
References
- 1. Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med. 2001;345 (4):266–275. [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364 (9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 3. Giudice LC. Endometriosis. N Engl J Med. 2010;362 (25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39 (4):535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Ostet Gynecol. 1927;14:422–469. [Google Scholar]
- 6. Gruenwald P. Origin of endometriosis form the mesenchyme of the celomic walls. Am J Obstet Gynecol. 1942;44:470–474. [Google Scholar]
- 7. Dmowski PW, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):245–263. [DOI] [PubMed] [Google Scholar]
- 8. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25 (8):2082–2086. [DOI] [PubMed] [Google Scholar]
- 9. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82(6):1501–1508. [DOI] [PubMed] [Google Scholar]
- 10. Rier SE. The potential role of exposure to environmental toxicants in the pathophysiology of endometriosis. Ann N Y Acad Sci. 2002;955:201–212. [DOI] [PubMed] [Google Scholar]
- 11. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15 (10):587–607. [DOI] [PubMed] [Google Scholar]
- 12. Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193 (2):371–380. [DOI] [PubMed] [Google Scholar]
- 13. Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137 (3):327–331. [DOI] [PubMed] [Google Scholar]
- 14. Vigano P, Somigliana E, Vignali M, Busacca M, Blasio AM. Genetics of endometriosis: current status and prospects. Front Biosci. 2007;12:3247–3255. [DOI] [PubMed] [Google Scholar]
- 15. Grechukhina O, Petracco R, Popkhadze S, et al. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med. 2012;4 (3):206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bischoff F, Simpson JL. Genetics of endometriosis: heritability and candidate genes. Best Pract Res Clin Obstet Gynaecol. 2004;18 (2):219–232. [DOI] [PubMed] [Google Scholar]
- 17. Nasu K, Kawano Y, Tsukamoto Y, et al. Aberrant DNA methylation status of endometriosis: epigenetics as the pathogenesis, biomarker and therapeutic target. J Obstet Gynaecol Res. 2011;37 (7):683–695. [DOI] [PubMed] [Google Scholar]
- 18. Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9 (16):2395–2402. [DOI] [PubMed] [Google Scholar]
- 20. Ng HH, Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev. 1999;(2):158–163. [DOI] [PubMed] [Google Scholar]
- 21. Robertson KD. DNA methylation and chromatin-unraveling the tangled web. Oncogene. 2002;21 (35):5361–5379. [DOI] [PubMed] [Google Scholar]
- 22. Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. [DOI] [PubMed] [Google Scholar]
- 23. Izawa M, Taniguchi F, Terakawa N, Harada T. Epigenetic aberration of gene expression in endometriosis. Front Biosci (Elite Ed). 2013;5:900–10. [DOI] [PubMed] [Google Scholar]
- 24. Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertil Steril. 2009;92 (4):1246–1249. [DOI] [PubMed] [Google Scholar]
- 25. Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30 (1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xue Q, Lin Z, Yin P, et al. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92 (8):3261–3267. [DOI] [PubMed] [Google Scholar]
- 27. Xue Q, Lin Z, Cheng YH, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77 (4):681–687. [DOI] [PubMed] [Google Scholar]
- 28. Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59(7 suppl):1693s–1700s. [PubMed] [Google Scholar]
- 29. Risberg B, Karlsson K, Abeler V, Lagrelius A, Davidson B, Karlsson MG. Dissociated expression of Bcl-2 and Ki-67 in endometrial lesions: diagnostic and histogenetic implications. Int J Gynecol Pathol. 2002;21 (2):155–160. [DOI] [PubMed] [Google Scholar]
- 30. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril. 2007;87 (1):24–32. [DOI] [PubMed] [Google Scholar]
- 31. Christmann M, Verbeek B, Roos WP, Kaina B. O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry. Biochim Biophys Acta. 2011;1816 (2):179–190. [DOI] [PubMed] [Google Scholar]
- 32. Peggs AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res. 2000;462 (2-3):83–100. [DOI] [PubMed] [Google Scholar]
- 33. Sekine Y, Ikeda O, Hayakawa Y, et al. DUSP22/LMW-DSP2 regulates estrogen receptor-alpha-mediated signaling through dephosphorylation of Ser-118. Oncogene. 2007;26 (41):6038–6049. [DOI] [PubMed] [Google Scholar]
- 34. Uchida F, Uzawa K, Kasamatsu A, et al. Overexpression of CDCA2 in human squamous cell carcinoma: correlation with prevention of G1 phase arrest and apoptosis. PLoS One. 2013;8 (2):e56381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang P, Zhao Y, Sun XH. Notch-regulated periphery B cell differentiation involves suppression of e protein function. J Immunol. 2013;191 (2):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. So T, Croft M. Regulation of PI-3-kinase and Akt signaling in T lymphocytes and other cells by TNFR family molecules. Front Immunol. 2013;4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schall TJ, Lewis M, Koller KJ, et al. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990;61 (2):361–370. [DOI] [PubMed] [Google Scholar]
- 38. Dang DT, Pevsner J, Yang VW. The biology of the mammalian Krüppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32 (11-12):1103–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh H, Aplin JD . Adhesion molecues in endometrial epithelium: tissue integrity and embryo implantation. J Anat. 2009;215 (1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golias C, Batistatou A, Bablekos G, et al. Physiology and pathophysiology of selectins, integrins, and IGSF cell adhesion molecules focusing on inflammation. A paradigm model on infectious endocarditis. Cell Commun Adhes. 2011;18(3):19–32. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, He X, Yu Q, Eng C. Androgen receptor-induced tumor suppressor, KLLN, inhibits breast cancer growth and transcriptionally activates p53/p73-mediated apoptosis in breast carcinomas. Hum Mol Genet. 2013;22 (11):2263–2272. [DOI] [PubMed] [Google Scholar]
- 42. Jacobs DI, Hansen J, Fu A, et al. Methylation alterations at imprinted genes detected among long-term shiftworkers. Environ Mol Mutagen. 2013;54 (2):141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodhe J, Kavanagh E, Joseph B. TAp73β-mediated suppression of cell migration requires p57Kip2 control of actin cytoskeleton dynamics. Oncotarget. 2013;4 (2):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80 (1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ebata A, Suzuki T, Takagi K, et al. Oestrogen-induced genes in ductal carcinoma in situ: their comparison with invasive ductal carcinoma. Endocr Relat Cancer. 2012;19 (4):485–496. [DOI] [PubMed] [Google Scholar]
- 46. Miglori V, Müller J, Phalke S, et al. Symmetric dimethylation of H3R2 is a newly identified histone mark that supports euchromatin maintenance. Nat Struct Mol Biol. 2012;19 (2):136–144. [DOI] [PubMed] [Google Scholar]
- 47. Yang J, Kiefer S, Rauchman M. Characterization of the gene encoding mouse retinoblastoma binding protei-7, a component of chromatin-remodeling complexes. Genomics. 2002;80 (4):407–415. [DOI] [PubMed] [Google Scholar]
- 48. Zhang TF, Yu SQ, Wang ZY. RbAp46 inhibits estrogen-stimulated progression of neoplastigenic breast epithelial cells. Anticancer Res. 2007;27 (5A):3205–3209. [PubMed] [Google Scholar]
- 49. Buck-Koehntop BA, Stanfield RL, Ekiert DC, et al. Molecular basis for recognition of methylated and specific DNA sequences by the zinc finger protein Kaiso. Proc Natl Acad Sci USA. 2012;109 (38):15229–15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van De Stolpe A, Van Der Saag PT. Intercellular adhesion molecule-1. J Mol Med (Berl). 1996;74(1):13–33. [DOI] [PubMed] [Google Scholar]
- 51. Zhang H, Niu Y, Feng J, Guo H, Ye X, Cui H. Use of proteomic analysis of endometriosis to identify different protein expression in patients with endometriosis versus normal controls. Fertil Steril. 2006;86(2):274–282. [DOI] [PubMed] [Google Scholar]
- 52. Wong W, Dye DE, Coombe DR. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol. 2012;2012:340296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93 (6):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim JJ, Taylor HS, Lu Z, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13 (5):323–332. [DOI] [PubMed] [Google Scholar]
- 55. Borghese B, Barbaux S, Mondon F, et al. Research resource: genome-wide profiling of methylated promoters in endometriosis reveals a subtelomeric location of hypermethylation. Mol Endocrinol. 2010;24 (9):1872–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allen C, Hopewell S, Prentice A, Gregory D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst. 2009;(2):CD004753. [DOI] [PubMed] [Google Scholar]
- 57. Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst. 2009;(1):CD005997. [DOI] [PubMed] [Google Scholar]
- 58. Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG. 2008;115 (7):818–822. [DOI] [PubMed] [Google Scholar]
- 59. Rodgers AK, Falcone T. Treatment strategies for endometriosis. Expert Opin Pharmacother. 2009;9 (2):243–255. [DOI] [PubMed] [Google Scholar]