Abstract
Preterm premature rupture of membranes (PPROM) occurs in 1% to 2% of births. Impact of PPROM is greatest in low- and middle-income countries where prematurity-related deaths are most common. Recent investigations identify cytokine and matrix metalloproteinase activation, oxidative stress, and apoptosis as primary pathways to PPROM. These biological processes are initiated by heterogeneous etiologies including infection/inflammation, placental bleeding, uterine overdistention, and genetic polymorphisms. We hypothesize that pathways to PPROM overlap and act synergistically to weaken membranes. We focus our discussion on membrane composition and strength, pathways linking risk factors to membrane weakening, and future research directions to reduce the global burden of PPROM.
Keywords: preterm premature rupture of membranes, chorioamnion, fetal membranes, intraamniotic infection, abruption, oxidative stress, inflammation, preterm birth, PPROM, chorioamnionitis
Introduction
Fetal membranes are a resilient tissue, designed to withstand the insults of a lengthy pregnancy. However, they also ultimately give way to rupture and labor. In 1% to 2% of pregnancies, fetal membranes rupture preterm and outside of the context of labor, a problem known as preterm premature rupture of membranes (PPROM).1 The impact of PPROM is greatest in low- and middle-income countries where the majority of childhood deaths associated with prematurity occur.2 When compared to high-income countries, PPROM occurs with a similar frequency but is associated with greater maternal and neonatal morbidity and mortality.3 Maternal morbidity often arises from an associated intrauterine infection (chorioamnionitis) that complicates both PPROM and premature rupture of membranes (PROM) at term. Perinatal mortality, which largely occurs from prematurity and infectious complications, is high in low- and middle-income countries with a range from 55 of 1000 to 520 of 1000 births.4-7
A better understanding of the pathophysiology leading to PPROM is imperative to reduce the global burden of prematurity and its associated neonatal and maternal consequences. Several clinical conditions are associated with PPROM including infection/inflammation, decidual bleeding (abruption), uterine overdistention (eg, twins), genetic predispositions, and cigarette smoking. Although these conditions occur at different stages of gestation and affect pregnancies variably, their pathways to membrane degradation and ultimate rupture overlap (Figure 1). Recent investigations identify matrix metalloproteinases (MMPs), cytokines, apoptosis, and oxidative stress as primary mechanisms in these processes. We hypothesize that most cases of PPROM result from the synergistic actions of several activated pathways to biochemically weaken the membranes. These factors can act in synergy to cross a biomechanical threshold leading to pathologic rupture of membranes.
Figure 1.
Factors contributing to chorioamnion weakening. Considerable overlap exists among the biological mechanisms and pathways to PPROM initiated by different clinical conditions. This diagram lists factors contributing to chorioamnion weakening by clinical risk factor and demonstrates commonalities among these pathways. Pathway factors including infection and inflammation, placental bleeding (abruption), and genetic variants have been linked with PPROM. Common biological mediators are noted for uterine overdistention and cigarette smoking and may explain the increased risk of PPROM among women with these conditions. Common themes among pathways include MMP activation, cytokine and chemokine activation and oxidative stress, all leading to collagen weakening. With the initiation of multiple pathways, weakening is accelerated, ultimately leading to membrane rupture. PPROM indicates preterm premature rupture of membranes; MMP, matrix metalloproteinase.
The aim of this review is to reveal connections among pathways implicated in PPROM. First, we provide an overview of the anatomy of the fetal membranes and studies of its biomechanical strength. Next, we discuss biological mechanisms and clinical risk factors implicated in PPROM, emphasizing shared pathways. We also review the role of progesterone to prevent PPROM in the context of recent clinical trials. Finally, we discuss interesting areas for further research that arise from commonalities between these pathways to enable the next steps to reducing PPROM and the global burden of prematurity.
Membrane Composition and Strength
Anatomy
The chorioamnion consists of 2 fetal membranes that enclose the amniotic cavity: the chorion and the amnion. This membrane functions to contain and regulate amniotic fluid volume around the fetus, selectively transport molecules, and protect the fetus from vaginal bacteria.8 The amnion is a thin avascular layer derived from extraembryonic ectoderm and avascular mesoderm attached to the underlying chorion (Figure 2). The amnion has 5 distinct layers.9 Studies of the chorioamnion at term suggest that the amnion, while thinner, is more robust than the chorion with greater membrane strength and lower likelihood of rupture.10,11 Within the amniotic epithelium, intracellular cytoskeletal proteins and intermediate filaments contribute to shear strength.12 The highly folded basal surface of the amniotic epithelium forms a tight interface with the basement membrane, anchoring the descending filaments into the underlying layers. These descending filaments include type I and type III collagen, which are susceptible to degradation by MMPs (Figure 2). The myofibroblast layer secretes anionic proteoglycans and type I and type III collagen for the 2 adjacent layers: the compact and spongy layers. Differing structures of collagen tissue organization in the compact and spongy layers may relate to their respective biochemical properties.9,12,13 Although controversial, the compact layer may provide resistance to shear stresses through the presence of elastin.14,15 The spongy layer, a layer prone to edema and rich with hyaluronan, cushions the underlying chorion by sliding over its surface.13,16 Collectively these layers provide the amnion with its strength and integrity.
Figure 2.
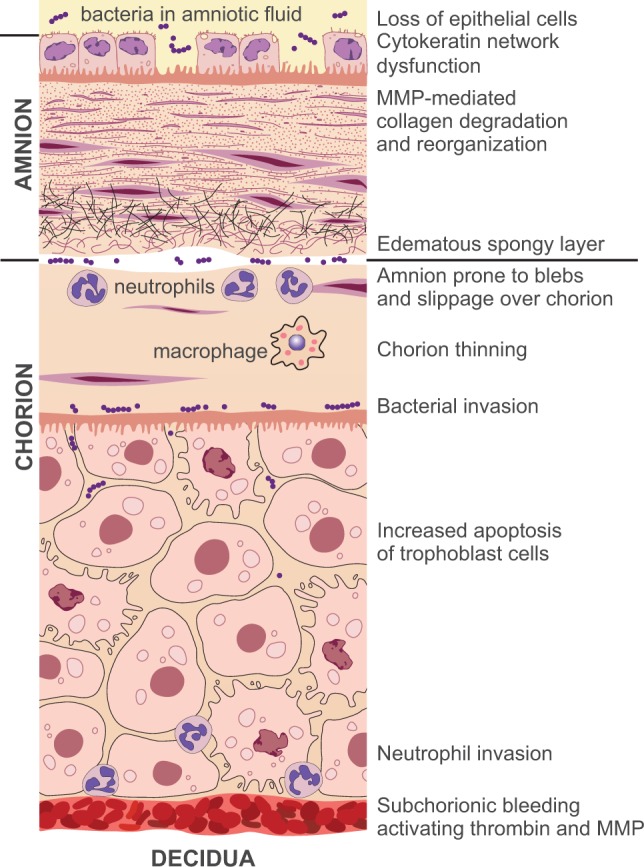
Structural changes with membrane weakening. We depict structural changes that occur with membrane degradation, using the infection and bleeding pathways as examples. Bacteria invade the space between amnion and chorion, infect the amniotic fluid, or enter via the placenta. Blood accumulates between the chorion and decidua. The most important structural changes occur in the amnion, the stronger of the 2 layers. Cytokeratins and other cytostructural genes become downregulated leading to a loss of cytostructural support and tensile strength in the amnion. Amniotic epithelial cells become pyknotic and slough leaving a “punched out” appearance in the membrane. Collagen throughout the amnion degrades and reorganizes with activation of MMPs. The spongy layer of the amnion becomes edematous and prone to slippage over the chorion creating blebs that result in separation of the membranes. Trophoblast cells undergo apoptosis and the chorion thins. Neutrophilic invasion occurs both in the reticular layer and near the interface with decidua. These processes occur through inflammatory activation of the myometrium, MMP activation, and bacterial proteolysis. Exposure to activated MMPs leads to collagen reorganization and degradation throughout the amnion including derangement of the anchoring descending collagen from the basement membrane. MMP indicates matrix metalloproteinase.
The 3 layers of the chorion, the reticular layer, the basement membrane, and trophoblast cells make up the bulk of the chorioamnion membrane. The chorion also contributes to the strength and elasticity of the membranes. Laminin stabilizes the membrane and fibronectin allows for adherence of the chorion to the decidua. Microfibrils, elastin, and collagen also contribute to the elasticity of the chorion.17,18 Accommodation is an integral part of chorioamnion function, and membranes with PPROM demonstrate decreased elasticity.19 The chorion undergoes active remodeling throughout pregnancy and is susceptible to apoptotic destruction, changes in MMP activity, and prostaglandin production. The interface between the overlying amnion and the chorion is prone to blebs and slippage allowing for easy separation of the 2 membranes upon examination (Figure 2). In the embryonic stage, the chorion is of uniform thickness throughout. With implantation, chorion trophoblasts invade the underlying decidua at the site of the future placental disk, whereas the remaining trophoblastic villi progressively atrophy forming the chorion laeve. This process continues until 16 weeks gestation resulting in a gradual spread of the chorion laeve to cover 70% of the surface of the chorionic sac.20 Biomechanics of the membrane may therefore depend on the distance of the sampled membrane from the placental disk due to varying trophoblastic composition. This is an important observation in the critical evaluation of biomechanical studies.
Membrane Biomechanics
Membrane biomechanics are impacted by both the complexity of the layers of the chorioamnion and the orientation of collagen fibrils. When compared to other collagen-based tissues (eg, aorta), fetal membranes are physiologically under high stress/strain and are loaded relatively closer to the failure threshold.21 Assessment of membrane biomechanical properties is important, as membranes are placed under constant stretch from approximately 28 weeks gestation onward due to the expanding uterus.19 Membrane tensile strength is variable across the tissue with notable differences between intact membranes and those that spontaneously ruptured.22-24 Improved use of placental mapping further identified heterogeneity within the chorioamnion with the weakest region consistently overlying the cervix in both term and preterm membranes.24,25 Histologically, this weak area represents a unique “zone of altered morphology” suggesting its vulnerability to eventual rupture. Not surprisingly, membranes overlying the cervix are exposed to different environmental conditions than unexposed membranes. Taking into account, the sampling site is important when considering the results of biomechanical studies of membrane strength.
Studies to evaluate chorioamnion strength vary in design and technique. “Axial testing” occurs when membranes are suspended in 1 or 2 axes (uniaxial or biaxial testing) and are subjected to sequential stretch until rupture occurs. This type of testing may best approximate physiologic conditions of membrane rupture in pregnancy. Biaxial testing demonstrates that pressures required to produce membrane rupture readily exceed those contributed by physiologic intrauterine pressures alone.26 Although technically challenging, “burst testing” involves progressive loading of the membranes with saline or air thereby replicating the tension placed on membranes with a dilated cervix. Burst pressure needed to cause rupture peaks prior to 38 weeks and declines with advancing gestation, suggesting that parturition-associated changes may contribute to membrane weakening.27 “Puncture testing” examines the membranes ability to withstand variously shaped blunt objects. Estimations of membrane strength are, therefore, dependent on the method of biomechanical study, the distance from the cervix or membrane rupture site, and the gestational conditions. Table 1 demonstrates the heterogeneity of study designs reporting the force required to rupture membranes. To better place new biomechanical data into context, future studies should document gestational age, mode of delivery, presence or absence of labor, and sampled membrane relation to the rupture site, placental disk, and cervical os.
Table 1.
Summary of Studies Evaluating Force Required to Rupture Fetal Membranes.a,b
| Study | Population | Sampling | Failure Value | ||||
|---|---|---|---|---|---|---|---|
| Size | Gestation | Conditions | Size | Placental Region Sampled | |||
| Uniaxial testing | |||||||
| Oxlund et al10 | 7 | Term | Labor | 40 × 40 mm | “Halfway between placental edge and rupture site” | 0.95 N (237.7 N/m) | |
| Burst Testing | |||||||
| Polishuk et al28 | 68c | Term | Uncomplicated at 6, 12, and 24 hours postdelivery | 54 mm FD | Unknown | 0.21 kg/cm (201.1 N/m) | |
| 10c | Preterm | Uncomplicated at 6, 12, and 24 hours postdelivery | 54 mm FD | Unknown | 0.26 kg/cm (255.0 N/m) | ||
| MacLachlan26 | 126 | 56 Term 17 Preterm 53 Unknown | 73 SVD 53 CD | 20 mm FD | (1) At site of rupture (2) “Halfway” (3) Distal to rupture | 393 mm Hgd (261.98 N/m) | |
| Lavery and Miller29 | 20 | Term | Uncomplicated | 76.2 mm FD | Unknown | 60 mm Hg (152.39 N/m) | |
| Lavery and Miller30 | 66 | 54 Term 12 Preterm | Uncomplicated term Preterm PPROM | 76.2 mm FD | Unknown | 40 mm Hgd (101.59 N/m) | |
| Puncture Testing | |||||||
| El Khwad et al24 | 12 | Term | Elective term CD | 25 mm FD 3.2 mm PD | Cervical zone (within 10 cm of cervix) Non-cervical zone | 4.98 ± 1.38 N 9.07 ± 2.61 N | |
| Oyen et al31 | 32 | 18 Term 6 Preterm 8 Multiples | 9 Term unlabored CD 9 Term labored SVD 6 Preterm unknown 8 Multiples unknown | 20 mm FD 3.2 mm PD | “Away from clinical rupture site” | 4.04 ± 1.52 N (term) 5.09 ± 1.30 N (preterm) | |
| Arikat et al11 | 8 | Term | SVD | 25 mm FD 10 mm PD | 1) Within 5 cm of area overlying cervical os 2) Outside of 5 cm from area overlying cervical os | 9.70 ± 2.42 N (154.54 N/m) | |
| Oyen et al33 | 78c | 39 Term 14 Preterm | 20 SVD 19 CD, no labor 7 SVD 7 CD, no labor | 20 mm FD 3.2 mm PD | “Away from clinical rupture site” | 3.47 ± 1.57 N(term SVD) 4.10 ± 1.62 N(term CD) | |
Abbreviations: FD, Foramen diameter: the diameter of the ring which secures the membrane specimen in puncture and burst testing studies; PD, probe diameter: the diameter of the probe in puncture testing studies; SVD, spontaneous vaginal delivery; CD, cesarean delivery; PPROM, preterm premature rupture of membranes.
a Note the heterogeneity of gestational ages and conditions under which fetal membranes were collected. Also included are the size and location of sampled membrane and the averaged maximum force recorded prior to membrane rupture (failure value).
b Adapted from Joyce et al.21
c Authors report number of samples obtained, not patients enrolled. These studies may have sampled multiple specimens from 1 placenta.
d Pooled failure value among all samples. Data were pooled only when differentiation between preterm and term was not reported.
Biomechanical studies have also examined the sequential effects of force applied to the amnion alone, chorion alone, and the full thickness chorioamnion.11,32 The amnion is significantly stronger than the chorion, yet it only represents 20% of the overall membrane thickness.10,11 The amnion strength also varies with gestational age and with labor, while chorion strength remains.33 Video analysis of puncture testing from membranes obtained after term vaginal deliveries indicates that membrane rupture begins with distention of the intact membrane, followed by amnion separation from the choriodecidua, choriodecidual failure (chorion rupture) permitting further distention of the now isolated amnion, and ultimately amnion rupture.11 This observation is further supported by studies that note a thinner chorion among samples from PPROM compared to the chorion after preterm labor, preterm birth without preterm labor, and at term.34 Pathways active in weakening the chorion may represent the earliest biological events leading to PPROM.
Biological Mechanisms Implicated in PPROM
Matrix Metalloproteinases and the “Zone of Altered Morphology”
In the 1990s, term placentas were evaluated to better understand the histology of rupture sites compared to the remainder of chorioamnion. Extracellular matrix of samples obtained from term membrane rupture sites exhibited marked edema, disruption, and chorionic thinning when compared to samples obtained distal to the rupture site.15 It was proposed that this “zone of altered morphology” was related to disorganization and disruption of collagen fibrils.17
Collagen is susceptible to degradation by MMPs, a family of zinc enzymes activated at term, in the setting of PPROM or infection.9,35 The MMP can degrade many types of extracellular proteins including collagen and can cleave cell surface receptors, release ligands-stimulating apoptosis (eg, FAS ligand), and activate chemokines and cytokines. The results of MMP activation and other biological mechanisms contributing to PPROM are shown in Figure 2. Tissue inhibitors of metalloproteinases (TIMPs) act as an MMP counterbalance to prevent enzymatic degradation of the membranes. Although other enzymatic processes, like oxidative stress, also contribute to extracellular matrix degradation, MMP activity represents the best-studied mechanism associated with PPROM.
Several MMPs contribute to enzymatic degradation of collagen in the setting of lower TIMP activity. Cervical cells such as fibroblasts, smooth muscle cells, and granulocytes release MMPs. Although the MMP-9 association with PPROM is best characterized, MMP-8 activity is also elevated in PPROM and correlates with chorioamnionitis, funisitis, and poor neonatal outcomes.36,37 Furthermore, activities of MMP-1, -2, -3, -10, -11, -13, and -14 are also elevated in the amniotic fluid and fetal membranes of women with PPROM.38 By-products of collagen cleavage (matrikines) are currently under investigation, as they may be involved in neutrophil chemotaxis and upregulation of the inflammatory response.39
The biochemistry of membrane rupture involves a series of complex interactions: enzymatic activation resulting in degradation of extracellular matrix, release of cytokine mediators, increased apoptotic remodeling, and prostaglandin release. The amnion’s strength and delayed failure compared to the chorion may be related to the varying compositions of extracellular matrix and susceptibility to biochemical processes. Furthermore, preconditioning by varying risk factors (ie, infection, tobacco smoke, and oxidative stress) may increase the chorioamnion susceptibility to various degradative and apoptotic pathways. Future studies of membrane degradation should consider these conditions in their analyses.
Apoptosis and Oxidative Stress
Apoptosis, a normal part of growth and development, is enhanced by cytokines, like tumor necrosis factor α (TNF-α). When initiated prematurely, apoptosis can lead to PPROM.24,25,34,40 Apoptosis is measured through a variety of surrogate markers such as Terminal deoxynucleotidyl transferase 2′-deoxyuridine 5′-triphosphate nick end labeling staining of DNA fragments, telomere length, and caspase activation. Studies consistently show that fetal membranes from women with PPROM demonstrate higher rates of apoptosis than women with preterm labor and intact membranes.34,38,41
Preterm premature rupture of membranes-associated apoptotic pathways occur through either a TNF-α factor receptor (TNFR)/Fas-mediated or p53/Bax pathway.37,39,42 Binding of TNF or Fas ligand triggers the TNFR/Fas-mediated pathway, ultimately leading to caspase activation. In the p53 pathway, Bax is activated and Bcl-2 (anti-apoptotic protein) is suppressed resulting in mitochondrial membrane damage, release of cytochrome c, and caspase-9 activation. Infection is a recognized initiator of these pathways and has been suggested to upregulate apoptotic genes.43 More recently, cigarette smoke exposure to fetal membranes has also been shown to inhibit Bcl-2 and increase apoptosis and oxidative stress.44
Oxidative stress represents another well-established pathway to apoptosis and is associated with both PPROM and collagen weakening.45,46 Reactive oxygen species (ROS) are unstable molecules released from mitochondria with normal cellular respiration and by immune cells during bacterial killing. Reactive oxygen species are capable of widespread membrane damage through a variety of mechanisms: cleavage of collagen, induction of MMP-9, direct damage to DNA, release of catalytic enzymes, and initiation of lipid peroxidation. Neutrophils, recruited to intrauterine infection, may release hypochlorous acid, an ROS that causes DNA strand breaks, initiates lipid peroxidation, inhibits TIMP-1 activity, and compromises repair mechanisms.47
Studies of antioxidants (vitamins C and E) to target the pathway of oxidative stress show mixed results. Although lower plasma vitamin C levels are associated with PPROM, supplementation of vitamins C and E in a single, large clinical trial showed a paradoxical increase in PPROM rates (4.6% vs 1.7%; relative risk [RR] 2.68; P = .025).48-50 α-Lipoic acid, another antioxidant, decreased membrane weakening from thrombin in vitro.51 Phytophenols (plant antioxidants) also reduced MMP-9 and prostaglandins in an ex vivo inflammatory model.52 Blocking oxidative stress induction of MMP may yield new therapies that would have value across several PPROM pathways.
Clinical Risk Factors Implicated in PPROM
Infection and Inflammation
Inflammation of the fetal membranes either via amniotic fluid infection or via chorioamnionitis is associated with nearly half of all PPROM cases.9 The presence of bacteria in the amniotic cavity is estimated to occur in 18% to 38% of all cases of PPROM.53-58 Specifically, the presence of vaginal bacteria in the uterus appears to contribute to both PPROM and cases of early spontaneous preterm labor.59,60 Among women with intact membranes and spontaneous preterm labor, the presence of microbes within amniotic fluid is highly associated with eventual PPROM (odds ratio [OR] = 27).61 How bacteria access the pregnant uterus remains unclear but likely occurs through trafficking from the lower genital tract or in some cases transplacentally (eg, Listeria monocytogenes). Microbial and nutritional factors contributing to alterations in the immunologic or metabolic microenvironment of the upper vagina and cervix may play a role in facilitating bacterial trafficking into the choriodecidua.
Conflicting data exist regarding the association between vaginal bacteria species and the risk of PPROM.62-65 The Vaginal Infections in Pregnancy Study prospectively followed more than 13 000 women enrolled at 24 to 26 weeks to delivery. For nearly all microbes studied, no association with PPROM was seen including Group B Streptococcus (GBS), Chlamydia trachomatis, Ureaplasma urealyticum, and bacterial vaginosis. Only the recovery of Trichomonas vaginalis was associated with PPROM.66 However, recent molecular studies using 16 S quantitative polymerase chain reaction identified vaginal microbes such as Atopobium vaginae and Sneathia/Leptotrichia within amniotic fluid after PPROM.59 Although individual species were not studied for their RR for PPROM, an inverse correlation was observed between total bacterial abundance and gestational age at delivery.59 The recent identification of an ornithine rhamnolipid pigment in GBS as an important virulence factor determining whether GBS can penetrate the chorioamnion illustrates that microbial factors also play a role in preterm birth risk.67 The risk of PPROM likely involves a combination of microbial pathogenicity, expression of specific virulence factors, abundance of the microbial species, and gestational age of exposure to the amniotic cavity.
Once bacteria enter the choriodecidua, degradation of collagen can begin through direct proteolysis or through activation of MMP (Figure 2). Several strains implicated in PPROM directly lyse collagen including GBS and T. vaginalis. Bacterial activation of the innate immune response within the chorioamnion is broad based including proinflammatory cytokines (interleukin [IL]-1β, TNF-α, and IL-6), chemokines (macrophage chemotactic protein 1 or CCL2), and macrophage inflammatory protein 1 or CCL3.68 Of the cytokines, TNF-α may be the strongest mediator of MMP activation.69-72 Proinflammatory chemokines also induce MMP-2 activation. Both TNF-α and IL-1β are associated with biomechanical membrane weakening in vitro when applied to full thickness membranes.73 Infection-induced MMP activation is likely the result of multiple inflammatory effectors and pathways.74-77
Alternative mechanisms for activating MMP involve prostaglandin production by the amnion and chorion. Prostaglandin E2 can be released directly from the membranes in response to inflammatory cytokines. Alternatively, precursors can be released from arachidonic acid in response to vaginal bacteria.9 Encouraging results come from in vitro studies of indomethacin and phytophenols demonstrating decreased expression of cyclooxygenase (COX) 2 and decreased release of prostaglandins leading to decreased activity of MMPs.52,73,75 Despite promising animal models, the single randomized controlled trial of COX inhibitors to reduce the risk of preterm labor is disappointing; COX inhibitors were associated with an increase in preterm birth and PPROM.78,79
A recent study suggested a novel mechanism for infection-associated PPROM that involves downregulation of genes critical for tensile strength within the chorioamnion.80 In a nonhuman primate model of an early GBS choriodecidual infection, there was a significant downregulation of multiple cytokeratin and other genes critical for maintenance of chorioamnion tensile strength including cytokeratins (CK3, CK6A, CK7, CK8, CK14, CK15, CK16, CK19, and CK24), collagens and collagen-binding proteins (COL1A2, COL7A1, COL5A1, and LUM), and components of the intracellular matrix (laminins and desmoplakin). Perturbations in the cytokeratin network within amniocytes were also evident by immunofluorescence and transmission electron micrography. Weakening of the tensile strength of the amnion appears to be an early event after choriodecidual infection, and understanding how to prevent or reverse this process may be necessary to prevent PPROM.
As we begin to understand how pathogens exploit the fetal membranes to inhibit an innate immune response, we remain limited by an incomplete understanding of the complex microbiology within the amniotic fluid, chorioamnion, and choriodecidual space. The introduction of molecular techniques to identify uncultivable bacteria within the amniotic fluid, placental tissue, and fetal membranes from women in preterm labor demonstrates that more than 60% of placental tissues and fetal membranes are infected with 2 or more bacterial species.81 Similar studies support that microbial communities contributing to PPROM are more complex and diverse than previously understood.81-83 The recent identification of intracellular bacteria in the placental basal plate in both preterm (<28 weeks) and term births suggests that the placenta may itself be a source of bacteria that could invade the amniotic fluid or trigger preterm labor.84 These data suggest that there may be commensal communities of bacteria within the placenta, which play a role in preventing or promoting bacterial trafficking and PPROM. Exploring this microbial diversity within the amniotic fluid, fetal membranes, and placenta may lead to further insight into inflammatory causes of PPROM.
Abruption and Thrombin Mediators
Placental abruption is a strong risk factor for PPROM. The coagulation cascade can be connected to many biological mechanisms implicated in PPROM: activation of MMP, inflammation, oxidative stress, and apoptosis.85-89 The coagulation cascade begins soon after vessel injury with the exposure of blood to proteins like tissue factor. Tissue factor is highly expressed in the choriodecidua.90 Thrombin, an early product of the coagulation cascade, is a marker of abruption. In a small case–control study, elevated maternal plasma levels of thrombin–antithrombin complexes were associated with PPROM.91
Much of the association between abruption and PPROM has previously been attributed to inflammation. Thrombin activates MMP (MMP-1 and MMP-9) and induces cytokines/chemokines (IL-8 and CCL2) in the chorioamnion or decidua.85,92,93 However, recent studies also demonstrate that thrombin directly weakens fetal membranes in a dose-dependent manner. This appears to be mediated through MMP-9 activity and polyadenosine diphosphate ribose polymerase (PARP) cleavage. Polyadenosine diphosphate ribose polymerase is a family of proteins involved in DNA repair and is often associated with apoptosis, whereas the same effects can be seen with administration of TNF-α and IL-1β to chorioamnion explants; only thrombin has these effects on the amnion in isolation.73,94 Of interest, pretreatment with the antioxidant α-lipoic acid of amnion explants later exposed to thrombin inhibits weakening of fetal membranes, suggesting that oxidative stress may also mediate the thrombin pathway to PPROM.51 These data demonstrate several pathways activated by placental bleeding to weaken fetal membranes, both inflammatory and noninflammatory.
Cigarette Smoking
Smoking is a well-known clinical risk factor for PPROM, but recent studies suggest that smoking-associated PPROM is restricted to early gestational ages. In a large retrospective Canadian study of nearly 18 000 women, smoking more than 10 cigarettes per day was significantly associated with PPROM less than 28 weeks (OR 5.3, 95% confidence interval [CI] 2.2-12.7). The OR decreased as the gestational categories approached term (term: OR 3.2, 95% CI 0.92-11.0).95 Another retrospective Australian study including approximately 4500 preterm births found an association between smoking and PPROM 27 to 33 weeks but not closer to term.96 These findings suggest that the biological mechanism linking smoking and PPROM differentially affects fetal membranes at early gestational ages, unlike other risk factors for PPROM. The biochemical effect of smoking on membranes is under studied. Recent findings link cigarette chemicals with increased apoptosis (activated caspase 3) and oxidative stress in chorioamnion ex vivo.44 Interestingly, the antioxidant capacity of amniotic fluid increases in the third trimester, adding biologic mechanism to the decreased association of cigarette smoking and PPROM near term.97 Further research is needed to determine whether the antioxidant capacity of membranes parallel that of amniotic fluid.
Uterine Overdistention
Uterine overdistention results from rapid uterine growth following multiple gestations (ie, twins) or polyhydramnios. Polyhydramnios, excessive accumulation of amniotic fluid, occurs secondary to a variety of conditions including fetal anomalies, maternal diabetes, hydrops fetalis, or idiopathic etiologies. Women with uterine anomalies (eg, bicornuate uterus) may also experience overdistention, as anomalous uteri may have a smaller capacity to carry a pregnancy. Not only are multiple gestations affected more frequently by PPROM than singleton pregnancies (7%-8% vs 2%-4%), but PPROM also occurs at earlier gestations.9 Of PPROM cases prior to 24 weeks, 26% are multiple gestations.98 This suggests that both the rate of uterine distension and the total uterine volume contribute to pathologic membrane rupture.
An ex vivo model of myometrial and placental tissues demonstrates that mechanical stretch, as with uterine overdistention, increases inflammation, upregulates MMPs and increases catabolism of collagen. Mechanical stretch of myometrial cells upregulated messenger RNA expression of COX-2 and the oxytocin receptor, both of which are associated with prostaglandin-driven uterine activity.99,100 Further studies support increased cytokines (IL-8) and chemokines (CCL2) with myometrial stretch.101,102 Although an ex vivo study demonstrated that progesterone inhibited increased chemokine IL-8 and MMP-1 from cyclic stretch of human decidual cells,103 a clinical trial of progesterone (17α-hydroxyprogesterone caproate) administration to women with twins did not reduce preterm birth.104 This discrepancy may be a function of inappropriate dosing. Alternatively in vivo uterine stretch may present unaccounted for factors preventing a progesterone effect. Myometrial stretch is also associated with increases in prostaglandin production, another activator of MMP.99,105 These studies demonstrate that myometrial stretch leads to myometrial activation, upregulation of chemokines, and activation of MMPs, common factors among pathways to PPROM. Further research is needed to determine whether these factors contribute to PPROM associated with uterine overdistention.
Genetic Predisposition
Connective tissue weakening due to single gene defects, as in Ehlers-Danlos syndrome, represents a unique pathway to PPROM with an exceptionally high risk of rupture.106,107 The initial report of women with Ehlers-Danlos syndrome found that 78% of 18 affected women delivered prematurely; PPROM occurred in 13 of the 14 preterm births.107 Interestingly, the risk doubled among women with an affected fetus (50%) compared to women with an unaffected fetus (20%). The mutations in Ehlers-Danlos syndrome affect collagen or collagen processing, likely substantially lowering the biomechanical threshold for rupture. Case reports link PPROM to other connective tissue disorders like restrictive dermopathy and osteogenesis imperfecta type II.106 Notably, there is no associated risk of PPROM with Marfan syndrome, a genetic disorder with mutations in fibrillin 1 leading to abnormal collagen structure.108 Fetal genetics are a strong risk factor for PPROM, particularly in connective tissue disorders with defects in several collagen genes (COL1A1, COL1A2, and COL5A1), cartilage-associated protein (CRTAP), leucine proline-enriched proteoglycan (leprecan) 1 (LEPRE1), and zinc metallopeptidase STE24 (ZMPSTE24).106
More recently, single-nucleotide polymorphisms (SNPs) were investigated as predisposing factors to PPROM. Studies targeted women of African ancestry in their investigations due to a disproportionate number of preterm births. Compelling evidence exists that a functional SNP in the promoter of the SERPINH1 gene (−656 C/T), enriched in women of West African ancestry, leads to a reduction in stable fibrillar collagen thus increasing the risk of PPROM.110 The SERPINH1 −656 T allele was significantly increased in African American neonates born from pregnancies with PPROM in both an initial and a subsequent case–control study (combined P value < .001). Modulation by SNPs of MMP (MMP1, MMP8, and MMP9) and other immune or apoptosis genes (TNF-α, IL-10, CD14, and Fas) are also implicated in PPROM.110-116
Several other genetic loci are hypothesized to contribute to the risk of preterm birth and PPROM. A large genetic association study in a homogenous Chilean population was performed to investigate the association between PPROM and 775 SNPs in 190 candidate genes.117 After correction for multiple testing, the maternal carriage of TIMP2 rs2277698 SNP (adjusted OR 2.12; P < .001) remained significantly associated with PPROM. Interestingly, no associations with fetal SNP in this population could withstand multiple hypothesis correction.
Recurrence of PPROM and Progesterone
Although spontaneous preterm birth recurs in approximately 25% of subsequent pregnancies, reports of PPROM recurrence vary significantly (20%-32%).118-121 The use of progesterone among some at-risk populations is associated with a reduction in spontaneous preterm birth.122-127 However, there is little evidence to support that it specifically reduces the risk of recurrent PPROM. In 2003, publication of 2 trials of women at risk of preterm birth suggested that progesterone supplementation reduces the rate of a subsequent preterm birth. The first trial did not report whether the index preterm birth occurred after PPROM or spontaneous preterm labor.123 The latter trial excluded women developing PPROM in the index pregnancy from their analysis,124 for which they were highly criticized.128 Three additional randomized controlled trials of progesterone to delay preterm birth were published in 2007.125-127 Of the 3 studies, 1 reported the rate of PPROM among women receiving vaginal progesterone (37 of 309; 12.0%) versus controls (38 of 302; 12.6%), which was not statistically different.125 Not surprisingly, the use of progesterone after the occurrence of PPROM does not delay preterm birth as the chorioamnion does not contain nuclear progesterone receptors.129,130 Therefore, any impact of progesterone on membrane function is likely indirect or mediated by nongenomic pathways.
The mechanisms by which progesterone prolongs pregnancy in women at high risk of preterm birth possibly include oxytocin antagonism, support of cervical integrity, anti-inflammatory effects, and a reduction in gap–junction formation.131,132 One study cultured fetal membranes in vitro and demonstrated progesterone inhibition of apoptosis by reducing caspase-3 activity in the fetal membranes at baseline and also after TNF-α stimulation. Interestingly, progesterone did not inhibit lipopolysaccharide induction of apoptosis in this study suggesting that progesterone does not block all apoptotic pathways in the membranes.133 Despite the paucity of data supporting the use of progesterone as secondary prophylaxis for recurrent PPROM, its use in women with a history of PPROM to prevent preterm labor is reasonable given the large contribution of PPROM to preterm labor.
Future Directions
Over the last 15 years, studies identified new pathways to membrane weakening and PPROM including thrombin, oxidative stress, and likely apoptosis. Improved microbial detection techniques demonstrate increased diversity of microbes contributing to PPROM as well as a higher prevalence of microbes in association with PPROM than previously thought. The activation of MMPs by infection, thrombin, ROS, and mechanical stretch implies their critical role in membrane weakening as a common downstream mediator for several pathways. However, integration of the many pathways leading to PPROM in a unifying model is challenging, as most studies studied a single biological mechanism or risk factor in isolation. Here, we propose that PPROM occurs through the cumulative effect of several activated pathways leading to a common downstream process of MMP and cytokine activation.
New drugs that have anti-inflammatory or antioxidant properties that target PPROM and preterm birth pathways are being actively investigated in vitro and in animal models. The investigation of microbial communities within the upper and lower genital tract is another area likely to yield new strategies for PPROM prevention. The discovery of microbes within the placental basal plate of normal pregnancies suggests that we must further define which organisms might normally reside within placental tissues. Commensal microbial communities within the vagina, cervix, and placenta may play an important role in preventing the trafficking of pathogenic bacteria into the amniotic fluid. Identification of new pathogenic factors involved in breach of the chorioamnion, such as the ornithine rhamnolipid pigment of GBS, may lead to vaccine targets. Further study of the heterogeneity in the human inflammatory response to these microbes (eg, SERPINH1 −656 C/T) will better identify women at risk of PPROM.
PPROM represents a common end point for several unique pathologic events. Like preterm birth, complex pathways are involved in PPROM that likely act synergistically to weaken the membranes and predispose to rupture. Future research should integrate the study of membrane biomechanics with MMP and cytokine activation, oxidative stress, and apoptosis to better understand the sequence and relative importance of biological events leading to membrane weakening according to clinical risk factors.
Acknowledgments
We would like to thank Jan Hamanishi for her help in preparing the figures.
Footnotes
Authors’ Notes: All authors worked as a writing group and contributed to the ideas, writing, and editing of the manuscript. No human activities were associated with this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Allergy and Infectious Diseases, and National Center for Research Resources of the National Institute of Health under award numbers (R01AI100989, R21AI109222, K08AI067910, K12HD001264, R56AI070749). The authors are grateful for support past and present from the March of Dimes (21-FY06-77, 21-FY08-562,) Global Alliance for the Prevention of Prematurity and Stillbirth (GAPPS 12007), Bill and Melinda Gates Foundation, University of Washington Institute for Translational Health Research, and the Washington State Obstetrical Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
References
- 1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371 (9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379 (9832):2151–2161. [DOI] [PubMed] [Google Scholar]
- 3. Ye G, Jiang Z, Lu S, Le Y. Premature infants born after preterm premature rupture of membranes with 24-34 weeks of gestation: a study of factors influencing length of neonatal intensive care unit stay. J Matern Fetal Neonatal Med. 2011;24 (7):960–965. [DOI] [PubMed] [Google Scholar]
- 4. Khashoggi TY. Outcome of pregnancies with preterm premature rupture of membranes. Saudi Med J. 2004;25 (12):1957–1961. [PubMed] [Google Scholar]
- 5. Stewart CJ, Tregoning SK, Moller G, Wainwright H. Preterm prelabour rupture of the membranes before 28 weeks: better than feared outcome of expectant management in Africa. Eur J Obstet Gynecol Reprod Biol. 2006;126 (2):186–192. [DOI] [PubMed] [Google Scholar]
- 6. Noor S, Nazar AF, Bashir R, Sultana R. Prevalence of PPROM and its outcome. J Ayub Med Coll Abbottabad. 2007;19 (4):14–17. [PubMed] [Google Scholar]
- 7. Obi SN, Ozumba BC. Pre-term premature rupture of fetal membranes: the dilemma of management in a developing nation. J Obstet Gynaecol. 2007;27 (1):37–40. [DOI] [PubMed] [Google Scholar]
- 8. Brace RA. Physiology of amniotic fluid volume regulation. Clin Obstetrics Gynecol. 1997;40 (2):280–289. [DOI] [PubMed] [Google Scholar]
- 9. Parry S, Strauss JF III. Premature rupture of the fetal membranes. N Engl J Med. 1998;338 (10):663–670. [DOI] [PubMed] [Google Scholar]
- 10. Oxlund H, Helmig R, Halaburt JT, Uldbjerg N. Biomechanical analysis of human chorioamniotic membranes. Eur J Obstet Gynecol Reprod Biol. 1990;34 (3):247–255. [DOI] [PubMed] [Google Scholar]
- 11. Arikat S, Novince RW, Mercer BM, et al. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol. 2006;194 (1):211–217. [DOI] [PubMed] [Google Scholar]
- 12. Ockleford C, Malak T, Hubbard A, et al. Confocal and conventional immunofluorescence and ultrastructural localisation of intracellular strength-giving components of human amniochorion. J Anat. 1993;183 (pt 3):483–505. [PMC free article] [PubMed] [Google Scholar]
- 13. Bourne G. The foetal membranes. A review of the anatomy of normal amnion and chorion and some aspects of their function. Postgrad Med J. 1962;38:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta. 1998;19 (1):1–11. [DOI] [PubMed] [Google Scholar]
- 15. Malak TM, Bell SC. Structural characteristics of term human fetal membranes: a novel zone of extreme morphological alteration within the rupture site. Br J Obstet Gynaecol. 1994;101 (5):375–386. [DOI] [PubMed] [Google Scholar]
- 16. Meinert M, Eriksen GV, Petersen AC, Helmig RB, Laurent C, Uldbjerg N, et al. Proteoglycans and hyaluronan in human fetal membranes. American journal of obstetrics and gynecology. 2001;184 (4):679–85. [DOI] [PubMed] [Google Scholar]
- 17. Malak TM, Ockleford CD, Bell SC, Dalgleish R, Bright N, Macvicar J. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta. 1993;14 (4):385–406. [DOI] [PubMed] [Google Scholar]
- 18. Hieber AD, Corcino D, Motosue J, et al. Detection of elastin in the human fetal membranes: proposed molecular basis for elasticity. Placenta. 1997;18 (4):301–312. [DOI] [PubMed] [Google Scholar]
- 19. Parry-Jones E, Priya S. A study of the elasticity and tension of fetal membranes and of the relation of the area of the gestational sac to the area of the uterine cavity. Br J Obstet Gynaecol. 1976;83 (3):205–212. [DOI] [PubMed] [Google Scholar]
- 20. Benirschke K, Kaufman P, Baergen RN. Anatomy and Pathology of the Human Membranes. Pathology of the Human Placenta. 5th ed New York: Springer Science + Business Media, Inc; 2006:321–379. [Google Scholar]
- 21. Joyce EM, Moore JJ, Sacks MS. Biomechanics of the fetal membrane prior to mechanical failure: review and implications. Eur J Obstet Gynecol Reprod Biol. 2009;144 (suppl 1):S121–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Artal R, Sokol RJ, Neuman M, Burstein AH, Stojkov J. The mechanical properties of prematurely and non--prematurely ruptured membranes. Methods and preliminary results. Am J Obstet Gynecol. 1976;125 (5):655–659. [DOI] [PubMed] [Google Scholar]
- 23. Manabe Y, Himeno N, Fukumoto M. Tensile strength and collagen content of amniotic membrane do not change after the second trimester or during delivery. Obstet Gynecol. 1991;78 (1):24–27. [PubMed] [Google Scholar]
- 24. El Khwad M, Stetzer B, Moore RM, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72 (3):720–726. [DOI] [PubMed] [Google Scholar]
- 25. El Khwad M, Pandey V, Stetzer B, et al. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13 (3):191–195. [DOI] [PubMed] [Google Scholar]
- 26. Maclachlan TB. A Method for the investigation of the strength of the fetal membranes. Am J Obstet Gynecol. 1965;91:309–313. [DOI] [PubMed] [Google Scholar]
- 27. Rangaswamy N, Abdelrahim A, Moore RM, et al. Biomechanical characteristics of human fetal membranes. Preterm fetal membranes are stronger than term fetal membranes [in French]. Gynecol Obstet Fertil. 2011;39 (6):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polishuk WZ, Kohane S, Pernaio A. The physical properties of fetal membranes. Obstet Gynecol. 1962;20 (2):204–210. [PubMed] [Google Scholar]
- 29. Lavery JP, Miller CE. The viscoelastic nature of chorioamniotic membranes. Obstet Gynecol. 1977;50 (4):467–472. [PubMed] [Google Scholar]
- 30. Lavery JP, Miller CE. Deformation and creep in the human chorioamniotic sac. Am J Obstet Gynecol. 1979;134 (4):366–375. [DOI] [PubMed] [Google Scholar]
- 31. Oyen ML, Cook RF, Calvin SE. Mechanical failure of human fetal membrane tissues. J Mater Sci Mater Med. 2004;15 (6):651–658. [DOI] [PubMed] [Google Scholar]
- 32. Schober EA, Kusy RP, Whitley JQ, Savitz DA. Effect of thickness on the fracture characteristics of fetal membranes. J Mater Sci Mater Med. 1994;5 (3):130–137. [Google Scholar]
- 33. Oyen ML, Calvin SE, Landers DV. Premature rupture of the fetal membranes: is the amnion the major determinant? Am J Obstet Gynecol. 2006;195 (2):510–515. [DOI] [PubMed] [Google Scholar]
- 34. Canzoneri BJ, Feng L, Grotegut CA, Bentley RC, Heine RP, Murtha AP. The chorion layer of fetal membranes is prematurely destroyed in women with preterm premature rupture of the membranes. Reprod Sci. 2013;20(10):1246–1254. [DOI] [PubMed] [Google Scholar]
- 35. Strauss JF III. Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci. 2013;20 (2):140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biggio JR, Jr, Ramsey PS, Cliver SP, Lyon MD, Goldenberg RL, Wenstrom KD. Midtrimester amniotic fluid matrix metalloproteinase-8 (MMP-8) levels above the 90th percentile are a marker for subsequent preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192 (1):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Athayde N, Edwin SS, Romero R, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179 (5):1248–1253. [DOI] [PubMed] [Google Scholar]
- 38. Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11 (7):427–437. [DOI] [PubMed] [Google Scholar]
- 39. Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21 (3):467–478. [DOI] [PubMed] [Google Scholar]
- 40. Paavola LG, Furth EE, Delgado V, et al. Striking changes in the structure and organization of rat fetal membranes precede parturition. Biol Reprod. 1995;53 (2):321–338. [DOI] [PubMed] [Google Scholar]
- 41. Saglam A, Ozgur C, Derwig I, Unlu BS, Gode F, Mungan T. The role of apoptosis in preterm premature rupture of the human fetal membranes. Arch Gynecol Obstet. 2013;288 (3):501–505. [DOI] [PubMed] [Google Scholar]
- 42. Fortunato SJ, Menon R, Bryant C, Lombardi SJ. Programmed cell death (apoptosis) as a possible pathway to metalloproteinase activation and fetal membrane degradation in premature rupture of membranes. Am J Obstet Gynecol. 2000;182 (6):1468–1476. [DOI] [PubMed] [Google Scholar]
- 43. Fortunato SJ, Menon R, Lombardi SJ. Support for an infection-induced apoptotic pathway in human fetal membranes. Am J Obstet Gynecol. 2001;184 (7):1392–1397. [DOI] [PubMed] [Google Scholar]
- 44. Menon R, Fortunato SJ, Yu J, et al. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta. 2011;32 (4):317–322. [DOI] [PubMed] [Google Scholar]
- 45. Longini M, Perrone S, Vezzosi P, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin Biochem. 2007;40 (11):793–797. [DOI] [PubMed] [Google Scholar]
- 46. Woods JR., Jr Reactive oxygen species and preterm premature rupture of membranes-a review. Placenta. 2001;22 (suppl A):S38–S44. [DOI] [PubMed] [Google Scholar]
- 47. Barrera G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncology. 2012; 137289:21. doi:10.5402/2012/137289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plessinger MA, Woods JR, Jr, Miller RK. Pretreatment of human amnion-chorion with vitamins C and E prevents hypochlorous acid-induced damage. Am J Obstet Gynecol. 2000;183 (4):979–985. [DOI] [PubMed] [Google Scholar]
- 49. Mercer BM, Abdelrahim A, Moore RM, et al. The impact of vitamin C supplementation in pregnancy and in vitro upon fetal membrane strength and remodeling. Reprod Sci. 2010;17 (7):685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spinnato JA II, Freire S, Pinto e Silva JL, et al. Antioxidant supplementation and premature rupture of the membranes: a planned secondary analysis. Am J Obstet Gynecol. 2008;199(4):433. e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moore RM, Schatz F, Kumar D, et al. Alpha-lipoic acid inhibits thrombin-induced fetal membrane weakening in vitro. Placenta. 2010;31 (10):886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lim R, Barker G, Wall CA, Lappas M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol Hum Reprod. 2013;19 (7):451–462. [DOI] [PubMed] [Google Scholar]
- 53. Joaquin SF, Romero R, Espinoza J, et al. Prelabor rupture of membranes In: Reece EA, John CH, eds. Clinical Obstetrics: The Fetus and The Mother. 3rd ed Malden, MA: Blackwell Publishing; 2007:1130–1188. [Google Scholar]
- 54. Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol. 1979;54 (2):226–230. [PubMed] [Google Scholar]
- 55. Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159 (3):661–666. [DOI] [PubMed] [Google Scholar]
- 56. Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197(3):292. e1–e5. [DOI] [PubMed] [Google Scholar]
- 57. Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191 (4):1339–1345. [DOI] [PubMed] [Google Scholar]
- 58. Romero R, Baumann P, Gomez R, et al. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol. 1993;168 (6 pt 1):1654–1664. [DOI] [PubMed] [Google Scholar]
- 59. DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64 (1):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165 (4 pt 1):955–961. [DOI] [PubMed] [Google Scholar]
- 61. Guinn DA, Goldenberg RL, Hauth JC, Andrews WW, Thom E, Romero R. Risk factors for the development of preterm premature rupture of the membranes after arrest of preterm labor. Am J Obstet Gynecol. 1995;173 (4):1310–1315. [DOI] [PubMed] [Google Scholar]
- 62. McDonald HM, O'Loughlin JA, Jolley PT, Vigneswaran R, McDonald PJ. Changes in vaginal flora during pregnancy and association with preterm birth. J Infect Dis. 1994;170 (3):724–728. [DOI] [PubMed] [Google Scholar]
- 63. Regan JA, Chao S, James LS. Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. Am J Obstet Gynecol. 1981;141 (2):184–186. [DOI] [PubMed] [Google Scholar]
- 64. Alger LS, Lovchik JC, Hebel JR, Blackmon LR, Crenshaw MC. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstetrics Gynecol. 1988;159 (2):397–404. [DOI] [PubMed] [Google Scholar]
- 65. Ekwo EE, Gosselink CA, Woolson R, Moawad A. Risks for premature rupture of amniotic membranes. Int J Epidemiol. 1993;22 (3):495–503. [DOI] [PubMed] [Google Scholar]
- 66. Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity study group. Sex Transm Dis. 1997;24 (6):353–360. [DOI] [PubMed] [Google Scholar]
- 67. Whidbey C, Harrell MI, Burnside K, et al. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med. in press. 2013;210 (6):1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cobo T, Kacerovsky M, Palacio M, et al. Intra-amniotic inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. PloS ONE. 2012;7 (8):e43677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-alpha in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol. 2002;187 (5):1159–1162. [DOI] [PubMed] [Google Scholar]
- 70. Jacobsson B, Aaltonen R, Rantakokko-Jalava K, Morken NH, Alanen A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta obstetricia et gynecologica Scandinavica. 2009;88 (1):63–70. [DOI] [PubMed] [Google Scholar]
- 71. Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166 (5):1576–1587. [DOI] [PubMed] [Google Scholar]
- 72. Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15 (8):819–835. [DOI] [PubMed] [Google Scholar]
- 73. Kumar D, Schatz F, Moore RM, et al. The effects of thrombin and cytokines upon the biomechanics and remodeling of isolated amnion membrane, in vitro. Placenta. 2011;32(3):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li W, Unlugedik E, Bocking AD, Challis JR. The role of prostaglandins in the mechanism of lipopolysaccharide-induced proMMP9 secretion from human placenta and fetal membrane cells. Biol Reprod. 2007;76 (4):654–659. [DOI] [PubMed] [Google Scholar]
- 75. McLaren J, Taylor DJ, Bell SC. Prostaglandin E(2)-dependent production of latent matrix metalloproteinase-9 in cultures of human fetal membranes. Mol Hum Reprod. 2000. ;6(11):1033–1040. [DOI] [PubMed] [Google Scholar]
- 76. Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod. 2001;7(12):1187–1193. [DOI] [PubMed] [Google Scholar]
- 77. Kodali R, Hajjou M, Berman AB, et al. Chemokines induce matrix metalloproteinase-2 through activation of epidermal growth factor receptor in arterial smooth muscle cells. Cardiovasc Res. 2006;69 (3):706–715. [DOI] [PubMed] [Google Scholar]
- 78. Groom KM, Shennan AH, Jones BA, Seed P, Bennett PR. TOCOX--a randomised, double-blind, placebo-controlled trial of rofecoxib (a COX-2-specific prostaglandin inhibitor) for the prevention of preterm delivery in women at high risk. BJOG. 2005;112 (6):725–730. [DOI] [PubMed] [Google Scholar]
- 79. Khanprakob T, Laopaiboon M, Lumbiganon P, Sangkomkamhang US. Cyclo-oxygenase (COX) inhibitors for preventing preterm labour. Cochrane Database Syst Rev. 2012;10:CD007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vanderhoeven JP, Bierle CJ, Kapur RP, et al. Group B streptococcal infection of the choriodecidua induces dysfunction of the cytokeratin network in amniotic epithelium: a pathway to membrane weakening. PLoS Pathog. 2014;10(3):e1003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jones HE, Harris KA, Azizia M, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4 (12):e8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3 (8):e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47 (1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stout MJ, Conlon B, Landeau M, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208(3):226. e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stephenson CD, Lockwood CJ, Ma Y, Guller S. Thrombin-dependent regulation of matrix metalloproteinase (MMP)-9 levels in human fetal membranes. J Matern Fetal Neona. 2005;18 (1):17–22. [DOI] [PubMed] [Google Scholar]
- 86. Chen D, Dorling A. Critical roles for thrombin in acute and chronic inflammation. J Thromb Haemost. 2009;7 (suppl 1):122–126. [DOI] [PubMed] [Google Scholar]
- 87. Park KW, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons: role of neuronal NADPH oxidase. J Neurosci Res. 2008;86 (5):1053–1063. [DOI] [PubMed] [Google Scholar]
- 88. Lopez JJ, Salido GM, Gomez-Arteta E, Rosado JA, Pariente JA. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J Thromb Haemost. 2007;5 (6):1283–1291. [DOI] [PubMed] [Google Scholar]
- 89. Harger JH, Hsing AW, Tuomala RE, et al. Risk factors for preterm premature rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol. 1990;163 (1 pt 1):130–137. [DOI] [PubMed] [Google Scholar]
- 90. Erez O, Espinoza J, Chaiworapongsa T, et al. A link between a hemostatic disorder and preterm PROM: a role for tissue factor and tissue factor pathway inhibitor. J Matern Fetal Neona. 2008;21 (10):732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Maternal Fetal Med. 2001;10 (5):297–300. [DOI] [PubMed] [Google Scholar]
- 92. Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neona. 2002;11 (1):11–17. [DOI] [PubMed] [Google Scholar]
- 93. Matta P, Lockwood CJ, Schatz F, et al. Thrombin regulates monocyte chemoattractant protein-1 expression in human first trimester and term decidual cells. Am J Obstet Gynecol. 2007;196(3):268. e1–e8. [DOI] [PubMed] [Google Scholar]
- 94. Puthiyachirakkal M, Lemerand K, Kumar D, et al. Thrombin weakens the amnion extracellular matrix (ECM) directly rather than through protease activated receptors. Placenta. 2013;34 (10):924–931. [DOI] [PubMed] [Google Scholar]
- 95. England MC, Benjamin A, Abenhaim HA. Increased risk of preterm premature rupture of membranes at early gestational ages among maternal cigarette smokers. Am J Perinatol. 2013;30 (10):821–826. [DOI] [PubMed] [Google Scholar]
- 96. Henderson JJ, McWilliam OA, Newnham JP, Pennell CE. Preterm birth aetiology 2004-2008. Maternal factors associated with three phenotypes: spontaneous preterm labour, preterm pre-labour rupture of membranes and medically indicated preterm birth. J Matern Fetal Neona. 2012;25 (6):642–647. [DOI] [PubMed] [Google Scholar]
- 97. Burlingame JM, Esfandiari N, Sharma RK, Mascha E, Falcone T. Total antioxidant capacity and reactive oxygen species in amniotic fluid. Obstet Gynecol. 2003;101 (4):756–761. [DOI] [PubMed] [Google Scholar]
- 98. Falk SJ, Campbell LJ, Lee-Parritz A, et al. Expectant management in spontaneous preterm premature rupture of membranes between 14 and 24 weeks' gestation. J Perinatol. 2004;24 (10):611–616. [DOI] [PubMed] [Google Scholar]
- 99. Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod. 2004;10 (2):109–113. [DOI] [PubMed] [Google Scholar]
- 100. Terzidou V, Sooranna SR, Kim LU, Thornton S, Bennett PR, Johnson MR. Mechanical stretch up-regulates the human oxytocin receptor in primary human uterine myocytes. J Clin Endocrinol Metab. 2005;90 (1):237–246. [DOI] [PubMed] [Google Scholar]
- 101. Loudon JA, Sooranna SR, Bennett PR, Johnson MR. Mechanical stretch of human uterine smooth muscle cells increases IL-8 mRNA expression and peptide synthesis. Mol Hum Reprod. 2004;10 (12):895–899. [DOI] [PubMed] [Google Scholar]
- 102. Hua R, Pease JE, Sooranna SR, et al. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology. 2012;153 (1):481–491. [DOI] [PubMed] [Google Scholar]
- 103. Zhao Y, Koga K, Osuga Y, et al. Cyclic stretch augments production of neutrophil chemokines and matrix metalloproteinases-1 (MMP-1) from human decidual cells, and the production was reduced by progesterone. Am J Reprod Immunol. 2013;69 (5):454–462. [DOI] [PubMed] [Google Scholar]
- 104. Durnwald CP, Momirova V, Rouse DJ, et al. Second trimester cervical length and risk of preterm birth in women with twin gestations treated with 17-alpha hydroxyprogesterone caproate. J Matern Fetal Neona. 2010;23 (12):1360–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Manabe Y, Manabe A, Takahashi A. F prostaglandin levels in amniotic fluid during balloon-induced cervical softening and labor at term. Prostaglandins. 1982;23 (2):247–256. [DOI] [PubMed] [Google Scholar]
- 106. Anum EA, Hill LD, Pandya A, Strauss JF III. Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity. Placenta. 2009;30 (3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Barabas AP. Ehlers-Danlos syndrome: associated with prematurity and premature rupture of foetal membranes; possible increase in incidence. Br Med J. 1966;2 (5515):682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rossiter JP, Repke JT, Morales AJ, Murphy EA, Pyeritz RE. A prospective longitudinal evaluation of pregnancy in the Marfan syndrome. Am J Obstet Gynecol. 1995;173 (5):1599–1606. [DOI] [PubMed] [Google Scholar]
- 109. Rocnik EF, van der Veer E, Cao H, Hegele RA, Pickering JG. Functional linkage between the endoplasmic reticulum protein Hsp47 and procollagen expression in human vascular smooth muscle cells. J Biol Chem. 2002;277 (41):38571–38578. [DOI] [PubMed] [Google Scholar]
- 110. Wang H, Parry S, Macones G, et al. A functional SNP in the promoter of the SERPINH1 gene increases risk of preterm premature rupture of membranes in African Americans. Proc Natl Acad Sci U S A. 2006;103 (36):13463–13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fujimoto T, Parry S, Urbanek M, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277 (8):6296–6302. [DOI] [PubMed] [Google Scholar]
- 112. Kalish RB, Nguyen DP, Vardhana S, Gupta M, Perni SC, Witkin SS. A single nucleotide A>G polymorphism at position -670 in the Fas gene promoter: relationship to preterm premature rupture of fetal membranes in multifetal pregnancies. Am J Obstet Gynecol. 2005;192 (1):208–212. [DOI] [PubMed] [Google Scholar]
- 113. Kalish RB, Vardhana S, Normand NJ, Gupta M, Witkin SS. Association of a maternal CD14 -159 gene polymorphism with preterm premature rupture of membranes and spontaneous preterm birth in multi-fetal pregnancies. J Reprod Immunol. 2006;70 (1-2):109–117. [DOI] [PubMed] [Google Scholar]
- 114. Annells MF, Hart PH, Mullighan CG, et al. Interleukins-1, -4, -6, -10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women: Risk of preterm birth. Am J Obstet Gynecol. 2004;191 (6):2056–2067. [DOI] [PubMed] [Google Scholar]
- 115. Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, et al. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol. 1999;180 (5):1297–1302. [DOI] [PubMed] [Google Scholar]
- 116. Fuks A, Parton LA, Polavarapu S, et al. Polymorphism of Fas and Fas ligand in preterm premature rupture of membranes in singleton pregnancies. Am J Obstet Gynecol. 2005;193 (3 pt 2):1132–1136. [DOI] [PubMed] [Google Scholar]
- 117. Romero R, Friel LA, Velez Edwards DR, et al. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). Am J Obstet Gynecol. 2010;203(4):361. e1–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Asrat T, Lewis DF, Garite TJ, et al. Rate of recurrence of preterm premature rupture of membranes in consecutive pregnancies. Am J Obstet Gynecol. 1991;165 (4 pt 1):1111–1115. [DOI] [PubMed] [Google Scholar]
- 119. Ekwo EE, Gosselink CA, Moawad A. Unfavorable outcome in penultimate pregnancy and premature rupture of membranes in successive pregnancy. Obstet Gynecol. 1992;80 (2):166–172. [PubMed] [Google Scholar]
- 120. Lee T, Carpenter MW, Heber WW, Silver HM. Preterm premature rupture of membranes: risks of recurrent complications in the next pregnancy among a population-based sample of gravid women. Am J Obstet Gynecol. 2003;188 (1):209–213. [DOI] [PubMed] [Google Scholar]
- 121. Mercer BM, Goldenberg RL, Meis PJ, et al. The preterm prediction study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. the national institute of child health and human development maternal-fetal medicine units network. Am J Obstet Gynecol. 2000;183 (3):738–745. [DOI] [PubMed] [Google Scholar]
- 122. Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol. 1990;97 (2):149–154. [DOI] [PubMed] [Google Scholar]
- 123. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348 (24):2379–2385. [DOI] [PubMed] [Google Scholar]
- 124. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188 (2):419–424. [DOI] [PubMed] [Google Scholar]
- 125. O'Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30 (5):687–696. [DOI] [PubMed] [Google Scholar]
- 126. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH, Fetal Medicine Foundation Second Trimester Screening G. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357 (5):462–469. [DOI] [PubMed] [Google Scholar]
- 127. Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357 (5):454–461. [DOI] [PubMed] [Google Scholar]
- 128. Keirse MJ. Progesterone and preterm: seventy years of “deja vu” or “still to be seen”? Birth. 2004;31 (3):230–235. [DOI] [PubMed] [Google Scholar]
- 129. Briery CM, Veillon EW, Klauser CK, et al. Women with preterm premature rupture of the membranes do not benefit from weekly progesterone. Am J Obstet Gynecol. 2011;204(1):54. e1–e5. [DOI] [PubMed] [Google Scholar]
- 130. Merlino A, Welsh T, Erdonmez T, et al. Nuclear progesterone receptor expression in the human fetal membranes and decidua at term before and after labor. Reprod Sci. 2009;16 (4):357–363. [DOI] [PubMed] [Google Scholar]
- 131. Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. J Matern Fetal Neonatal Med. 2006;19 (12):763–772. [DOI] [PubMed] [Google Scholar]
- 132. Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371 (9607):164–175. [DOI] [PubMed] [Google Scholar]
- 133. Luo G, Abrahams VM, Tadesse S, et al. Progesterone inhibits basal and TNF-alpha-induced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17 (6):532–539. [DOI] [PubMed] [Google Scholar]



