Abstract
Background:
Spontaneous preterm birth is the leading cause of neonatal morbidity and mortality worldwide. The ability to examine the exact mechanisms underlying this syndrome in humans is limited. Therefore, the study of animal models is critical to unraveling the key physiologic mechanisms that control the timing of birth. The purpose of this review is to facilitate enhanced assimilation of the literature on animal models of preterm birth by a broad range of investigators.
Methods:
Using classical systematic and informatics search techniques of the available literature through 2012, a database of intact animal models was generated. Research librarians generated a list of articles using multiple databases. From these articles, a comprehensive list of Medical Subject Headings (MeSH) was created. Using mathematical modeling, significant MeSH descriptors were determined, and a MEDLINE search algorithm was created. The articles were reviewed for mechanism of labor induction categorized by species.
Results:
Existing animal models of preterm birth comprise specific interventions to induce preterm birth, as no animal model was identified that exhibits natural spontaneous preterm birth at an incidence comparable to that of the humans. A search algorithm was developed which when used results in a comprehensive list of agents used to induce preterm delivery in a host of animal species. The evolution of 3 specific animal models—sheep, mice, and rats—has demonstrated a clear shift in focus in the literature from endocrine to inflammatory agents of preterm birth induction.
Conclusion:
The process of developing a search algorithm to provide efficient access to information on animal models of preterm birth illustrates the need for a more precise organization of the literature to allow the investigator to focus on distinctly maternal versus fetal outcomes.
Keywords: preterm birth, animal models, obstetric labor
Introduction
Preterm birth is generally defined as a birth occurring at less than 37 weeks of gestation.1 The estimated worldwide incidence of preterm birth is approximately 12.9 million and is 9.6% of all births. The highest recorded rates of preterm birth are concentrated in Africa and North America.2,3 Using the most recent 2012 statistics, the incidence of preterm delivery remains 11.54% of all births in the United States.4 Despite advances in public health and medical interventions, including the use of progesterone for the prevention of preterm birth, only a modest decline in the preterm birth rate has been realized in recent years.5
Preterm birth is the major cause of neonatal morbidity and mortality. Most of this morbidity occurs in the 2% of newborns delivered before 32 weeks gestation and newborns with a very low birth weight (less than 1500 g). The most profound impairments of this group in early childhood include cerebral palsy, mental retardation, sensory impairments, and chronic lung disease. In later childhood, high rates of dysfunction in cognitive development, visual processing, academic progress, and executive function occur in preterm survivors.6 An increasing body of evidence suggests that preterm neonatal neurologic injury may be associated with in utero infection and inflammation, which may constitute up to 40% of spontaneous preterm births.7 Developing a better understanding of preterm birth pathophysiology may not only prolong gestation but may also lead to improved neonatal outcomes.
Experimental research in preterm birth is difficult to perform in humans and, therefore, the use of animal models is necessary to test hypotheses developed from clinical observational studies. As with most disease/physiologic processes in which animal models are used for research, one specific animal model is insufficient to completely replicate the physiology of human parturition. In terms of preterm birth, variations in animal physiology, response to different labor induction methods, and fetal physiology and maturation all play a role in determining model validity.8 The limitations of human studies to establish disease causality and of in vivo animal models to replicate human physiology support the use of animal models in an iterative manner. In this process, animal experimentation refines hypotheses to be tested in humans leading to further questions and more productive animal experimentation. In addition to the critical value or emphasis of replace, reduce, and refine, an integral part of this approach is the systematic definition of pathways and molecular mechanisms involved in the physiology of parturition, defined within an expandable, modifiable database. Informatics approaches have an important role in the critical step of integrating of human “-omics” data regarding preterm birth with the data derived from animal models.9
Systemic reviews of animal models for preterm birth research have been performed.10–13 These reviews focus on specific animal models and the distinctive properties and molecular pathways that are able to be examined with a chosen model. To date, no common resource exists that provides a complete listing of animal models used to research preterm birth. Additionally, a common search algorithm to aid in the identification of currently used animal models for preterm birth is lacking. The purpose of this study was to generate a comprehensive, updatable database of preterm birth animal models. MEDLINE, maintained by the US National Library of Medicine, is one of the most common bibliographic databases that biomedical researchers use to access peer-reviewed publications. MEDLINE is indexed using the Medical Subject Headings (MeSH) vocabulary. The 2013 edition of MeSH consists of 26 853 subject headings.14 In this study, we developed a systematic process that leveraged MeSH for identifying MEDLINE-indexed citations that are associated with animal models of preterm birth. The result was a process that enables continuous review of literature associated with preterm birth model organisms and offer suggestions that could be used to further improve the identification of literature associated with preterm birth model organisms.
Materials and Methods
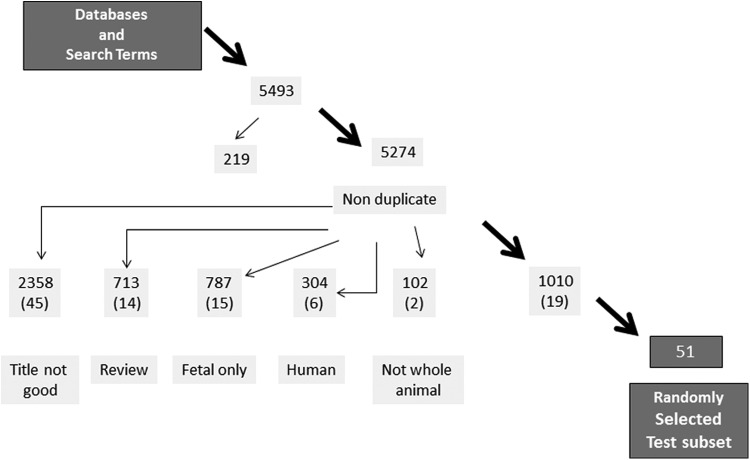
The goal of this study was to generate a searchable database for animal models of spontaneous preterm birth based on MEDLINE-indexed literature up to January 2012. The search algorithm was created based on information derived from a preliminary search for animal models of preterm birth that was compiled by research librarians from the Royal (Dick) School of Veterinary Studies, University of Edinburgh. For this preliminary search, the following databases were examined without language restriction on March 1, 2010: CAB Abstracts (1910-3/2010); Global Health (1973-3/2010); MEDLINE (1950 to February Week 3 2010); EMBASE (1980-week 8 2010); Web of Science (1900-3/2010); and BIOSIS Previews (1926-3/2010) and AGRICOLA (1970-3/2010). The following search descriptors were included alone or in combination with others: prematurity, short gestation, preterm, premature rupture and membranes, premature (obstetric) labor, premature parturition, low birth weight, chorioamnionitis, infants, sheep(s)/ovine, cow(s)/bovine, horse(s)/equine, dog(s)/canine, cat(s)/feline, mouse (mice), rat(s), guinea pig(s), marsupials, rabbits, animal models, biological models, and disease models. Initially, 5493 articles were identified using this search, and after duplicate articles were removed, the resulting 5274 abstracts were reviewed by at least one of the authors. One thousand and ten articles remained after articles were excluded because they were unrelated to preterm birth, exclusively about humans, review articles, not involving whole animals, or focused only on fetal management, physiology, or outcomes (Figure 1). A list of the articles are available in Supplement 1.
Figure 1.
Preliminary search results. A review of multiple databases generated 5274 journal articles, 1010 articles were reviewed for further author evaluation.
A secondary analysis to evaluate the quality and precision of the 1010 articles was performed. Fifty-one articles were randomly selected and appraised. A data extraction form that was piloted during the multiple database searches was used to ensure accuracy and consistency of data extraction. The following data were extracted from each article included in this study: species, mode of prematurity, and fetal status. Twenty-six (51%) articles described a whole animal model with a clearly defined method of preterm labor induction focused on maternal outcomes. The remainder of this sample was either not related to preterm birth (n = 16, 31%), focused on fetal outcomes (n = 6, 12%), or had no abstract available (n = 3, 6%) at the time of the review.
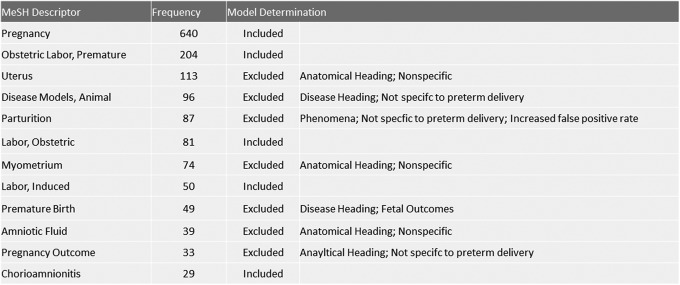
MEDLINE is an indexed bibliographic database for biomedical research. Using information gathered from the above-mentioned preliminary search, we developed an automated strategy that focused on maternal preterm birth induction, while eliminating articles representing in vitro experiments and those focusing on fetal outcomes. It was determined that 750 of the above-mentioned 1010 articles appeared in the MEDLINE database. A computer script written in the Ruby language was used to retrieve all of the MeSH descriptors for the 750 articles, leveraging the National Center for Biotechnology Information e-utilities.15 The frequency of unique descriptors associated with the 750-article set was then calculated. Since the data were significantly skewed, a Box-Cox transformation with a λ = −1 was used to normally distribute the data. The descriptors comprising the top 2.5% of frequencies were considered to be significantly associated with preterm birth induction. Significant MeSH descriptors are listed in Figure 2.
Figure 2.
Medical Subject Headings (MeSH) descriptors derived from preliminary Search. A list and critique of significant MeSH descriptors generated from the original multiple database search.
Based on information contained in the MeSH scope note or on empirical assessment of associated MEDLINE entries, MeSH descriptors were included or excluded to create a final search algorithm. The anatomical MeSH descriptors “Uterus,” “Myometrium,” and “Amniotic Fluid” were excluded because they were too broad. The scope of the “Premature Birth” MeSH descriptor emphasized fetal physiology and outcomes. The MeSH descriptors “Parturition” and “Pregnancy Outcome” were not specific to preterm labor induction and increased the rate of false-positive journal articles. The MeSH descriptor “Disease Models, Animal” was not found to be representative of many articles and did not always include intact animal models and was therefore excluded. The final algorithm (Appendix A) included the union of “Obstetric Labor, Premature,” “Labor, Obstetric,” “Labor, Induced,” and “Chorioamnionitis” with the intersection of “Animals” and “Pregnancy.” Additional exclusion descriptors were added to the search query to limit the number of articles using in vitro experiments and fetal outcomes. The final MEDLINE search retrieved 1370 original articles (performed on March 10, 2012). After duplicates were removed and records not related to animals were excluded, 1290 were screened for animal species (Supplement 2). The results of this process are displayed in Figure 3.
Figure 3.
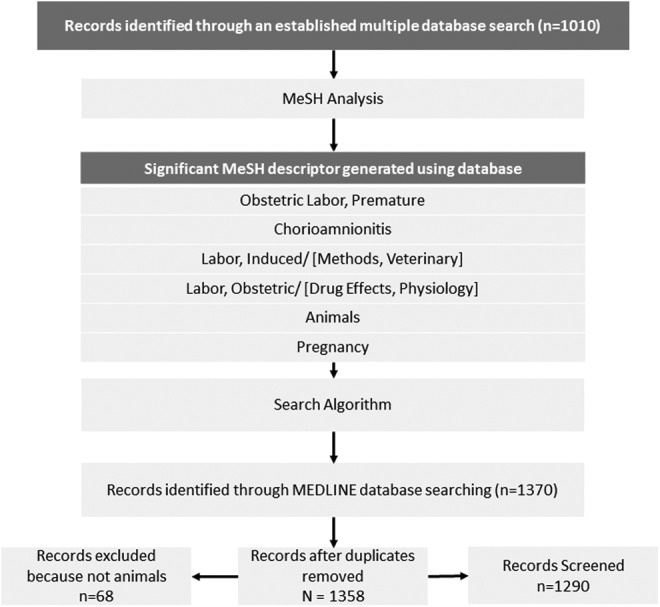
Use of algorithm to retrieve abstract on preterm birth. Medical Subject Headings (MeSH) headings were generated from the original database search. The specific MEDLINE search generated 1290 articles for further analysis.
To evaluate the search algorithm, articles were reviewed by species and focused on the most common animal species currently used in biomedical research of preterm birth—mice, rats, and sheep. For all species evaluated, we generated a list of sentinel articles that introduced a new method of preterm labor induction within the animal species. This study used publically available databases and did not involve humans.
Results
After a previous search and generation of a search algorithm, 1370 articles were identified in MEDLINE. After 12 duplicate articles were removed and 68 articles excluded because they did not involve animals, 1290 articles were reviewed for specific animal species. The major animal species identified in the search algorithm were rats (352 citations), sheep (237), cattle (201), mice (150), swine (131), and primates (66; Figure 4). The summation of these citations is greater than 1290 because multiple animals may be included into one citation. Because the focus of this study was more on animals commonly used in the experimental laboratory as opposed to domesticated animals, a more detailed analysis and review of papers was limited to sheep, mice, and rats.
Figure 4.
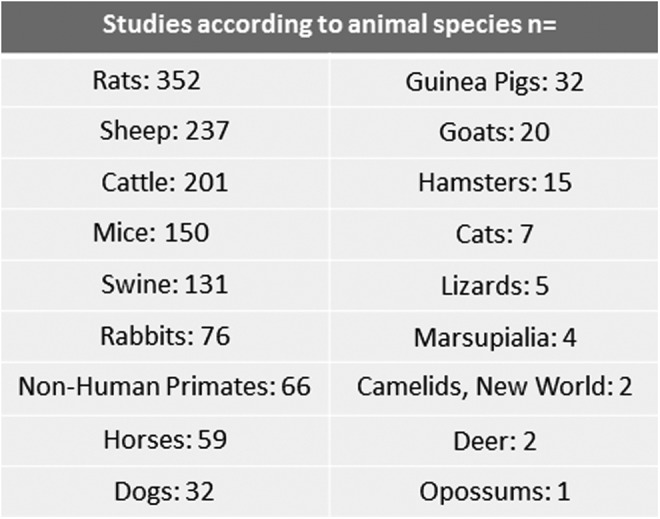
Animal models literature organized by species (n).
Reviewing the literature over time illustrates a change in which animal models have been used for the study of preterm birth (Figure 5). Classically, sheep was the first animal used in the study of parturition, and its use has been consistent over time. The rat was used increasingly during the 1980s and 1990s but has been used less frequently over the last decade. Significant use of mice began in the 1990s and has become the predominant animal for the study of preterm birth.
Figure 5.
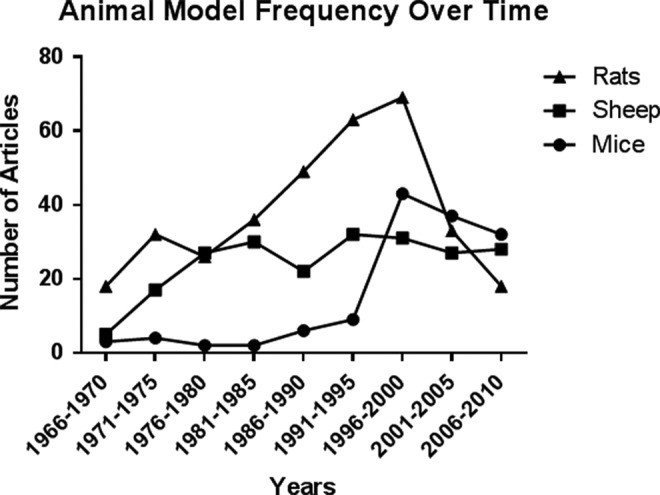
Animal model frequency of time. The variation in journal article counts organized in 5-year increments by species.
Sheep
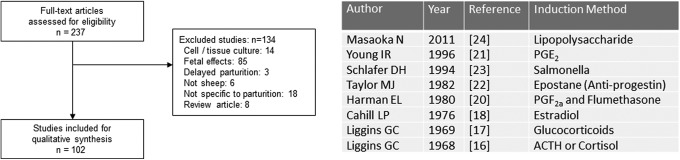
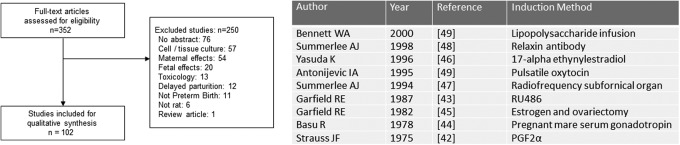
The animals’ large size, length of gestation, and the development of specific surgical techniques, such as placement of catheters in the fetus during gestation, contributed to the successful use of this animal model. Of the 237 articles identified in the original search, 102 (43% of the articles) were included for qualitative review. A comprehensive list of induction agents can be found in Figure 6. Sheep were one of the first animals used for identifying the mechanisms of parturition. It was through these experiments that the role of the fetal pituitary–adrenal axis was identified as an important part of parturition. Original research by Liggins16,17 confirmed that the use of cortisol or glucocorticoids could induce parturition in sheep.
Figure 6.
Sheep models of preterm birth. Review of full-text articles within the sheep Medical Subject Headings (MeSH) descriptor. Classification of sentinel induction method by year.
Further research focused on the hormonal control of labor. Early studies suggested that a single injection of estradiol benzoate administered to ewes results in parturition within 48 hours.18 Liggins and Grieves19 first implicated that an increase in the prostaglandin F2α (PGF) in the utero-ovarian vein of sheep occurred at the time of parturition. Other studies suggested administration of PGF late in gestation can also induce parturition in sheep, although less efficiently than glucocorticoids.20 Additionally, a continuous infusion of prostaglandin E2 was found to shorten the length of gestation.21 Finally, a reduction in progesterone late in gestation with an inhibitor of 3β-hydroxysteroid dehydrogenase activity induced delivery in 6 of 7 sheep.22
As the study of preterm birth shifted from hormonal control to an inflammation etiology, attempts were made to create an inflammation and/or infectious-induced model in sheep. Lipopolysaccharide (LPS) from Salmonella 23 or Escherichia coli 24 have been used to induce preterm birth in this species. The route of administration is either a constant intravenous infusion or via intra-amniotic infusion. The majority of the excluded articles (n = 85) pertained to preterm birth’s effect on the fetus.
Mice
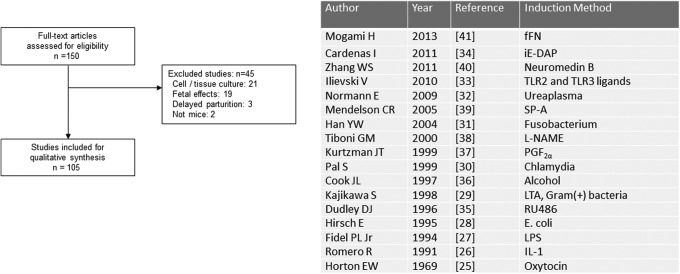
The mouse is one of the most common animals used in biomedical research. It is relatively inexpensive compared to other species, tolerates surgery, and is genetically modifiable.10 The mouse citations were the most specific for preterm birth (105 of 150, 70%; Figure 7). The majority of irrelevant articles were focused on tissue culture (n = 21) and fetal effects (n = 19). Research using mice for the study of preterm birth had been limited until the 1990s when inflammation and infection became more studied etiologies of preterm birth. Original research involving prostaglandins had a control group that delivered after injection with oxytocin.25 As preterm birth became associated with infection and inflammation, the first group used recombinant interleukin 1 in toll-like receptor 4 mutants to induce preterm birth.26 Since then, a number of different bacteria and bacterial antigens have been used to induce preterm birth in mice: LPS,27 E coli,28 lipoteichoic acid from gram-positive bacteria,29 Chlamydia,30 Fusobacterium,31 Ureaplasma,32 TLR2 and TLR3 ligands,33 and gamma-d-glutamyl-meso-diaminopimelic acid.34 Since systemic withdrawal of progesterone is essential to parturition in mice, the anti-progesterone RU486 has been used as the most common noninflammatory route for preterm birth induction in mice.35 Other more novel routes of induced preterm birth in mice include alcohol,36 PGF2α,37 L-NAME,38 SP-A,39 neuromedin B,40 and fetal fibronectin.41
Figure 7.
Mouse models of preterm birth. Review of full-text articles within the mouse Medical Subject Headings (MeSH) descriptor. Classification of sentinel induction method by year.
Rats
Compared to mice, rats have not been consistently described as ideal models for inflammation-induced preterm birth. One laboratory implanted intrauterine catheters and infused LPS42 directly. However, not all studies have reported premature delivery using infectious stimuli.43 The reasons why rats are not as sensitive to these stimuli compared to other rodents are unclear. In the studies conducted on rats, 352 citations were identified and after a detailed review of abstract and articles, 102 (29%) were identified as papers related to preterm birth induction (Figure 8). The majority of the papers were related to tissue bath experiments (n = 57), and effects on pregnant maternal physiology and postpartum breastfeeding toxicology (n = 87), or had no abstract (n = 76). Early work with rats focused on endocrine etiologies for induced preterm birth. One of the first uses of the rat model utilized PGF2α to cause a decline in serum progesterone levels, which lead to parturition.44 Similar to other rodents, RU486 will act as a progesterone antagonist and lead to induced preterm birth in rats.45 Also, estrogen stimulation with pregnant mare serum gonadotropin was able to stimulate prostaglandin production and lead to preterm birth.46 Similarly, ovariectomy and estrogen stimulation47 or estrogen stimulation alone48 induced delivery in late pregnant rats. The rat model was also used to delineate a role for in parturition as ablation of the subfornical organ in the brain or the administration of relaxin antibodies leads to preterm birth.49,50 Finally, continuous intravenous infusion of oxytocin can induce delivery in late-pregnant rats.51
Figure 8.
Rat models of preterm birth. Review of full-text articles within the rat Medical Subject Headings (MeSH) descriptor. Classification of sentinel induction method by year.
Uncommon Models
The search algorithm was able to detect less common animals of preterm birth (Figure 4). A large variety of animal species were identified: guinea pigs (n = 32), goats (n = 20), hamster (n = 15), marsupials (n = 4), cats (n = 7), lizards (n = 5), deer (n = 2), and opossum (n = 1). Guinea pigs have a unique advantage compared to the previously discussed sheep and murine models in that parturition occurs without an apparent change in maternal progesterone. This is true for humans and primates as well. Guinea pigs also have a longer gestation compared to rats and mice. Similar to the animals discussed previously, guinea pigs more recently have been used to model intra-amniotic or chorioamnionitis as mechanism of induced preterm birth. One unique method of preterm birth induction was a type I hypersensitivity reaction using ovalbumin-sensitized guinea pigs.52
For the other animals, the purpose of the research was not the prevention of spontaneous preterm birth. Many citations reviewed various methods of parturition induction in animals at term. In veterinary medicine, parturition is associated with the risk of infection and injury. This could immediately endanger the life of the dam or fetus or greatly affect the animal’s future reproductive potential. Mechanisms to shorten the length of parturition or even shorten gestation without risk to the fetus are highly desired. The focus of these papers was endocrine and hormonal regulation of parturition, for example, in goats induction of parturition with anti-progestins, prostaglandin analogs (at term), and dexamethasone. Interestingly, in lizards exogenous progesterone and indomethacin can delay parturition, while arachidonic acid and prostaglandins can induction parturition. Most of these models are for historical purposes and are not typically used today.
Discussion
The results of this study can serve as a systematic review of the literature for animal models of induced preterm birth based on an automated algorithm that may be useful for future similar reviews. Although multiple reviews are available in the current literature, these reviews tend to be either species specific or focus on precise methods of labor induction.10–13 Presently, a knowledge gap exists with regard to a comprehensive search tool for researchers investigating preterm birth within animal models. The relevant literature is distributed across MEDLINE citations and is not systematically categorized according to specific MeSH descriptors. In this review, an automated search algorithm was developed to leverage MEDLINE-indexed literature, which is one of the most commonly used digital resources for biomedical research. One of the advantages of using the MeSH-controlled vocabulary is that it reduces the variability of terminology used between authors in titles or abstracts. Another related advantage is that MeSH descriptors are applied to MEDLINE citations using a subject-expert mediated process. To underscore the value of the automated approach, the algorithm developed in this study was able to identify the sentinel articles that have impacted the field of premature birth specifically within the species of sheep, mice, and rats. This algorithm allows one to review the use of various animal models over time and the variation in induction methods across animal species. The results of this review also highlight the challenges in creating an effective search algorithm that can be used to robustly assist researchers to discover the plethora of labor induction models across multiple species. Even within species, depending on how the species is used in research, one can retrieve a large number of nonsignificant citations pertaining to maternal physiology or fetal prematurity complications. Some of these limitations may be caused by the lack of clearly identified keywords and the process used to classify articles. Using the results of this systemic review, recommendations are offered that may improve the ability to comprehensively search the literature.
The results of this study also point to the potential factors highlighting the evolution of animal models and the methods of preterm birth induction over time. Ultimately, the goal of this research is to determine the mechanisms that initiate labor, whether at term or earlier in gestation. The first historical sentinel article from Liggins16 illustrated that exogenous administration of corticosteroids induces preterm birth in sheep. Later research illustrated that maturation of the hypothalamic-pituitary axis may play a role in the initiation of labor. Throughout the 1970s and 1980s, research in whole animals models concentrated on endocrine etiologies of preterm birth initiation. In particular, the most common and successful mechanisms of preterm birth induction studied were with antiprogestational agents, epostane, and RU486 in sheep, mice, and rats, respectively.22,35,45 With the introduction of the fetal inflammatory response syndrome as a mechanism of preterm birth,26 researchers sought out new mechanisms of preterm birth induction. The most common agent was LPS. Interestingly, this method of preterm birth induction was most successful with mice, via intraperitoneal or intraamniotic administration. However, the use of LPS in sheep and rats has been limited and requires continuous infusions of LPS to induce labor successfully. The reasons for this species variation are unclear. Researchers in the last 2 decades have embraced an inflammatory mechanism to study preterm birth over endocrine changes, and this can be seen in increased use of the mouse model for the study of preterm birth. Increased enthusiasm for the mouse model may stem from their low cost and capacity for genetic modification.
Over the last 4 decades, parturition and preterm birth literature has evolved from a fetal induction of labor, to changes in estrogen and progesterone levels and signaling, to inflammation as the primary induction method of preterm birth research. Research in other risk factors or etiologies of preterm birth have been limited. Although the search algorithm developed in this study was successful in identifying animal models of preterm birth, it does have some limitations. Creating the final search algorithm involved striking a balance between sensitivity and precision, which are commonly used metrics for gauging the quality of information retrieval algorithms.53 The relationship between recall and precision is dependent on many variables, and searches that are designed to maximize recall may tend to retrieve irrelevant citations and vice versa.54 In other words, searches can be optimized, but no individual search may be a perfect representation of the knowledge embedded in the literature. Within the scope of this study, the precision and recall varied across the target species. Within the search algorithm, mice had the highest recall and precision, whereas the sheep and rat species had much lower recall and precision. This finding might be due to differences to how the respective animals have been historically used as compared to the focus of our search, which was to determine intact models of induced parturition. For example, it is only recently that sheep are used for inflammatory models of preterm birth, and rats have been used to generate tissue for in vitro experiments.
In order to assist investigators determine animal models of preterm birth induction, some recommendations can be made based on the findings of this study. First, authors should clearly define their work as an animal model with key words to help with literature search algorithms. This may help the National Library of Medicine indexers label the literature with the appropriate MeSH descriptors. To illustrate this point, the MeSH descriptor “Disease models, animal” was infrequently used to index those articles identified as relevant in this study. Second, this study revealed that the representation of preterm birth within the MeSH hierarchy is not unified under a single descriptor or within a single hierarchy. This is likely an artifact of the fact that the study of preterm birth generally falls into 2 major categories: (1) the prevention or treatment of maternal parturition (organized under the MeSH descriptor “Parturition” that is under the “Reproductive and Urinary Physiological Phenomena” MeSH descriptor hierarchy) or (2) the treatment of the complications to the newborn due to prematurity (organized under either the MeSH descriptor “Infant, Premature” that is under the “Persons” MeSH descriptor hierarchy). In the absence of a unifying MeSH descriptor, search algorithms must account for both sets of MeSH descriptors. Ultimately, the maturation of means to organize biomedical literature, such as developed in this study, may inspire the generation of preterm birth-specific queries akin to the Clinical Queries that are used to support evidence-based medicine queries.55
As with many other disease processes being studied, information on animal models of preterm birth is limited to review articles that summarize the field at a single point of time. This article is the beginning of an automated framework for obtaining information on animal models of preterm birth for investigators. In addition, it is a framework for getting up-to-date information to new investigators at the time of entry. As an example, a more recent search from 2012 to 2015 using the model generates 98 new citations illustrating that indeed articles could be identified moving forward (data not shown).
This framework could also be used to interrogate other data structures that utilize MESH headings, for example, GenBank, and could be a method of performing in silico analyses done to compare specific pathways in animals and humans that are relevant to human reproductive disease. A bioinformatic approach to the iterative study of animals and humans to understand disease is becoming of greater importance. For example, a recent article56 questioned the applicability of mouse models in inflammation-related human diseases. Those with limited knowledge of the field might accept the conclusion of that paper and not probe the literature to find that a published reanalysis of the data refuted that conclusion.57 We posit that the systematic on-line approach described herein will enhance the new investigator’s functional access to the relevant literature.
Conclusion
Preterm birth remains the leading cause of neonatal and long-term physiologic and developmental complications. Animals models can led to new insight about parturition because studies in humans are not possible. This study presents the first automated approach for tracking animal model literature from within MEDLINE for studying preterm birth. The promising results of this study, which can be viewed as a review of the current literature on animal model organisms for preterm birth, may be used for supporting the research endeavors of current and future generations of researchers in pursuit of novel therapies for the treatment of preterm birth.
Supplementary Material
Acknowledgments
We would like to thank Dr Ramkumar Menon (Past President, PREBIC) and Dr Michael Katz (March of Dimes Foundation) for advice and encouragement. We would also like to thank the librarians Fiona Brown and Marshall Dozier at the Royal (Dick) School of Veterinary Studies, University of Edinburgh, for getting us started on this work.
Appendix A
Search Strategy
Ovid MEDLINE(R)
Search Strategy:
Obstetric Labor, Premature/
Chorioamnionitis/
Labor, Induced/mt, ve [Methods, Veterinary]
Labor, Obstetric/de, ph [Drug Effects, Physiology]
1 or 2 or 3 or 4 (Pool all citations that include the above MeSH descriptors)
Animals/
Pregnancy/
5 and 6 and 7 (Pool all citations that are common to step 5 and animals and pregnancy)
Postpartum Period/
Journal Article/
Electromyography/
Myography/
Abortion, Veterinary/ or Abortion, Missed/ or Abortion, Spontaneous/
Stillbirth/
Fetal Monitoring/
Biography.mp.
Hernia, Diaphragmatic/
Midwifery/
Aggression/
Telemetry/
“United States Food and Drug Administration”/
8 not 9
22 and 10
23 not 11
24 not 12
25 not 13
26 not 14
27 not 15
28 not 16
29 not 17
30 not 18
31 not 19
32 not 20
33 not 21 (Discard citations that include 9-21)
34 not review.mp.
35 not case reports.mp.
36 not editorials.mp. (Discard reviews, case reports, and editorials)
remove duplicates from 37
Animal MeSH Descriptors:
Mice/
Rats/
Guinea Pigs/
Dogs/
Rabbits/
Sheep/ or Sheep, domestic/
Horses/
Cattle/
Goats/
Primates/ or Haplorhini/ or Catarrhini/ or Macaca/ or Macaca mulatta/ or Macaca fascicularis/ or Papio/ or Gorilla gorilla/ or Pan troglodytes/
Swine/ or Swine, miniature/
Opossums/
Marsupialia/ or Macropodidae/
Cricetinae/ or Phodopus/ or Mesocricetus/ (Hamster)
Camelids, New World
Cats/
Lizards/
Deer/
Footnotes
Authors’ Contribution: Brian W. Nielsen is the first author and contributed to preparation of the manuscript. Elizabeth A. Bonney organized the project, reviewed the original search, reviewed the articles generated by computerized search, and co-prepared the manuscript. Bradley D. Pearce designed the original systematic search. Leah Rae Donahue key reviewed the original search. Indra Neil Sarkar is the senior author and contributed to writing of the systematic search program and designing of theoretical framework. Preterm Birth International Collaborative (PREBIC), animal models group—participants in review of search articles and review of manuscript. The remaining PREBIC Animal models group members contributed to this work are as follows: Martin G. Frasch, MD, PhD, CHU Ste-Justine Research Center, l’Université de Montréal, QC, Canada and Centre de recherche en reproduction animale (CRRA), l’Université de Montréal, St-Hyacinthe, QC, Canada; Sam Mesiano, PhD, Case Western Reserve, Cleveland, Ohio; Indira Mysorekar, PhD, Washington University School of Medicine St Louis Missouri; Muriel Palmgren, PhD, Louisiana State University. Douglas Taylor, DVM, Emory University School of Medicine, Atlanta, Georgia.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online [appendices/data supplements/etc] are available at http://rs.sagepub.com/supplemental
References
- 1. ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol. 2012;119(6):1308–1317. [DOI] [PubMed] [Google Scholar]
- 2. Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin JA, Ventura SJ, Osterman MJK, Kirmeyer S, Mathews TJ, Wilson EC. Births: final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–71. [PubMed] [Google Scholar]
- 4. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20. [PubMed] [Google Scholar]
- 5. National Center for Health Statistics, final natality data. 2015; Web site www.marchofdimes.com/peristats.
- 6. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. Accessed September 3, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Bastek JA, Gomez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol. 2011;38(3):385–406. [DOI] [PubMed] [Google Scholar]
- 8. Bonney EA. Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside. Am J Reprod Immunol. 2013;69(6):567–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kriesberg N. Animals as Models. Web site http://ori.hhs.gov/education/products/ncstate/models.htm. Accessed September 3, 2015.
- 10. Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15(10):479–487. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integrat Comp Physiol. 2009;297(3): R525–R545. [DOI] [PubMed] [Google Scholar]
- 12. Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17(7):619–628. [DOI] [PubMed] [Google Scholar]
- 13. Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG. 2011;118(2):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. United States National Library of Medicine. Fact Sheet: Medical Subject Headings (MeSH®). 2013; Web site http://www.nlm.nih.gov/pubs/factsheets/mesh.html. Accessed September 3, 2015.
- 15. National Center for Biotechnology Information, United States National Library of Medicine. Entrez Programming Utilities Help. 2010; Web site http://www.ncbi.nlm.nih.gov/books/NBK25501/. Accessed September 3, 2015. [DOI] [PMC free article] [PubMed]
- 16. Liggins GC. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. J Endocrinol. 1968;42(2):323–329. [DOI] [PubMed] [Google Scholar]
- 17. Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45(4):515–523. [DOI] [PubMed] [Google Scholar]
- 18. Cahill LP, Knee BW, Lawson RA. Proceedings: Induction of parturition in sheep with a single dose of oestradiol benzoate. J Reprod Fertil. 1976;46(2):528–529. [DOI] [PubMed] [Google Scholar]
- 19. Liggins GC, Grieves S. Possible role for prostaglandin F 2 in parturition in sheep. Nature. 1971;232(5313):629–631. [DOI] [PubMed] [Google Scholar]
- 20. Harman EL, Slyter AL. Induction of parturition in the ewe. J Animal Sci. 1980;50(3):391–393. [DOI] [PubMed] [Google Scholar]
- 21. Young IR, Deayton JM, Hollingworth SA, Thorburn GD. Continuous intrafetal infusion of prostaglandin E2 prematurely activates the hypothalamo-pituitary-adrenal axis and induces parturition in sheep. Endocrinology. 1996;137(6):2424–2431. [DOI] [PubMed] [Google Scholar]
- 22. Taylor MJ, Webb R, Mitchell MD, Robinson JS. Effect of progesterone withdrawal in sheep during late pregnancy. J Endocrinol. 1982;92(1):85–93. [DOI] [PubMed] [Google Scholar]
- 23. Schlafer DH, Yuh B, Foley GL, Elssaser TH, Sadowsky D, Nathanielsz PW. Effect of Salmonella endotoxin administered to the pregnant sheep at 133-142 days gestation on fetal oxygenation, maternal and fetal adrenocorticotropic hormone and cortisol, and maternal plasma tumor necrosis factor alpha concentrations. Biol Reprod. 1994;50(6):1297–1302. [DOI] [PubMed] [Google Scholar]
- 24. Masaoka N, Watanabe M, Nakajima Y. The effects of sivelestat sodium hydrate on uterine contraction and the concentration of maternal and fetal blood cytokines in a sheep model of intra-amniotic infection induced by lipopolysaccharide. J Matern Fetal Neonat Med. 2011;24(8):1013–1018. [DOI] [PubMed] [Google Scholar]
- 25. Horton EW, Marley PB. An investigation of the possible effects of prostaglandins E-1, F-2alpha and F-2beta on pregnancy in mice and rats. Br J Pharmacol. 1969;36(1):188P–189P. [PMC free article] [PubMed] [Google Scholar]
- 26. Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165(4 pt 1):969–971. [DOI] [PubMed] [Google Scholar]
- 27. Fidel PL, Jr, Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170(5 pt 1):1467–1475. [DOI] [PubMed] [Google Scholar]
- 28. Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172(5):1598–1603. [DOI] [PubMed] [Google Scholar]
- 29. Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods. 1998;39(3):147–154. [DOI] [PubMed] [Google Scholar]
- 30. Pal S, Peterson EM, De La Maza LM. A murine model for the study of Chlamydia trachomatis genital infections during pregnancy. Infect Immun. 1999;67(5):2607–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72(4):2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thebaud B. A novel mouse model of Urea plasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 2009;65(4):430–436. [DOI] [PubMed] [Google Scholar]
- 33. Ilievski V, Hirsch E. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod. 2010;83(5):767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cardenas I, Mulla MJ, Myrtolli K, et al. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J Immunol. 2011;187(2):980–986. [DOI] [PubMed] [Google Scholar]
- 35. Dudley DJ, Branch DW, Edwin SS, Mitchell MD. Induction of preterm birth in mice by RU486. Biol Reprod. 1996;55(5):992–995. [DOI] [PubMed] [Google Scholar]
- 36. Cook JL, Randall CL. Early onset of parturition induced by acute alcohol exposure in C57BL/6 J mice: role of uterine PGE and PGF2alpha. Reprod Fertil Dev. 1997;9(8):815–823. [DOI] [PubMed] [Google Scholar]
- 37. Kurtzman JT, Spinnato JA, Goldsmith LJ, et al. Human chorionic gonadotropin exhibits potent inhibition of preterm delivery in a small animal model. Am J Obstet Gynecol. 1999;181(4):853–857. [DOI] [PubMed] [Google Scholar]
- 38. Tiboni GM, Giampietro F. Inhibition of nitric oxide synthesis causes preterm delivery in the mouse. Hum Reprod. 2000;15(8):1838–1842. [DOI] [PubMed] [Google Scholar]
- 39. Mendelson CR, Condon JC. New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol. 2005;93(2-5):113–119. [DOI] [PubMed] [Google Scholar]
- 40. Zhang WS, Xie QS, Wu XH, Liang QH. Neuromedin B and its receptor induce labor onset and are associated with the RELA (NFKB P65)/IL6 pathway in pregnant mice. Biol Reprod. 2011;84(1):113–117. [DOI] [PubMed] [Google Scholar]
- 41. Mogami H, Kishore AH, Shi H, Keller PW, Akgul Y, Word RA. Fetal fibronectin signaling induces matrix metalloproteases and cyclooxygenase-2 (COX-2) in amnion cells and preterm birth in mice. J Biol Chem. 2013;288(3):1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bennett WA, Terrone DA, Rinehart BK, Kassab S, Martin JN, Jr, Granger JP. Intrauterine endotoxin infusion in rat pregnancy induces preterm delivery and increases placental prostaglandin F2alpha metabolite levels. American journal of obstetrics and gynecology. 2000;182(6):1496–1501. [DOI] [PubMed] [Google Scholar]
- 43. Hirsch E, Filipovich Y, Romero R. Failure of E. coli bacteria to induce preterm delivery in the rat. J Negat Results Biomed. 2009;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Strauss JF, III, Sokoloski J, Caploe P, Duffy P, Mintz G, Stambaugh RL. On the role of prostaglandins in parturition in the rat. Endocrinology. 1975;96(4):1040–1043. [DOI] [PubMed] [Google Scholar]
- 45. Garfield RE, Gasc JM, Baulieu EE. Effects of the antiprogesterone RU 486 on preterm birth in the rat. Am J Obstet Gynecol. 1987;157(5):1281–1285. [DOI] [PubMed] [Google Scholar]
- 46. Basu R, Chatterjee A. Pregnant mare’s serum gonadotropin. IV. Induction of premature labor by pregnant mare’s serum gonadotropin and its prevention by using clomiphene or indomethacin. Fertil Steril. 1978;29(6):640–642. [PubMed] [Google Scholar]
- 47. Garfield RE, Puri CP, Csapo AI. Endocrine, structural, and functional changes in the uterus during premature labor. Am J Obstet Gynecol. 1982;142(1):21–27. [DOI] [PubMed] [Google Scholar]
- 48. Yasuda K, Furukawa M, Johnston JM. Effect of estrogens on plasma platelet-activating factor acetylhydrolase and the timing of parturition in the rat. Biol Reprod. 1996;54(1):224–229. [DOI] [PubMed] [Google Scholar]
- 49. Summerlee AJ, Wilson BC. Role of the subfornical organ in the relaxin-induced prolongation of gestation in the rat. Endocrinology. 1994;134(5):2115–2120. [DOI] [PubMed] [Google Scholar]
- 50. Summerlee AJ, Ramsey DG, Poterski RS. Neutralization of relaxin within the brain affects the timing of birth in rats. Endocrinology. 1998;139(2):479–484. [DOI] [PubMed] [Google Scholar]
- 51. Antonijevic IA, Leng G, Luckman SM, Douglas AJ, Bicknell RJ, Russell JA. Induction of uterine activity with oxytocin in late pregnant rats replicates the expression of c-fos in neuroendocrine and brain stem neurons as seen during parturition. Endocrinology. 1995;136(1):154–163. [DOI] [PubMed] [Google Scholar]
- 52. Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am J Obstet Gynecol. 2004;191(4):1356–1361. [DOI] [PubMed] [Google Scholar]
- 53. Lowe HJ, Barnett GO. Understanding and using the medical subject headings (MeSH) vocabulary to perform literature searches. JAMA. 1994;271(14):1103–1108. [PubMed] [Google Scholar]
- 54. McGill GSaMJ. Introduction to Moden Information Retrieval. New York, New York: McGraw-Hill International Book Company; 1983. [Google Scholar]
- 55. Wong SS, Wilczynski NL, Haynes RB, Ramkissoonsingh R, Hedges T. Developing optimal search strategies for detecting sound clinical prediction studies in MEDLINE. AMIA Annu Symp Proc. 2003:728–732. [PMC free article] [PubMed] [Google Scholar]
- 56. Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2015;112(4):1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.