Abstract
We investigated the ability of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), hydroxyl radical (·OH), and hypochlorous acid (HOCl), to overcome the defensive capacity of cumulus cells and elucidate the mechanism through which ROS differentially deteriorate oocyte quality. Metaphase II mouse oocytes with (n = 1634) and without cumulus cells (n = 1633) were treated with increasing concentration of ROS, and the deterioration in oocyte quality was assessed by the changes in the microtubule morphology and chromosomal alignment. Oocyte and cumulus cell viability and cumulus cell number were assessed by indirect immunofluorescence, staining of gap junction protein, and trypan blue staining. The treated oocytes showed decreased quality as a function of increasing concentrations of ROS when compared to controls. Cumulus cells show protection against H2O2 and ·OH insult at lower concentrations, but this protection was lost at higher concentrations (>50 μmol/L). At higher H2O2 concentrations, treatment dramatically influenced the cumulus cell number and viability with resulting reduction in the antioxidant capacity making the oocyte more susceptible to oxidative damage. However, cumulus cells offered no significant protection against HOCl at any concentration used. In all circumstances in which cumulus cells did not offer protection to the oocyte, both cumulus cell number and viability were decreased. Therefore, the deterioration in oocyte quality may be caused by one or more of the following: a decrease in the antioxidant machinery by the loss of cumulus cells, the lack of scavengers for specific ROS, and/or the ability of the ROS to overcome these defenses.
Keywords: hydrogen peroxide, reactive oxygen species, cumulus cells, oocyte quality, oxidative stress, and inflammation
Introduction
Oocytes are surrounded by tightly packed, highly organized layers of cumulus cells (CCs) that exist in spatial and temporal heterogeneity, forming the cumulus–oocyte complex (COC).1–3 In addition to their role in providing nutrition to the oocyte, the CCs provide a communication network between the oocyte and its extracellular microenvironment.1,2,4,5 The manner through which CCs communicate within the COC are specialized connections called gap junctions, which are aggregates of protein-based intercellular channels that directly connect adjacent cells, allowing the bidirectional movement of molecules.6 These channels known as connexins (Cx) have been described in many tissues; however, Cx37 is the only one identified on the oocyte and forms connection with CCs, while Cx43 is the main gap junction protein found on CCs. Loss of Cx proteins has been attributed to disrupted folliculogenesis, CC dysfunction, and altered cell and tissue viability.6–9 Therefore, Cxs are critical as they function as primary means of disseminating information to and from the oocyte and also serve the important function of anchoring the CCs within the COC to protect the oocyte. It is known that oocytes, under pathological conditions, protect themselves against the toxicity of reactive oxygen species (ROS) through a scavenging enzymatic (eg, catalase and glutathione peroxidase) and nonenzymatic antioxidant (eg, ascorbic acid and reduced glutathione) network provided by the surrounding CCs.10,11 Translational research has shown that patients with these conditions have higher rates of adverse reproductive outcomes and poor oocyte quality compared to those without such disorders, suggesting that inflammation may be central to the decrease in fertility potential.12–16
Reactive oxygen species such as superoxide (O2 ·−), hydroxyl radical (·OH), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl) are highly disruptive to cellular function.13,17,18 The major intracellular sources of H2O2 are spontaneous production, superoxide dismutase-catalyzed reaction of O2 ·−,19,20,71 generation through the mitochondrial electron transport chain, and the nicotinamide adenine dinucleotide phosphate oxidase system in the cellular plasma membrane.13,21,22,72 Hydrogen peroxide is found in physiologic concentrations ranging from 10 to 20 μmol/L and up to 100 μmol/L in pathologic circumstances.23 Exposure of mouse oocytes to higher H2O2 concentrations (200 μmol/L) completely inhibited cleavage and caused arrest of the zygote at the 1-cell stage.24,25 A link between the concentration of endogenous H2O2 and the occurrence of apoptosis in human embryos has been suggested.25–28 There are other ways that H2O2 can indirectly affect oocyte quality, for example, we have recently shown that ·OH generated by the H2O2-induced Fenton reaction caused instantaneous oocyte damage, which has been estimated indirectly in plasma at levels of 250 to 500 μmol/L.17,29 More recently, we have demonstrated that diffused intra-oocyte H2O2 is normally found in the oocyte and its microenvironment. This H2O2, in the presence of chloride, can subsequently trigger the catalytic activity of MPO generating the toxic oxidant, HOCl,20,30,73 a substance known to deteriorate oocyte quality.18 Activated neutrophils, the major cellular releaser of myeloperoxidase (MPO), generate around 150 to 425 μm HOCl/h, whereas at sites of inflammation, the HOCl level is estimated to reach as high as 5 mm.31 Previously, it has been shown that higher levels of MPO exist in the peritoneal and follicular fluids of women with inflammatory conditions such as endometriosis.32,33 Macrophages, neutrophils, and monocytes are the major cellular sources of MPO, which function to generate HOCl and other ROS.34 The current study investigates the ability of the antioxidant system of the CCs to deflect the H2O2-mediated oxidative damage from mouse oocytes and highlights the mechanism through which H2O2 deteriorates oocyte quality. Our results provide a previously undescribed mechanistic link between excess H2O2 accumulation and poor oocyte quality, namely, through the disassembly and decreased viability of the protective CC cloud, which allows the dismantling of the spindle and chromosomal alignment (CH). These effects may cause poor oocyte quality and thus poor reproductive outcomes, which are associated with various inflammatory conditions.
Materials and Methods
Materials
All the materials used were of highest grade of purity and without further purification. Hydrogen peroxide, sodium hypochlorite (NaOCl), ammonium ferrous sulfate (Fe(II)), human tubular fluid (HTF) media, anti-α tubulin antibody, fluorescein isothiocyanate (FITC)-conjugate antigoat antibody, propidium iodide (PI), 1% bovine serum albumin (BSA), 0.1% mol/L glycine, and 0.1% Triton X-100 were obtained from Sigma-Aldrich (St Louis, Missouri). Normal goat serum (2%) was from Invitrogen (Grand Island, New York), and 0.2% powder milk from grocery. Metaphase II (MII) oocytes (with and without CCs) from a B6C3F1 mouse crossed with a B6D2F1 mouse were obtained commercially (Embryotech Inc, Haverhill, MA) in cryopreserved straws using ethylene glycol-based slow freeze cryopreservation protocol. We used mature MII oocytes for the purpose of understanding the defense rendered by the CCs at the mature cell stage level. Although it is understood that there may be some loss of antioxidant defense during the expansion as a physiologic mechanism for preparation of ovulation, we believe that preexistence of intact gap junctions for delivery of antioxidant defense is essential for the prevention of spindle damage during maturation process in inflammatory states. It is known that the oocyte spindle repolymerizes to normal structure when incubated in media for 60 to 120 minutes at 37°C and 5% CO2 35,36 prior to induction of oxidative stress. This mechanism actually has helped support utilization of frozen oocytes for studying spindle damage. The use of frozen–thawed oocytes is well accepted, as many studies have been published in the past utilizing frozen–thawed oocytes and effects of oxidative stress on spindle morphology.37,38
Methods
Metaphase II mouse oocytes with and without CCs, in triplicate for each ROS experiment, were transferred from straws to phosphate-buffered saline (Dulbecco phosphate-buffered saline [PBS]) and washed to remove excess cryoprotectant for 3 minutes. Oocytes were then transferred to HTF media and incubated at 37°C and 5% CO 2 for 60 minutes to allow spindle repolymerization and attainment of normal oocyte architecture. The oocytes were then screened for the presence of the polar body confirming their MII stage. Ten to 20 oocytes from each group were discarded as they were found to be immature or displayed disrupted zona pellucidas. In each ROS experiment, MII oocytes with and without CCs were divided into 3 groups: H2O2, ·OH, and HOCl treatments.
For H2O2 treatment (experiments performed in triplicate), oocytes were divided into 4 different groups: (group 1, n = 620) oocytes without CCs incubated with increasing concentrations of H2O2 (10, 17, 25, 50, and 100 µmol/L), (group 2, n = 611) oocytes with CCs incubated with increasing concentrations of H2O2 (10, 17, 25, 50, and 100 µmol/L), (group 3, n = 62) untreated oocytes with CCs, and (group 4, n = 62) untreated oocytes without CCs.
For ·OH treatment (experiments performed in triplicate), oocytes were divided equally into 8 groups: oocytes with (group 1, n = 171) and without (group 2, n = 171) CCs treated with increasing concentrations of H2O2 (5, 10, and 20 µmol/L); oocytes with (group 3, n = 60) and without (group 4, n = 61) CCs treated with a fixed concentration of Fe(II), 100 µmol/L; oocytes with (group 5, n = 173) and without (group 6, n = 191) CCs preincubated with a fixed concentration of Fe (II), 100 µmol/L, and treated with increasing concentrations of H2O2 (5, 10, and 20 µmol/L), under these circumstances all H2O2 was converted to ·OH; and untreated oocytes with (group 7, n = 60) and without (group 8, n = 73) CCs. Due to the instability of ·OH and its instant effect on oocyte quality, all sets were exposed for less than 10 minutes. This short incubation time also eliminates the effect of H2O2 and Fe(II) alone as described previously.17 The concentration of Fe(II), 100 µmol/L, used in the current study to establish the ·OH generating system has been widely used in previous studies.39
For HOCl treatment (experiments performed in triplicate), oocytes were divided into 4 different groups: (group 1, n = 324) oocytes with CCs and (group 2, n = 391) oocytes without CCs treated with increasing concentrations of HOCl (10, 25, 50, and 100 µmol/L); and (group 3, n = 73) untreated oocytes with CCs and (group 4, n = 64) untreated oocytes without CCs. The treated and untreated oocytes were incubated with HOCl for 15 minutes to ensure maximum effect.
Immunofluorescence Staining and Fluorescence Microscopy
All treated and untreated oocytes were fixed in a solution prepared from 2% formaldehyde and 0.2% Triton X-100 for 30 minutes at 25°C.40 The fixed oocytes were treated with blocking solution (PBS, 0.2% powdered milk, 2% normal goat serum, 1% BSA, 0.1 mol/L glycine, and 0.1% Triton X-100) for 30 minutes, then washed with PBS for 3 minutes.17,40 Subsequently, the oocytes were subjected to indirect immunofluorescence staining by incubating in mouse primary anti-α tubulin antibody against the microtubule morphology (MT) for 60 minutes and secondary FITC-conjugated antigoat antibody for 30 minutes.40 The chromosomes were stained using PI and incubated for 15 minutes.40 Stained oocytes were loaded into an antifade agent on slides with 2 etched rings, and cover slips were affixed using nail varnish. The alterations in the MT and CH were compared with controls and scored by 3 blinded observers based on a previously published scoring system (Figure 1).18,41,42 Scores of 1 to 4 were assigned for both MT and CH alterations, with scores 1 and 2 combined for good outcomes meaning microtubules were organized in a barrel shaped with slightly pointed poles formed by organized microtubules crosswise from pole to pole, and chromosomes were normally arranged in a compact metaphase plate at the equator of the spindle.41,42 Scores of 3 and 4 signified poor outcomes and consisted of spindle length reduction, disorganization and/or complete spindle absence, and chromosome dispersion or aberrant condensation appearance.41,42 Images were obtained utilizing both immunofluorescence and confocal microscopy.
Figure 1.
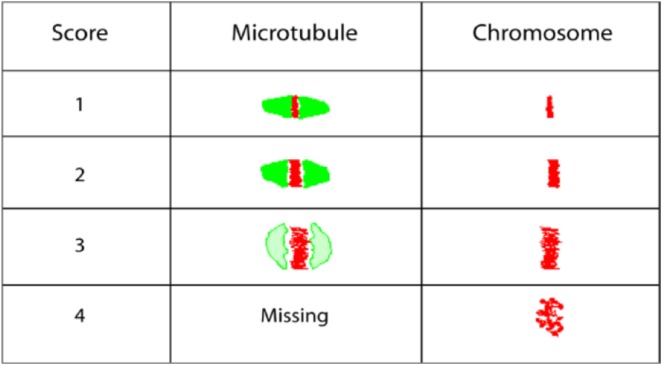
Schematic diagram demonstrating scoring system of microtubule morphology (MT) and chromosomal alignment (CH) alteration based on previous study by Choi et al41 and Banerjee et al.18
Confocal Microscopy, Assessment of Microtubules, and CH
Slides were examined with the Axiovert 25 inverted microscope (Zeiss, Thornwood, New York) using Texas Red (red) and FITC (green) fluorescent filters with excitation and emission wavelengths of 470 and 525 nm and 596 and 613 nm, respectively. Confocal images were obtained utilizing a Zeiss LSM 510 META NLO microscope (Zeiss, Germany). Oocytes were localized using a 10× magnification lens and spindle alterations assessed using 40× oil immersion lens. The MT was stained fluorescent green, which was distinct from the fluorescent red staining of the chromosomes. Following completion of the experiments, each oocyte was closely examined for spindle status by 3 independent observers blinded to the assigned treatment groups. Observers used comprehensive evaluation of the individual optical sections and the 3-dimensional reconstructed images.
Viability assay (measurement of COC viability)
We used oocytes with CCs (n = 100) exposed to 10 and 25 µmol/L of each ROS before fixing followed by addition of 10 µL of trypan blue dye (Sigma) into the HTF media for 4 minutes. The untreated oocytes were also subjected to trypan blue dye to determine the number of viable cells (the dye exclusion test) in the media. This test is based on the fact that living cells possess intact cell membranes that will keep out certain dyes (trypan blue and propidium), whereas dead cells will not. Both control and exposed groups were examined under the Axiovert 25 light microscope, and CCs were counted for staining with images obtained.
Connexin 43 immunostaining and fluorescence confocal microscopy
In this experiment, we grouped the MII mouse cumulus oocytes (n = 30) in 2 sets in the HTF culture media: (A) control cumulus oocytes and (B) cumulus oocytes incubated with 100 µmol/L H2O2 oocytes for 45 minutes. Then as mentioned earlier, oocytes were fixed in a solution prepared from 2% formaldehyde and 0.2% Triton X-100 for 30 minutes. The fixed oocytes were treated with blocking solution as described previously for 1 hour, and then washed with PBS for 3 to 5 minutes. Subsequently, the oocytes were subjected to indirect immunostaining for Cx43 by incubating them in monoclonal anti-Cx43 antibody produced in mouse (1:100; C8093, from Sigma-Aldrich) over night at 4°C followed by secondary FITC-conjugated antigoat antibody (1:50) for 30 minutes (green color). The chromosomes were stained using PI (1:50) and incubated for 15 minutes to count the granulosa cells before and after H2O2 treatment. Stained oocytes were loaded into an antifade agent on slides with 2 etched rings, and cover slips were placed using nail varnish.
Solutions Preparation
The H2O2 solution was prepared fresh in phosphate buffer (PH 7.4), while the concentrations of the working solutions were determined spectrophotometrically (extinction coefficient of 43.6 mol/L−1 cm−1 at 240 nm).43,44
The HOCl was prepared as described previously with some modifications.45 Briefly, a stock solution of HOCl was prepared by adding 1 mL of NaOCl solution to 40 mL of 154 mmol/L NaCl, and the pH was adjusted to around 3 by adding HCl. The concentration of active total chlorine species in solution, expressed as [HOCl]T (where [HOCl]T = [HOCl] + [Cl2] + [Cl3 −] + [OCl−]) in 154 mmol/L NaCl, was determined by converting all the active chlorine species to OCl− by adding a bolus of 40 μL of 5 mol/L NaOH and measuring the concentration of OCl−. The concentration of OCl− was determined spectrophotometrically at 292 nm (∊ = 362 mol/L−1 cm−1). As HOCl is unstable, the stock solution was freshly prepared on a daily basis, stored on ice, and used within 1hour of preparation. For further experimentation, dilutions were made from the stock solution using 200 mmol/L phosphate buffer, pH 7.0, to give working solutions of lower HOCl concentrations.46 During and after the preparation process, all solutions were kept on ice to minimize decomposition.
Statistical Analysis
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc, Chicago, Illinois). One-way analysis of variance procedures were performed to compare the percentage of oocytes with poor outcomes (scores 3 and 4) for MT and CH between controls and oocytes treated with various oxidant concentrations. No transformation was used because a good portion of the data ranged between 0.2 and 0.8. The Tukey post hoc procedure was used for pairwise comparisons among treatment groups. Statistical significance was indicated by P < .05. Independent t tests were conducted to compare the cumulus and noncumulus oocytes for each oxidant concentration.
Results
Effect of ROS on Cumulus Enclosed and Denuded Oocytes
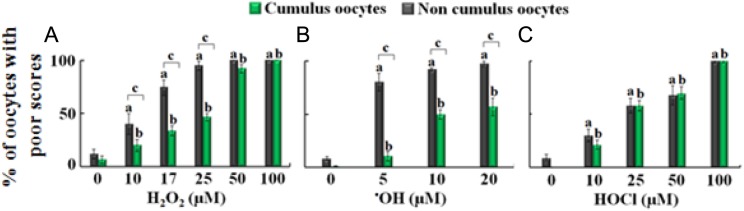
The majority of both cumulus-enclosed and denuded control oocytes had good scores (98% and 90%, respectively; Figure 2). Exposure to H2O2, ·OH, and HOCl resulted in various detrimental effects on oocyte quality as assessed by the poor scoring of MT and CH. These detrimental effects depended on the presence or absence of CCs, relative strength of the oxidizing agent, and its concentration. As shown in Figures 2A and 3 (upper panel), in the absence of CCs, increasing H2O2 concentrations (10, 17, 25, 50, and 100 µmol/L) were associated with significant increases in poor scores of MT (45%, 74.4%, 93.5%, 100%, and 100%, respectively; P < .001). In contrast as shown in Figure 3 (lower panel), in the presence of CCs, the frequency of poor MT scores also increased when subjected to similar concentrations of H2O2 but to a lesser extent: 19.8% (P > .05), 33.9% (P < .05), 46.7% (P < .001), 96.7% (P < .001), and 100% (P < .001), respectively (Figures 2A and 3). Similar results were observed for CH. A similar trend was observed for oocytes treated with increasing concentration of H2O2 in the presence of fixed amounts of Fe(II), 100 µmol/L. Under these circumstances, H2O2 was immediately converted to ·OH in a 1:1 ratio.17 Poor scores of MT were higher in the noncumulus groups at 5, 10, and 20 μmol/L (80.3%, 92.2%, and 96.6%, respectively, P < .001) compared to oocytes with CCs (10.5%, P > .05; 49.8%, P < .001; and 56.6%, P < .001, respectively), suggesting CC protection (P < .001; Figure 2B).
Figure 2.
The effect of increasing concentration of hydrogen peroxide (H2O2), hydroxyl radical (·OH), and hypochlorous acid (HOCl) on microtubule morphology (MT) of metaphase-II mouse oocytes in the absence (gray bars) and the presence (green bars) of cumulus cells. A, The percentage of oocytes with poor scores in MT treated with 0, 10, 17, 25, 50, and 100 µmol/L H2O2. B, The percentage of oocytes with poor scores in MT treated with 0, 5, 10, and 20 µmol/L ·OH. C, The percentage of oocytes with poor scores in MT treated with 0, 10, 25, 50, and 100 µmol/L HOCl. One-way analysis of variance (ANOVA) and independent t test employing SPSS 21.0 were used for statistical analysis. a P < .05 noncumulus oocytes as compared to control, b P < .05 cumulus oocytes as compared to control, and c P < .05 cumulus compared to noncumulus oocytes at each concentration. The experiments were conducted with 3 replications, and the error bars represent the standard error of the mean. (The color version of this figure is available in the online version at http://rs.sagepub.com/)
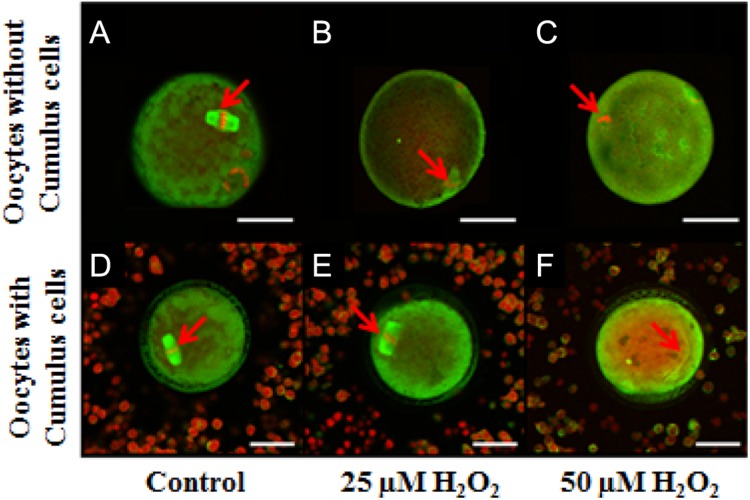
Figure 3.
Images showing the effect of different hydrogen peroxide (H2O2) concentrations on oocyte quality obtained using Confocal Zeiss LSM 510 META NLO microscope. A, Control oocyte without cumulus cells with normal microtubule morphology (MT) and chromosomal alignment (CH). B, Oocyte without cumulus cells with altered MT and CH exposed to 25 µmol/L H2O2. C, Oocyte without cumulus cells treated with 50 µmol/L H2O2. D, Control oocyte with cumulus cells with normal MT and CH. E, Oocyte with cumulus cells with normal MT and CH exposed to 25 µmol/L H2O2. F, Oocyte with cumulus cells treated with 50 µmol/L H2O2 with altered MT and CH. Scale bars: 1 pixel, 5 mm for images A-C and 1 pixel, 3 mm for images D-F. Red arrows show the MT and the CH alterations. The experiments were conducted with 3 replications.
In contrast to H2O2 and ·OH, nonsignificant independent t tests revealed that CCs do not offer protection against HOCl at concentrations of 10 to 100 μmol/L (Figure 2C). Poor scores were noted following increasing HOCl concentrations (10, 25, 50, and 100 μmol/L) for both cumulus (20.7%, 57.9%, 66.1%, and 100%, respectively) and noncumulus oocytes (30%, 58.3%, 64.6%, and 100%, respectively) compared to controls (P < .001). Thus, antioxidant machinery provided by CCs may have selective protection against ROS.
Effect of ROS on Cumulus Cell Number, Dispersion, and Cx43
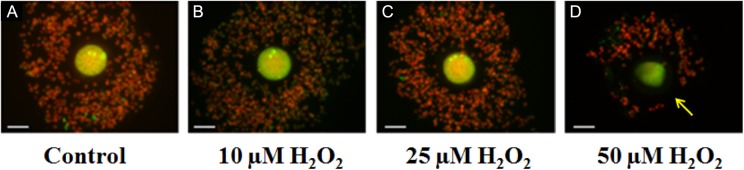
To determine the mechanism by which different concentrations of ROS overwhelm the protective antioxidant machinery provided by CCs, we next investigated the effect of increasing concentrations of H2O2, ·OH, and HOCl on COC organization, CC number, and cumulus–CC interaction through staining of gap junction protein, Cx43, utilizing confocal imaging as well as cumulus oocytes viability using trypan blue dye exclusion method. As shown in Figure 4A, untreated oocytes were surrounded by tightly packed highly organized layers of CCs. After treatment with lower concentrations of H2O2 (<25 µmol/L), the COC remained intact and organized with similar number of CCs observed as compared to controls, reflecting the preservation of oocyte quality (Figure 4B and C). Treatment with high H2O2 concentrations changed the organized compact CC mass into a dispersed structure of cells (Figure 4D). In some cases, these clouds of CCs are small, scattered, and remain loosely linked to the oocyte (Figure 4). Collectively, these alterations in the oocyte microenvironments upon exposure of the oocytes with CCs to higher ROS concentrations could explain the damaging effect of H2O2 and ·OH to oocyte quality. As shown in Figure 5A, the control COC, stained for Cx43 (green) and CH (red), showed organized clustering of CCs surrounding the oocyte. Oocyte with CCs exposed to a higher H2O2 concentration (eg, 100 µmol/L) showed significant decrease in the CCs number or in some cases complete removal of the CCs compared with untreated COCs (data not shown). However, the intensity of Cx43 staining appears similar to control.
Figure 4.
Images of cumulus–oocyte complexes as a function of increasing concentrations of hydrogen peroxide (H2O2) obtained using a microscope-mounted Axiocam camera with Axiovision software (Zeiss). Panel A is a control of cumulus–oocyte complex (COC) with normal microtubule morphology (MT), chromosomal alignment (CH), and good organized cluster of cumulus cells surrounding the oocyte. Panels B-D, oocytes treated with 10, 25, and 50 µmol/L H2O2, respectively. Scale bar: 1 pixel, 2 mm. Arrow shows decrease in cumulus cells. The experiments were conducted with 3 replications.
Figure 5.
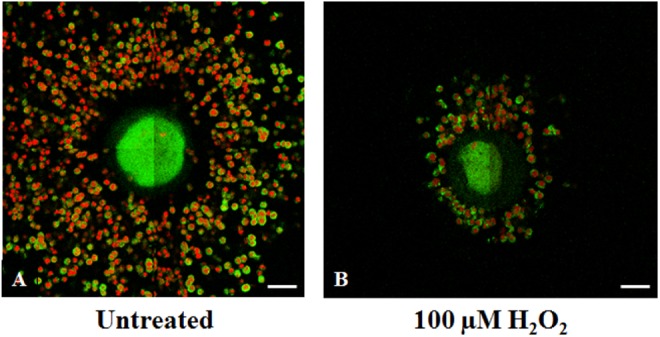
Metaphase-II mouse cumulus–oocyte complexe (COC) images obtained with Confocal Zeiss LSM 510 META NLO microscope showed the effect of hydrogen peroxide (H2O2) on Connexin (Cx) 43 density (n = 20). A, Control COC stained for Cx43 (green) with chromosomal alignment (CH; red) with organized clustering of cumulus cells surrounding the oocyte. B, COC exposed to 100 µmol/L of H2O2 with significant decrease in the cumulus cells number with decrease in Cx43 staining compared with untreated COC. Results depict observations from 3 experiments. Scale bar: 1 pixel, 2 mm. (The color version of this figure is available in the online version at http://rs.sagepub.com/)
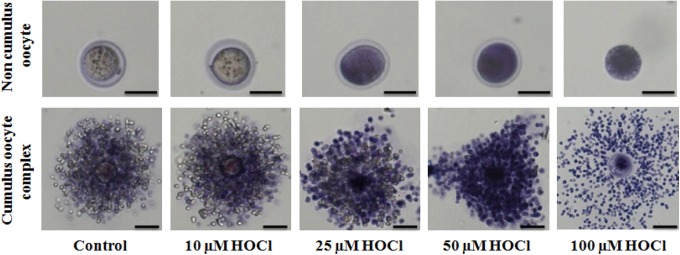
Finally, the trypan blue dye exclusion method was used to determine the viability of the cells after exposure to HOCl and to explain the failure of CCs to protect oocyte against HOCl. Oocytes without CCs exposed to higher HOCl concentrations (>25 µmol/L) had a higher intensity of staining compared with untreated controls and oocytes exposed to lower concentrations of HOCl (Figure 6, upper panels). Cumulus oocytes showed greater intensity of staining and significant decrease in CC number at higher concentrations of HOCl, specifically at 100 µmol/L (Figure 6, lower panels). This signifies that exposure to HOCl rendered the CCs nonviable, which could explain the failure of the CCs to provide antioxidant defense as trypan blue staining was similar between cumulus and noncumulus oocytes (Figure 6). Collectively, the mechanism through which CCs lose their ability to defend against the effects of ROS is largely through the partial or complete loss of CCs, which can be explained by the loss of CC viability.
Figure 6.
Images of oocytes without (upper panel) and with (lower panel) cumulus cells as a function of increasing concentrations of hypochlorous acid (HOCl) obtained using a microscope-mounted Axiocam camera with Axiovision software (Zeiss). Trypan blue viability staining with increased stain uptake in oocytes exposed to 0, 10, 25, 50, and 100 µmol/L HOCl). Scale bars: 1 pixel, 4 mm for images A-C and 1 pixel, 2 mm for images D-F. The experiments were conducted with 3 replications.
Discussion
In this work, we investigated the ability of CCs to protect the oocyte against ROS and elucidate the mechanism and details of this action. Our results showed that oocytes, with and without the surrounding CCs, treated with increasing concentrations of various ROS exhibited deterioration in oocyte quality as a function of concentration, when compared to untreated controls. Cumulus cells demonstrated protection against H2O2 and ·OH insult at low concentrations, but this protection was lost at higher concentrations. Cumulus cells offered no statistically significant protection against HOCl at any concentration. In all circumstances in which CCs did not offer protection to the oocyte, both CC number and viability were decreased as judged by confocal immunofluorescence and viability staining. Collectively, the deterioration in oocyte quality may be caused by a decrease in the antioxidant machinery of the COC by loss of CCs or the lack of scavengers for specific ROS, and/or the ability of the ROS to overwhelm these defenses.
Hydrogen peroxide is known to generate cellular toxicity both independently and through its involvement in the production of other ROS.20,47,48 Hydroxyl radical, produced by the H2O2-driven Fenton reaction, is known to be associated with disorders of iron overload, such as endometriosis and hemochromatosis, in which reproductive failure linked to oxidative stress is common.17,47,49–51 Our current investigation supports the notion that CC’s, the major components of the cellular layers directly surrounding the oocyte, provide protection against ROS only to a certain extent. Indeed, oocyte exposure to low concentrations of H2O2 in the absence or in the presence of Fe(II), where H2O2 is instantly converted to ·OH, induced little or no effect on the organized arrangement of surrounding CCs. Under these circumstances, the oocyte’s protection against H2O2/·OH insult is due to the antioxidant machinery provided by the CCs rather than that provided locally by the oocyte. This conclusion is based on a significant decrease in the percentage of oocytes with poor scores in the presence versus absence of CCs as a function of increasing concentration of H2O2/·OH (Figure 2A and B). In addition to a number of nonenzymatic small molecule antioxidants that are known to be present in the intact COC, there are a number of enzymes responsible for H2O2 detoxification including catalase, glutathione peroxidase, and peroxiredoxin.52–56 These enzymes display the capacity to scavenge lower concentrations of both H2O2/·OH and protect the oocytes from their damaging effects.52,53,55
We have also shown that treatment with high H2O2 and ·OH concentrations changed the organized compact CC mass into a dispersed structure of cells. With high but physiological concentrations of these oxidants, the compact clouds of CCs are scattered and remain loosely linked to the oocyte. At pathological H2O2 and ·OH concentrations, the CCs are stretched further and finally disconnected from the oocyte. Once the CCs are dispersed or disconnected from the oocyte, the oocyte has lost its protection and therefore becomes as susceptible to ROS insult as noncumulus oocytes.2,57 The loss of CC protection is due to the partial or complete loss of CC number, which could be caused by either the loss of CC viability or by the disruption of CC–cell interactions (Cx43). Our results show the major factor is the loss of CC viability, which may explain the mechanism for oxidative stress associated reproductive failure.
Hydrogen peroxide is also known to be involved in the production of HOCl mediated by MPO.20,30,46 At all concentrations tested, HOCl treatment was harmful and directly affected the viability and the number of CCs through its ability to react with a range of biological molecules, particularly those with thiol, thiolether, heme proteins, and amino groups leading to tissue injury.58,59 Recently, we showed that HOCl, in a feedback mechanism, degraded the heme ring in MPO, which released free iron46 and led to ·OH generation.17,47 In contrast to treatment with H2O2 and ·OH, CCs showed no significant sign of protection against HOCl at any concentration. We have recently shown that ONOO−, like HOCl, mediates damage to MT and CH alignment.40 After exposure to both reagents, CCs were stripped from the oocyte, and oocyte viability was significantly compromised. Such compromise could occur either due to the deficiency of specific enzymatic and nonenzymatic antioxidants that help to scavenge ROS throughout the female reproductive tract.60,61
The CCs undergo cytodifferentiation, proliferation, and expansion and are important during early oocyte growth and development, maturation, ovulation, and fertilization.2,62–64 Most infertility disorders are associated with decline in CC number, spindle abnormalities, and an altered cumulus oocyte association, leading to poor oocyte quality, as well as poor reproductive outcomes.4,22,26,27,65–67 Therefore, the presence of CCs maintained in correct organization relative to the oocyte appears necessary for the protection and function of the oocyte. Fatehi et al have demonstrated that intact CCs during in vitro fertilization protected bovine oocytes against oxidative stress and improved first cleavage.67 In addition, incomplete denudation of oocytes prior to intracytoplasmic sperm injection (ICSI) enhances embryo quality and blastocyst development.67 Furthermore, the removal of CCs before complete oocyte maturation showed a premature migration with partial exocytosis of cortical granules68 as well as adversely affects early embryonic development.69,70
It has also been shown that apoptosis rates of human CC from morphologically abnormal oocytes were significantly higher than morphologically normal oocytes examined under transmission electron microscopy.2 An increase in CC apoptosis has also been associated with immaturity of human oocytes, impaired fertilization, and suboptimal embryo development.2 The mutual dependency of the oocyte and the CCs involves a complex and varied set of interactions, and the functionality of COC depends on the individual competence and cooperation of both the CCs and the oocyte.2
In conclusion, the intact arrangement of viable, functional CCs around the oocyte is paramount to the quality and reproductive capacity of the oocyte. Enhancement in the production or defective elimination of ROS and subsequent oxidative stress may be associated with infertility through a mechanism that involves the COC dysfunction and deterioration in oocyte quality. The mechanisms through which the different ROS affect oocyte quality are through CC apoptosis, causing decreased number of CCs or decrease in CC viability. H2O2 like ·OH, decreased the viability of CCs; however at high concentrations decreased the number as well. HOCl, like ONOO−, stripped the CCs from the oocyte as well as dissolved the zona pellucida. The severity of the insult of ROS on cumulus–cumulus and cumulus–oocyte interaction depends mainly on the bioavailability of the antioxidant machinery provided by CCs and the scavenging ability of these antioxidants. When increasing ROS concentrations overwhelm the antioxidant machinery provided by the oocyte and/or CCs, the mechanism of damage is most likely to be similar in both CCs and oocyte.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health grant RO1HL066367 and the Children’s Hospital of Michigan. Dr Iyad Ali is a Fulbright Senior Research Scholar in Dr H. M. A-S laboratory.
References
- 1. Motta PM, Nottola SA, Pereda J, Croxatto HB, Familiari G. Ultrastructure of human cumulus oophorus: a transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum Reprod. 1995;10(9):2361–2367. [DOI] [PubMed] [Google Scholar]
- 2. Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16(10):715–725. [DOI] [PubMed] [Google Scholar]
- 3. D’Alessandris C, Canipari R, Di Giacomo M, et al. Control of mouse cumulus cell-oocyte complex integrity before and after ovulation: plasminogen activator synthesis and matrix degradation. Endocrinology. 2001;142(7):3033–3040. [DOI] [PubMed] [Google Scholar]
- 4. Wang Q, Chi MM, Schedl T, Moley KH. An intercellular pathway for glucose transport into mouse oocytes. Am J Physiol Endocrinol Metab. 2012;302(12):E1511–E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashimoto S, Saeki K, Nagao Y, Minami N, Yamada M, Utsumi K. Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes. Theriogenology. 1998;49(8):1451–1463. [DOI] [PubMed] [Google Scholar]
- 6. Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226(2):167–179. [DOI] [PubMed] [Google Scholar]
- 7. Goodenough DA, Simon AM, Paul DL. Gap junctional intercellular communication in the mouse ovarian follicle. Novartis Foundation Symp. 1999;219:226–235; discussion 35-40. [DOI] [PubMed] [Google Scholar]
- 8. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180. [DOI] [PubMed] [Google Scholar]
- 9. Lee RK, Li SH, Lu CH, Ho HY, Chen YJ, Yeh HI. Abnormally low expression of connexin 37 and connexin 43 in subcutaneously transplanted cryopreserved mouse ovarian tissue. J Assist Reprod Genet. 2008;25(9-10):489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51(1):57–64. [DOI] [PubMed] [Google Scholar]
- 11. Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Effect of lactate dehydrogenase activity and isoenzyme localization in bovine oocytes and utilization of oxidative substrates on in vitro maturation. Theriogenology. 1999;51(3):541–550. [DOI] [PubMed] [Google Scholar]
- 12. Goud PT, Goud AP, Joshi N, Puscheck E, Diamond MP, Abu-Soud HM. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil Steril. 2014;102(1):151–159.e5. [DOI] [PubMed] [Google Scholar]
- 13. Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008;44(7):1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S, Chakravarty B. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. J Hum Reprod Sci. 2012;5(2):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Papaleo E, Ottolina J, Vigano P, et al. Deep pelvic endometriosis negatively affects ovarian reserve and the number of oocytes retrieved for in vitro fertilization. Acta Obstet Gynecol Scand. 2011;90(8):878–884. [DOI] [PubMed] [Google Scholar]
- 16. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaeib F, Banerjee J, Maitra D, Diamond MP, Abu-Soud HM. Impact of hydrogen peroxide-driven Fenton reaction on mouse oocyte quality. Free Radic Biol Med. 2013;58:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banerjee J, Maitra D, Diamond MP, Abu-Soud HM. Melatonin prevents hypochlorous acid-induced alterations in microtubule and chromosomal structure in metaphase-II mouse oocytes. J Pineal Res. 2012;53(2):122–128. [DOI] [PubMed] [Google Scholar]
- 19. Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS lett. 1974;42(1):68–72. [DOI] [PubMed] [Google Scholar]
- 20. Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(7):1199–1234. [DOI] [PubMed] [Google Scholar]
- 21. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine . 2nd ed Oxford: Clarendon Press; 1989:1–20 [Google Scholar]
- 22. Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9(3):338–347. [DOI] [PubMed] [Google Scholar]
- 23. Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS lett. 2000;486(1):10–13. [DOI] [PubMed] [Google Scholar]
- 24. Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod. 2000;62(6):1745–1753. [DOI] [PubMed] [Google Scholar]
- 25. Liu L, Keefe DL. Cytoplasm mediates both development and oxidation-induced apoptotic cell death in mouse zygotes. Biol Reprod. 2000;62(6):1828–1834. [DOI] [PubMed] [Google Scholar]
- 26. Fujino Y, Ozaki K, Yamamasu S, et al. DNA fragmentation of oocytes in aged mice. Hum Reprod. 1996;11(7):1480–1483. [DOI] [PubMed] [Google Scholar]
- 27. Perez GI, Tilly JL. Cumulus cells are required for the increased apoptotic potential in oocytes of aged mice. Hum Reprod. 1997;12(12):2781–2783. [DOI] [PubMed] [Google Scholar]
- 28. Velez-Pardo C, Morales AT, Del Rio MJ, Olivera-Angel M. Endogenously generated hydrogen peroxide induces apoptosis via mitochondrial damage independent of NF-kappaB and p53 activation in bovine embryos. Theriogenology. 2007;67(7):1285–1296. [DOI] [PubMed] [Google Scholar]
- 29. Korotkova EI, Misini B, Dorozhko EV, Bukkel MV, Plotnikov EV, Linert W. Study of OH radicals in human serum blood of healthy individuals and those with pathological schizophrenia. Int J Mol Sci. 2011;12(1):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tahboub YR, Galijasevic S, Diamond MP, Abu-Soud HM. Thiocyanate modulates the catalytic activity of mammalian peroxidases. J Biol Chem. 2005;280(28):26129–26136. [DOI] [PubMed] [Google Scholar]
- 31. Kettle AJ, Winterbourn CC. Assays for the chlorination activity of myeloperoxidase. Methods Enzymol. 1994;233:502–512. [DOI] [PubMed] [Google Scholar]
- 32. Riley CF, Moen MH, Videm V. Inflammatory markers in endometriosis: reduced peritoneal neutrophil response in minimal endometriosis. Acta Obstet Gynecol Scand. 2007;86(7):877–881. [DOI] [PubMed] [Google Scholar]
- 33. Lamaita RM, Pontes A, Belo AV, et al. Evaluation of N-acetilglucosaminidase and myeloperoxidase activity in patients with endometriosis-related infertility undergoing intracytoplasmic sperm injection. J Obstet Gynaecol Res. 2012;38(5):810–816. [DOI] [PubMed] [Google Scholar]
- 34. Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukocyte Biol. 2005;77(5):598–625. [DOI] [PubMed] [Google Scholar]
- 35. Larman MG, Minasi MG, Rienzi L, Gardner DK. Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod Biomed Online. 2007;15(6):692–700. [DOI] [PubMed] [Google Scholar]
- 36. Rienzi L, Martinez F, Ubaldi F, et al. Polscope analysis of meiotic spindle changes in living metaphase II human oocytes during the freezing and thawing procedures. Human Reprod. 2004;19(3):655–659. [DOI] [PubMed] [Google Scholar]
- 37. Eroglu A, Toth TL, Toner M. Alterations of the cytoskeleton and polyploidy induced by cryopreservation of metaphase II mouse oocytes. Fertility Steril. 1998;69(5):944–957. [DOI] [PubMed] [Google Scholar]
- 38. Lindley EM, Jacobson JD, Corselli J, King A, Chan PJ. Cryopreservation of human cumulus cells for co-cultures and assessment of DNA damage after thawing using the comet assay. J Assist Reprod Genet. 2001;18(10):534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohashi Y, Kan Y, Watanabe T, Honda Y. Redox silencing of the Fenton reaction system by an alkylitaconic acid, ceriporic acid B produced by a selective lignin-degrading fungus, Ceriporiopsis subvermispora. Org Biomol Chem. 2007;5(5):840–847. [DOI] [PubMed] [Google Scholar]
- 40. Banerjee J, Shaeib F, Maitra D, et al. Peroxynitrite affects the cumulus cell defense of metaphase II mouse oocytes leading to disruption of the spindle structure in vitro. Fertil Steril. 2013;100(2):578–584. e1. [DOI] [PubMed] [Google Scholar]
- 41. Choi WJ, Banerjee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factor-alpha-induced alterations in metaphase II mouse oocyte spindle structure. Fertil Steril. 2007;88(4 suppl):1220–1231. [DOI] [PubMed] [Google Scholar]
- 42. Boiso I, Marti M, Santalo J, Ponsa M, Barri PN, Veiga A. A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 2002;17(7):1885–1891. [DOI] [PubMed] [Google Scholar]
- 43. Sliskovic I, Abdulhamid I, Sharma M, Abu-Soud HM. Analysis of the mechanism by which tryptophan analogs inhibit human myeloperoxidase. Free Radic Biol Med. 2009;47(7):1005–1013. [DOI] [PubMed] [Google Scholar]
- 44. Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 45. Wang L, Bassiri M, Najafi R, et al. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6:e5. [PMC free article] [PubMed] [Google Scholar]
- 46. Maitra D, Shaeib F, Abdulhamid I, et al. Myeloperoxidase acts as a source of free iron during steady-state catalysis by a feedback inhibitory pathway. Free Radic Biol Med. 2013;63:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prousek J. Fenton chemistry in biology and medicine. Pure Appl Chem. 2007;79(12):2325–2338. [Google Scholar]
- 48. Crichton RR, Wilmet S, Legssyer R, Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorg Biochem. 2002;91(1):9–18. [DOI] [PubMed] [Google Scholar]
- 49. Defrere S, Lousse JC, Gonzalez-Ramos R, Colette S, Donnez J, Van Langendonckt A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008;14(7):377–385. [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi K, Mandai M, Toyokuni S, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008;14(1):32–40. [DOI] [PubMed] [Google Scholar]
- 51. Iizuka M, Igarashi M, Abe Y, Ibuki Y, Koyasu Y, Ikuma K. Chemical assay of iron in ovarian cysts: a new diagnostic method to evaluate endometriotic cysts. Gynecol Obstet Invest. 1998;46(1):58–60. [DOI] [PubMed] [Google Scholar]
- 52. Spolarics Z, Wu JX. Role of glutathione and catalase in H2O2 detoxification in LPS-activated hepatic endothelial and Kupffer cells. Am J Physiol. 1997;273(6 pt 1):G1304–G1311. [DOI] [PubMed] [Google Scholar]
- 53. Dayer R, Fischer BB, Eggen RI, Lemaire SD. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii. Genetics. 2008;179(1):41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goyer A, Haslekas C, Miginiac-Maslow M, et al. Isolation and characterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. Eur J Biochem. 2002;269(1):272–282. [DOI] [PubMed] [Google Scholar]
- 55. Sikka SC. Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J Androl. 2004;25(1):5–18. [DOI] [PubMed] [Google Scholar]
- 56. Leyens G, Verhaeghe B, Landtmeters M, Marchandise J, Knoops B, Donnay I. Peroxiredoxin 6 is upregulated in bovine oocytes and cumulus cells during in vitro maturation: role of intercellular communication. Biol Reprod. 2004;71(5):1646–1651. [DOI] [PubMed] [Google Scholar]
- 57. Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reprod Suppl. 2001;121(5):647–653. [DOI] [PubMed] [Google Scholar]
- 58. Lapenna D, Cuccurullo F. Hypochlorous acid and its pharmacological antagonism: an update picture. Gen Pharmacol. 1996;27(7):1145–1147. [DOI] [PubMed] [Google Scholar]
- 59. Vissers MC, Winterbourn CC. Oxidation of intracellular glutathione after exposure of human red blood cells to hypochlorous acid. Biochem J. 1995;307(pt 1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14(4):345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Agarwal A, Allamaneni SSR. Oxidants and antioxidants in human fertility. Middle East Fertil Soc J. 2004;9(3):187–197. [Google Scholar]
- 62. Diaz FJ, O’Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299(1):91–104. [DOI] [PubMed] [Google Scholar]
- 63. Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13(6):1035–1048. [DOI] [PubMed] [Google Scholar]
- 64. Vanderhyden BC, Macdonald EA, Nagyova E, Dhawan A. Evaluation of members of the TGFbeta superfamily as candidates for the oocyte factors that control mouse cumulus expansion and steroidogenesis. Reproduction. 2003;61:55–70. [PubMed] [Google Scholar]
- 65. Wang Q, Frolova AI, Purcell S, et al. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010;5(12):e15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician’s perspective. Reprod Biomed Online. 2005;11(5):641–650. [DOI] [PubMed] [Google Scholar]
- 67. Fatehi AN, Roelen BA, Colenbrander B, et al. Presence of cumulus cells during in vitro fertilization protects the bovine oocyte against oxidative stress and improves first cleavage but does not affect further development. Zygote. 2005;13(2):177–185. [DOI] [PubMed] [Google Scholar]
- 68. Galeati G, Modina S, Lauria A, Mattioli M. Follicle somatic cells influence pig oocyte penetrability and cortical granule distribution. Mol Reprod Dev. 1991;29(1):40–46. [DOI] [PubMed] [Google Scholar]
- 69. Wongsrikeao P, Kaneshige Y, Ooki R, et al. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reprod Domest Anim. 2005;40(2):166–170. [DOI] [PubMed] [Google Scholar]
- 70. Wongsrikeao P, Otoi T, Murakami M, et al. Relationship between DNA fragmentation and nuclear status of in vitro-matured porcine oocytes: role of cumulus cells. Reprod Fertil Dev. 2004;16(8):773–780. [DOI] [PubMed] [Google Scholar]
- 71. Gutteridge JM, Halliwell B. Comments on review of Free Radicals in Biology and Medicine, second edition. Free Radical Biology and Medicine. 1992;12(1):93–95. [DOI] [PubMed] [Google Scholar]
- 72. Sakellariou GK, Vasilaki A, Palomero J, et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18(6):603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khan SN, Shaeib F, Najafi T, et al. Diffused Intra-oocyte Hydrogen peroxide activates myeloperoxidase and deteriorates oocyte quality. PLoS One. 2015;10(7):e0132388 doi: 10.1371/journal.pone.0132388. [DOI] [PMC free article] [PubMed] [Google Scholar]