Abstract
Matrix metalloproteinase 1 (MMP-1) is an activator of protease-activated receptor 1 (PAR-1), which is known to mediate the release of endothelin 1 (ET-1) in endothelial cells and activate the RhoA kinase (ROCK) pathway. Recently, we reported increased serum and vascular MMP-1 in women with preeclampsia and hypothesized that the action of MMP-1 on PAR-1 might have vasoconstrictive effects. Resistance-sized omental arteries obtained from normal pregnant women were mounted on a myograph system and perfused with MMP-1 in a dose range of 0.025 to 25 ng/mL or with angiotensin II (Ang II) in a dose range of 0.001 to 10 µmol/L in the presence of intraluminal MMP-1 (2.5 ng/mL) perfusion. Angiotensin II dose response was also performed with omental arteries from women with preeclampsia. Matrix metalloproteinase 1 caused dose-dependent vasoconstriction in endothelium-intact, but not in endothelium-denuded, vessels from normal pregnant women, which was blocked by inhibitors of PAR-1 and ET-1 type A receptor blocker. Intraluminal perfusion with a constant amount of MMP-1 enhanced vessel reactivity to Ang II, which was blocked by inhibitors of PAR-1, ROCK, and ET-1. Enhanced vascular reactivity to Ang II was observed in endothelium-intact, but not in endothelium-denuded, arteries of women with preeclampsia. Inhibitors of PAR-1, ROCK, and ET-1 blocked enhanced vascular reactivity to Ang II in endothelium-intact preeclamptic arteries. These data demonstrate that MMP-1 has potent vasoconstrictor effects and the ability to enhance vascular reactivity to vasoconstrictor hormones, which are mediated by an endothelial PAR-1, ROCK, and ET-1 pathway. Increased circulating levels of MMP-1 and its increased expression in systemic vessels of women with preeclampsia may contribute to the development of maternal hypertension.
Keywords: preeclampsia, matrix metalloproteinase 1, protease-activated receptor 1, vasoconstriction, hypertension
Introduction
Preeclampsia is a hypertensive disorder that complicates 5% to 7% of all pregnancies,1 resulting in significant maternal and fetal morbidity and mortality.2 It is associated with altered placental oxygenation,3 oxidative stress,4–6 activation of circulating leukocytes,7–10 neutrophil infiltration of the vasculature,11,12 and endothelial and vascular smooth muscle dysfunction.11,13–15 The cause of preeclampsia remains unknown, and the only definitive treatment is delivery.
Matrix metalloproteinase 1 (MMP-1) is an interstitial collagenase classically considered active during tissue remodeling but was recently shown to activate protease-activated receptor 1 (PAR-1).16–19 Matrix metalloproteinase 1 is secreted as an inactive proform by the endothelium,20 neutrophils,21 monocytes, and vascular smooth muscle.22 Pro-MMP-1 becomes catalytically active by inflammatory oxidants23 and extracellular proteinases.24 Recently, we showed significant increases in plasma concentrations of both proform and activated form of MMP-1 in women with preeclampsia.25 The sources of elevated MMP-1 production were localized via immunohistochemistry of preeclamptic omental fat sections to the vasculature, which showed significantly greater staining for MMP-1 in the endothelium, vascular smooth muscle, and infiltrating leukocytes. Additionally, we showed that MMP-1 messenger RNA and protein expression were higher in omental arteries of patients with preeclampsia versus normal pregnant patients.
Several complications of preeclampsia may be explained by the ability of MMP-1 to activate PAR-1. Coagulation abnormalities are mediated by PAR-1, and activation of PAR-1 on endothelial cells causes them to contract, resulting in protein leakage and edema.26 Activation of endothelial PAR-1 also results in the release of endothelin 1 (ET-1),27 which is a potent vasoconstrictor,28 so PAR-1 activation may play a role in hypertension. One of the signaling pathways through which PAR-1 acts is RhoA kinase (ROCK).26,29,30 Inhibition of ROCK is effective in abolishing enhanced contractile response to angiotensin II (Ang II) of small resistance-sized omental arteries obtained from women with preeclampsia,31 and it abates hypertension in mice,32 which suggests a possible mechanism by which MMP-1 working through PAR-1 may enhance vascular tone via ROCK.
In this study, we tested a novel hypothesis that MMP-1 contributes to hypertension in preeclampsia via a PAR-1, ROCK, ET-1 pathway. To test this hypothesis, we used omental arteries obtained from normal pregnant women or women with preeclampsia and a pressure myograph system to record real-time changes in vessel diameter in response to MMP-1. A dose–response contraction curve was established to physiologic concentrations of active MMP-1, and enhancement of vascular reactivity was evaluated by perfusing MMP-1 through the lumen of normal arteries in conjunction with progressively increasing concentration of Ang II. Enhanced vascular response to Ang II was also evaluated for preeclamptic arteries, and the mechanism of action was dissected with inhibitors of PAR-1, ROCK, or ET-1.
Materials and Methods
Study Participants
Omental fat samples (approximately 20 × 40 × 20 mm) were collected from normal pregnant women and women with preeclampsia undergoing term cesarean section at MCV Hospitals, Virginia Commonwealth University Medical Center. The study population’s clinical characteristics including age, systolic and diastolic pressures, body mass index (BMI), gestational age at delivery, and birth weight are summarized in Table 1. The Office of Research Subjects Protection of Virginia Commonwealth University approved this study, and all participants gave informed consent.
Table 1.
Clinical Characteristics of Patient Groups.a
| Variable | Normal Pregnant (n = 31) | Preeclamptic (n = 7) |
|---|---|---|
| Maternal age, y | 29.1 ± 6.1 | 30.0 ± 7.8 |
| Prepregnancy BMI, kg/m2 | 29.7 ± 6.6 | 28.9 ± 8.4 |
| BMI at sample collection, kg/m2 | 34.4 ± 7.4 | 38.8 ± 8.0 |
| Systolic blood pressure, mm Hg | 125.2 ± 14.9 | 169.3 ± 15.1b |
| Diastolic blood pressure, mm Hg | 76.4 ± 9.6 | 101.0 ± 8.0b |
| Proteinuria, mg/24 h | ND | 166.7 ± 115.5, n = 3 |
| Dipstick | ND | 3.0 ± 1.4, n = 4 |
| Primiparous | 20 | 4 |
| Multiparous | 11 | 3 |
| Gestational age, weeks | 38.9 ± 1.2 | 33.1 ± 6.3b |
| Infant birth weight, g | 3459 ± 522 | 1964 ± 1424b |
| Vessel lumen diameter, µm | 303 ± 85 | 335 ± 141 |
Abbreviations: BMI, body mass index; ND, not detected; SD, standard deviation.
aValues are mean ± SD.
b P < .001.
Activation of Matrix Metalloproteinase 1
Pro-MMP-1 (Calbiochem, San Diego, California) was activated using the organomercurial protocol described in the manufacturer’s product data sheet. Pro-MMP-1 (400 ng/100 µL) was incubated in tris-triton-calcium buffer containing 1 mmol/L p-amino phenyl mercuric acetate (APMA; Calbiochem) for 2 hours at 37°C. The product was followed by ultrafiltration at 4°C to remove the APMA using a Microcon Ultracel Filter device YM-10 (molecular weight 10 000; Millipore, Billerica, MA). To prevent sticking and allow for maximum MMP-1 recovery, the flow-through filter was first treated with 100 μL of 1 mg/mL bovine serum albumin for 30 minutes at 37°C.
Myograph Experiments
The omental fat sample—a tissue abundant in resistance-sized vessels that contribute to total peripheral vascular resistance—was placed in Dulbecco phosphate-buffered saline (D-PBS; Gibco Invitrogen, Carlsbad, California) on a silicone dissection dish precooled to 4°C. A 10-mm length of an omental artery 200 to 500 µm in diameter was dissected and mounted on glass microcannulas of a myograph system (Model 110P, Danish Myo Technologies [DMT], Denmark, the Netherlands) as described previously.31 For some experiments, the endothelium was denuded by passing a glass cannula through the lumen. The vessel was immersed in 10 mL of D-PBS and secured at both ends using two 11-O silk suture ties. The myograph chamber temperature was set at 37°C, and the vessel pressures were maintained at constant inlet (45 mm Hg) and outlet (42 mm Hg) to achieve flow through the vessel. The vessel was monitored optically by a charge-coupled device camera (XC-73CE; Sony, Japan), and changes in lumen diameter were recorded in real time by the DMT software.
After a period of stabilization, endothelium-intact arteries from normal pregnant women and women with preeclampsia were challenged with 60 μmol/L potassium chloride to assess vessel reactivity and viability. Endothelium-denuded arteries from normal pregnant women and women with preeclampsia were exposed to an additional challenge of acetylcholine (10 µmol/L) to verify successful removal of the endothelium. Matrix metalloproteinase 1 was perfused through the vessel lumen of endothelium-intact (n = 12), as well as endothelium-denuded (n = 4), omental arteries of normal pregnant women at 10-minute intervals in 10-fold stepwise increases in concentration (0.025-25 ng/mL). The MMP-1 dose response was repeated with perfusion of a specific PAR-1 inhibitor (10 µmol/L, SCH-79797; Tocris, Ellisville, Missouri; n = 5) or 5 µmol/L ET-1 type A (ETA) receptor blocker (BQ-123; Sigma-Aldrich, St Louis, Missouri; n = 5). An Ang II dose response, based on values reported by other investigators studying in vitro vascular effects of Ang II in omental and pregnancy vessels,33–37 was run alone in 10-fold increments (0.001-10 µmol/L; n = 11) and in the presence of 2.5 ng/mL of MMP-1 perfused through the vessel lumen (n = 11). Angiotensin II doses were added at 10-minute intervals. The Ang II dose response in the presence of MMP-1 was repeated with perfusion of 10 µmol/L SCH-79797 (n = 4), 10 µmol/L ROCK inhibitor (n = 4, Y-27632 dihydrochloride; Tocris), or 5 µmol/L BQ-123 (n = 4; Sigma-Aldrich). Angiotensin II dose response was done alone and in the presence of perfusion with PAR-1 (n = 5), ROCK (n = 5), or ET-1 (n = 5) inhibitors in endothelium-intact omental arteries of women with preeclampsia. Angiotensin II dose response was also done in endothelium-denuded arteries of women with preeclampsia (n = 2). In some cases, inhibitors were given first to assure that inhibition was not due to vessel fatigue. The vessel was contracted with potassium chloride after each treatment to assess viability and recharge intracellular calcium stores and at the end of experimental treatments to verify vessel viability.
Data Analysis
Demographic data are presented as mean ± standard deviation (SD) and were analyzed for significance using a t test. The myograph experiment data are presented as mean ± standard error (SE) and were analyzed by 2-way analysis of variance with Bonferroni multiple comparisons test using a statistical software program (Prism 4; GraphPad Software, San Diego, California). A P < .05 was considered statistically significant.
Results
Demographic Data
Demographic data for 31 normal pregnant women and 7 participants with preeclampsia are shown in Table 1. Maternal age and BMI were matched, while the preeclamptic group showed significant elevation in systolic and diastolic blood pressures that accompany the disease. Results for proteinuria were also recorded by 24-hour urine measurement or dipstick. The mean lumen diameters of omental resistance arteries studied for normal pregnant women and participants with preeclampsia were 303 ± 85 µm and 335 ± 141 µm, respectively, and not statistically different.
Matrix Metalloproteinase 1 Dose Response
As shown in Figure 1, when MMP-1 was perfused through the vessel lumen in endothelium-intact human omental arteries, it caused dose-dependent vasoconstriction ranging from an average decrease of 5 µm with 0.025 ng/mL MMP-1 to an average decrease of 40 µm with 25 ng/mL MMP-1. Significant vasoconstriction was present at 0.25 ng/mL (P < .01). In endothelium-denuded vessels, luminal MMP-1 perfusion did not elicit contraction at any dose.
Figure 1.
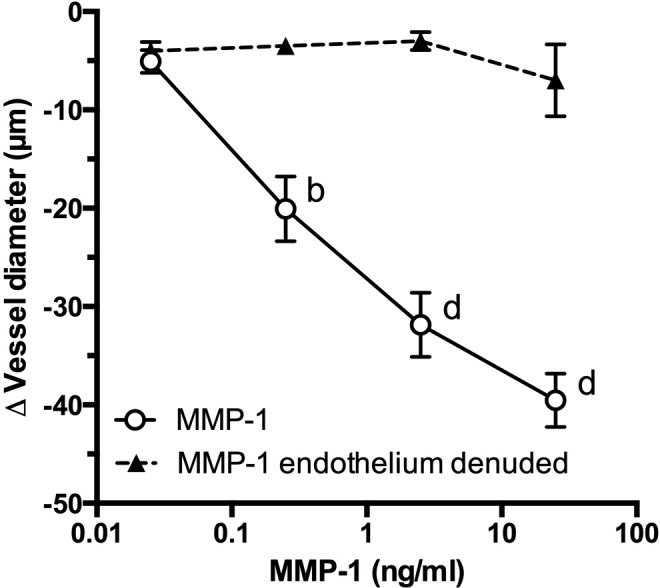
Role of endothelium in matrix metalloproteinase 1 (MMP-1)–induced vasoconstriction of pregnancy blood vessels. Matrix metalloproteinase 1 perfusion through the lumen of endothelium-intact small omental arteries obtained from normal pregnant women caused dose-dependent vasoconstriction (0.25-25 ng/mL, n = 12). Endothelium-denuded vessels did not constrict or otherwise respond to MMP-1 perfusion (n = 4). b P < .01, d P < .0001 compared to MMP-1 endothelium-denuded arteries for that dose.
Role of PAR-1 and ET-1
Coperfusion of a PAR-1 inhibitor with MMP-1 in endothelium-intact omental arteries abolished MMP-1–induced vasoconstriction (Figure 2). Coperfusion of an ETA receptor blocker with MMP-1 also abolished MMP-1–induced vasoconstriction.
Figure 2.
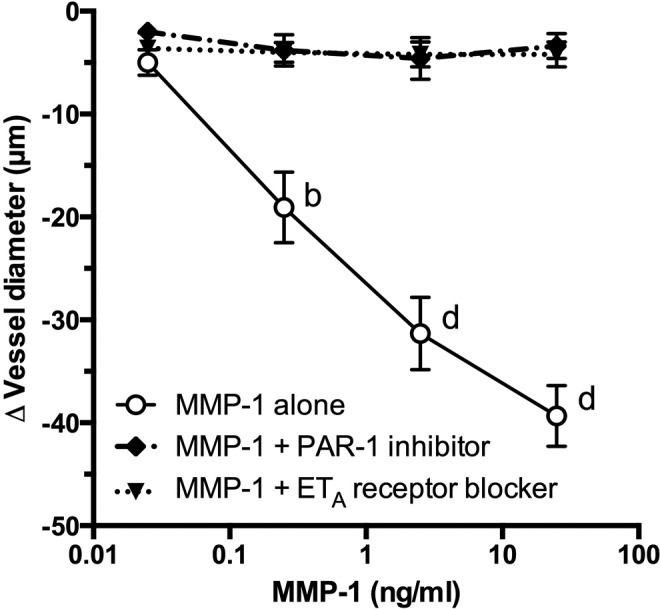
Role of protease-activated receptor 1 (PAR-1) and endothelin 1 (ET-1) in matrix metalloproteinase 1 (MMP-1)–mediated vasoconstriction. In endothelium-intact human omental arteries, coperfusion of a specific PAR-1 inhibitor (n = 5) or a specific ET-1 type A (ETA) receptor blocker (n = 5) inhibited MMP-1–induced vasoconstriction (n = 12). b P < .01, d P < .0001 compared to inhibitory treatments for that dose.
Matrix Metalloproteinase 1-Mediated Enhancement of Vascular Reactivity to Ang II
As shown in Figure 3, the dose response to Ang II alone in endothelium-intact vessels ranged from a 4 µm mean decrease in vessel diameter with 0.001 µmol/L Ang II and up to a 14 µm mean decrease with 1 µmol/L Ang II. Repeating the Ang II dose–response test with activated MMP-1 coperfusing through the lumen at 2.5 ng/mL resulted in a significant dose–response enhancement of vascular reactivity to treatment with Ang II. Significantly enhanced response started at 0.01 µmol/L Ang II (P < .0001). Coperfusion of a PAR-1 inhibitor with Ang II plus MMP-1 abolished MMP-1–enhanced vascular reactivity to Ang II. Similarly, the ROCK inhibitor and ETA receptor blocker abolished enhanced vascular reactivity to Ang II induced by MMP-1.
Figure 3.
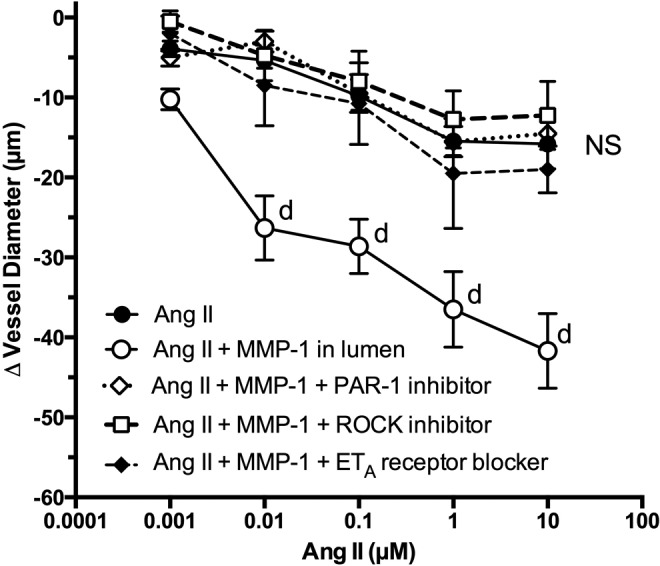
Vascular reactivity to Ang II in endothelium-intact omental arteries from normal pregnant women. Coperfusion of MMP-1 (2.5 ng/mL) through the vessel lumen significantly enhanced vascular reactivity to Ang II. Perfusion of a specific PAR-1 inhibitor (n = 4), a specific ROCK inhibitor (n = 4), or a specific ETA receptor blocker (n = 4) abolished MMP-1–mediated enhancement of vascular reactivity to Ang II (n = 11). d P < .0001 compared to Ang II alone and inhibitory treatments for that dose. Ang II indicates angiotensin II; ETA, endothelin 1 type A; MMP-1, matrix metalloproteinase 1; NS, nonsignificant for treatment effects; PAR, protease-activated receptor 1; ROCK, RhoA kinase.
Inhibition of PAR-1 Pathway in Preeclamptic Arteries
Treatment of endothelium-intact arteries from patients having preeclampsia with Ang II produced a hypercontractile response in a dose-dependent fashion (Figure 4, panel A). Significantly enhanced vasoconstriction was present at 0.01 µmol/L Ang II when compared to endothelium-intact arteries from normal pregnant patients. When Ang II dose response was tested in endothelium-denuded arteries from women with preeclampsia, the enhanced response was not present (Figure 4, panel A). Inhibition of PAR-1 prevented the enhanced response of endothelium-intact preeclamptic arteries (panel B), restoring the Ang II dose–response curve to that seen in untreated normal pregnant vessels. Inhibition of ROCK or blockade of ETA receptors also effectively abolished the enhanced contractile response to Ang II. The data for ROCK inhibition in preeclamptic arteries were published previously31 and are shown here for the sake of completeness.
Figure 4.
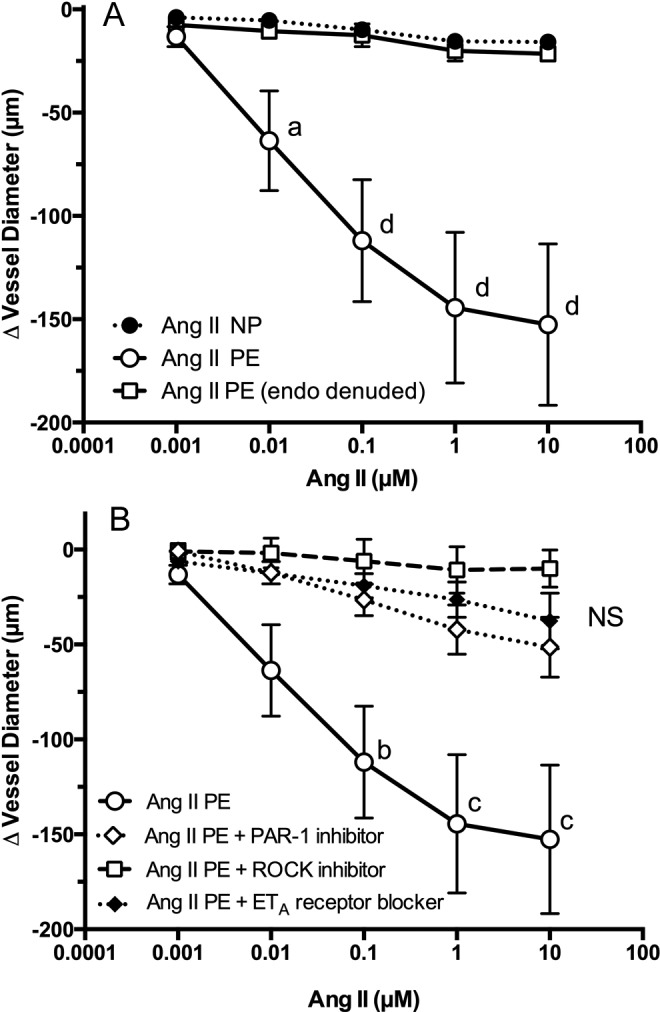
Vascular reactivity to Ang II in omental arteries obtained from women with preeclampsia. The vasoconstrictive response to Ang II was significantly enhanced in preeclamptic omental arteries (n = 5) as compared with normal pregnant arteries (n = 11; panel A). The enhanced response of preeclamptic arteries was abolished by removal of the endothelium (n = 2, panel A) or coperfusion with inhibitors of PAR-1 (n = 5), ROCK (n = 5), or ET-1 (n = 4; panel B). a P < .05, b P < .01, c P < .001, d P < .0001 compared to other treatments for that dose. Ang II indicates angiotensin II; ET-1, endothelin 1; NS, nonsignificant for treatment effects; PAR, protease-activated receptor; ROCK, RhoA kinase.
Discussion
In this study, we demonstrated that luminal perfusion of MMP-1 elicited a potent and dose-dependent vasoconstrictive response in endothelium-intact human omental arteries. Significant vasoconstriction was present at a dose of MMP-1 as low as 0.25 ng/mL. Removal of the endothelium or blockade of endothelium-derived signaling by coperfusion with inhibitors of PAR-1 or ET-1 prevented vasoconstriction by MMP-1. Perfusion of 2.5 ng/mL of activated MMP-1, a dose within the concentration range of active MMP-1 in the circulation of women with preeclampsia,25 significantly enhanced vascular reactivity to Ang II in intact normal pregnant omental arteries, similar to enhanced response of preeclamptic arteries. Coperfusion with inhibitors of PAR-1, ROCK, or ET-1 blocked the enhanced response of preeclamptic arteries as well as the enhanced response of normal pregnant arteries perfused with MMP-1. The enhanced vascular response of preeclamptic arteries was abolished when the endothelium was removed. These data demonstrate that vascular effects of MMP-1 are mediated via an endothelial PAR-1, ROCK, ET-1 pathway and suggest this pathway plays an important role in mediating hypertension in preeclampsia.
Matrix metalloproteinase 2, which is elevated in the maternal circulation of women with preeclampsia,38 has also been found to mediate vasoconstriction. It operates through a different mechanism than MMP-1 and PAR-1 by cleaving big ET-1 to form ET-1.39,40 Given the proposed model, these effects could be additive to MMP-1’s activation of endothelial PAR-1 and increased production of ET-1.
Matrix metalloproteinase 1 is best known as a collagenase. However, in recent years, it has been shown to activate the thrombin receptor, PAR-1, by cleaving the N-terminal peptide sequence just 2 amino acids distal to the thrombin cleavage site.16–19 Protease-activated receptor 1 is expressed on the surface of endothelial cells, and its activation by MMP-1, thrombin, or other serine/threonine proteases results in endothelial cell activation as evidenced by release of chymase, interleukin 8, and P-selectin from the Weibel–Palade bodies within the endothelial cells.41,42,43 Activation of PAR-1 on endothelial cells also results in the release of ET-1,44 which as shown in this study mediates vasoconstrictive effects of MMP-1.
In preeclampsia, levels of MMP-125 and ET-145,46 are significantly greater than that in normal pregnancy. With regard to MMP-1, plasma concentrations are 3-fold higher in women with preeclampsia than normal pregnant women, with 17% in the active form as opposed negligible amounts in the active form for normal pregnant women. In preeclampsia, the circulating amount of active MMP-1 is 7 to 8 ng/mL,25 so the vasoconstrictive effects we observed for MMP-1 at concentrations 0.25 and 2.5 ng/mL are well within the physiologic range for preeclampsia as is our test amount of 2.5 ng/mL perfused through the vessel lumen to enhance vascular reactivity to Ang II.
Neutrophils can produce MMP-1,21,47 thus, activated neutrophils in the maternal circulation are a potential source of elevated plasma levels of MMP-1 in preeclampsia. Another contributor is the vascular smooth muscle, which releases MMP-1 in response to neutrophils and neutrophil products.25 Matrix metalloproteinase 1 in turn increases endothelial ET-1 from endothelial cells. Our recent findings of greater gene and protein expression of MMP-1 and PAR-1 in omental fat vessels from women with preeclampsia support a role for MMP-1 activation of PAR-1 in the pathophysiology of preeclampsia.25
Matrix metalloproteinase 1 is activated by reactive oxygen species,23 so increased circulating levels of active MMP-1 in preeclampsia may be the result of pro–MMP-1 being exposed to increased placental secretion of lipid peroxides,5,6 as it passes through the intervillous space. Another source of activated MMP-1 is the vascular smooth muscle because it secretes MMP-1 and is infiltrated by activated neutrophils, which release reactive oxygen species, such as myeloperoxidase.48 The activated MMP-1 is then released into the circulation and/or works in an autocrine or paracrine fashion on local smooth muscle and endothelial PAR-1.
These data demonstrated that MMP-1 not only has potent vasoconstrictor effects by itself but also has the ability to enhance vascular reactivity to vasoconstrictor hormones. These effects are mediated by an endothelial PAR-1, ROCK, ET-1 pathway, with ET-1 being the final mediator of vasoconstriction. The enhanced vascular reactivity to Ang II elicited by MMP-1 in vitro may explain why women who go on to develop preeclampsia have an enhanced blood pressure response to Ang II in vivo49 because elevated circulating levels of active MMP-1 heighten their vascular sensitivity to vasoconstrictive hormones, which eventually results in hypertension.
These new data could provide novel avenues for treatment by targeting PAR-1, ROCK, or ETA receptor. SCH 530348 (vorapaxar), an oral PAR-1 antagonist designed as an antithrombotic, was recently approved for clinical use in the United States.50 The ROCK inhibitors have shown promise in animal models of hypertension51 and improved hemodynamics in clinical trials of human pulmonary arterial hypertension.52 The ETA/B antagonists have shown clinical efficacy in the treatment of pulmonary arterial hypertension.53 Additionally, MMP-1 inhibitors54 may be useful for the treatment of preeclampsia.
Acknowledgments
The authors gratefully acknowledge the clinicians of VCU Medical Center Department of Obstetrics and Gynecology for diligent collection of omental fat samples.
Footnotes
Authors’ Note: Drs Mishra and Nugent contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH Grant R01 HL069851 (SWW).
References
- 1. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1–7. [DOI] [PubMed] [Google Scholar]
- 2. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–644. [DOI] [PubMed] [Google Scholar]
- 3. Huppertz B, Weiss G, Moser G. Trophoblast invasion and oxygenation of the placenta: measurements versus presumptions. J Reprod Immunol. 2014;101-102:74–79. [DOI] [PubMed] [Google Scholar]
- 4. Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222(3):222–235. [DOI] [PubMed] [Google Scholar]
- 5. Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):93–104. [DOI] [PubMed] [Google Scholar]
- 6. Walsh SW, Vaughan JE, Wang Y, Roberts LJ II. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14(10):1289–1296. [DOI] [PubMed] [Google Scholar]
- 7. Clark P, Boswell F, Greer IA. The neutrophil and preeclampsia. Semin Reprod Endocrinol. 1998;16(1):57–64. [DOI] [PubMed] [Google Scholar]
- 8. Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185(4):792–797. [DOI] [PubMed] [Google Scholar]
- 9. Greer IA, Haddad NG, Dawes J, Johnstone FD, Calder AA. Neutrophil activation in pregnancy-induced hypertension. Br J Obstet Gynaecol. 1989;96(8):978–982. [DOI] [PubMed] [Google Scholar]
- 10. Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–86. [DOI] [PubMed] [Google Scholar]
- 11. Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44(1):72–77. [DOI] [PubMed] [Google Scholar]
- 12. Cadden KA, Walsh SW. Neutrophils, but not lymphocytes or monocytes, infiltrate maternal systemic vasculature in women with preeclampsia. Hypertens Pregnancy. 2008;27(4):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(5):1200–1204. [DOI] [PubMed] [Google Scholar]
- 14. Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):17–31. [DOI] [PubMed] [Google Scholar]
- 15. Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196(1):48. e1–e8. [DOI] [PubMed] [Google Scholar]
- 16. Pei D. Matrix metalloproteinases target protease-activated receptors on the tumor cell surface. Cancer Cell. 2005;7(3):207–208. [DOI] [PubMed] [Google Scholar]
- 17. Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–313. [DOI] [PubMed] [Google Scholar]
- 18. Ahn HS, Chackalamannil S, Boykow G, Graziano MP, Foster C. Development of proteinase-activated receptor 1 antagonists as therapeutic agents for thrombosis, restenosis and inflammatory diseases. Curr Pharm Des. 2003;9(28):2349–2365. [DOI] [PubMed] [Google Scholar]
- 19. Trivedi V, Boire A, Tchernychev B, et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137(2):332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanemaaijer R, Koolwijk P, le Clercq L, de Vree WJ, van Hinsbergh VW. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Effects of tumour necrosis factor alpha, interleukin 1 and phorbol ester. Biochem J. 1993;296(pt 3):803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grab DJ, Nyarko E, Barat NC, Nikolskaia OV, Dumler JS. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clin Vaccine Immunol. 2007;14(11):1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu Y, Hojo Y, Ikeda U, Takahashi M, Shimada K. Interaction between monocytes and vascular smooth muscle cells enhances matrix metalloproteinase-1 production. J Cardiovasc Pharmacol. 2000;36(2):152–161. [DOI] [PubMed] [Google Scholar]
- 23. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. [DOI] [PubMed] [Google Scholar]
- 24. Nagase H, Suzuki K, Enghild JJ, Salvesen G. Stepwise activation mechanisms of the precursors of matrix metalloproteinases 1 (tissue collagenase) and 3 (stromelysin). Biomed Biochim Acta. 1991;50(4-6):749–754. [PubMed] [Google Scholar]
- 25. Estrada-Gutierrez G, Cappello RE, Mishra N, et al. Increased expression of matrix metalloproteinase-1 in systemic vessels of preeclamptic women: a critical mediator of vascular dysfunction. Am J Pathol. 2011;178(1):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86(1):298–307. [PubMed] [Google Scholar]
- 27. Eto M, Barandier C, Rathgeb L, et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res. 2001;89(7):583–590. [DOI] [PubMed] [Google Scholar]
- 28. Schini VB, Vanhoutte PM. Endothelin-1: a potent vasoactive peptide. Pharmacol Toxicol. 1991;69(5):303–309. [DOI] [PubMed] [Google Scholar]
- 29. Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL. G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem. 1995;270(42):24631–24634. [DOI] [PubMed] [Google Scholar]
- 30. Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–1814. [DOI] [PubMed] [Google Scholar]
- 31. Mishra N, Nugent WH, Mahavadi S, Walsh SW. Mechanisms of enhanced vascular reactivity in preeclampsia. Hypertension. 2011;58(5):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao X, Luo T, Luo X, Tang Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens Res. 2014;37(9):803–810. [DOI] [PubMed] [Google Scholar]
- 33. Abramovich DR, Page KR, Wright F. Effect of angiotensin II and 5-hydroxytryptamine on the vessels of the human foetal cotyledon. Br J Pharmacol. 1983;79(1):53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maigaard S, Forman A, Andersson KE. Differential effects of angiotensin, vasopressin and oxytocin on various smooth muscle tissues within the human uteroplacental unit. Acta Physiol Scand. 1986;128(1):23–31. [DOI] [PubMed] [Google Scholar]
- 35. Mak KK, Gude NM, Walters WA, Boura AL. Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. Br J Obstet Gynaecol. 1984;91(2):99–106. [DOI] [PubMed] [Google Scholar]
- 36. Tulenko TN. The actions of prostaglandins and cyclo-oxygenase inhibition on the resistance vessels supplying the human fetal placenta. Prostaglandins. 1981;21(6):1033–1043. [DOI] [PubMed] [Google Scholar]
- 37. Aalkjaer C, Danielsen H, Johannesen P, Pedersen EB, Rasmussen A, Mulvany MJ. Abnormal vascular function and morphology in pre-eclampsia: a study of isolated resistance vessels. Clin Sci (Lond). 1985;69(4):477–482. [DOI] [PubMed] [Google Scholar]
- 38. Narumiya H, Zhang Y, Fernandez-Patron C, Guilbert LJ, Davidge ST. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens Pregnancy. 2001;20(2):185–194. [DOI] [PubMed] [Google Scholar]
- 39. Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85(10):906–911. [DOI] [PubMed] [Google Scholar]
- 40. Abdalvand A, Morton JS, Bourque SL, Quon AL, Davidge ST. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension. 2013;61(2):488–493. [DOI] [PubMed] [Google Scholar]
- 41. Goerge T, Barg A, Schnaeker EM, et al. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66(15):7766–7774. [DOI] [PubMed] [Google Scholar]
- 42. Urata H, Boehm KD, Philip A, et al. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J Clin Invest. 1993;91(4):1269–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of Weibel-Palade bodies. Histochem Cell Biol. 2002;117(2):113–122. [DOI] [PubMed] [Google Scholar]
- 44. Marsen TA, Simonson MS, Dunn MJ. Thrombin induces the preproendothelin-1 gene in endothelial cells by a protein tyrosine kinase-linked mechanism. Circ Res. 1995;76(6):987–995. [DOI] [PubMed] [Google Scholar]
- 45. Greer IA, Leask R, Hodson BA, Dawes J, Kilpatrick DC, Liston WA. Endothelin, elastase, and endothelial dysfunction in pre-eclampsia. Lancet. 1991;337(8740):558. [DOI] [PubMed] [Google Scholar]
- 46. Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71(6):1675–1677. [DOI] [PubMed] [Google Scholar]
- 47. Mousa AA, Cappello RE, Estrada-Gutierrez G, et al. Preeclampsia is associated with alterations in DNA methylation of genes involved in collagen metabolism. Am J Pathol. 2012;181(4):1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shukla J, Walsh SW. Neutrophil release of myeloperoxidase in systemic vasculature of obese women may put them at risk for preeclampsia. Reprod Sci. 2015;22(3):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52(11):2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poole RM, Elkinson S. Vorapaxar: first global approval. Drugs. 2014;74(10):1153–1163. [DOI] [PubMed] [Google Scholar]
- 51. Lohn M, Plettenburg O, Kannt A, et al. End-organ protection in hypertension by the novel and selective Rho-kinase inhibitor, SAR407899. World J Cardiol. 2015;7(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fukumoto Y, Yamada N, Matsubara H, et al. Double-blind, placebo-controlled clinical trial with a rho-kinase inhibitor in pulmonary arterial hypertension. Circ J. 2013;77(10):2619–2625. [DOI] [PubMed] [Google Scholar]
- 53. Lee YH, Song GG. Meta-analysis of randomized controlled trials of bosentan for treatment of pulmonary arterial hypertension. Korean J Intern Med. 2013;28(6):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498. [DOI] [PubMed] [Google Scholar]


