Abstract
Background:
Gene therapy is a potentially effective non-surgical approach for the treatment of uterine leiomyoma. We demonstrated that targeted adenovirus vector, Ad-SSTR-RGD-TK/GCV, was highly effective in selectively inducing apoptosis and inhibiting proliferation of human leiomyoma cells in vitro while sparing normal myometrial cells.
Study design:
An in-vivo study, to compare efficacy and safety of modified adenovirus vector Ad-SSTR-RGD-TK/GCV versus untargeted vector for treatment of leiomyoma.
Materials and methods:
Female nude mice were implanted with rat leiomyoma cells subcutaneously. Then mice were randomized into three groups. Group 1 received Ad-LacZ (marker gene), Group 2 received untargeted Ad-TK, and Group 3 received the targeted Ad-SSTR-RGD-TK. Tumors were measured weekly for 4 weeks. Then mice were sacrificed and tissue samples were collected. Evaluation of markers of apoptosis, proliferation, extracellular matrix, and angiogenesis was performed using Western Blot & Immunohistochemistry. Statistical analysis was done using ANOVA. Dissemination of adenovirus was assessed by PCR.
Results:
In comparison with the untargeted vector, the targeted adenoviral vector significantly shrank leiomyoma size (P < 0.05), reduced expression of proliferation marker (PCNA) (P < 0.05), induced expression of apoptotic protein, c-PARP-1, (P < 0.05) and inhibited expression of extracellular matrix-related genes (TGF beta 3) and angiogenesis-related genes (VEGF & IGF-1) (P < 0.01). There were no detectable adenovirus in tested tissues other than leiomyoma lesions with both targeted and untargeted adenovirus.
Conclusion:
Targeted adenovirus, effectively reduces tumor size in leiomyoma without dissemination to other organs. Further evaluation of this localized targeted strategy for gene therapy is needed in appropriate preclinical humanoid animal models in preparation for a future pilot human trial.
Keywords: uterine leiomyoma, gene therapy, adenovirus vectors, thymidine kinase/apoptosis
Introduction
Uterine leiomyomas (UL; also known as uterine fibroids) are the most common gynecological tumors in reproductive-age, occurring in 70% of caucasian women and 80% of African American women by the age of 50 years. Uterine leiomyomas are the most common clinical indication for hysterectomy, oftentimes prematurely ending a woman’s reproductive life.1,2 They commonly cause excessive uterine bleeding, anemia, pelvic discomfort, urinary incontinence, preterm labor, and recurrent pregnancy loss.3,4
Despite its high prevalence, currently there are limited therapeutic options readily available for the management of UL, without compromising initial or subsequent chances of achieving a healthy and safe pregnancy for women who want to preserve their fertility.5
Uterine leiomyomas are ideal candidates for direct delivery of therapeutic gene-based vectors because of their localized nature and accessibility through imaging and endoscopic methods. Gene therapy involves various strategies to deliver genetic material to targeted cells in achieving therapeutic benefits.6,7 Suicide gene therapy is a frequently applied method that involves delivery of the herpes simplex virus 1 thymidine kinase gene (HSV1TK) followed by delivery of a nontoxic guanosine analog, ganciclovir (GCV). The GCV is phosphorylated by HSV1TK and mammalian cellular kinases to form a toxic, triphosphorylated form (GCVTP).8 This toxic product, in turn, inhibits DNA synthesis and blocks the cell cycle, ultimately leading to cell death via apoptosis.9,10 Phase 1 trials for various human cancers have utilized the adenovirus thymidine kinase (TK)/GCV protocol and demonstrated its safety as a therapeutic agent.11
Our previous in vitro and in vivo (Eker rat model) studies have demonstrated that untargeted adenoviral vectors are able to infect uterine leiomyoma cells and severely inhibit cell proliferation, resulting in an increased number of apoptotic cells and the regression of UL tumors; however, minimal but detectable leakage to the liver and uterus have been reported.7,12,13
To optimize the approach of treating ULs by targeting therapeutic genes, we tested several modified adenoviral vectors in the UL cell line to identify the most selective and efficient virus for targeting human leiomyoma (HuLM) cells.12 The adenovirus serotype 5 used for gene therapies binds to the coxsackie-adenovirus receptor (CAR). Several clinical trials for tumor treatment using adenovirus gene therapy have shown unimpressive results, which may be due to the poor or absent expression of the coxsackie receptor in primary tumor cells as a consequence of increased aggressiveness of the tumor cells or higher activity of the MAPK pathway.14 Microarray studies of UL tissues have demonstrated that CAR is downregulated in leiomyomas in comparison to myometrium, where it might have a role in myometrial contractions.15 A promising approach used to circumvent this dependence on CAR and to enhance transduction efficiency is the genetic modification of the adenovirus fiber with an arginine–glycine–aspartic acid (RGD-4C) motif.16 The fiber-modified Ad5-RGD-Luc vector is constructed through the insertion of a short peptide (21 amino acids) composed of arginine, glycine, and aspartate (RGD) into the H1 loop of the wild fiber knob domain.16,17 The addition of this peptide modification in the H1 loop of the fiber domain allows the virus a port of entry via cellular integrins rather than through the CAR.16
Previous reports on gene therapy for ovarian and other similar cancer cell lines have shown an increase in infectivity through CAR-independent transduction, achieving higher gene expression by an increasingly greater magnitude in primary tumor cells.16,18,19 Our data show that Ad5-RGD-luc, as compared with the Ad5-luc viruses, enhanced transduction efficiency in leiomyoma cells.12
An additional reason for the low transduction of adenoviral vectors is the presence of antivirus-neutralizing antibodies. This limitation has been reported to be partially overcome by modification of the adenovirus using RGD-4C.20,21
Evaluation of the safety and efficacy of a gene therapy candidate is demonstrated by the level, persistence, and location of the transgene expression. Earlier work reported the construction of an RGD-4C infectivity-enhanced bicistronic type 5 adenoviral vector, Ad-SSTR-RGD-TK, which encodes 2 transgenes, a herpes simplex virus TK and the human somatostatin receptor subtype 2 (SSTR), expressed from a cytomegalovirus early promoter.21 The SSTR is an imaging cassette that permits assessment of transduction in vivo. A phase I clinical trial in women diagnosed with ovarian cancer has been successfully completed using 109 to 1012 pfu/day doses in determining the safety, clinical outcomes, and biological effects of Ad-RGD-TK-SSTR.22,23
We previously demonstrated that targeted adenovirus vector, Ad-SSTR-RGD-TK, followed by GCV, was highly effective in selectively inducing apoptosis and inhibiting proliferation of HuLM cells while sparing normal human myometrial cells.24
In this work, we aim to evaluate the efficacy and safety of the targeted adenovirus–human somatostatin receptor subtype 2-arginine, glycine and aspartate-thymidine kinase/ganciclovir (Ad-SSTR-RGD-TK/GCV) in vivo.
Materials and Methods
Animals
Weight-matched female nude mice (Harlan Sprague Dawley, Indianapolis, Indiana), 6 weeks old, received subcutaneous implantation of 1.7 mg, 90 days sustained-release 17 β estradiol pellets (Innovative Research of America, Sarasota, Florida) as we described previously.25 The pellets were implanted 4 days before the subcutaneous injection of the cells to allow steady state tissue estrogen distribution. These mice were maintained in a specific pathogen germ-free environment. Mice were handled and cared for in accordance with guidelines from the National Institutes of Health and the Association for the Accreditation of Laboratory Animal Care-accredited facilities, and all protocols involving the use of these animals received prior approval by the local Institutional Animal Care and Use Committees. Eker rat tumor-derived ELT-3 rat leiomyoma cells (10 million cells/mouse) were then subcutaneously implanted in the right flank region of each animal. Animals were examined for tumor formation twice a week; tumor dimensions were measured using an electronic slide caliper; and tumor volume was calculated by the following formula: (length × width × depth × 0.52). After tumors were visible and palpable (tumor volume ranged from 50 to 100 mm3, 4 weeks after cell implantation), mice were randomized into 3 treatment groups (6 mice/group). Group 1 received Ad-Lac Z (marker gene), group 2 received untargeted first-generation herpes simplex virus thymidine kinase gene (Ad-TK), and group 3 received the leiomyoma-targeted Ad-SSTR-RGD-TK. Vectors were delivered by a single direct intratumoral injection (3 × 1010 pfu/cm3) followed by a 5-day regimen of GCV treatment (25 mg/kg/d intraperitoneally). Tumors were measured weekly for 4 weeks. Animals were examined for any obvious signs of toxicity such as lethargy, body weight reduction, difficulty in mobility, and changes in food and water intake. Mice were killed at the end of the experiment by CO2, euthanasia and tissue samples from tumors as well as other body organs were collected to assess the efficacy and safety of the targeted vector.
Western Blot
Tissues were lysed with a lysis buffer (Cellytic-M; Sigma, Sigma Co, St. Louis, MO, USA) containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, Indiana), and protein was extracted using a standard laboratory method. Protein concentrations were determined using a bicinchoninic acid (BCA) protein assay reagent (Thermo Scientific, Inc, Rockford, Illinois). Samples were diluted with 4× sodium dodecyl sulfate (SDS) loading buffer containing beta-mercaptoethanol. Equal amounts of protein (10 µg) for each sample were separated by SDS-polyacrylamide gel electrophoresis and electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, Massachusetts). Proteins were detected by immunoblotting followed by enhanced chemiluminescence detection (Amersham Biosciences, GE Healthcare, Piscataway, New Jersey). Chemiluminescence signals were detected by Bio-Rad imager ChemiDoc MP System (Bio-Rad, Ramsey, New Jersey). Membranes were immunoblotted with primary antibodies against proliferating cell nuclear antigen (PCNA; 1:500), BCL-2 (1:500), cleaved poly (ADP-ribose) (PAR) polymerase (c-PARP-1; 1:500), transforming growth factor β3 (TGF-β3; 1:500), vascular endothelial growth factor (VEGF; 1:500) and insulin like growth factor 1 (IGF-1; 1:500). Anti-β-actin (1:5000; Santa Cruz Biotech, Santa Cruz, California) was used as the loading control. Membranes were washed and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. The intensity of each protein band was determined using Bio-Rad imager software and normalized against the values obtained for β-actin.
Immunohistochemistry
Freshly obtained tumor samples were fixed in 10% formalin solution for 24 hours and transferred to 70% ethanol. Tissues were then embedded in paraffin wax according to the embedding machine manufactures’ instructions, and 4-μm sections were prepared. Antigen retrieval was achieved by heating sections in 95°C citrate buffer for 10 minutes. Sections were incubated overnight at 4°C with specific antibodies against PCNA (1:200), c-PARP-1 (1:200), TGF-β3 (1:200), VEGF (1:200), and IGF-1 (1:200; Santa Cruz Biotech). Treated sections were then washed and incubated for 30 minutes with a secondary antibody (HRP-conjugated; ImmPRESS; Vector Labs, Burlingame, California) and then washed and incubated in peroxidase substrate solution (diaminobenzene; Vector Labs, Burlingame, California) until desired stain intensity developed. The negative control was treated using the same methodology as the other samples except that no primary antibody was added to the slides. The degree of expression was scored by counting positive cells, against the total number of the cells in 3 random high-power fields (total number 1200 cells) for each tissue section (representing 1 animal). The percentage expressions were calculated for 3 animals per group and were included in these calculations. We also used H-score to assess staining intensity where the fractions of negative (score 0), weakly positive (score 1), positive (score 2), strongly positive (score 3), and very strongly positive (score 4) cells were estimated by blinded observer, and the fractions were multiplied with the scores and summed, the total being the H-score.
Safety Study
To evaluate the safety of local delivery of the gene therapy approach, animals were examined upon autopsy for gross evidence of toxicity. In addition, distant dissemination of the adenovirus was also assessed in treated mice. Tissue samples were collected from (1) tumors themselves; (2) tissues surrounding the injected tumors; and (3) the spleen, kidney, lung, heart, brain, uterus, ovary, fallopian tubes, and liver after the mice were killed. Blinded histopathological assessment of representative samples from these organs was provided by an animal pathologist. In addition, these tissues were tested for the presence of adenovirus by polymerase chain reaction (PCR) amplification of an adenovirus-specific E4 region sequence (forward primer: TGTGACTGATTGAGCGGTG; reverse primer: CCCATTTAACACGCCATGCA)26,27 performed on purified DNA from the leiomyoma lesions as well as from multiple organs.
Statistical Analyses
All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, North Carolina). All data are presented as the mean ± standard error of the mean for all tested animals per group. Data were checked for normality using the Shapiro-Wilk test. Data were analyzed using the 1-way analysis of variance test and were considered to be statistically significant if P ≤ .05. Significant differences were determined by Tukey honestly significant difference post hoc test.
Results
Targeted Adenovirus Shrinks Leiomyoma
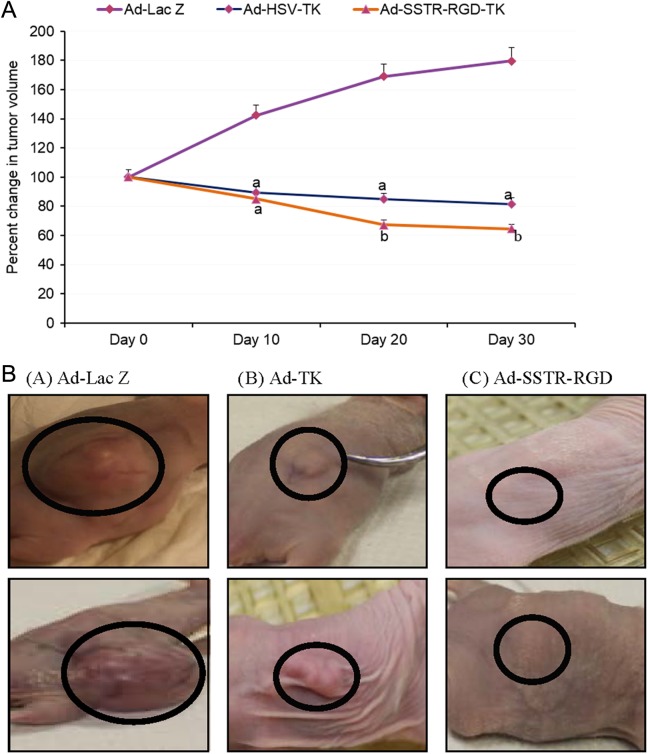
As shown in Figure 1, treatment of leiomyoma with Ad-SSTR-RGD-TK/GCV yielded a significant decrease in the size of leiomyoma lesions by 23.4% ± 3.9% and 91.8% ± 0.8% as compared with Ad-TK and Ad-LacZ, respectively (P < .05). The shrinkage of leiomyoma with the targeted Ad-SSTR-RGD-TK/GCV was superior to the untargeted Ad-TK. Representative mice from each treatment group are shown in Figure 1B.
Figure 1.
A, Effect of direct intratumor injection of adenovirus on preexisting subcutaneous leiomyoma lesions in nude mice. Animals were inoculated with the ELT3 cells subcutaneously. After tumors were palapble, animals were treated with intratumoral injection with the corresponding agent. Tumor volume was measured weekly for a period of 4 weeks after the start of treatment. a: significant difference at P value <.05; b: Significant difference at P value <.01. B, Representative mouse from each treatment group is shown; Ad-Lac Z (A), Ad-TK (B), or ADSSTR-RGD (C). Treatment of leiomyoma with Adenovirus –human somatostatin receptor subtype 2- arginine, glycine and aspartate-thymidine kinase / ganciclovir (Ad-SSTR-RGD-TK/GCV) yielded a significant decrease in the size of leiomyoma lesions by 23.4% ± 3.9% and 91.8% ± 0.8% as compared with Ad-TK and Ad-LacZ, respectively (P < .05).
Targeted Adenovirus Induces Apoptosis in Leiomyoma
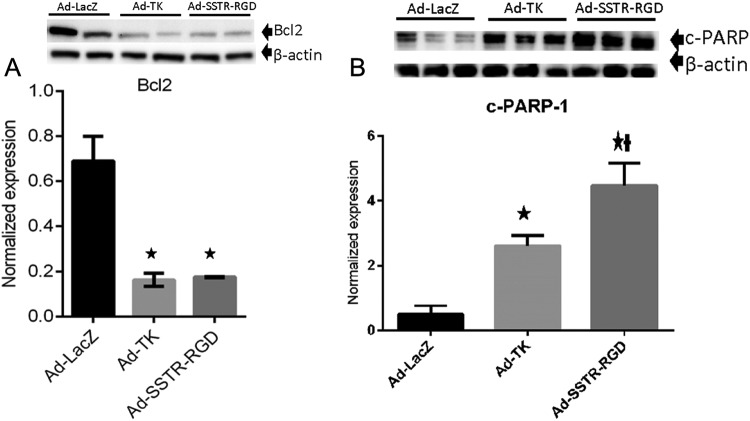
Treatment of leiomyoma with the targeted Ad-SSTRRGD-TK/GCV when compared to Ad-LacZ yielded a significant decrease (P < .05) in the expression of antiapoptosis protein (Bcl-2; Figure 2A). Also, treatment of leiomyoma with the targeted Ad-SSTRRGD-TK/GCV when compared to both Ad-TK and Ad-LacZ yielded a significant increase (P < .05) in the expression of death substrate c-PARP-1 protein (Figures 2B and 3A).
Figure 2.
A, Adenovirus vectors induce significant decreases in expression of antiapoptosis protein (Bcl2). * indicates significant differences from Ad-LacZ at P < .05. Data are presented as mean ± standard error of the mean for all tested animals per group (n = 3). B, Adenovirus vectors induce significant increases in expression of proapoptosis protein (cleaved poly (ADP-ribose) polymerase -1 [c-PARP]). * indicates significant differences from Ad-LacZ at P <.05. † indicates significant differences between Ad-TK and Ad-SSTR-RGD at P < .05. Data are presented as mean ± standard error of the mean for all tested animals per group (n = 3).
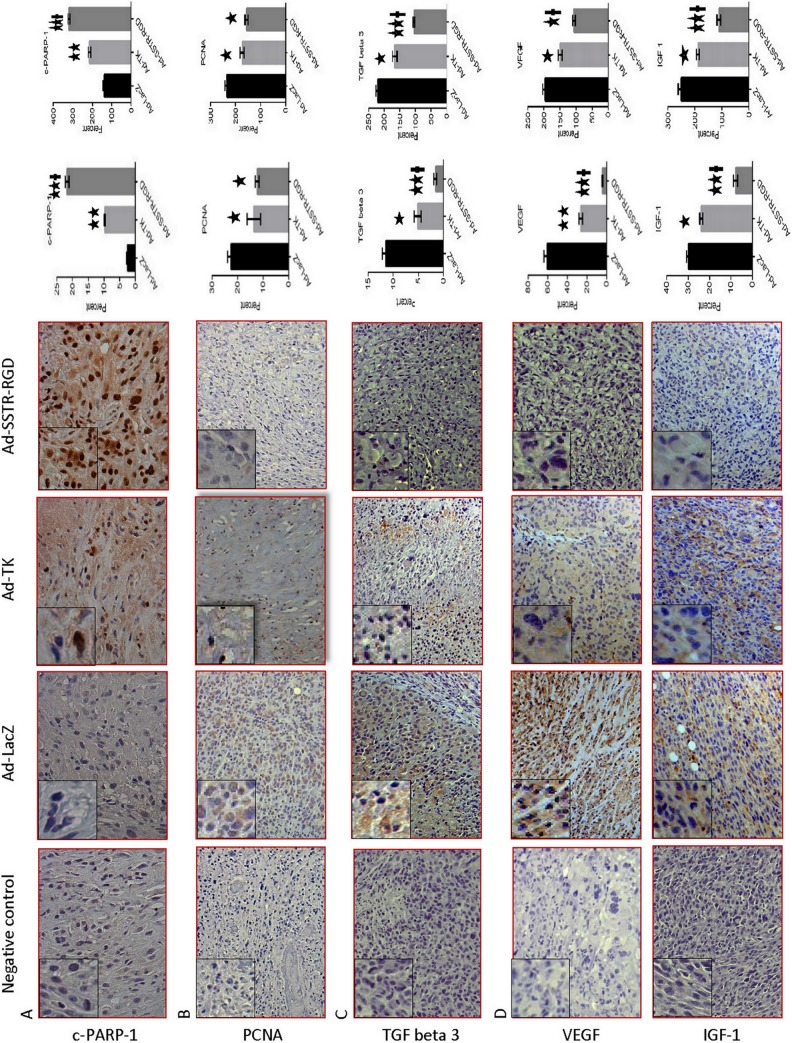
Figure 3.
Immunohistochemical staining (hematoxylin-eosin stain) of leiomyoma lesions from Ad-LacZ, Ad-TK, and Ad-SSTR-RGD-treated nude mice. (A) Ad-SSTR-RGD increases the death substrate Poly ADP-Ribose Polymerase (PARP1) protein; magnification: ×40; (B) Ad-SSTR-RGD decreases Proliferating Cell Nuclear Antigen (PCNA); magnification: ×20; (C) Ad-SSTR-RGD decreases transforming growth factor β-3 (TGFβ-3); magnification: ×20; (D) Ad-SSTR-RGD decreases vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1); magnification: ×20. The degree of expression was scored by the percentage of positive cells in which positive cells counted against the total number of the cells in 3 random high-power fields (total number 1200 cells) for each tissue section (representing 1 animal) and also by the H-score to assess staining intensity, where the fractions of negative (score 0), weakly positive (score 1), positive (score 2), strongly positive (score 3), and very strongly positive (score 4) cells were estimated by blinded observer. The fractions were multiplied with the scores and summed, the total being the H-score. The degree of expressions were calculated for 3 animals per group and were included in these calculations. The left bar represents the percentage of positive cells and the right bar represents the H-score. Data are presented as mean ± standard error of the mean for all tested animals per group. * and ** indicate significant differences from Ad-LacZ at P < .05 and P < .01, respectively. † indicates significant differences between Ad-TK and Ad-SSTR-RGD at P < .05.
Targeted Adenovirus Inhibits the Proliferation in Leiomyoma
Treatment of leiomyoma lesions with Ad-SSTRRGD-TK/GCV compared to Ad-TK and Ad-LacZ, yielded a significant decrease (P < .05) in the expression of the PCNA (Figures 4 and 3B). These results suggest that the targeted Ad-SSTRRGD-TK/GCV vector has superior capabilities, as compared with the untargeted Ad-TK vector, in reducing cell proliferation in leiomyoma.
Figure 4.
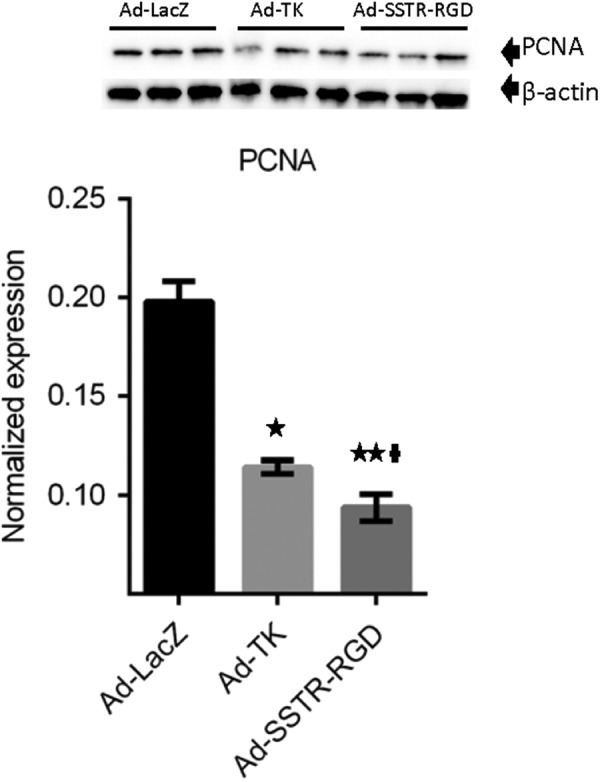
Adenovirus vectors induce significant decreases in expression of proliferating cell nuclear antigen (PCNA). * and ** indicate significant differences from Ad-LacZ at P < .05 and P < .01, respectively. † indicates significant differences between Ad-TK and Ad-SSTR-RGD at P < .05. Data are presented as mean ± standard error of the mean for all tested animals per group (n = 3).
Targeted Adenovirus Inhibits the Expression of Extracellular Matrix-Related Genes
As shown in Figures 5 and 3C, treatment of leiomyoma with the targeted Ad-SSTRRGD-TK/GCV when compared to both the untargeted Ad-TK and the Ad-LacZ yielded a significant decrease (P < .01) in the expression of the TGF-β3.
Figure 5.
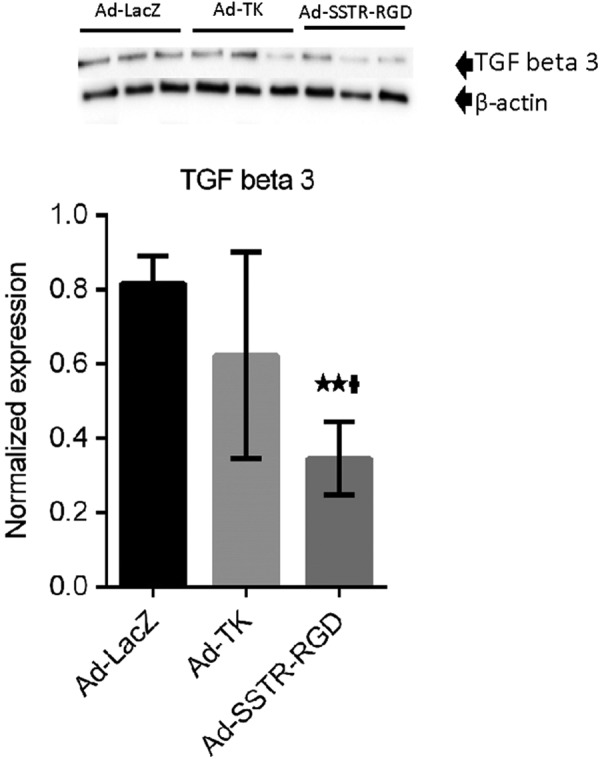
Adenovirus vectors induce significant decreases in expression of transforming growth factor β3 (TGF-β3). ** indicates significant differences from Ad-LacZ at P < .01. † indicates significant differences between Ad-TK and Ad-SSTR-RGD at P < .05. Data are presented as mean ± standard error of the mean for all tested animals per group (n = 3).
Targeted Adenovirus Inhibits the Expression of Angiogenesis-Related Genes
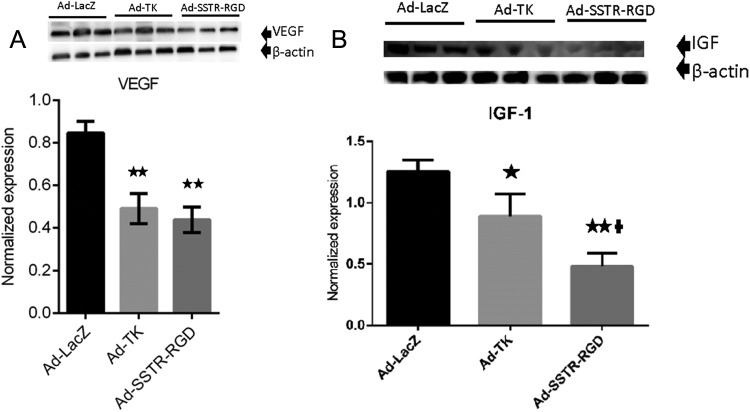
Treatment of leiomyoma with the targeted Ad-SSTRRGD-TK/GCV when compared to Ad-LacZ yielded a significant decrease (P < .01) in the expression of VEGF (Figures 6A and 3D) and also significant decreases (P < .01) in the expression of IGF-1 when compared to both the untargeted Ad-TK and the Ad-LacZ (Figures 6B and 3D). These results suggest that the targeted Ad-SSTRRGD-TK/GCV vector has superior capabilities, when compared to the untargeted Ad-TK vector, in reducing angiogenesis in leiomyoma.
Figure 6.
A, Adenovirus vectors induce significant decreases in expression of vascular endothelial growth factor (VEGF). ** indicates significant differences from Ad-LacZ at P < .01. Data are presented as mean ± standard error of the mean for all tested animals per group (n = 3). B, Adenovirus vectors induce significant decreases in expression of insulin-like growth factor 1 (IGF-1). * and ** indicate significant differences from Ad-LacZ at P < .05 and P < .01, respectively. † indicates significant differences between Ad-TK and Ad-SSTR-RGD at P < .05. Data are presented as mean ± standard error of the mean for all tested animals per group (n = 3).
Safety and Toxicity Evaluation of Targeted Versus Untargeted Adenovirus
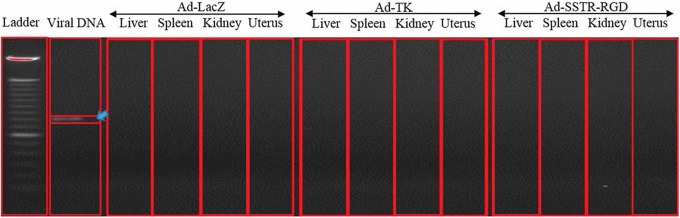
Histopathological examination revealed that all tissues appeared normal with no evident microscopic tissue damage (data not shown) and no observed evidence of gross toxicity, necrosis, or changes in the morphology of the vital organs including liver, kidney, lung, spleen, brain, uterus, and ovary. Adenovirus DNA was not detected in any of the tested tissues (uterus, ovary, liver, spleen, lung, and kidney) except in the inoculated leiomyomas with both targeted and untargeted adenovirus (Figure 7).
Figure 7.
Polymerase chain reaction (PCR) amplification of adenovirus DNA 4 weeks postintratumor injection of adenovirus vectors. The adenovirus was only detectable in the tumor tissues (arrow). The following organs tested negative for the presence of the adenovirus: liver, spleen, kidney and uterus.
Discussion
Uterine leiomyomas continue to pose a major health challenge, inpart due to the lack of effective, nonsurgical, localized therapeutic options. A therapeutic option that could considerably reduce leiomyomas in size and reverse associated morbidity without compromising the future fertility potential of the patient would be highly desirable.5 The radical surgical option of total hysterectomy continues to be the mainstay method of treatment for this very common premenopausal disorder. Gene therapy offers a potentially safe, effective, localized, and nonsurgical method of treatment for women with symptomatic uterine leiomyomas which can be administered as outpatient procedure by direct injection in leiomyoma lesions under ultrasound guidance. Women with multiple leiomyomas will need multiple injections. In our mouse model, all mice harbored 1 leiomyoma lesion, so treating multiple lesions concurrently will require additional evaluation. Nude mice ovarian function is compromised,28 therefore the endogenous E2 is minimal, it wouldn’t support robust fibroid growth and hence the need for subcutaneous estrogen pellets.
We, along with others, have reported on the efficacy of the Ad-TK/GCV system in several malignant and nonmalignant disorders.27,29 In previous studies, we have shown that the gene therapy approach using untargeted adenoviral vectors can effectively reduce leiomyoma cell proliferation in vitro as well as reduce tumor volume in vivo in the Eker rat model of uterine leiomyomas.6,7,13,27
In spite of these encouraging results, one of the limitations of this vector is its dependence on CAR for effective gene transfer, as CAR expression is generally reduced in tumor cells, including fibroids.14 Consequently, nontargeted cells, which express high levels of CAR, will potentially sequester a large number of recombinant virions, leaving the tumor cells, which express low levels of CAR, poorly transduced.16,30 An obvious solution would be to administer a higher dose of the vector; however, this approach could ultimately lead to an increased risk of toxicity and the initiation of adverse immune responses against the vector. Furthermore, our previous work suggested that untargeted adenoviral vectors directly injected into leiomyoma lesions did leak out and reach some distant organs, for example, liver.13 Therefore, to enhance the targeting ability of the vector, we modified it by reducing its dependency on CAR for transduction. The addition of an RGD-4C motif has been shown to enhance in vivo targeting abilities of adenoviral vectors.31,32 Dmitriev et al have shown that a recombinant adenoviral vector containing fibers with the RGD motif in the H1 loop demonstrated superior transduction via a CAR-independent mechanism of targeted cell entry in ovarian cancer cells.16 Previously, we showed that fiber-modified Ad5-RGD-Luc yielded higher reporter gene activity in HuLM cells and lower activity in both normal uterine smooth muscle cells (HM9) and immortalized liver cells (THLE3) when compared to that induced by the unmodified Ad5-luc vector.12 This result indicated that modified Ad5-RGD is a promising candidate for use as a vector in targeted gene therapy for uterine leiomyomas. We have also demonstrated in previous work that targeted adenovirus vector, Ad-SSTR-RGD-TK followed by GCV, was highly effective in selectively inducing apoptosis and inhibiting proliferation of HuLM cells while sparing normal human myometrial cells.24
In our current study, we compared Ad-SSTR-RGD-TK/GCV with Ad-TK to evaluate the effect of the modified vector on tumor growth in a nude mice model. Our results indicate that the shrinkage in tumor size was significantly superior following transduction of Ad-SSTR-RGD-TK/GCV when compared to untargeted Ad-TK.
BCL-2 is a 24-KD cytosolic protein localized in the mitochondria and perinuclear membrane.33 The BCL-2 protein has been well described for its ability to extend the life span of cells and to promote cell replication.34,35 Elevation in the BCL-2 gene in vivo or in vitro is preventative in the apoptosis of cells.36,37 BCL-2 is abundantly expressed in leiomyomas when compared to normal myometrium.38 We observed a significant reduction in BCL-2 expression in tumors injected with Ad-SSTR-RGD-TK/GCV when compared to Ad-TK or Ad-LacZ.
In our work, we observed a significant increase in c-PARP expression in tumors injected with Ad-SSTR-RGD-TK/GCV when compared to Ad-TK or Ad-LacZ. The PARP can be activated in cells experiencing stress and/or DNA damage. Activated PARP can deplete the adenosine triphosphate (ATP) of a cell in an attempt to repair the damaged DNA. Depletion of ATP in a cell leads to lysis and cell death (necrosis). The PARP also has the ability to induce programmed cell death, via the production of PAR, which stimulates mitochondria to release apoptosis-inducing factor.39 Cleavage of PARP by caspases to produce c-PARP is a central step of intrinsic apoptosis. It is believed that normal cleavage occurs in systems where DNA damage is extensive. In these cases, more energy would be invested in repairing damage than is feasible so that energy is instead retrieved for other cells in the tissue through programmed cell death.40
The PCNA is a 36-kD nuclear protein that is synthesized in dividing cells and a well-established proliferation marker. We observed a significant reduction in PCNA expression in leiomyoma lesions treated with AD-SSTR-RGD-TK, and this reduction was significantly more remarkable than that observed in leiomyoma lesions treated with Ad-TK. This observation confirms that the RGD-fiber modification enhanced the transduction characteristics of this vector and increased its targeting efficiency toward HuLM cells.
Transforming growth factor β are multifunctional peptides that regulate growth and differentiate in a variety of cells. Increased expression of TGF-β3 may contribute to the growth of human leiomyoma.41 We observed a significant reduction in TGF-β3 expression in tumors injected with Ad-SSTR-RGD-TK/GCV when compared to Ad-TK or Ad-LacZ.
The ability of tumors to recruit new blood vessels is an absolute requirement for tumor growth, and the expression of several angiogenesis factors, such as VEGF, have been shown to be estrogen responsive.42 Angiogenic factors, such as VEGF, its receptors, and endothelial growth factor receptors (EGF-R) may be involved in tumor angiogenesis.43 Inhibition of the expression of angiogenic factors therefore constitutes another potential mechanism for the inhibition of tumor growth. We observed a significant reduction in VEGF expression in tumors injected with Ad-SSTR-RGD-TK/GCV when compared to Ad-TK or Ad-LacZ.
Untargeted adenovirus delivered by direct intratumor injection into leiomyoma lesions did not have any significant effect on Eker rat liver function and had minimal but detectable leakage in the liver and uterus.13 In this study, we aimed to assess whether targeting modification of adenovirus by making it tumor specific would alleviate such limitation. To evaluate the safety of local delivery of the gene therapy approach with targeted adenovirus Ad-SSTRRGD-TK/GCV, local and distant dissemination of the adenovirus was assessed in different organs of the leiomyoma mouse model. We collected organs such as the spleen, kidney, lung, heart, brain, uterus, ovary, and liver. We found that both targeted and untargeted adenovirus did not leak to other organs. However, one limitation of the study is the anatomical position of the leiomyoma lesions within the nude mice leiomyoma model. Within this model, leiomyoma lesions were subcutaneous and, therefore, not in the normal anatomical position within the myometrium. Our next step will include a study designed to verify these data in an authentic model such as Eker rat prior to embarking on a future human trial.
Another theoretical concern in this experimental approach is the possibility of germ line transmission of viral genes via the oocyte DNA. In our previous work, when Ad-LacZ was delivered directly to the ovary, no adenoviral or LacZ gene or gene products were detected in resulting pups, which suggests no germ line transmission.44
In conclusion, the targeted Ad-SSTR-RGD system induced superior shrinkage in leiomyoma when compared to untargeted Ad-TK, and it inhibited cell proliferation while inducing apoptosis and inhibited the expression of extracellular matrix and angiogenesis-related genes. These changes were significantly more prominent in tumors injected with the targeted Ad-SSTR-RGD when compared to the untargeted Ad-TK or Ad-LacZ. This study has generated important preclinical data for the development of leiomyoma-targeted gene therapy as a potential therapeutic approach for the safe, nonsurgical treatment of uterine leiomyomas.
Footnotes
Authors’ Note: M.A. substantially contributed toward the conceptualization and design of this study including data acquisition, analysis, and interpretation. He also drafted the manuscript and remained actively involved in its final approval for publication. S.S., S.N., S.M., N.E., and M.A.-L. participated in data acquisition, analysis, and interpretation. They also participated in drafting and critical review of the manuscript. They were actively involved in the final approval of the manuscript for publication. L.S., M.E., N.I., D.C., and M.D. participated in the analysis and interpretation of data and critical review of the manuscript and were actively involved in the final approval of the manuscript for publication. A.A.-H. made substantial contributions in the conceptualization, design, and interpretation of the data. He was involved in drafting the manuscript and provided a critical review of its intellectual content as well as final approval for publication. Moreover, he provided the necessary funding and laboratory space to conduct this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Institute of Child Health and Human Development, National Institutes of Health [R01 HD046228].
References
- 1. Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41(7):483–490. [PubMed] [Google Scholar]
- 2. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. [DOI] [PubMed] [Google Scholar]
- 3. Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104(2):393–406. [DOI] [PubMed] [Google Scholar]
- 4. Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298. [DOI] [PubMed] [Google Scholar]
- 5. Segars JH, Parrott EC, Nagel JD, et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update. 2014;20(3):309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hassan MH, Othman EE, Hornung D, Al-Hendy A. Gene therapy of benign gynecological diseases. Adv Drug Deliv Rev. 2009;61(10):822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hassan M, Zhang D, Salama S, et al. Towards fibroid gene therapy: adenovirus-mediated delivery of herpes simplex virus 1 thymidine kinase gene/ganciclovir shrinks uterine leiomyoma in the Eker rat model. Gynecol Obstet Invest. 2009;68(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tasciotti E, Zoppe M, Giacca M. Transcellular transfer of active HSV-1 thymidine kinase mediated by an 11-amino-acid peptide from HIV-1 Tat. Cancer Gene Ther. 2003;10(1):64–74. [DOI] [PubMed] [Google Scholar]
- 9. Reid R, Mar EC, Huang ES, Topal MD. Insertion and extension of acyclic, dideoxy, and ara nucleotides by herpesviridae, human alpha and human beta polymerases. A unique inhibition mechanism for 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1988;263(8):3898–3904. [PubMed] [Google Scholar]
- 10. Robe PA, Princen F, Martin D, et al. Pharmacological modulation of the bystander effect in the herpes simplex virus thymidine kinase/ganciclovir gene therapy system: effects of dibutyryl adenosine 3’,5’-cyclic monophosphate, alpha-glycyrrhetinic acid, and cytosine arabinoside. Biochem Pharmacol. 2000;60(2):241–249. [DOI] [PubMed] [Google Scholar]
- 11. Hemminki A, Zinn KR, Liu B, et al. In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J Natl Cancer Inst. 2002;94(10):741–749. [DOI] [PubMed] [Google Scholar]
- 12. Hassan MH, Khatoon N, Curiel DT, Hamada FM, Arafa HM, Al-Hendy A. Toward gene therapy of uterine fibroids: targeting modified adenovirus to human leiomyoma cells. Hum Reprod. 2008;23(3):514–524. [DOI] [PubMed] [Google Scholar]
- 13. Hassan MH, Salama SA, Zhang D, et al. Gene therapy targeting leiomyoma: adenovirus-mediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in Eker rat model. Fertil Steril. 2010;93(1):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anders M, Ding R, Lipner EM, Balmain A, McCormick F, Korn WM. Inhibition of the MAPK pathway up-regulates the human coxsackie and adenovirus receptor (CAR) and increases the infectivity of cancer cells with adenovirus. Proc Am Assoc Cancer Res. 2001;42:703. [Google Scholar]
- 15. Tsibris JC, Segars J, Coppola D, et al. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72(12):9706–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cripe TP, Dunphy EJ, Holub AD, et al. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001;61(7):2953–2960. [PubMed] [Google Scholar]
- 18. Kasono K, Blackwell JL, Douglas JT, et al. Selective gene delivery to head and neck cancer cells via an integrin targeted adenoviral vector. Clin Cancer Res. 1999;5(9):2571–2579. [PubMed] [Google Scholar]
- 19. Vanderkwaak TJ, Wang M, Gomez-Navarro J, et al. An advanced generation of adenoviral vectors selectively enhances gene transfer for ovarian cancer gene therapy approaches. Gynecol Oncol. 1999;74(2):227–234. [DOI] [PubMed] [Google Scholar]
- 20. Blackwell JL, Li H, Gomez-Navarro J, et al. Using a tropism-modified adenoviral vector to circumvent inhibitory factors in ascites fluid. Hum Gene Ther. 2000;11(12):1657–1669. [DOI] [PubMed] [Google Scholar]
- 21. Hemminki A, Belousova N, Zinn KR, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther. 2001;4(3):223–231. [DOI] [PubMed] [Google Scholar]
- 22. Kimball KJ, Preuss MA, Barnes MN, et al. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin Cancer Res. 2010;16(21):5277–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim KH, Dmitriev I, O’Malley JP, et al. A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer. Clin Cancer Res. 2012;18(12):3440–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nair S, Curiel DT, Rajaratnam V, Thota C, Al-Hendy A. Targeting adenoviral vectors for enhanced gene therapy of uterine leiomyomas. Hum Reprod. 2013;28(9):2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang D, Al-Hendy M, Richard-Davis G, et al. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am J Obstet Gynecol. 2010;202(3):289. e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alvarez RD, Barnes MN, Gomez-Navarro J, et al. A cancer gene therapy approach utilizing an anti-erbB-2 single-chain antibody-encoding adenovirus (AD21): a phase I trial. Clin Cancer Res. 2000;6(8):3081–3087. [PubMed] [Google Scholar]
- 27. Salama SA, Kamel M, Christman G, Wang HQ, Fouad HM, Al-Hendy A. Gene therapy of uterine leiomyoma: adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir treatment inhibits growth of human and rat leiomyoma cells in vitro and in a nude mouse model. Gynecol Obstet Invest. 2007;63(2):61–70. [DOI] [PubMed] [Google Scholar]
- 28. Rebar RW, Morandini IC, Erickson GF, Petze JE. The hormonal basis of reproductive defects in athymic mice: diminished gonadotropin concentrations in prepubertal females. Endocrinology. 1981;108(1):120–126. [DOI] [PubMed] [Google Scholar]
- 29. Ketola A, Maatta AM, Pasanen T, Tulimaki K, Wahlfors J. Osteosarcoma and chondrosarcoma as targets for virus vectors and herpes simplex virus thymidine kinase/ganciclovir gene therapy. Int J Mol Med. 2004;13(5):705–710. [PubMed] [Google Scholar]
- 30. Nakayama M, Both GW, Banizs B, et al. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology. 2006;350(1):103–115. [DOI] [PubMed] [Google Scholar]
- 31. Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15(6):542–546. [DOI] [PubMed] [Google Scholar]
- 32. Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–380. [DOI] [PubMed] [Google Scholar]
- 33. Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94(7):1506–1512. [DOI] [PubMed] [Google Scholar]
- 34. Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129–1132. [DOI] [PubMed] [Google Scholar]
- 35. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. [DOI] [PubMed] [Google Scholar]
- 36. Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80(4):879–886. [PubMed] [Google Scholar]
- 37. Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124(1-2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsuo H, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82(1):293–299. [DOI] [PubMed] [Google Scholar]
- 39. Yu SW, Andrabi SA, Wang H, et al. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A. 2006;103(48):18314–18319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plenat F. Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem. 2009;57(4):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86(2):913–920. [DOI] [PubMed] [Google Scholar]
- 42. Hyder SM, Stancel GM, Chiappetta C, Murthy L, Boettger-Tong HL, Makela S. Uterine expression of vascular endothelial growth factor is increased by estradiol and tamoxifen. Cancer Res. 1996;56(17):3954–3960. [PubMed] [Google Scholar]
- 43. Sanci M, Dikis C, Inan S, Turkoz E, Dicle N, Ispahi C. Immunolocalization of VEGF, VEGF receptors, EGF-R and Ki-67 in leiomyoma, cellular leiomyoma and leiomyosarcoma. Acta Histochem. 2011;113(3):317–325. [DOI] [PubMed] [Google Scholar]
- 44. Ghadami M, El-Demerdash E, Salama SA, et al. Toward gene therapy of premature ovarian failure: intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR(-/-) FORKO mice. Mol Hum Reprod. 2010;16(4):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]