Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine disorder characterized by chronic oligoanovulation and hyperandrogenism and associated with insulin resistance, type 2 diabetes, and cardiovascular risk. In recent years, genetic studies have linked PCOS to a dinucleotide marker D19S884 in the fibrillin 3 gene. Fibrillins make up the major component of microfibrils in the extracellular matrix (ECM) and interact with molecules in the ECM to regulate transforming growth factor β (TGF-β) signaling. Therefore, variations in fibrillin 3 and subsequent dysregulation of TGF-β may contribute to the pathogenesis of PCOS. Here, we review the evidence from genetic studies supporting the role of TGF-β in PCOS and describe how TGF-β dysregulation may contribute to (1) the fetal origins of PCOS, (2) reproductive abnormalities in PCOS, and (3) cardiovascular and metabolic abnormalities in PCOS.
Keywords: polycystic ovary syndrome, TGF-β, fibrillin, genetics, fetal origins
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder that affects 5% to 10% of reproductive-aged women.1 Genetic studies have linked PCOS to allele 8 (A8) of D19S884, a dinucleotide repeat marker in intron 55 of the fibrillin 3 gene.2–4 Other dinucleotide repeat polymorphisms have been shown to function as both transcriptional5–12 and splicing enhancers.13–16 In Stewart et al, we showed that while the D19S884 containing genomic fragment has promoter activity, this activity is not A8 specific4; however, the impact of D19S884 allele status on splicing of the FBN3 transcript has not been evaluated to date.
Although the exact mechanisms by which the D19S884 fibrillin 3 variant contributes to the etiology of PCOS are yet to be fully elucidated, much can be inferred from what is known about fibrillins in general. There are 3 homologous fibrillin genes: fibrillin 1, 2, and 3. Fibrillin 3 is more recently discovered and less well characterized than fibrillin 1 or 2. Fibrillins make up the major component of microfibrils in the extracellular matrix (ECM). Although fibrillins were initially recognized as purely structural proteins, more recent studies reveal that fibrillins also have important functional roles, interacting with molecules in the ECM to regulate the local bioavailability of the transforming growth factor-β (TGF-β) superfamily in tissues throughout the body. In fact, TGF-β dysregulation due to mutations in fibrillin 1 has been shown to contribute to cardiovascular and other connective tissue abnormalities found in Marfan syndrome.17,18 Given that fibrillin 3 shares the same binding domains as fibrillins 1 and 2, it is likely that fibrillin 3 performs similar molecular functions as fibrillins 1 and 2, which include binding to and regulating members of the TGF-β superfamily.19 TGF-β dysregulation due to variants in fibrillin 3 could contribute to the pathogenesis of reproductive and cardiometabolic abnormalities in PCOS, via similar mechanisms as in fibrillins 1 and 2.
The TGF-β superfamily signaling pathway regulates many important cellular processes, including cell proliferation, differentiation, and survival.20 The TGF-β superfamily of ligands includes inhibins, activins, anti-Müllerian hormone (AMH), growth and differentiation factors (GDFs), bone morphogenetic proteins (BMPs), and the TGF-β family which consists of 3 primary isoforms: TGF-β1, TGF-β2, and TGF-β3.21 TGF-β1, originally called TGF-β, was the founding member of the TGF-β family. Many cell types, including macrophages, secrete TGF-β. Many of these cells also express TGF-β receptors on their surface, allowing TGF-β to act in an autocrine manner.22 TGF-β signaling is initiated when a TGF-β superfamily ligand binds to a TGF-β type II receptor.20 Each class of ligand binds to a specific type II receptor, which then recruits and phosphorylates a specific type I receptor. The type I receptor then phosphorylates receptor-regulated SMADs (R-SMADs), which subsequently bind to the coSMAD SMAD4. The R-SMAD/coSMAD complexes accumulate in the nucleus where they act as transcription factors and regulate gene expression.20
Members of the TGF-β ligand family are secreted in an inactive latent form complexed to their propeptide latency-associated peptide and at times also complexed to latent TGF-β binding proteins (LTBPs), which share homology with the fibrillins. Once secreted, the latent TGF-β complexes then bind to the fibrillin in microfibrils and become incorporated into the ECM.23 This sequestration of TGF-β by the ECM appears to be critical for the regulation of TGF-β activity.23 In tissues throughout the body, various fibrillins bind to TGF-β, holding it in an inactive complex in the ECM until it is needed, at which point it is released by proteases and activated.23 Once TGF-β is released from the ECM, it is able to exert its effects locally by binding to nearby receptors on the surface of cells.20 Thus, alterations in fibrillin could prevent the normal binding and/or release of TGF-ß and other ligands of the TGF-β superfamily from the ECM, leading to dysregulated TGF-β activity, which then adversely affects the development and function of various organs throughout the body.17
Many TGF-ß superfamily members are expressed in the ovary and have been implicated in the pathogenesis of abnormal follicle development and hyperandrogenism in PCOS, including activins, inhibins, AMH, and BMPs.24–28 Although less is known about the role of the TGF-β family, TGF-β1, TGF-β2, and TGF-β3, in the pathogenesis of PCOS, reproductive dysfunction has been demonstrated in knockout mice at all levels of the TGF-β1 signaling pathway.29 In addition, follistatin, which binds to and regulates members of the TGF-β superfamily like fibrillins do, has also been implicated in PCOS.2,26,30 Further, the PCOS ovary is characterized by a thickening of the ovarian capsule and stroma due to increased collagen deposition and fibrous tissue, which can be attributed to the TGF-β superfamily members as they are known to regulate collagen synthesis and have been implicated in fibrosis.31 This article reviews the evidence from genetic studies supporting the role of TGF-β in PCOS and describes how TGF-β dysregulation may contribute to (1) the fetal origins of PCOS, (2) reproductive abnormalities in PCOS, and (3) cardiovascular and metabolic abnormalities in PCOS.
Genetic Studies Supporting the Role of TGF-β in PCOS
In a screen of 37 candidate genes for PCOS, the 2 loci (fibrillin 3 [FBN3] and follistatin [FST]), with the strongest evidence for linkage and/or association with PCOS encode proteins that are implicated in signaling by the TGF-β superfamily. As is with many variants associated with complex genetic diseases, the fibrillin 3 variant, the dinucleotide repeat polymorphism D19S884, is in a noncoding portion of the fibrillin 3 gene. This has made it difficult to determine its functional significance using traditional molecular biologic techniques. One approach that has proven useful for determining the importance of such variants is the examination of genotype–phenotype associations. We have demonstrated that women with PCOS, who possess the fibrillin 3 PCOS-associated allele D19S884 A8, 1 or 2 A8 alleles, have significantly increased fasting insulin levels and homeostasis model assessment of insulin resistance (HOMA-IR) values compared to women with PCOS who posses all other alleles of D19S884.32 In addition, the fibrillin 3 variant, D19S884 A8, has also been associated with β-cell dysfunction and alterations in basal glucose homeostasis.33,34
The PCOS-associated locus mapping to the gene encoding follistatin has not been fine mapped, and therefore the functional variant has not yet been identified. However, although much less is known about the specific follistatin variants that are associated with PCOS, follistatin has been implicated in PCOS.2,26,30 Circulating follistatin levels are higher, and activin A levels are lower in women with PCOS compared to controls and may contribute to impaired folliculogenesis in PCOS.26,35 Follistatin binds to and inactivates the TGF-β superfamily ligand activin, impairing activin’s functions that include stimulating the synthesis and secretion of follicle-stimulating hormone (FSH) by pituitary gonadotrophs, promoting ovarian follicular development, inhibiting androgen production, and increasing insulin secretion. Since both fibrillins and follistatin affect TGF-β signaling by sequestering and thereby biologically inactivating TGF-β superfamily ligands, variants in both fibrillin 3 and follistatin may act in a complementary manner to alter the TGF-β-signaling pathway in PCOS.
FBN3 maps to chromosome 19p13.2 and encodes an ECM protein, fibrillin 3.2,3 In a candidate gene screen, the strongest evidence for both linkage and association was observed with markers mapping intron 55 of the FBN3 gene. Specifically, the strongest evidence for association with PCOS was with A8 of D19S884. These findings have been confirmed in 2 additional samples of families collected by us and by 2 of 3 independent case–control studies.3,4,33,36–38 Fine mapping of the Chr19p13.2 PCOS susceptibility locus, including sequencing the coding regions of all genes within 100 KB of D19S884, did not reveal any markers with stronger evidence for association than D19S884.
Although D19S884 could be a “proxy” for association with another variant, this is unlikely given the very limited linkage disequilibrium in the vicinity of D19S884 in both our cohort and in the caucasian HapMap population. In fact, D19S884 maps within a recombination hot spot according to both HAPMAP and Perlegen cohorts (http://genome.ucsc.edu). Also, no haplotypes in the D19S884 region had stronger evidence for association than D19S884, further evidence against an unidentified variant in this region. These findings suggest that D19S884 is a functional variant rather than a proxy for a functional variant. Other dinucleotide repeat polymorphisms have been shown to function as transcriptional5–8,10–12,39 and splicing enhancers.13–16 Stewart et al showed that while the D19S884 containing genomic fragment has promoter activity, this activity is not A8 specific.4 D19S884, therefore, may affect splicing of FBN3 messenger RNA.
In our initial candidate gene screen, FST showed the strongest evidence for linkage with PCOS.2 However, mutation screening of the coding region of FST followed by testing for association did not show evidence for association between FST and PCOS in our families,30 and follow-up linkage analysis did not find any further evidence for linkage.30 Sequence analysis and association studies of coding variants by other investigators have also failed to identify a PCOS-associated FST variant.40,41 However, a broader analysis of the entire genomic region of FST including 20 KB upstream and downstream of the gene, and all intronic sequences in a cohort of 633 women with PCOS and 574 controls showed strong evidence for association with variants in intron 1. The strongest evidence of association was with rs3756498 (chi-square = 14.4, P = .0001; M. Urbanek, PhD, personal communications, January 2010).
Furthermore, we have found that genetic partitioning of PCOS families based on D19S884 A8 status strengthens the evidence for a role of follistatin in the etiology of PCOS. Both fibrillins and follistatin are believed to affect TGF-β signaling by sequestering and thereby biologically inactivating TGF-β ligand/ligands. Therefore, these genes may act in a complementary manner to alter this signaling pathway. We used genetic partitioning of multiplex PCOS families to test this hypothesis. However, for FBN3, the A8+ families had very strong evidence for linkage (77% Identity by descent [IBD], P = .0007), while the A8- families had no evidence for linkage with PCOS (IBD = 53%, P = .53). The converse was true for FST: A8+ families have no evidence for linkage (IBD = 48%, P = .84), while A8- families have significant evidence for linkage (IBD = 62%, P = .014; M. Urbanek, PhD, personal communications, October 2006). These findings support our hypothesis that the TGF-β-signaling pathway is important in the etiology of PCOS and that 2 genes in this pathway, FBN3 and FST, contribute independently to PCOS.
The TGF-β-signaling pathway plays a critical role in the development of multiple tissues or cells including folliculogenesis. Given the importance of folliculogenesis in the development of PCOS, Sproul et al tested for association between PCOS and genetic variation in 4 members of the TGF-β-signaling pathway (GDF9, BMP15, AMH, and AMHR) in a cohort of 355 PCOS cases and 198 controls.42 Sproul et al tested for association with 5 small nucleotide polymorphisms (SNPs) in GDF9, 2 SNPs in BMP15, 3 SNPs in AMH, and 3 SNPs in AMHR2 and found no evidence for association between any of the variants and PCOS although variants in GDF9 were associated with hirsutism scores. Similarly, Kevenaar et al found no evidence for association between the AMH lle 49 Ser coding variant and the AMHR2 −482A>G variant and PCOS in a cohort of 331 women with PCOS and 3635 population-based controls.27 However, among women with PCOS, the AMH 49 Ser allele was associated with a lower rate of polycystic ovaries, lower number of follicles, and lower androgen levels. Therefore, the sample of SNPs in AMH does not appear to increase the risk of PCOS, but they may still be important in determining the severity of the PCOS phenotype among women diagnosed with PCOS. Furthermore, while these studies do not support a strong role for these genes in the etiology of PCOS, it should be noted that given the relatively small sample sizes, the studies were not powered to detect loci with the small effect sizes generally observed in association studies of complex traits like PCOS, and the effect of these genes will need to be further examined in larger cohorts.
Genetic analysis of ACVR1 that encodes ALK2, the type 1 receptor shared by the AMH/BMP class of TGF-β-signaling pathway, tested for association between 7 SNPs and PCOS in a cohort of 359 women with PCOS and 3543 population-based controls and 30 normo-ovulatory women.43 An SNP, rs17798043, mapping upstream of the first exon was significantly associated with PCOS, while multiple SNPs within the gene were associated with AMH levels and/or follicle number.
Although genetic variation in multiple members of the TGF-β-signaling pathway has been evaluated, the selection of genes has been somewhat arbitrary, and in most cases the analysis has been underpowered. Thus, a more systematic and better-powered analysis of the pathway is required. Genome-wide association studies (GWAS) of PCOS do not identify any members of the TGF-β-signaling pathway among their top signals.44 Given the significant penalty for multiple testing required by GWAS, a more targeted analysis focused on members of the TGF-β-signaling pathway may be a more informative approach for assessing the impact of the TGF-β pathway on PCOS.
In conclusion, the TGF-β superfamily signaling pathway appears to be important in the etiology of PCOS as the 2 genes with the strongest evidence for association with PCOS, FBN3 and FST, are implicated in the TGF-β superfamily signaling pathway. Genetic variants of other components of the TGF-β superfamily signaling pathway have also been investigated in PCOS with mixed results. SNPs in GDF9, BMP15, AMH, and AMHR were not associated with PCOS, although GDF-9 variants were associated with hirsutism, and an AMH variant was associated with a less severe PCOS phenotype among women with PCOS. Additionally, PCOS has been significantly associated with an SNP in ACVR1, which encodes ALK2, the type 1 receptor shared by the AMH/BMP class of TGF-β-signaling pathway. However, the GWAS studies do not identify any members of the TGF-β-signaling pathway among their top signals. Although the TGF-β-signaling pathway may not contribute the strongest genetic signals to PCOS, as larger sample sizes for genetic studies are assembled, members of the TGF-β-signaling pathway may attain significant evidence for association with PCOS. Furthermore, given that PCOS is a complex disorder, environmental, endocrine, and metabolic factors are also likely to play a prominent role as genetic factors in the etiology of PCOS. Indeed, both genetic and environmental factors may contribute to dysregulated TGF-β signaling in PCOS.
Role of TGF-β in the Fetal Origins of PCOS
Why Fibrillin 3 was a Good Candidate Gene?
Genomic mapping can identify loci involved in diseases; however, further research is required to identify how or why these loci are involved. This is true for the dinucleotide repeat microsatellite marker D19S884, which showed familial linkage with PCOS.4 It maps to intron 55 of the fibrillin 3 gene and because of fibrillin 3’s potential roles, fibrillin 3 itself was targeted for further investigation by a number of researchers.19,38,45,46
Fibrillins 1 to 3 are extracellular matrix (ECM) glycoproteins that form elastin fibers or extracellular microfilaments.47,48 Fibrillins 1 and 2 also bind latent TGF-β binding proteins (LTBPs). Little research has been conducted on fibrillin 3 directly; however, because of its structural homology to fibrillins 1 and 2, it is assumed it also binds LTBPs. Fibrillins and LTBPs therefore sequester TGF-β in tissues and regulate its local bioavailability and action in the following manner.49 TGF-β is first synthesized as latent TGF-β that is inactive and complexed to its propeptide (also called the latency-associated peptide). The combination of latent TGF-β complexed to its propeptide is known as small latent complex. The LTBPs (1, 3 or 4) chaperone the formation of the small latent complexes and in the process form complexes with them in the endoplasmic reticulum; these tripartite complexes are known as the large latent complexes. Upon secretion, the large latent complexes bind to fibrillins in the ECM and incorporate TGF-β into the ECM. Thus, in tissues throughout the body, fibrillins retain TGF-βs in these inactive complexes until TGF-βs are released and activated by the action of proteases and integrins.50
In stroma, TGF-β stimulates fibroblast function including production and deposition of collagen in both normal and fibrotic tissues.21,51–58 Additionally, in the fibrosis of many organs, TGF-β activity is enhanced.55,57,59 Thus, increased stroma and collagen is invariably due to increased TGF-β activity.
It was stated recently that “the ovaries of women with polycystic ovary syndrome show all the hallmarks of increased TGF-β activity.”46 This was based upon the PCOS ovary phenotype as first described,60 in which increased amounts of stroma and tunica were observed. This was later quantitated showing PCOS ovaries having more ovarian capsule or tunica albuginea containing more collagen and increased thicknesses of the cortical and subcortical stroma.31 These are not the only stromal tissues altered in PCOS ovaries. The theca interna that develops around each antral follicle is a stromal tissue and is abnormal with elevated capacity to produce steroid hormones.61,62
Initial efforts to identify a role of fibrillin 3 in human control and PCOS ovaries and in bovine ovaries were not entirely successful. Very little expression of fibrillin 3 was observed in adult ovaries,38,45 even when using sensitive real-time polymerase chain reaction. There is one study in which fibrillin 3 was localized to adult human ovaries by immunohistochemistry.19 Fibrillin 3 was observed in stroma near “transitional” follicles. Not all ovaries, nor all such areas were positive for fibrillin 3 though.
A tagging and functional SNP analysis of the fibrillin 3 gene in a case–cohort study found no evidence of any linkage with PCOS.38 In this study38 and in another case–control study,36 even the dinucleotide repeat microsatellite marker D19S884 was not linked with PCOS. Thus, these earlier studies were not successful at identifying how the D19S884 or fibrillin 3 could be involved in the etiology of PCOS. Other alternative hypotheses had to be considered.
Evidence of Fetal Origins of PCOS
Abnormalities in the growth and development of fetal organs could lead to disease later in life. Indeed, much of ovarian development, including the establishment of the ovarian follicle pool for a woman’s lifetime, occurs during fetal life. Thus, the actions of fibrillin 3 variants in the fetal ovary could lead to permanent alterations in ovarian stroma that persist to adulthood and manifest as PCOS. Evidence from a variety of discoveries suggests that there is a fetal cause of PCOS. Treatment of pregnant sheep63 and nonhuman primates64 with androgens produces a PCOS phenotype in the offspring. Adult PCOS ovaries have more primordial follicles65 that are formed during fetal life. Interestingly, androgenization of a fetus also increases the pool of primordial follicles in adult ovaries.66 Congenital adrenal hyperplasia in humans also leads to symptoms of PCOS later in life.67,68 Recent epidemiological data have shown a linkage between weight and ponderal index at birth with symptoms of PCOS at 30 years of age.69 These facts collectively suggest that PCOS can be caused by perturbations in fetal life.
Linkage of Genetic Causes and Fetal Origins of PCOS
In a landmark study, fibrillin 3 was found to be expressed in the developing stroma of human and bovine fetal ovaries (Figure 1). It was highly expressed in the first trimester and declined soon after as gestation progressed.46 This study mechanistically combined 3 important observations about PCOS: (1) the fetal origins of PCOS, (b) the genetic studies suggesting that fibrillin 3 could be a candidate gene in PCOS, and (c) the PCOS ovarian phenotype with altered stromal compartments (tunica albuginea, cortical stroma, theca interna); the hallmarks of enhanced TGF-β activity. It was concluded that “since fibrillins are stromal matrices and since the ovarian stromal compartments are altered in women with PCOS, fibrillin 3 expression in the developing fetal ovary, via the activity of TGF-β to regulate stromal formation and function, could predispose an individual to PCOS in later life.”46
Figure 1.
Immunolocalization of fibrillin 3 in (A) human (10 weeks of gestation) and (B) bovine (crown-rump length of 13 cm equivalent to 86 days of gestation) fetal ovaries as conducted and reported previously. 33 Fibrillin 3 is stained red in (A) and green in (B). Fibrillin 3 co-localizes to COUP-TF11 positive stromal cells (red) in (B). In both sections (A, B), nuclei are counterstained with 4',6-diamidino-2-phenylindole (blue). Bar: 50 µm.
Soon afterward further evidence linking TGF-β activity and fetal origins of PCOS was obtained.70 Using a primate model of PCOS in which mothers are androgenized and the offspring develop features of PCOS,64 the promoter regions of genes in adipose tissue were examined to identify those that were differentially epigenetically altered (methylated) between control and PCOS monkeys.70 Pathway analysis identified that many of the genes that were differentially and epigenetically regulated belonged to the TGF-β signaling pathway. Since in this model the only intervention to cause PCOS symptoms is during fetal development, evidence is accumulating that links a fetal origin and TGF-β signaling pathway.
Collectively, these exciting concepts require additional research and discoveries even if only to understand the ovarian phenotype. What role does the microsatellite marker D19S884 play, if any, on expression or activity of fibrillin 3 and how? Is altered expression of fibrillin 3 during fetal development sufficient in itself to alter the epigenetics of the TGF-β-signaling pathway, and if so is this the cause of increased stroma and collagen deposition in the adult PCOS ovary? What roles do androgens play in these processes?
It is interesting to note that fibrillin 3 expression during fetal development is not confined to the ovary as it is also expressed in other fetal organs during development.71 Therefore, changes in fibrillin 3 activity in other organs could also potentially alter TGF-β-signaling pathways in those tissues as well.
Role of TGF-β in Reproductive Abnormalities in PCOS
The direct implication of TGF-β and its signaling pathways in reproductive abnormalities which characterize PCOS, including, anovulation and ovarian/adrenal hyperandrogenism, is primarily inferential. As discussed above, increased ovarian stroma and a thickened ovarian stroma may be signs or sequelae of local TGF-β dysregulation. There are few studies in humans that directly target the local or systemic effects of TGF-β dysregulation in women with PCOS. Mammals express 3 of the 5 TGF-β isoforms: TGF-β1, TGF-β2, and TGF-β3.72 We have examined circulating levels of TGF-β1 and TGF-β2 in women with PCOS and found significant genotypic correlations with A8 of D19S884.73 For the purpose of this article, we have additionally examined in this cohort of women with PCOS the correlation of these cytokines with circulating testosterone levels (Figure 2) and found a significant correlation between circulating TGF-β2 levels and testosterone in controls but not in PCOS women. There was no significant correlation between circulating TGF-β1 levels and testosterone in controls or women with PCOS. One possible explanation for this is that circulating TGF-β levels may not accurately reflect local TGF-β dysregulation. Indeed, the effects of TGF-β are more likely local, rather than systemic. A recent microarray study of granulosa cells from women with PCOS and controls found differential expression of genes involved in TGF-β signaling (insulin-like growth factor 2 receptor [IGF2R] increase and hyaluronan synthase 2 [HAS2] decreased).74
Figure 2.
Correlation between circulating levels of TGF-β1 and TGF-β2 and testosterone levels in women with and without polycystic ovary syndrome (PCOS; Panels A-D).
There is likely an association between ovarian size and morphology and ovarian hyperandrogenism and anovulation. This is reflected in the ultrasound diagnostic criteria for PCOS75; and even within PCOS, a larger ovary may be associated with a more severe reproductive phenotype.76 The mechanism between the ovarian composition and the reproductive pathology may be biomechanical, in which the turgor of the ovary due to excessive stroma/connective tissue is so great that no follicle can expand to develop into a dominant follicle.77 Further, the continued proliferation and/or presence of preantral follicles that are primarily androgenic leads to a vicious circle of hyperandrogenic anovulation.
There are several lines of evidence that suggest this biomechanical mechanism plays a significant role in PCOS. The first goes back to the proposed primary surgical treatment of the ovary in PCOS by a wedge resection as pioneered by Stein and Leventhal in their original description of the syndrome.60 Many women subsequently ovulated and conceived. This may be due to a primary decompression of the ovary, through a removal of tunica and stroma, allowing normal expansion of follicles and the opportunity to ovulate. The fact that lesser degrees of ovarian reduction, such as that achieved by bilateral or even unilateral ovarian diathermy78,79 or by transvaginal laser ablation of the ovaries,80 result in similar restoration of ovulation suggests a common mechanism. Further, the imputed direct correlation between the degree of ovarian reduction and spontaneous ovulation, that is, the more ovary removed, the longer ovulation persists, suggests a dose–response relationship.81 Aging is associated with a shrinkage of the ovary, a loss of preantral follicles, a drop in circulating AMH levels, and, in women with PCOS, an increased rate of ovulation.82–84
The mechanism behind the benefits of ovarian destruction/reduction on ovulation is not understood. There does appear to be an immediate decline in circulating androgen levels,85 much has been noted after bilateral oophorectomy.86 Further, in most studies there appears to be minimal impact on insulin sensitivity and metabolic parameters through ovarian diathermy.87 Although insulin-sensitizing agents do improve insulin sensitivity and ovulatory frequency,88,89 the 2 processes may be unrelated. A recent study of rosiglitazone, in a rat model of androgen-induced polycystic ovaries, found that this agent lowered TGF-β and connective tissue growth factor (CTGF) levels in the ovary and circulation of the rats.90
A second line of evidence supporting a primary relationship between the composition of the ovary is the effects of the surrounding matrix in studies of in vitro follicle maturation. When the matrix surrounding the follicle is too rigid, a PCOS-like preantral follicle develops with the follicular environment, mimicking the hyperandrogenic milieu of a PCOS preantral follicle.91 When these in vitro follicles are cultured in a less rigid alginate matrix, they are more likely to grow rapidly, develop an antrum, have a normal follicular fluid steroidal milieu, and yield developmentally competent oocytes.92
Finally, there are multiple other related phenotypic characteristics in women with PCOS, which suggest a hyperproliferation of connective tissue. These include the development of acanthosis nigricans, the development of abdominal striae, and the association of PCOS with nonalchoholic fatty liver disease (NAFLD), ranging from development of steatosis, to steatohepatitis, and finally fibrosis with subsequent cirrhosis steatohepatosis (NASH),93 though these latter stages have been rarely reported in women with PCOS. Although insulin resistance/hyperinsulinemia is often implicated in the etiology of these disorders, TGF-β dysregulation may also play a role.
There are other human diseases in which specific organs are plagued by fibrosis, such as idiopathic pulmonary fibrosis, which have not been linked to women with PCOS. Fibrogenic growth factors, including TGF-β dysregulation, have been implicated in the development of fibrosis in this disorder.94 Because these factors and/or their pathways signal through tyrosine kinase receptors, treatment with tyrosine kinase inhibitors has been proposed as a potential treatment.95,96 Although the results to date have been mainly disappointing because of little benefit and elevated risk of adverse events in response to treatment, there is at least one study with a proof of concept decrease in lung rigidity.97 Nonetheless, the promise of developing agents with more specific inhibition of fibrogenesis, without associated effects on immune and inflammatory response holds not only for primary fibrosing conditions but also for the treatment of hyperandrogenism and anovulation in women with PCOS.
In summary, it is hypothesized that in PCOS, alterations in local TGF-β signaling within the ovary lead to structural changes (eg, increased ovarian stroma and tunica characteristic of PCOS) that promote functional changes (eg, increased production of androgens by the stromal tissue of the theca interna that surrounds each antral follicle and inability of a follicle to expand and develop into a dominant follicle due to the excessive stroma and turgor of the ovary), resulting in the PCOS reproductive phenotype of chronic hyperandrogenic anovulation. As the TGF-β signaling system is a complex multicomponent system regulated by multiple factors, there are many potential causes of dysregulated TGF-β signaling in PCOS, including variants in fibrillin 3 such as A8 of D19S884. Regarding the question, is one of the TGF-β family cytokines elevated specifically in the PCOS stroma, this is yet to be studied and is an important area for future research. Additionally, as many members of the TGF-ß superfamily (eg, BMPs, AMH, activins, inhibins and GDFs) are involved in the regulation of folliculogenesis, it is likely that some of these TGF-β-related cytokines could also be involved in TGF-β signaling dysregulation in PCOS.
Role of TGF-β in Cardiovascular and Metabolic Abnormalities in PCOS
Variations in fibrillin 3 and subsequent dysregulation of TGF-β may contribute to the pathogenesis of cardiovascular and metabolic abnormalities in PCOS. The fibrillin 3 gene encodes an ECM protein that is homologous to the more extensively studied fibrillin 1.98 Mutations in fibrillin 1 result in Marfan syndrome, an autosomal dominant systemic connective tissue disorder that is associated with an increased incidence of aortic root aneurysm and subsequent life-threatening aortic dissection.18,99 Excessive activation of TGF-β has been implicated in the pathogenesis of Marfan syndrome. Increased TGF-β activity has been demonstrated in the cardiovascular tissues, lung, and dura of mice with abnormal fibrillin 1.100–103 In these mouse models of Marfan syndrome, blocking TGF-β activity with neutralizing antibodies or the angiotensin receptor blocker, losartan, normalizes lung development and prevents cardiac valvular disease. Therefore, TGF-β dysregulation may also be an important therapeutic target for reducing vascular disease in women with PCOS.
Targeting TGF-β dysregulation may be particularly important in PCOS as women with PCOS are at increased risk of impaired glucose tolerance and type 2 diabetes due to underlying insulin resistance. Excessive TGF-β activity has been implicated in the pathogenesis of arterial disease in patients with altered glucose metabolism.104 Cells in the vessel wall express the isoforms TGF-β1, TGF-β2, and TGF-β3, which regulate cell differentiation, cell proliferation, cell migration, production of the ECM, and immune cell functions. As a potent regulator of vascular cell responses, TGF-β plays an important role in atherosclerosis and vascular remodeling.105 Dysregulated TGF-β activity may contribute to atherosclerosis by stimulating smooth muscle cells in the vasculature to proliferate and synthesize collagen. TGF-β also regulates endothelial cells, macrophages, and T cell responses in the vasculature. In endothelial cells, TGF-β regulates the expression of genes that promote inflammation, such as interleukin 6. TGF-β may also have a direct etiologic role in hypertension, as TGF-β1 stimulates the synthesis of endothelin 1, a potent vasoconstrictor that plays an important role in the regulation of vascular tone. TGF-β also reduces stimulation of nitric oxide (NO) synthase expression by cytokines and promotes the degradation of NO synthase.105
Finally, TGF-ß1 stimulates renin release from the renal juxtaglomerular cells, activating the renin–angiotensin–aldosterone system (RAAS) and increasing the production of angiotensin II that further stimulates TGF-β1 expression.106 Angiotensin II also induces endothelial dysfunction, which gradually leads to overt atherosclerosis by promoting altered vasoreactivity, hypercoagulability, and increased infiltration of inflammatory cells into the vessel. Activation of RAAS induces a proinflammatory and fibrogenic state that affects vascular tone and structure and leads to vascular and myocardial fibrosis, reduced arterial compliance, impaired cardiac remodeling, and perivascular inflammation.107,108 This is relevant for PCOS as one study showed that aldosterone levels are significantly greater in women with PCOS compared to healthy women.109 In the same study, aldosterone levels correlated with C-reactive protein, a measure of inflammation, and intima media thickness, an early marker of atherosclerosis. Furthermore, we showed that women with PCOS who have A8 of D19S884 in the fibrillin 3 gene have higher aldosterone levels than women with PCOS who do not have A8,73 and A8 is associated with higher levels of fasting insulin and homeostasis model assessment for insulin resistance in women with PCOS.33 Therefore, women with PCOS, especially those with altered fibrillin 3, may be particularly susceptible to metabolic and cardiovascular complications due to TGF-β dysregulation.
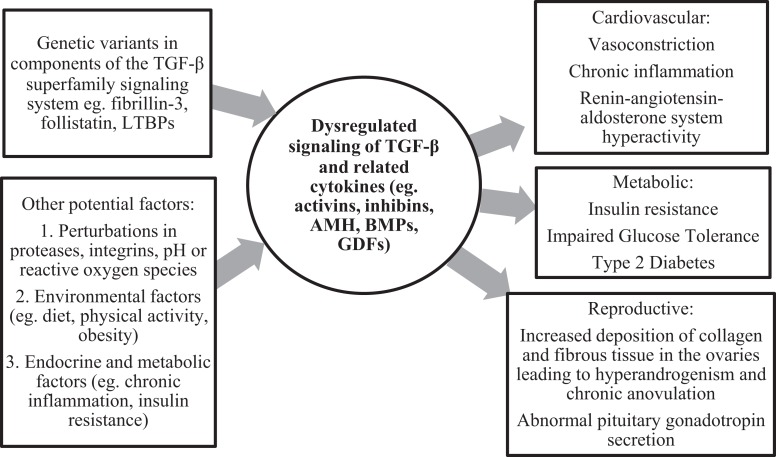
The mechanisms regulating TGF-β signaling are complex and incompletely understood. Although fibrillin 3 is minimally expressed in the adult ovary, and there appears to be no difference in fibrillin expression in PCO versus non-PCO ovaries, it is biologically plausible that altered fibrillin 3 expression in other adult tissues, such as the pituitary, contributes to dysregulated TGF-β signaling in adult women with PCOS.38 It is also possible that the fibrillin 3 gene variant associated with PCOS causes functional changes in the structure of fibrillin 3, without affecting its expression. In addition, as the TGF-β-signaling system is a complex multicomponent system regulated by multiple factors, there are several other things that could be driving dysregulated TGF-β family and superfamily signaling in adult women with PCOS, such as abnormalities in follistatin and other components of the TGF-β signaling system such as LTBPs. Proteases, integrins, pH, and reactive oxygen species have also been shown to activate TGF-β.110–112 Indeed, perturbations of these factors can lead to dysregulated TGF-β signaling, resulting in several complications including inflammation and fibrosis.113,114 Other factors, such as environmental, endocrine, and metabolic factors, may also play a role in dysregulated TGF-β signaling in PCOS (Figure 3).
Figure 3.
Proposed mechanisms for TGF-β-mediated cardiovascular, metabolic, and reproductive disease in polycystic ovary syndrome (PCOS).
Conclusions
There is increasing evidence that the TGF-β-signaling pathway plays a critical role in the development of PCOS. In recent years, many have hypothesized that the increased stromal tissues observed in PCOS ovaries are due to TGF-β dysregulation, resulting in increased production and deposition of collagen. Indeed, TGF-β dysregulation is implicated in the fibrosis of many organs and tissues. Genetic studies have linked PCOS to a dinucleotide marker D19S884 in the gene for fibrillin 3, which regulates TGF-β activity. Fibrillin 3 has been recently shown to be expressed in the developing stroma of human and bovine fetal ovaries, further supporting the role of TGF-β in the fetal origins of PCOS. Additionally, TGF-β dysregulation may also contribute to cardiovascular and metabolic abnormalities in PCOS, as an intact TGF-β-signaling pathway is critical for the normal development and function of multiple organs and tissues. Although these animal studies and genetic studies strongly suggest that dysregulated TGF-β signaling and subsequent alterations in the ECM may contribute to the pathogenesis of PCOS, there is a lack of clear evidence for actual changes in TGF-β signaling in the ovaries of patients with PCOS. Thus, future research is needed to characterize TGF-β signaling in the ovaries of patients with PCOS. In conclusion, the TGF-β regulatory pathway appears to play a critical role in the development of PCOS and may be an important therapeutic target for PCOS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Raja-Khan N, Legro RS. Diagnosis and management of polycystic ovary syndrome. J Clin Outcomes Manage. 2005;12(4):218–227. [Google Scholar]
- 2. Urbanek M, Legro RS, Driscoll DA, et al. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96(15):8573–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urbanek M, Woodroffe A, Ewens KG, et al. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90(12):6623–6629. [DOI] [PubMed] [Google Scholar]
- 4. Stewart DR, Dombroski BA, Urbanek M, et al. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91(10):4112–4117. [DOI] [PubMed] [Google Scholar]
- 5. Fornoni A, Lenz O, Striker LJ, Striker GE. Glucose induces clonal selection and reversible dinucleotide repeat expansion in mesangial cells isolated from glomerulosclerosis-prone mice. Diabetes. 2003;52(10):2594–2602. [DOI] [PubMed] [Google Scholar]
- 6. Ferrand PE, Parry S, Sammel M, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8(5):494–501. [DOI] [PubMed] [Google Scholar]
- 7. Fenech AG, Billington CK, Swan C, et al. Novel polymorphisms influencing transcription of the human CHRM2 gene in airway smooth muscle. Am J Respir Cell Mol Biol. 2004;30(5):678–686. [DOI] [PubMed] [Google Scholar]
- 8. Dolan-O'Keefe M, Chow V, Monnier J, Visner GA, Nick HS. Transcriptional regulation and structural organization of the human cytosolic phospholipase A(2) gene. Am J Physiol Lung Cell Mol Physiol. 2000;278(4):L649–657. [DOI] [PubMed] [Google Scholar]
- 9. Huang X, Vaag A, Carlsson E, Hansson M, Ahren B, Groop L. Impaired cathepsin L gene expression in skeletal muscle is associated with type 2 diabetes. Diabetes. 2003;52(9):2411–2418. [DOI] [PubMed] [Google Scholar]
- 10. Hata R, Akai J, Kimura A, Ishikawa O, Kuwana M, Shinkai H. Association of functional microsatellites in the human type I collagen alpha2 chain (COL1A2) gene with systemic sclerosis. Biochem Biophys Res Commun. 2000;272(1):36–40. [DOI] [PubMed] [Google Scholar]
- 11. Rothenburg S, Koch-Nolte F, Rich A, Haag F. A polymorphic dinucleotide repeat in the rat nucleolin gene forms Z-DNA and inhibits promoter activity. Proc Natl Acad Sci U S A. 2001;98(16):8985–8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gebhardt F, Zanker KS, Brandt B. Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem. 1999;274(19):13176–13180. [DOI] [PubMed] [Google Scholar]
- 13. Gabellini N. A polymorphic GT repeat from the human cardiac Na+Ca2+ exchanger intron 2 activates splicing. Eur J Biochem. 2001;268(4):1076–1083. [DOI] [PubMed] [Google Scholar]
- 14. Hui J, Stangl K, Lane WS, Bindereif A. HnRNP L stimulates splicing of the eNOS gene by binding to variable-length CA repeats. Nat Struct Biol. 2003;10(1):33–37. [DOI] [PubMed] [Google Scholar]
- 15. Hui J, Reither G, Bindereif A. Novel functional role of CA repeats and hnRNP L in RNA stability. RNA. 2003;9(8):931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stangl K, Cascorbi I, Laule M, et al. High CA repeat numbers in intron 13 of the endothelial nitric oxide synthase gene and increased risk of coronary artery disease. Pharmacogenetics. 2000;10(2):133–140. [DOI] [PubMed] [Google Scholar]
- 17. Gelb BD. Marfan's syndrome and related disorders--more tightly connected than we thought. N Engl J Med. 2006;355(8):841–844. [DOI] [PubMed] [Google Scholar]
- 18. Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366(9501):1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010;58(10):903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–178. [DOI] [PubMed] [Google Scholar]
- 21. Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98(2):257–265. [DOI] [PubMed] [Google Scholar]
- 22. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. [DOI] [PubMed] [Google Scholar]
- 23. Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J. Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev. 1999;10(2):99–117. [DOI] [PubMed] [Google Scholar]
- 24. Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab. 2005;90(10):5582–5587. [DOI] [PubMed] [Google Scholar]
- 25. Fleming R, Harborne L, MacLaughlin DT, et al. Metformin reduces serum mullerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fertil Steril. 2005;83(1):130–136. [DOI] [PubMed] [Google Scholar]
- 26. Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homburg R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16(12):2552–2556. [DOI] [PubMed] [Google Scholar]
- 27. Kevenaar ME, Laven JS, Fong SL, et al. A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2008;93(4):1310–1316. [DOI] [PubMed] [Google Scholar]
- 28. Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146(4):1883–1892. [DOI] [PubMed] [Google Scholar]
- 29. Ingman WV, Robker RL, Woittiez K, Robertson SA. Null mutation in transforming growth factor beta1 disrupts ovarian function and causes oocyte incompetence and early embryo arrest. Endocrinology. 2006;147(2):835–845. [DOI] [PubMed] [Google Scholar]
- 30. Urbanek M, Wu X, Vickery KR, et al. Allelic variants of the follistatin gene in polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85(12):4455–4461. [DOI] [PubMed] [Google Scholar]
- 31. Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37(2):59–77. [DOI] [PubMed] [Google Scholar]
- 32. Urbanek M. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3(2):103–111. [DOI] [PubMed] [Google Scholar]
- 33. Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007;92(11):4191–4198. [DOI] [PubMed] [Google Scholar]
- 34. Yalamanchi SK, Sam S, Cardenas MO, Holaday LW, Urbanek M, Dunaif A. Association of fibrillin-3 and transcription factor-7-like 2 gene variants with metabolic phenotypes in PCOS. Obesity (Silver Spring). 2012;20(6):1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16(4):668–672. [DOI] [PubMed] [Google Scholar]
- 36. Villuendas G, Escobar-Morreale HF, Tosi F, Sancho J, Moghetti P, San Millan JL. Association between the D19S884 marker at the insulin receptor gene locus and polycystic ovary syndrome. Fertil Steril. 2003;79(1):219–220. [DOI] [PubMed] [Google Scholar]
- 37. Tucci S, Futterweit W, Concepcion ES, et al. Evidence for association of polycystic ovary syndrome in caucasian women with a marker at the insulin receptor gene locus. J Clin Endocrinol Metab. 2001;86(1):446–449. [DOI] [PubMed] [Google Scholar]
- 38. Prodoehl MJ, Hatzirodos N, Irving-Rodgers HF, et al. Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod. 2009;15(12):829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang X, Vaag A, Carlsson E, Hansson M, Ahren B, Groop L. Impaired cathepsin L gene expression in skeletal muscle is associated with type 2 diabetes. Diabetes. 2003;52(9):2411–2418. [DOI] [PubMed] [Google Scholar]
- 40. Liao WX, Roy AC, Ng SC. Preliminary investigation of follistatin gene mutations in women with polycystic ovary syndrome. Mol Hum Reprod. 2000;6(7):587–590. [DOI] [PubMed] [Google Scholar]
- 41. Jones MR, Wilson SG, Mullin BH, Mead R, Watts GF, Stuckey BG. Polymorphism of the follistatin gene in polycystic ovary syndrome. Mol Hum Reprod. 2007;13(4):237–241. [DOI] [PubMed] [Google Scholar]
- 42. Sproul K, Jones MR, Mathur R, Azziz R, Goodarzi MO. Association study of four key folliculogenesis genes in polycystic ovary syndrome. BJOG. 2010;117(6):756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kevenaar ME, Themmen AP, van Kerkwijk AJ, et al. Variants in the ACVR1 gene are associated with AMH levels in women with polycystic ovary syndrome. Hum Reprod. 2009;24(1):241–249. [DOI] [PubMed] [Google Scholar]
- 44. Chen ZJ, Zhao H, He L, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–59. [DOI] [PubMed] [Google Scholar]
- 45. Prodoehl MJ, Irving-Rodgers HF, Bonner WM, et al. Fibrillins and latent TGFbeta binding proteins in bovine ovaries of offspring following high or low protein diets during pregnancy of dams. Mol Cell Endocrinol. 2009;307(1-2):133–141. [DOI] [PubMed] [Google Scholar]
- 46. Hatzirodos N, Bayne RA, Irving-Rodgers HF, et al. Linkage of regulators of TGF-beta activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25(7):2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramirez F, Pereira L. The fibrillins. Int J Biochem Cell Biol. 1999;31(2):255–259. [DOI] [PubMed] [Google Scholar]
- 48. Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115(pt 14):2817–2828. [DOI] [PubMed] [Google Scholar]
- 49. Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J Cell Biochem. 2012;113(2):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ono RN, Sengle G, Charbonneau NL, et al. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem. 2009;284(25):16872–16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. LeClair R, Lindner V. The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor beta signaling. Trends Cardiovasc Med. 2007;17(6):202–205. [DOI] [PubMed] [Google Scholar]
- 52. Christner PJ, Ayitey S. Extracellular matrix containing mutated fibrillin-1 (Fbn1) down regulates Col1a1, Col1a2, Col3a1, Col5a1, and Col5a2 mRNA levels in Tsk/+ and Tsk/Tsk embryonic fibroblasts. Amino Acids. 2006;30(4):445–451. [DOI] [PubMed] [Google Scholar]
- 53. Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004;16(8):873–880. [DOI] [PubMed] [Google Scholar]
- 54. Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chapman HA. Disorders of lung matrix remodeling. J Clin Invest. 2004;113(2):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183(4):225–237. [DOI] [PubMed] [Google Scholar]
- 57. Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22(suppl 1):S73–S78. [DOI] [PubMed] [Google Scholar]
- 58. Prud'homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87(11):1077–1091. [DOI] [PubMed] [Google Scholar]
- 59. Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27(3):309–320. [DOI] [PubMed] [Google Scholar]
- 60. Stein IF, Leventhal ML. Amenorhea associated with bilateral polcystic ovaries. American Journal of Obstetrics and Gynaecology. 1935;29:181–191. [Google Scholar]
- 61. Nelson VL, Legro RS, Strauss JF, 3rd , McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–957. [DOI] [PubMed] [Google Scholar]
- 62. Nelson VL, Qin KN, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(12):5925–5933. [DOI] [PubMed] [Google Scholar]
- 63. Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78(4):636–647. [DOI] [PubMed] [Google Scholar]
- 64. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 65. Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. [DOI] [PubMed] [Google Scholar]
- 66. Forsdike RA, Hardy K, Bull L, et al. Disordered follicle development in ovaries of prenatally androgenized ewes. J Endocrinol. 2007;192(2):421–428. [DOI] [PubMed] [Google Scholar]
- 67. Hague WM, Adams J, Rodda C, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf). 1990;33(4):501–510. [DOI] [PubMed] [Google Scholar]
- 68. Barnes RB, Rosenfield RL, Ehrmann DA, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79(5):1328–1333. [DOI] [PubMed] [Google Scholar]
- 69. Davies MJ, March WA, Willson KJ, Giles LC, Moore VM. Birthweight and thinness at birth independently predict symptoms of polycystic ovary syndrome in adulthood. Hum Reprod. 2012;27(5):1475–1480. [DOI] [PubMed] [Google Scholar]
- 70. Xu N, Kwon S, Abbott DH, et al. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One. 2011;6(11):e27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sabatier L, Miosge N, Hubmacher D, Lin G, Davis EC, Reinhardt DP. Fibrillin-3 expression in human development. Matrix Biol. 2011;30(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Raja-Khan N, Kunselman AR, Demers LM, Ewens KG, Spielman RS, Legro RS. A variant in the fibrillin-3 gene is associated with TGF-beta and inhibin B levels in women with polycystic ovary syndrome. Fertil Steril. 2010;94(7):2916–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaur S, Archer KJ, Devi MG, Kriplani A, Strauss JF, 3rd , Singh R. Differential gene expression in granulosa cells from polycystic ovary syndrome patients with and without insulin resistance: identification of susceptibility gene sets through network analysis. J Clin Endocrinol Metab. 2012;97(10):E2016–E2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9(6):505–514. [DOI] [PubMed] [Google Scholar]
- 76. Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A. Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab. 2005;90(5):2571–2579. [DOI] [PubMed] [Google Scholar]
- 77. Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28(1):3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Farquhar C, Lilford RJ, Marjoribanks J, Vandekerckhove P. Laparoscopic “drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2005;(3):CD001122. [DOI] [PubMed] [Google Scholar]
- 79. Balen AH, Jacobs HS. A prospective study comparing unilateral and bilateral laparoscopic ovarian diathermy in women with the polycystic ovary syndrome [see comments]. Fertil Steril. 1994;62(5):921–925. [DOI] [PubMed] [Google Scholar]
- 80. Zhu W, Fu Z, Chen X, et al. Transvaginal ultrasound-guided ovarian interstitial laser treatment in anovulatory women with polycystic ovary syndrome: a randomized clinical trial on the effect of laser dose used on the outcome. Fertil Steril. 2010;94(1):268–275. [DOI] [PubMed] [Google Scholar]
- 81. Donesky BW, Adashi EY. Surgically induced ovulation in the polycystic ovary syndrome: wedge resection revisited in the age of laparoscopy. Fertil Steril. 1995;63(3):439–463. [DOI] [PubMed] [Google Scholar]
- 82. Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod. 2000;15(1):24–28. [DOI] [PubMed] [Google Scholar]
- 83. Elting MW, Kwee J, Korsen TJ, Rekers-Mombarg LT, Schoemaker J. Aging women with polycystic ovary syndrome who achieve regular menstrual cycles have a smaller follicle cohort than those who continue to have irregular cycles. Fertil Steril. 2003;79(5):1154–1160. [DOI] [PubMed] [Google Scholar]
- 84. Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94(12):4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Armar NA, McGarrigle HH, Honour J, Holownia P, Jacobs HS, Lachelin GC. Laparoscopic ovarian diathermy in the management of anovulatory infertility in women with polycystic ovaries: endocrine changes and clinical outcome. Fertil Steril. 1990;53(1):45–49. [DOI] [PubMed] [Google Scholar]
- 86. Chang RJ, Laufer LR, Meldrum DR, et al. Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab. 1983;56(5):897–903. [DOI] [PubMed] [Google Scholar]
- 87. Lemieux S, Lewis GF, Ben-Chetrit A, Steiner G, Greenblatt EM. Correction of hyperandrogenemia by laparoscopic ovarian cautery in women with polycystic ovarian syndrome is not accompanied by improved insulin sensitivity or lipid-lipoprotein levels. J Clin Endocrinol Metab. 1999;84(11):4278–4282. [DOI] [PubMed] [Google Scholar]
- 88. Azziz R, Ehrmann D, Legro RS, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86(4):1626–1632. [DOI] [PubMed] [Google Scholar]
- 89. Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2010;(1):CD003053. [DOI] [PubMed] [Google Scholar]
- 90. Miao ZL, Guo L, Wang YX, et al. The intervention effect of Rosiglitozone in ovarian fibrosis of PCOS rats. Biomed Environ Sci. 2012;25(1):46–52. [DOI] [PubMed] [Google Scholar]
- 91. West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75(6):916–923. [DOI] [PubMed] [Google Scholar]
- 93. Baranova A, Tran TP, Birerdinc A, Younossi ZM. Systematic review: association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33(7):801–814. [DOI] [PubMed] [Google Scholar]
- 94. Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9(3):111–116. [DOI] [PubMed] [Google Scholar]
- 95. Antoniu SA, Kolb MR. Intedanib, a triple kinase inhibitor of VEGFR, FGFR and PDGFR for the treatment of cancer and idiopathic pulmonary fibrosis. IDrugs. 2010;13(5):332–345. [PubMed] [Google Scholar]
- 96. Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181(6):604–610. [DOI] [PubMed] [Google Scholar]
- 97. Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–1087. [DOI] [PubMed] [Google Scholar]
- 98. Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83(3):461–472. [DOI] [PubMed] [Google Scholar]
- 99. Dietz HC, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of Marfan syndrome. Am J Med Genet C Semin Med Genet. 2005;139C(1):4–9. [DOI] [PubMed] [Google Scholar]
- 100. Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33(3):407–411. [DOI] [PubMed] [Google Scholar]
- 101. Jones KB, Myers L, Judge DP, Kirby PA, Dietz HC, Sponseller PD. Toward an understanding of dural ectasia: a light microscopy study in a murine model of Marfan syndrome. Spine (Phila Pa 1976). 2005;30(3):291–293. [DOI] [PubMed] [Google Scholar]
- 102. Ng CM, Cheng A, Myers LA, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114(11):1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Coucke PJ, Willaert A, Wessels MW, et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38(4):452–457. [DOI] [PubMed] [Google Scholar]
- 105. Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26(8):1712–1720. [DOI] [PubMed] [Google Scholar]
- 106. Suthanthiran M, Li B, Song JO, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: a novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci U S A. 2000;97(7):3479–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rocha R, Stier CT., Jr Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001;12(7):308–314. [DOI] [PubMed] [Google Scholar]
- 108. Williams JS, Williams GH. 50th anniversary of aldosterone. J Clin Endocrinol Metab. 2003;88(6):2364–2372. [DOI] [PubMed] [Google Scholar]
- 109. Cascella T, Palomba S, Tauchmanova L, et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2006;91(11):4395–4400. [DOI] [PubMed] [Google Scholar]
- 110. Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. [DOI] [PubMed] [Google Scholar]
- 111. Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10(9):1077–1083. [DOI] [PubMed] [Google Scholar]
- 112. Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87(8-9):601–615. [DOI] [PubMed] [Google Scholar]
- 113. Yu Q, Stamenkovic I. Cell surface-localized matrix metall-oproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- 114. Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124(1-2):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]