Abstract
Lysyl oxidases (LOXs) are enzymes involved in collagen deposition, extracellular membrane remodeling, and invasive/metastatic potential. Previous studies reveal an association of LOXs and endometriosis. We aimed to identify the mechanisms activated by upregulation of lysyl oxidases (LOX) in endometriotic cells and tissues. We hypothesized that LOX plays a role in endometriosis by promoting invasiveness and epithelial to mesenchymal transition (EMT).
Methods:
The LOX protein expression levels were measured by immunohistochemistry in lesions and endometrium on a tissue microarray (TMA) and in endometrial biopsies from patients and controls during the window of implantation (WOI). Estradiol regulation of LOX expression was determined by quantitative polymerase chain reaction (qPCR). Proliferation, invasion, and migration assays were performed in epithelial (endometrial epithelial cell), endometrial (human endometrial stromal cell), and endometriotic cell lines (ECL and 12Z). Pathway-focused multiplex qPCR was used to determine transcriptome changes due to LOX overexpression.
Results:
LOX protein was differentially expressed in ovarian versus peritoneal lesions. During WOI, LOX levels were higher in luminal epithelium of patients with endometriosis-associated infertility compared to controls. Invasive epithelial cell lines expressed higher levels of LOX than noninvasive ones. Transfection of LOX into noninvasive epithelial cells increased their migration in an LOX inhibitor-sensitive manner. Overexpression of LOX did not fully induce EMT but the expression of genes related to fibrosis and extracellular matrix remodeling were dysregulated.
Conclusions:
This study documents that expression of LOX is differentially regulated in endometriotic lesions and endometrium. A role for LOX in mediating proliferation, migration, and invasion of endometrial and endometriotic cells was observed, which may be implicated in the establishment and progression of endometriotic lesions.
Keywords: endometriosis, endometrium, lysyl oxidase, infertility, epithelial to mesenchymal transition
Introduction
Endometriosis is characterized by growth of endometrial tissue outside the uterine cavity, causing severe pelvic pain and, often, infertility, defined as diminished ability or inability to conceive.1 Preliminary studies in our laboratory and others independently point to a possible role of lysyl oxidases (LOXs) in endometriosis. The LOX gene expression was shown to be significantly increased in endometriosis-like lesions in animal models of the disease2,3 and in human endometriotic lesions compared to the normal endometrium of women with endometriosis.4,5 Lysyl oxidases are a family of copper-dependent extracellular amine oxidases composed of 5 isoforms (LOX and LOX-like LOXL1-4) encoded by 5 different genes. These isoforms are 70% homologous at the protein level and share a conserved C-terminal domain.6 In addition to a role in collagen and elastin cross-linking and in stabilization and stiffness of the extracellular matrix, LOXs have been ascribed roles in cell growth regulation, differentiation, and cell migration of cancer as well as in normal cells.7–10 Expression of LOXs has been correlated with in vitro and in vivo tumor invasion capacity and with the metastatic phenotype of cancer cell lines.11–17 The LOX family members cooperate with the transcription factor SNAIL to downregulate expression of E-cadherin thus promoting epithelial to mesenchymal transition (EMT), a molecular process that underlies fibrotic and metastatic cellular phenotypes.18,19 Interestingly, LOXL1, a LOX family member, is one of the most downregulated genes in the endometrium during the window of implantation (WOI).20,21 Also, LOXL4 is located in 10q23.3, near a region significantly linked to endometriosis,22 and recently, single-nucleotide polymorphisms in this gene were found to be associated with endometriosis and infertility.23
It has been estimated that up to 50% of patients with endometriosis will have fertility problems.24 The pathogenesis of endometriosis-associated infertility remains an unsolved issue in reproductive medicine, although a number of molecular pathways involving defects in the functional interaction of integrin-extracellular membrane ECM ligands (eg, fibronectin, collagen IV, and collagen I) have been implicated.25–27 Studies of the underlying mechanisms involved in endometriosis and endometriosis-associated infertility would offer the possibility of identifying specific biomarkers and new treatment modalities for both conditions. Although neither endometriosis nor infertility is a life-threatening disease, living with chronic, severe physical pain and/or the emotional distress of failed motherhood is known to inflict substantial negative impact in the quality of life of millions of women around the world.28–31
This study was conducted to investigate the expression levels of LOX in endometriotic cell lines and in tissues (lesions and eutopic endometrium from patients and controls) as well as during WOI in endometrial biopsies obtained from women with endometriosis-associated infertility and fertile controls. We hypothesized that upregulation of LOX levels can lead to aberrant proliferative, migratory, and invasive behaviors, and cause the dysregulation of ECM remodeling changes in endometrium. We report herein that LOX is aberrantly expressed in ectopic and eutopic endometrium from patients with endometriosis and that overexpression increased the proliferative and invasive phenotype of epithelial cells in vitro; however, LOX expression alone was not able to fully induce EMT. These findings may help advance the current understanding of the mechanisms at play in endometriosis, infertility, and other menstrual disorders.
Materials and Methods
All protocols involving tissue collection were approved by the institutional review board (IRB) Committee of Ponce Health Sciences University—School of Medicine (PHSU).
Endometriosis Tissue Microarray
Deidentified formalin-fixed paraffin-embedded (FFPE) endometrium and endometriosis tissues were obtained from a collaborating pathology laboratory after protocol approval by the PHSU IRB Committee. Average age of patients and controls represented in the TMA was 35 and 42 years old, respectively. All biopsies were evaluated by a pathologist (MG) who confirmed diagnosis and stage of the menstrual cycle of eutopic endometrial samples using Noyes criteria.32 A total of 164 core biopsies obtained from 83 tissue blocks were used to construct a TMA at the Moffitt Cancer Center Pathology Department. From most blocks, 2 different core biopsies were included in the TMA. The total number of cores on the TMA was 29 ovarian endometriosis, 16 fallopian tube endometriosis, 34 peritoneal endometriosis, 4 skin (umbilical) endometriosis, 7 gastrointestinal (GI), 22 eutopic proliferative endometrium of patients with endometriosis (EE), 14 control proliferative phase endometrium (PE), and 38 control secretory phase endometrium (SE). Control endometria were obtained from women with uterine myomas, abnormal uterine bleeding, or enlarged/prolapsed uterus.
Immunohistochemistry Analysis of LOX Protein Expression in Endometrium and Endometriotic Samples on a TMA
Ten-μm sections of the TMA block were cut for immunostaining. The endometriotic and eutopic endometrial tissue sections on the TMA were stained using the LSAB+ Kit (Dako, Carpinteria, California) following the manufacturer’s recommendations and as previously described.33 Briefly, after dewaxing and rehydration, antigen retrieval and removal of endogenous peroxidase activity were conducted using standard methods. A TMA section was incubated for 1 hour at room temperature (RT) with anti-human LOX antibody (1:800 dilution, Novus Biologicals, Littleton, Colorado, cat# NB100-2530), followed by 30-minute incubation at RT with the secondary biotinylated antibody. After development with diaminobenzidine, the slide was counterstained with hematoxylin. Positive controls were sections of tonsils. The slide was scanned to produce a virtual image using the VENTANA Virtuoso image and workflow management software (Ventana Medical Systems, Inc; Tucson, Arizona). Immunostaining intensity was blindly scored by 2 investigators in nuclei of epithelium, glands, and stroma using a 0 to 3 score system: 0 = no staining, 1 = weak, 2 = moderate, and 3 = strong staining.
Immunohistochemistry of LOX in Endometrial Biopsies During WOI
Deidentified FFPE endometrial biopsies of women were obtained by reproductive endocrinologists (PB and NB) during mid-secretory phase (postovulatory day +7 to +10), that is during WOI, as part of the infertility workup of the patients at the infertility clinics. We included in our analysis women younger than 41 years of age with regular menstrual cycles, with a surgical diagnosis of endometriosis (n = 5; group 1), and fertile women who were undergoing infertility treatment due to tubal ligation or male factor infertility (n = 6; group 2 —controls). Endometrial tissue was obtained during office by curettage with a Pipelle curette following standard procedures, fixed with 10% formalin, then dehydrated and embedded in paraffin. Only biopsies that contained stroma, glands, and epithelium were analyzed. Samples were dated for endometrial development by a pathologist according to Noyes criteria32 to exclude those that were not in the secretory phase. The immunolocalization of LOX protein was conducted using anti-LOX antibody (1:800 dilution) previously described on the TMA methodology.
Cell Culture
The stromal endometriotic cell line ECL (Hs832cT; CRL-7566) was obtained from American Type Culture Collection (ATCC; Manassas, Virginia) and cultured following their recommendations.34 We also tested expression of LOX in 2 primary stromal endometriotic cell lines (PEC and PED) isolated at our laboratory from 1 ovarian and 1 peritoneal lesion, respectively (Bello et al, unpublished data). The endometriotic epithelial cell line 12Z was obtained as part of a collaboration with Dr Fazleabas and Dr Starzinski.35 This cell line has been extensively characterized by Banu et al.36 As controls, we used the invasive human endometrial stromal cell (HESC) line (CRL-4003; ATCC), noninvasive epithelial cells (endometrial epithelial cells–EECs, which have recently been shown to have Michigan Cancer Foundation - 7 (MCF7) characteristics by DNA fingerprinting),37 HES (human EECs; shown to have HeLa characteristics by DNA fingerprinting analysis38), HeLa cells, human uterine fibroblast cells, and the highly invasive breast carcinoma cells Hs578T (ATCC). All cells were maintained at 37°C in a humidified incubator with 5% CO2 and cultured using recommended media as described previously.39,40 For the hormone experiments, 1 million cells were cultured in 6-well tissue culture dishes until 90% confluence at which time medium was replaced by fresh serum-deprived media (1% charcoal-dextran stripped fetal bovine serum). After 24 hours, cells were incubated with 10−8 mol/L estradiol (E2) in serum-deprived media for 24 hours. Control cells received media plus vehicle (0.1% ethanol). Cells were washed and trypsinized for RNA and protein isolation as described subsequently. All experiments were repeated 3 times.
Quantitative Polymerase Chain Reaction
Basal and E2-regulated LOX gene expression levels were measured using TaqMan Gene Expression Assays (LOX: Hs00184700_m1; Applied Biosystems, Foster City, California) at 24, 48, and 72 hours. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH: Hs99999905_m1) or ribosomal protein S17 (RPS17; Hs00734303_g1) was used as control for normalization purposes. Quantitative polymerase chain reaction (qPCR) reactions were performed in triplicate on an iCycler (Bio-Rad, Hercules, California). Gene expression levels were calculated according to the 2−ΔΔCt method as described by Livak and Schmittgen.41
Analysis of Estrogen Response Elements in LOX Genomic Sequence
The genomic sequences of LOX (NM_001178102) plus the 50kb upstream promoter sequence were downloaded from the UCSC genome Browser (genome.ucsc.edu, build hg19) and scanned for putative estrogen response elements (EREs) using the position weight matrix (PWM) constructed from 48 experimentally identified EREs (15 bp in length)42 and PWMs in the TRANFAC database, respectively. A subsequence was declared a putative binding site when the P value of its log-likelihood ratio score was ≤.0001.43 In addition, the 50kb upstream sequences were checked for EREs using publically available ChIP data sets.44,45
In Vitro Cell Proliferation Assays (5-Bromo-2′-deoxyuridine Incorporation Assay)
Proliferation rates of endometriotic, endometrial, and cancer cell lines were determined using the 5-bromo-2′-deoxyuridine (BrdU) cell proliferation enzyme-linked immunosorbent assay (ELISA) assay kit (Millipore, Billerica, Massachusetts) according to the manufacturer’s specifications. Briefly, HESC (20 000 cells/well), EEC (40,000 cells/well), and 12Z (20 000 cells/well) were cultured in complete media in triplicate. Following incubation in serum-deprived media for 24 hours at 37°C in 5% CO, BrdU labeling solution was added to cells alone and in combination with 10 μmol/L of LOX pan inhibitor β-aminopropionitrile (β-APN; Sigma Aldrich, St Louis, Missouri) and incubated for another 48 hours. After removal of the culture medium, the cells were fixed, permeabilized, and DNA denatured. Cell proliferation was assayed by quantification of BrdU that is incorporated into the newly synthesized DNA of replicating cells. The BrdU levels were measured in an ELISA plate reader (MRX Revelation, Dynex Technologies, Inc, Chantilly, Virginia) by colorimetric detection using anti-BrdU antibody followed by the mouse immunoglobulin G peroxidase conjugate. The signal was developed with tetramethylbenzidine solution in darkness. Spectrophotometric detection was performed at 450 nm with a reference wavelength at 595 nm. Experiments were performed in triplicate.
In Vitro Cell Migration Assay (Scratch Assay)
The basal migration capacity of endometriotic, endometrial, and cancer cell lines was measured by Scratch assays. The cells were seeded and cultured in 6-well plates until they reached ∼90% to 100% of confluence and then incubated 24 hours with deprived media as described.46 A scratch was made in the middle of the well using a 100 μL sterile pipette tip, and cells were further grown in deprived media for an additional 72 hours. Photos were taken at different time points (0, 8, 24, 48, and 72 hours) after the initial scratch was made (Olympus Light Microscope, 4-100X, Olympus, Center Valley, Pennsylvania). Measurements of the area without cell growth were taken using TScratch software (Computational Science and Engineering Laboratory, Zurich, Switzerland).47 Percentage of invasion was determined by dividing the average area of the scratch measured at 0 hours by the average of the scratch measured at 24, 48, and 72 hours, then multiplied by 100. The fold expression index was calculated by dividing the percentage of invasion for each cell line by the percentage of invasion of the noninvasive control cells (EEC) as described earlier.48 HESC, EEC, ECL, and 12Z cells were also incubated with or without 2.5 to 30 µmol/L of the LOX inhibitor, β-aminopropionitrile (βAPN; Sigma-Aldrich, St Louis, Missouri) for up to 72 hours to determine the effects of blocking LOX in the wound healing capacity of the cells. Experiments were conducted 3 times in duplicate.
Matrigel Cell Invasion Assays
Invasion capacity of endometriotic, endometrial, and cancer cell lines was measured using the BioCoat Matrigel Invasion Chambers (BD Biosciences, San Jose, California). Cell suspensions (2.5 × 104 cells in 500 μL of complete culture medium) were added to chambers (control and matrigel) and incubated overnight in a humidified tissue culture incubator at 37°C, 5% CO2 atmosphere. After incubation, the medium was removed and the noninvading cells were removed by scrubbing the insert membrane with a cotton swab. The cells were then stained using Diff-Quik Kit (Dade Behring, Inc, Newark, Delaware) and counted in 8 different fields under light microscopy (40X objective; Olympus Microscope, Olympus, Center Valley, Pennsylvania). Percentage of invasion was determined by dividing the mean of cells invading the Matrigel insert membrane by the mean of cells invading the control insert membrane, then multiplying by 100. The invasion index was assessed by dividing the percentage of invasion of transfected cells by the percentage of invasion of the control cells as described by the manufacturer. Experiments were conducted in duplicate.
Transfection of LOX Into Noninvasive Epithelial Cancer Cells
To determine the in vitro functional and biological roles of LOX, the noninvasive epithelial cancer cell line EEC was transfected with pCMV-XL5-LOX or with empty vector (pCMV-XL5; complementary DNA Clones, Origene, Rockville, Maryland) using Lipofectamine 2000 transfection reagent and protocol (Invitrogen, Grand Island, New York). Briefly, EEC cells were seeded in a 6-well tissue culture plate at ∼2 × 105 cells/well in 2 mL of culture medium. Once the cells reached approximately 80% to 90% of confluence, they were cultured in medium without antibiotic 24 hours prior to the transfection. Lipofectamine/DNA complexes were generated with 2 μg of DNA suspended in Opti-MEM I Reduced Serum Medium (Invitrogen) and incubated for 30 minutes at RT. The cells were then incubated with the complexes for 24 hours at 37°C under 5% CO2, and then serum-free medium was replaced for complete fresh medium after 24 hours of the transfections. Expression of LOX was assessed by qPCR as described earlier. Downstream experiments (proliferation, invasion, and gene expression profiling) were performed 48 hours after transfections as described earlier.
RT2 Profiler Pathway-Focused Gene Expression Profiling of LOX Transfectants
Total RNA was isolated from LOX-transfected cells using RNAeasy kit (Qiagen, Valencia, California) following standard protocols. After DNase treatment, RNA was quantified using a Biophotometer (Eppendorf, Hauppauge, New York). Total RNA (1 µg) was reverse transcribed using the RT2 First Strand Synthesis Kit according to the manufacturer’s protocol (C-03/330401; SABiosciences, QIAGEN; Frederick, Maryland). Complementary DNA (cDNA) was added to RT2 SYBR Green/Fluorescein qPCR Master Mix (PA-011-12/330512; SABiosciences, QIAGEN; Frederick, Maryland) according to the manufacturer’s protocol. The Human Fibrosis Array (HFA; PAHS-120A-12; SABiosciences, QIAGEN) and Human EMT (PAHS-090A-12; SABiosciences, QIAGEN) were used to identify the panel of genes regulated by overexpression of LOX. Complementary DNAs were added to each well of the HFA or EMT PCR plate, and qPCR reactions were performed in triplicate on an iCycler (Bio-Rad, Hercules, California) following the manufacturer’s protocol. The PCR Array Data Analysis Web Portal was used as a tool to analyze gene expression results.
Statistical Analyses
Basal gene expression is reported as relative units (RU; 2−ΔCt) normalized against expression of GAPDH or RPS17. Gene expression levels in cells treated with E2 were calculated according to the 2−ΔΔCt method, relative to the expression in media plus vehicle (CV).41 For tissues, after normalization with housekeeping gene, mean fold-expression changes (2−ΔΔCt) ± standard error of the mean relative to the mean expression level in endometrial biopsies from women without endometriosis were reported. Nonparametric independent sample t test (Wilcoxon) or analysis of variance (ANOVA) with Dunn posttest was conducted to determine statistical significance of differences among study groups, using independent sample. Wilcoxon tests were done to assess statistical significance of selected differences between groups. Statistical significance of the differences in proportions of endometrial tissues with strong immunostaining was assessed by 2-sided Fisher exact test. Statistical significance level was set at P < .05. SPSS15 (SPSS Inc, Chicago, IL) or GraphPad Prism (Chicago, Illinois) were used for the statistical analysis.
Results
Protein Expression of LOX in Endometrial and Endometriotic Tissues on a TMA
Expression of LOX protein was significantly higher in both glands and stroma of ovarian lesions compared to those obtained from the peritoneum (Figure 1). In glands, LOX protein expression in skin endometriosis was also significantly higher than that of peritoneal lesions (Figure 1A). Only in stroma, LOX expression was significantly higher in ovarian lesions compared to fallopian tube endometriosis (Figure 1B). In addition, we observed a significantly higher expression of LOX protein in glands and stroma of proliferative endometrium of patients with endometriosis compared to those from controls.
Figure 1.
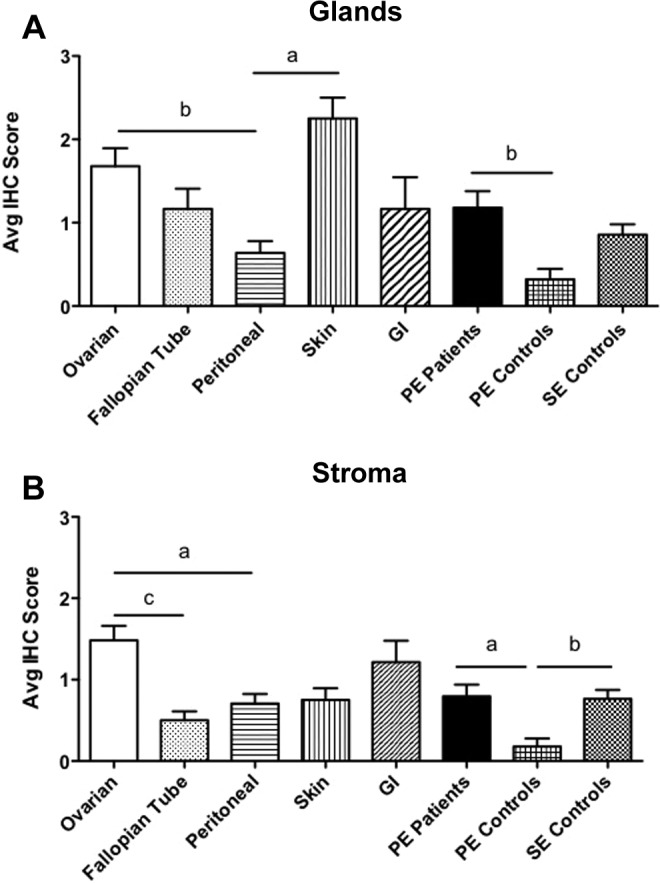
Immunostaining intensity of lysyl oxidase (LOX) protein in endometrial and endometriotic tissues in a TMA. A total of 164 formalin-fixed paraffin-embedded endometrial and endometriotic tissues on a Tissue Microarray were analyzed by immunohistochemistryIHC. Using the intensity scale (3 = strong, 2 = moderate, 1 = weak, and 0 = no staining), 2 independent observers evaluated and blindly scored the immunostaining intensity of LOX in glands (Figure 1A) and stroma (Figure 1B). In order to use analysis of variance with post hoc Bonferroni contrasts, we used the natural logarithm of the intensity score plus 1 to approach normalization. One-way analysis of variance was carried out comparing the mean scores among 6 endometriotic lesion locations and the eutopic endometrium from patients and controls. Analysis was conducted separately for stroma and glands. The results are summarized graphically, depicting the mean intensity scores and error bars and the statistical significance level among them (a, P < .05; b, P < .01; c, P < .001).
Expression of LOX Protein During WOI in Endometrium of Infertile Women With Endometriosis Compared to Fertile Women
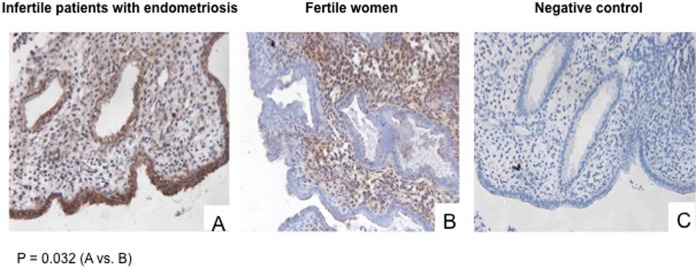
The LOX protein expression levels in the endometrium (eg, stroma, glandular epithelium, and luminal epithelium) during WOI was determined using immunohistochemistry (IHC) in the endometrium of patients with endometriosis-associated infertility (group 1) or in those of fertile women (group 2). All biopsies in these 2 groups were obtained from women who had not received any infertility treatment. We observed a significantly higher level of expression of LOX in the luminal epithelium of the endometrium obtained from patients with endometriosis-associated infertility (G1; representative results shown in panel A of Figure 2) compared to fertile controls (G2; representative results shown in panel B of Figure 2; P = .032). A negative control is shown in panel C. The LOX-specific staining was mostly observed in the cytoplasm of the endometrial epithelial cells. No significant differences in protein expression levels in the glandular and stromal compartments between the groups were observed (data not shown).
Figure 2.
Lysyl oxidase (LOX) protein expression during window of implantation (WOI). Representative immunohistochemical staining and localization of LOX in patients with endometriosis-associated infertility and fertile women. A, Infertile patients with endometriosis (G1); B, fertile women (G2); C, negative control (no primary antibody).
In Vitro Basal and E2-Regulated LOX Gene Expression
We determined the basal messenger RNA (mRNA) expression levels of LOX in endometriotic, endometrial, and cancer cell lines using qPCR. Figure 3 shows basal gene expression levels of LOX in cells expressed as relative units after normalization with the housekeeping gene (RPS17). LOX was highly expressed by all the stromal endometrial and endometriotic cell lines studied. The epithelial endometriotic cell line 12Z expressed LOX at higher levels than its counterparts, the epithelial cell lines HeLa and HES. The LOX expression was highest in the primary endometriotic cell lines PEC and PED and also the endometriotic cell line ECL (the 3 of them stromal). E2 did not significantly modulate LOX mRNA expression in any of the cell lines studied, which correlates with the absence of EREs in the LOX promoter region (data not shown).
Figure 3.
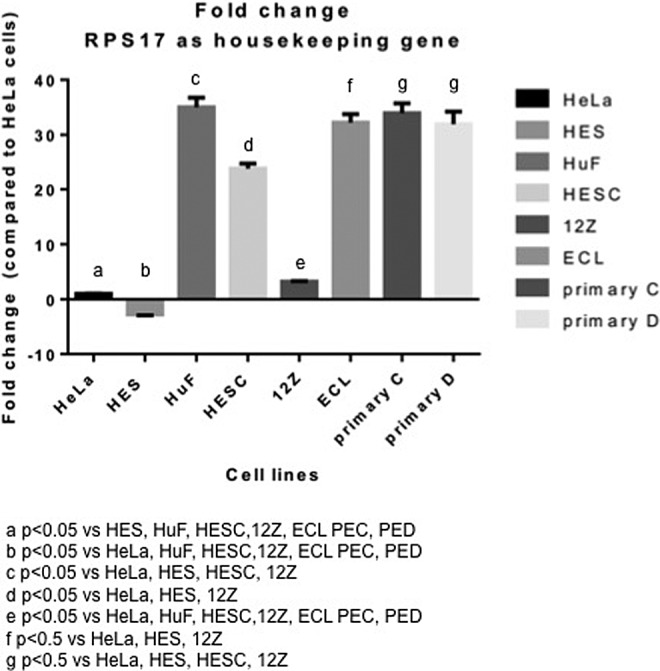
Basal expression of lysyl oxidase (LOX) in human endometrial and endometriotic cells. Basal gene expression levels of LOX were determined by quantitative polymerase chain reaction (qPCR) using RPS17 as the reference gene. Results from triplicates are shown as fold change relative to HeLa cells.
Effects of LOX on Proliferation Rates of Endometrial and Endometriotic Cells
The role of LOX in modulation of proliferation rates was determined by measuring incorporation of BrdU in endometrial stromal (HESC) and endometriotic (12Z) cells in the presence or absence of the LOX pan inhibitor (β-APN; Figure 4). 12Z cells had a significantly higher rate of proliferation compared to HESC cells (P ≤ .05). Treatment with β-APN significantly reduced the proliferation rates of HESC and 12Z cells by 28.7% (P = .038) and 31.7% (P = .012), respectively.
Figure 4.
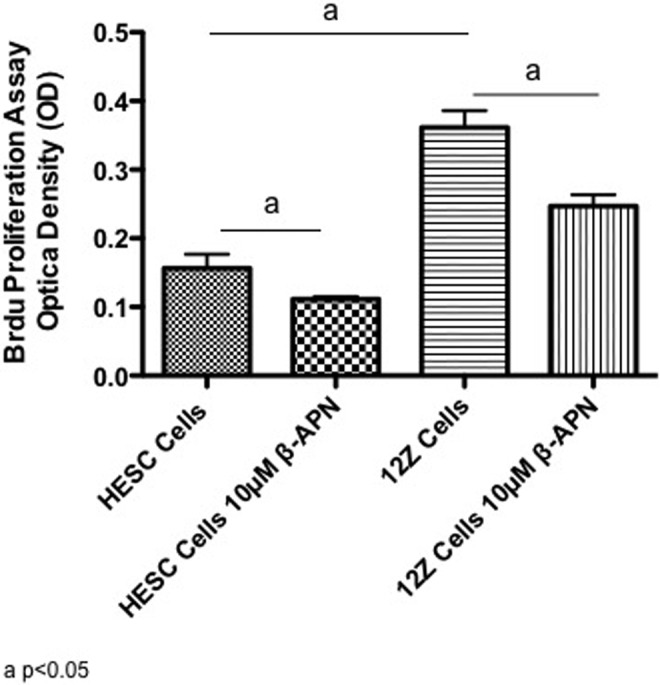
Effects of lysyl oxidase (LOX) inhibition on the proliferation of endometrial and endometriotic cells. The BrDU assay was performed in human endometrial stromal cell (HESC) and 12Z cells to determine the effects of 10 μmol/L of the LOX inhibitor β-aminopropionitrile (β-APN) on proliferation rates. Results from 3 experiments in triplicate are shown.
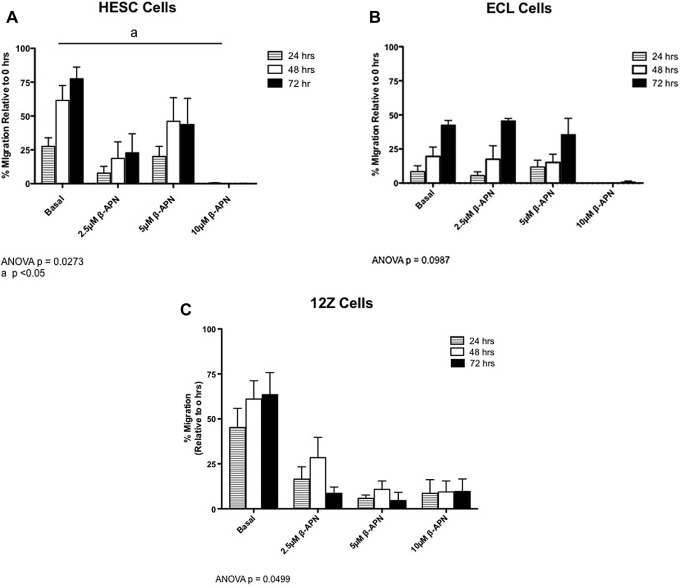
Effects of LOX on Migration Index of Endometrial and Endometriotic Cell Lines
Scratch assays were performed to determine the migration capacity of endometrial and endometriotic cell lines (Figure 5, panels A, B, and C). At 72 hours, the basal migration capacity into the wound was 78% for HESC cells (Figure 5A, P = .0273), 43% for ECLs (Figure 5B, P = .0987), and 63% for 12Z (Figure 5C, P = .0499). Culture of HESC, ECL, and 12Z cells in the presence of β-APN reduced their migration index in a time and dose-dependent manner. Treatment with 10 μmol/L of β-APN significantly decreased the migration capacity of HESC cells. 12Z cells had the highest basal migration capacity at 24 hours and were sensitive to the lower dose of β-APN (2.5 μmol/L) at 24 hours. However, the higher dose of 10 μmol/L was not sufficient to completely abolish their migration rate.
Figure 5.
Effects of lysyl oxidase (LOX) inhibition on the migration of endometrial and endometriotic cells. Migration capacity of endometrial and endometriotic cell lines (human endometrial stromal cell [HESC], endometriotic cell line [ECL], and 12Z cells) in the presence or absence of a LOX inhibitor was measured by Scratch assay after adding different concentrations of β-APN (2.5, 5, and 10 µmol/L) at 24, 48, and 72 hours. Open scratch areas were measured using Tscratch and reported as percentage of migration relative to 0 hour. Each experiment was done 3 times in duplicate. P < .05 is considered statistically significant.
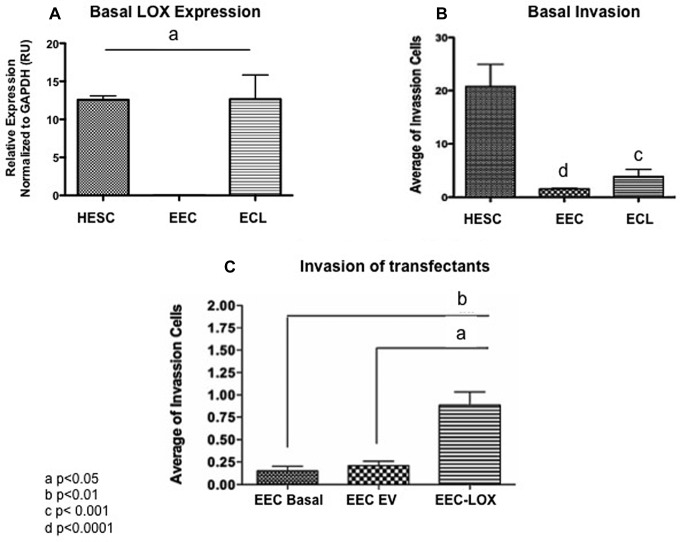
Effect of LOX in Invasion Capacity of Transfected Noninvasive Epithelial Cells
To measure the effect of LOX in invasion capacity, the noninvasive epithelial cell line MCF7 was transfected with LOX or empty vector and migration measured using BioCoat Matrigel Invasion chambers. Figure 6A shows the basal expression of LOX of the 3 cell lines tested. Compared to other cell lines (eg, HESC and ECL), the EEC cells do not express LOX and are poorly invasive (Figure 6A and B). LOX overexpression significantly increased the invasion capacity of the transfected EEC cells (ANOVA, P = .0010) when compared to the wild-type (P ≤ .01) and empty vector (P ≤ .05) invasion averages (Figure 6C).
Figure 6.
Effects of lysyl oxidase (LOX) expression in the invasive capacity of transfected EEC. A, Basal expression levels of LOX in cell lines. B, Basal invasion capacity of cell lines measured by BioCoat Matrigel Invasion. C, Migration of wild type, empty vector, and LOX-transfected endometrial epithelial cell (EECs). EECs overexpressing LOX showed a significantly (P ≤ .001) increased invasion capacity when compared to the basal EEC/MCF7 cells. A significantly (P ≤ .05) increased invasion capacity of EEC/MCF7 overexpressing LOX was obtained when compared to the EEC/MCF7 empty vector cells. The invasion capacity of the transfectants was significantly different compared to empty vector or basal controls (analysis of variance [ANOVA], P = .0010). Each experiment was done in duplicate.
Effect of LOX on the Transcriptome of Transfected Epithelial Cells
The molecular profile of transfected EEC cells was determined using the Human Fibrosis (HF) and the Human EMT Superarrays as described in detail in Methods section. LOX overexpression caused significant downregulation in gene expression levels of Actin, α 2, smooth muscle, and aorta (ACTA2), chemokine [C-C motif] ligand 11 (CCL11), Fas ligand (FASLG), interleukin 10 (IL10), Mothers Against Decapentaplegic (SMAD6), transforming growth factor beta receptor 1 (TGFBR1), and tissue inhibitor metalloproteinase 3 (TIMP3) in the HF array (Table 1). The same experiment with the EMT array showed that LOX overexpression caused a significant upregulation in gene expression of regulator of G-protein signaling (RGS2) and downregulation in the expression of desmoyokin (AHNAK [a nucleoprotein]), Cadherin 1 (CDH1 [E-cadherin; borderline significant]), Glycogen synthase kinase 3 beta (GSK3B), Notch homolog 1, translocation-associated (NOTCH1), and Vacuolar protein sorting 13 homolog A (VPS13A; Table 1). Other downregulated genes included CDH2, SNAI1, SNAI2, and SNAI3 (SNAIL homolog 1-3), but the changes in expression levels did not reach statistical significance. Thus, LOX overexpression induced changes in very few genes, including marginal downregulation of CDH1 (not significant, P = .057) that is critical to EMT but without upregulation of CDH2 gene (encoding N-Cadherin).
Table 1.
Molecular Profile of EEC Overexpressing LOX in Human Fibrosis and EMT Super Arrays.
| Human Fibrosisb and EMTc LOX Overexpression Upregulation (>2-fold) | Human Fibrosisb and EMTc LOX Overexpression Downregulation (<.05-fold) | ||||
|---|---|---|---|---|---|
| Gene | Fold Change | P value | Gene | Fold Change | P value |
| LOX b | 173.6278 | .106482 | ACTA2 b | 0.2982 | .001396 |
| RGS2 c | 4.1524 | .001894 | AHNAK c | 0.3857 | .003407 |
| CCL11 b | 0.3925 | .020233 | |||
| CDH1 c | 0.6893 | .057056 | |||
| CDH2 c | 0.86 | .538845 | |||
| FASLG b | 0.4149 | .03351 | |||
| GSK3B c | 0.5418 | .009628 | |||
| IL10 b | 0.1019 | .012177 | |||
| NOTCH1 c | 0.4833 | .009478 | |||
| SMAD6 b | 0.4804 | .041835 | |||
| SNAI1 c | 0.7271 | .279479 | |||
| SNAI2 c | 0.4034 | .071564 | |||
| SNAI3 c | 0.5675 | .067249 | |||
| TGFBR1 b | 0.4284 | .000328 | |||
| TIMP3 b | 0.3954 | .00697 | |||
| VPS13A c | 0.4785 | .017679 | |||
Abbreviations: HF, Human Fibrosis; EMT, Epithelial to Mesenchymal Transition; EEC, endometrial epithelial cell; LOX, lysyl oxidase; RGS2, regulator of G-protein signaling; ACTA, actin, α 2, smooth muscle, and aorta; AHNAK, CCL11, chemokine [C-C motif] ligand 11; FASLG, Fas ligand; GSK3B, glycogen synthase kinase 3 beta; IL10, interleukin 10; NOTCH1, Notch homolog 1, translocation-associated; SNA1, SNA2, SNA3, SNAIL homolog 1-3; TGFBR1, transforming growth factor beta receptor 1; TIMP3, tissue inhibitor metalloproteinase 3; VPS13A, vacuolar protein sorting 13 homolog A.
aSignificant results are shown in bold.
bHF Array (SABiosciences, QIAGEN, Frederick, Maryland).
cEpithelial to Mesenchymal Transition RT2 Profiler Arrays (SABiosciences, QIAGEN, Frederick, MD).
Discussion
We have previously observed high levels of LOX expression in endometriotic lesions of a rat model of endometriosis.2 These findings have been replicated by others using both rats and human endometriotic tissues.3–5 We hypothesized that LOX expression could also be dysregulated in the eutopic endometrium of women with endometriosis due to high levels of estradiol and contributing to their proliferative and invasive phenotype.49,50 This study represents the first investigation of the LOX protein expression pattern in human endometrium and endometriosis and of its potential role in modulating cellular functions (eg, proliferation, migration, and invasion) in endometrial and endometriotic cell lines. Inhibition of LOX function has been proposed as a potential therapy for cancer,51–53 thus investigations on the role of this enzyme in the etiology of endometriosis are of potential translational relevance.
We aimed to measure LOX-specific immunostaining on an endometriosis-focused TMA and observed significantly different levels of expression in the different types of endometriosis, being higher in ovarian versus peritoneal disease in both glands and stroma. We also observed significantly higher expression of LOX in both glands and stroma in eutopic proliferative endometrium from women with endometriosis versus proliferative endometrium from controls. The LOX plays role in extracellular matrix stability, integrity, and function as well as modulating focal adhesion, tissue stiffness, and integrin signaling; thus, it is possible that sustained LOX function may have an impact in the normal function of the endometrium.54
Next, we investigated the clinical relevance of LOX in endometriosis-associated infertility by measuring its levels of expression and localization in eutopic endometrium during the WOI by IHC. We observed that the expression of LOX protein was higher in the luminal endometrial epithelium of women with endometriosis-associated infertility compared to fertile controls. The mechanisms involved in the infertility problems seen in patients with endometriosis are likely to be a combination of physiological and hormonal defects.26,55 It has also been proposed that the chronic inflammatory process resulting from endometriosis could alter the endometrial environment (soil) or the embryo quality (seed).56–60 The high expression of LOX in the luminal epithelium shown here correlates with a possible impact in the interactions between the embryo and the endometrium during implantation or may lead to aberrant decidualization.61–63 Interestingly, Talbi et al showed that LOXL1 is downregulated during the WOI.20 In addition, several studies suggests that LOX is a potent “chemokine” inducing the migration of immune cells such as human monocytes and CD11b+ myeloid cells, thus potentiating the inflammatory milieu where LOX family members are expressed at high levels.64,65 Finally, we have recently uncovered a genetic association between genomic variants in LOXL4 and endometriosis-associated infertility.23 Evidently, more samples are needed to validate these results and to understand the mechanisms whereby aberrant expression and/or function of LOX could affect the function of luminal endometrium and possibly implantation.66–68
The LOX is normally found in various tissues including brain, prostate, and uterus and has been shown to be upregulated by several growth factors (fibroblast growth factor-2, insulin-like growth factor, prostaglandin E, and transforming growth factor β [TGF-β]), cytokines, and inflammatory molecules also known to be upregulated in endometriosis.69–71 In order to assess the possibility that estradiol may be upregulating LOX, we next determined basal and E2-regulated LOX expression levels in cell lines. We observed that LOX mRNA was high in the stromal endometrial (HESC) endometriotic (ECL, PEC, and PED) stromal cell lines studied except for the epithelial 12Z cells. Treatment with E2 did not significantly regulate the expression of LOX in vitro, which was in accord with bioinformatic analyses of the regulatory regions of the LOX did not identify any EREs. Pelvic endometriosis is characterized by altered levels of proinflammatory factors in the peritoneum and within the ectopic endometriotic lesions that can modulate gene expression in organs of the pelvic area, such as the GI tract and the uterus.72 Although LOX expression has been shown to be modulated by inflammation, its role in the endometrium, both in normal and pathological settings, is unknown. Relevant to endometriosis, overexpression of LOX could lead to abnormal “surface to surface” communication between underlying peritoneal epithelium and ectopic endometrium in pelvic endometriosis, but more studies are necessary to understand the role of LOX on interactions between refluxed menstrual tissue and peritoneal surfaces.
Our in vitro results support the notion that LOX enzymatic function promotes cellular processes relevant to the endometriotic phenotype. We showed that LOX is highly expressed in endometriotic stromal and epithelial cell lines, and that it increases cellular proliferation, invasion, and migration. Addition of the LOX inhibitor β−APN led to significant decreases in proliferation and migration of HESC and 12Z cells. Because β−APN is a pan-inhibitor of LOX function, the contribution of other LOX isoforms to the invasive phenotype needs to be characterized further. Transfection experiments showed that increased expression of LOX by a noninvasive epithelial cell line was able to induce an invasive phenotype, thus confirming a role of LOX in promoting invasiveness de novo, via molecular mechanisms that were investigated next.
The LOXs have been implicated in ECM remodeling, invasion/metastasis, and fibrotic diseases.73,74 Therefore, we also assessed the effects of overexpressing LOX in the expression of key genes involved in EMT and fibrosis. Overexpression of LOX significantly upregulated the expression of only 1 gene: regulator of G-protein signaling (RGS2) and downregulated the expression of few genes (16 in total), including marginal downregulation of CDH1 (P = .057). Downregulation of CDH1 (E-cadherin) with concomitant upregulation of CDH2 (N-cadherin, a mesenchymal cell marker) are hallmarks of EMT pathway. This cadherin switch leading the cells to a mesenchymal phenotype may be mediated by SNAI1 nuclear localization. However, we did not observe the full activation of EMT: CDH1 changes were minimal and without upregulation of CDH2. Also, LOX expression did not cause a significant downregulation of SNAIL isoforms (SNAI1, SNAI2, and SNAI3) mRNA levels. Another key gene in this pathway is glycogen synthase kinase 3 beta (GSK3B), which has been reported to sequester and degrade SNAI1, inhibiting its nuclear localization and leading to CDH1 downregulation. GSK3B was downregulated by LOX, which suggests that available SNAI1 could go into the nucleus to induce the EMT process by blocking CDH1 expression. However, we did not observe a cadherin switch, and therefore can conclude that the SNAIL/CDH1 EMT pathway is not activated by LOX.18 Interestingly, the only gene upregulated by LOX expression, RGS2, plays roles in cell migration, chemokine receptor signaling, and carcinogenesis,75,76 and has been shown to be expressed predominantly in stromal cells at implantation sites in mice, suggesting a role during implantation.76,77
One interesting finding was the downregulation of Notch homolog 1 by LOX overexpression. NOTCH1 plays a major role in development and mediates a survival signal in the uterine endometrium in response to chorionic gonadotropin (CG) for the establishment of a successful pregnancy. This gene has been shown to be downregulated in the eutopic endometrium of baboons and women with endometriosis and is associated with blunted decidualization.78,79 Induction of NOTCH1 during the WOI in response to CG exerts anti-apoptotic effects and induces stromal cell proliferation and decidualization, critical for implantation.79 NOTCH1 downregulation may be thus be critical for the transition of stromal fibroblast to decidual cells, which is essential for the establishment of a successful pregnancy.80 Downregulation of NOTCH1 could result in decreased proliferation and defects in differentiation, which needs to be studied further.81
Overexpression of LOX in epithelial cells led to significant downregulation of the following genes: ACTA2 that normally plays a role in motility, structure, and integrity of the cell; CCL11 has been reported to be upregulated in the endometrium of baboons with endometriosis compared with the endometrium of disease-free animals82; FASLG may induce apoptosis of T lymphocytes and produce an immunotolerant environment for the development of ectopic implants83; IL10 has negative effects in immunoregulation and its downregulation could lead to increased inflammation84; SMAD6 functions as a negative regulator of BMP and TGF-β/activin signaling85; TIMP3 is one of the genes that are modulated by PGE2 receptors in endometriosis; selective inhibition of EP2 and EP4 suppresses expression and/or activity of metalloproteinase proteins and increases expression of TIMP1-4 proteins, decreasing migration and invasion of endometriotic cell lines86; and VPS13A that plays roles in controlling protein trafficking through the trans-Golgi network to endosomes, lysosomes, and the plasma membrane.87
In summary, the gene profiles observed in LOX transfectants are not fully compatible with activation of EMT mechanisms as we hypothesized. However, these results suggest that LOX may be implicated in a partial or early EMT and of fibrotic processes and that other factors are required to fully induce remodeling of the extracellular matrix. In conclusion, we found upregulation of the expression of LOX in cell lines, lesions, and in endometrium of women with endometriosis-associated infertility during the WOI. We provide evidence for a role of LOX in promoting proliferation, migration, and invasion. Additional in vitro and in vivo studies using animal models already available (baboons, rhesus macaques) would need to be conducted to test the hypothesis that LOX overexpression causes stiffness of luminal epithelium leading to defects in implantation.
Acknowledgments
We thank Dr Anna Starzinski-Powitz (Institut fur Zellbiologie und Neurowissenschaft, Frankfurt am Main, Germany) for kindly providing the 12Z cells used in this study. We are grateful of Sonia Abac, RN, for her role in patient recruitment; Jessica Fourquet for management of the patient registry at the Endometriosis Research Program, and Elvin Estrada in the molecular and in vitro techniques. Also we acknowledge Southern Pathology Services in Ponce Puerto Rico, for access to blocks that were used to construct the TMA, pathology consultation by Dr Adalberto Mendoza, and technical help provided histotechnicians. We acknowledge the role of Dr Miosotis Garcia in the development of the endometriosis TMA. Gene analysis for ERE, PRE were conducted by Katherine Burns, PhD & Leping Li, PhD, National Institute of Environmental Health Sciences (NIEHS)-NIH, Research Triangle Park, North Carolina.
Footnotes
Authors’ Note: This work was performed at Ponce Health Sciences University—School of Medicine & Ponce Research Institute, Ponce, Puerto Rico & Michigan State University, Grand Rapids, Michigan
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported by NICHD grants #R01-HD050559 (IF), #3R01-HD050559-01A1S1 (LR), and #1F31HD056964-01A1 (PB); NIGMS grants #S06-GM08239-20 (IF) and R25GM082406 (PHSU RISE Program; PB and AR); NIMHD grant #MD007579 (PHSU Molecular Biology and Genomics Core) and #R25MD007607 (Post-doctoral Master of Science in Clinical and Translational Research Program, MSc/Hispanic Clinical and Translational Research Education and Career Development Program, University of Puerto Rico). This research was also supported in by grant # U54 HD 40093 (ATF) and Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (grant #ES101765) to Dr Leping Li. The construction of the Tissue Microarray was funded by grant #5U56 CA126379.
References
- 1. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;89(6):1603. [DOI] [PubMed] [Google Scholar]
- 2. Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril. 2007;87(5):1180–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konno R, Fujiwara H, Netsu S, et al. Gene expression profiling of the rat endometriosis model. Am J Reprod Immunol. 2007;58(4):330–343. [DOI] [PubMed] [Google Scholar]
- 4. Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88(6):1505–1533. [DOI] [PubMed] [Google Scholar]
- 5. Blassioli Dentillo D, Meola J, Rosa ESJC, et al. Deregulation of LOXL1 and HTRA1 gene expression in endometriosis. Reprod Sci. 2010;17(11):1016–1023. [DOI] [PubMed] [Google Scholar]
- 6. Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. [DOI] [PubMed] [Google Scholar]
- 7. Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19-20):2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molnar J, Fong KS, He QP, et al. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta. 2003;1647(1-2):220–224. [DOI] [PubMed] [Google Scholar]
- 9. Lazarus HM, Cruikshank WW, Narasimhan N, Kagan HM, Center DM. Induction of human monocyte motility by lysyl oxidase. Matrix Biol. 1995;14(9):727–731. [DOI] [PubMed] [Google Scholar]
- 10. Li W, Liu G, Chou IN, Kagan HM. Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. J Cell Biochem. 2000;78(4):550–557. [PubMed] [Google Scholar]
- 11. Dairkee SH, Ji Y, Ben Y, Moore DH, Meng Z, Jeffrey SS. A molecular ‘signature’ of primary breast cancer cultures; patterns resembling tumor tissue. BMC Genomics. 2004;5(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. [DOI] [PubMed] [Google Scholar]
- 13. Erler JT, Giaccia AJ. Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res. 2006;66(21):10238–10241. [DOI] [PubMed] [Google Scholar]
- 14. Kirschmann DA, Seftor EA, Fong SF, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62(15):4478–4483. [PubMed] [Google Scholar]
- 15. Kirschmann DA, Seftor EA, Nieva DR, Mariano EA, Hendrix MJ. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat. 1999;55(2):127–136. [DOI] [PubMed] [Google Scholar]
- 16. Payne SL, Fogelgren B, Hess AR, et al. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 2005;65(24):11429–11436. [DOI] [PubMed] [Google Scholar]
- 17. Akiri G, Sabo E, Dafni H, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63(7):1657–1666. [PubMed] [Google Scholar]
- 18. Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. Embo J. 2005;24(19):3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moreno-Bueno G, Salvador F, Martin A, et al. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011;3(9):528–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. [DOI] [PubMed] [Google Scholar]
- 21. Savaris RF, Hamilton AE, Lessey BA, Giudice LC. Endometrial gene expression in early pregnancy: lessons from human ectopic pregnancy. Reprod Sci. 2008;15(8):797–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Painter JN, Nyholt DR, Morris A, et al. High-density fine-mapping of a chromosome 10q26 linkage peak suggests association between endometriosis and variants close to CYP2C19. Fertil Steril. 2011;95(7):2236–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruiz LA, Dutil J, Ruiz A, et al. Single-nucleotide polymorphisms in the lysyl oxidase-like protein 4 and complement component 3 genes are associated with increased risk for endometriosis and endometriosis-associated infertility. Fertil Steril. 2011;96(2):512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598. [DOI] [PubMed] [Google Scholar]
- 25. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trinder J, Cahill DJ. Endometriosis and infertility: the debate continues. Hum Fertil (Camb). 2002;5(1 suppl):S21–S27. [DOI] [PubMed] [Google Scholar]
- 27. Stoikos CJ, Salamonsen LA, Hannan NJ, O’Connor AE, Rombauts L, Dimitriadis E. Activin A regulates trophoblast cell adhesive properties: implications for implantation failure in women with endometriosis-associated infertility. Hum Reprod. 2010;25(7):1767–1774. [DOI] [PubMed] [Google Scholar]
- 28. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–373. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao X, Yeh YC, Outley J, Simon J, Botteman M, Spalding J. Health-related quality of life burden of women with endometriosis: a literature review. Curr Med Res Opin. 2006;22(9):1787–1797. [DOI] [PubMed] [Google Scholar]
- 30. Fourquet J, Baez L, Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril. 2011;96(1):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fourquet J, Gao X, Zavala D, et al. Patients’ report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263. [DOI] [PubMed] [Google Scholar]
- 33. Colon-Diaz M, Baez-Vega P, Garcia M, et al. HDAC1 and HDAC2 are Differentially Expressed in Endometriosis. Reprod Sci. 2012;19(5):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borahay MA, Lu F, Ozpolat B, et al. Mullerian inhibiting substance suppresses proliferation and induces apoptosis and autophagy in endometriosis cells in vitro. ISRN Obstet Gynecol. 2013;2013:361489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159(5):1839–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banu SK, Lee J, Starzinski-Powitz A, Arosh JA. Gene expression profiles and functional characterization of human immortalized endometriotic epithelial and stromal cells. Fertil Steril. 2008;90(4):972–987. [DOI] [PubMed] [Google Scholar]
- 37. Korch C, Spillman MA, Jackson TA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol. 2012;127(1):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kniss DA, Summerfield TL. Discovery of HeLa Cell Contamination in HES Cells: Call for Cell Line Authentication in Reproductive Biology Research. Reprod Sci. 2014;21(8):1015–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–2296. [DOI] [PubMed] [Google Scholar]
- 40. Hombach-Klonisch S, Kehlen A, Fowler PA, et al. Regulation of functional steroid receptors and ligand-induced responses in telomerase-immortalized human endometrial epithelial cells. J Mol Endocrinol. 2005;34(2):517–534. [DOI] [PubMed] [Google Scholar]
- 41. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 42. Jin VX, Leu YW, Liyanarachchi S, et al. Identifying estrogen receptor alpha target genes using integrated computational genomics and chromatin immunoprecipitation microarray. Nucleic Acids Res. 2004;32(22):6627–6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li L. GADEM: a genetic algorithm guided formation of spaced dyads coupled with an EM algorithm for motif discovery. J Comput Biol. 2009;16(2):317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38(11):1289–1297. [DOI] [PubMed] [Google Scholar]
- 45. Welboren WJ, van Driel MA, Janssen-Megens EM, et al. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 2009;28(10):1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007 2007;2(2):329–333. [DOI] [PubMed] [Google Scholar]
- 47. Gebäck T, Schulz MM, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46(4):265–274. [DOI] [PubMed] [Google Scholar]
- 48. Nam HJ, Park YY, Yoon G, Cho H, Lee JH. Co-treatment with hepatocyte growth factor and TGF-beta1 enhances migration of HaCaT cells through NADPH oxidase-dependent ROS generation. Exp Mol Med. 2010;42(4):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103. [DOI] [PubMed] [Google Scholar]
- 51. Reynaud C, Gleyzal C, Jourdan-Le Saux C, Sommer P. Comparative functional study of the lysyl oxidase promoter in fibroblasts, Ras-transformed fibroblasts, myofibroblasts and smooth muscle cells. Cell Mol Biol (Noisy-le-grand). 1999;45(8):1237–1247. [PubMed] [Google Scholar]
- 52. Nishioka T, Eustace A, West C. Lysyl oxidase: from basic science to future cancer treatment. Cell Struct Funct. 2012;37(1):75–80. [DOI] [PubMed] [Google Scholar]
- 53. Cox TR, Erler JT. Lysyl oxidase in colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2013;305(10):G659–G666. [DOI] [PubMed] [Google Scholar]
- 54. Baker AM, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32(14):1863–1868. [DOI] [PubMed] [Google Scholar]
- 55. Senapati S, Barnhart K. Managing endometriosis-associated infertility. Clin Obstet Gynecol. 2011;54(4):720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vassiliadis S, Relakis K, Papageorgiou A, Athanassakis I. Endometriosis and infertility: a multi-cytokine imbalance versus ovulation, fertilization and early embryo development. Clin Dev Immunol. 2005;12(2):125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simon C, Gutierrez A, Vidal A, et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9(4):725–729. [DOI] [PubMed] [Google Scholar]
- 58. Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90(2):247–257. [DOI] [PubMed] [Google Scholar]
- 59. Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2002;8(1):95–103. [DOI] [PubMed] [Google Scholar]
- 60. Lessey BA. Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci. 2002;955:265–280; discussion 293–265, 396–406. [DOI] [PubMed] [Google Scholar]
- 61. Van Sinderen M, Cuman C, Gamage T, et al. Localisation of the Notch family in the human endometrium of fertile and infertile women. J Mol Histol. 2014;45(6):697–706. [DOI] [PubMed] [Google Scholar]
- 62. Cuman C, Menkhorst E, Winship A, et al. Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction. 2014;147(3):R75–R86. [DOI] [PubMed] [Google Scholar]
- 63. Tanriverdi G, Denir S, Ayla S, et al. Notch signaling pathway in cumulus cells can be a novel marker to identify poor and normal responder IVF patients. J Assist Reprod Genet. 2013;30(10):1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lazarus HM, Cruikshank WW, Narasimhan N, Kagan HM, Center DM. Induction of human monocyte motility by lysyl oxidase. Matrix Biol. 1995;14(9):727–731. [DOI] [PubMed] [Google Scholar]
- 65. Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stilley JA, Birt JA, Sharpe-Timms KL. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res. 2012;349(3):849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Holoch KJ, Lessey BA. Endometriosis and infertility. Clin Obstet Gynecol. 2010;53(2):429–438. [DOI] [PubMed] [Google Scholar]
- 69. Trackman PC, Graham RJ, Bittner HK, Carnes DL, Gilles JA, Graves DT. Inflammation-associated lysyl oxidase protein expression in vivo, and modulation by FGF-2 plus IGF-1. Histochem Cell Biol. 1998;110(1):9–14. [DOI] [PubMed] [Google Scholar]
- 70. Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(8):5240–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. [DOI] [PubMed] [Google Scholar]
- 72. Rojas-Cartagena C, Appleyard CB, Santiago OI, Flores I. Experimental intestinal endometriosis is characterized by increased levels of soluble TNFRSF1B and downregulation of Tnfrsf1a and Tnfrsf1b gene expression. Biol Reprod. 2005;73(6):1211–1218. [DOI] [PubMed] [Google Scholar]
- 73. Gao Y, Xiao Q, Ma H, et al. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci U S A. 2010;107(44):18892–18897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baker AM, Cox TR, Bird D, et al. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103(5):407–424. [DOI] [PubMed] [Google Scholar]
- 75. Moratz C, Harrison K, Kehrl JH. Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods Enzymol. 2004;389:15–32. [DOI] [PubMed] [Google Scholar]
- 76. Hurst JH, Mendpara N, Hooks SB. Regulator of G-protein signalling expression and function in ovarian cancer cell lines. Cell Mol Biol Lett. 2009;14(1):153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang ZP, Ni H, Yang ZM, Wang J, Tso JK, Shen QX. Expression of regulator of G-protein signalling protein 2 (RGS2) in the mouse uterus at implantation sites. Reprod. 2003;126(3):309–316. [DOI] [PubMed] [Google Scholar]
- 78. Afshar Y, Jeong JW, Roqueiro D, et al. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012;26(1):282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Afshar Y, Stanculescu A, Miele L, Fazleabas AT. The role of chorionic gonadotropin and Notch1 in implantation. J Assist Reprod Genet. 2007;24(7):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Afshar Y, Miele L, Fazleabas AT. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012;153(6):2884–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mitsuhashi Y, Horiuchi A, Miyamoto T, Kashima H, Suzuki A, Shiozawa T. Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology. 2012;60(5):826–837. [DOI] [PubMed] [Google Scholar]
- 82. Hastings JM, Jackson KS, Mavrogianis PA, Fazleabas AT. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol Reprod. 2006;75(2):176–182. [DOI] [PubMed] [Google Scholar]
- 83. Selam B, Kayisli UA, Akbas GE, Basar M, Arici A. Regulation of FAS ligand expression by chemokine ligand 2 in human endometrial cells. Biol Reprod. 2006;75(2):203–209. [DOI] [PubMed] [Google Scholar]
- 84. Portelli M, Pollacco J, Sacco K, Schembri-Wismayer P, Calleja-Agius J. Endometrial seedlings. A survival instinct? Immunomodulation and its role in the pathophysiology of endometriosis. Minerva Ginecol. 2011;63(6):563–570. [PubMed] [Google Scholar]
- 85. NCBI. National Center for Biotechnology Information. Web site http://www.ncbi.nlm.nih.gov/gene/4091. Updated April 27, 2015. Accessed May 1, 2015.
- 86. Lee J, Banu SK, Subbarao T, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol. 2011;332(1-2):306–313. [DOI] [PubMed] [Google Scholar]
- 87. NCBI. National Center for Biotechnology Information. Web site http://www.ncbi.nlm.nih.gov/gene/23230. Updated April 10, 2015. Accessed May 1, 2015.