Abstract
Objective:
We seek to characterize the effect of progesterone metabolites on spontaneous and oxytocin-induced uterine contractility.
Study Design:
Spontaneous contractility was studied in mouse uterine horns after treatment with progesterone, 2α-hydroxyprogesterone, 6β-hydroxyprogesterone (6β-OHP), 16α-hydroxyprogesterone (16α-OHP), or 17-hydroxyprogesterone caproate (17-OHPC) at 10−9 to 10−6 mol/L. Uterine horns were exposed to progestins (10−6 mol/L), followed by increasing concentrations of oxytocin (1-100 nmol/L) to study oxytocin-induced contractility. Contraction parameters were compared for each progestin and matched vehicle control using repeated measures 2-way analysis of variance. In vitro metabolism of progesterone by recombinant cytochrome P450 3A (CYP3A) microsomes (3A5, 3A5, and 3A7) identified major metabolites.
Results:
Oxytocin-induced contractile frequency was decreased by 16α-OHP (P = .03) and increased by 6β-OHP (P = .05). Progesterone and 17-OHPC decreased oxytocin-induced contractile force (P = .02 and P = .04, respectively) and frequency (P = .02 and P = .03, respectively). Only progesterone decreased spontaneous contractile force (P = .02). Production of 16α-OHP and 6β-OHP metabolites were confirmed in all CYP3A isoforms tested.
Conclusion:
Progesterone metabolites produced by maternal or fetal CYP3A enzymes influence uterine contractility.
Keywords: 16α-hydroxyprogesterone, 6β-hydroxyprogesterone, 17α-hydroxyprogesteone caproate, preterm labor, progesterone
Introduction
Preterm birth affects 1 of 9 neonates born in the United States, often resulting in increased infant morbidity and mortality.1 Infant mortality is most commonly caused by complications from extreme prematurity or low birth weight, particularly less than 28 weeks.2 A history of prior preterm birth and shortened cervical length have been identified as 2 risk factors for prematurity that can be targeted with pharmacotherapy.3
Progesterone and its analogues are the primary therapeutic options for the prevention of preterm birth. Investigations of 17-hydroxyprogesterone caproate (17-OHPC) have demonstrated efficacy in the prevention of recurrent preterm birth in singleton pregnancies.4 Further, micronized progesterone has been proposed to decrease the rates of preterm birth and neonatal morbidity in pregnant women with shortened cervical length.5 The mechanisms underlying the decreased rate of preterm birth are not well elucidated. Progesterone has been shown to decrease spontaneous contractions in myometrial tissue while increasing the threshold for stimulation.6 Studies have also demonstrated decreased uterine contraction amplitude (force) after treatment with progesterone in an in vitro human myometrial model.7 However, clinical studies have been inconsistent, with some suggesting decreased contractility8 and others finding no improvement in the rates of preterm labor.4
Metabolism of progesterone in the maternal–fetal dyad may produce molecules with biologic activity. The cytochrome P450 3A (CYP3A) family of enzymes is a major source of xenobiotic metabolism in both the mother and the fetus. The ontogeny of CYP3A involves a change in expression from CYP3A7 during the fetal period to CYP3A4 and CYP3A5 after the first year of life. Cytochrome P450 3A is the dominant enzyme for metabolic oxidation of xenobiotics in the fetus, comprising 50% of the total cytochrome P450 content in the fetal liver.9 Cytochrome P450 3A metabolic activity has been characterized and is known to catalyze hydroxylation of the steroid ring structure at specific sites.10 Due to the prominence of CYP3A isoforms in both maternal and fetal livers, we hypothesize that it may play a role in the production of progesterone derivatives with biologic activity. In this study, we examine whether CYP3A metabolites of progesterone have nongenomic (acute) effects on spontaneous and oxytocin-induced uterine contractile force or frequency.
Materials and Methods
Selection of Progestins
Cytochrome P450 3A has nicotinamide adenine dinucleotide phosphate-oxidase (NADPH)-dependent high catalytic activity at the 2-, 4-, 6β-, 16α-, and 16β-positions on the steroid ring structure.10 Monohydroxylated derivatives of progesterone at these sites were considered potential products of CYP3A metabolism. Recombinant CYP3A4 metabolism of progesterone has previously been demonstrated to produce 6β-hydroxyprogesterone (6β-OHP) and 16α-hydroxyprogesterone (16α-OHP).11 Three candidate metabolites were used in the experimental protocol: 4-pregnen-2α-ol-3,20-dione (2α-OHP), 4-pregnen-6β-ol-3,20-dione (6β-OHP), and 4-pregnen-16α-ol-3,20-dione (16α-OHP). Progesterone and 17-OHPC (4-pregnen-17-ol-3,20-dione caproate) were also selected for testing. All progestins were obtained from a chemical supply vendor (Steraloids, Newport, Rhode Island).
All progestins were solubilized in ethanol and then diluted in phosphate-buffered saline to the desired molar concentration. Progesterone, 17-OHPC, and 6β-OHP had an ethanol concentration of 0.001% at the 10−6 mol/L dose (0.0001% at 10−7 mol/L, 0.00001% at 10−8 mol/L, and 0.000001% at 10−9 mol/L), while 2α-OHP and 16α-OHP had an ethanol concentration of 0.004% at the 10−6 mol/L dose (0.0004% at 10−7 mol/L, 0.00004% at 10−8 mol/L, and 0.000004% at 10−9 mol/L). The concentration of ethanol decreased parallel to the progestin dose in order to minimize the effect of the solvent on uterine contractility.
Experimental Model
Approval for the use of a murine model of uterine contractility was obtained through the Duke University Institutional Animal Care & Use Committee (IACUC). Virgin nonpregnant wild-type C57BL/6J female mice (Jackson Labs, Bar Harbor, Maine) were obtained at 10 to 12 weeks of age and fed a standard diet. Mice were euthanized by an IACUC-approved protocol, and each of the 2 uterine horns were removed. A 1 × 0.5 cm segment of the uterine horn was suspended using 4-0 silk suture between a stainless steel wire hook connected to a Radnoti force displacement transducer (Radnoti LLC, Monrovia, California) and a glass hook inside an organ bath that served as an anchor. The bath was filled with modified Krebs buffer (118 mmol/L NaCl, 4.8 M KCl, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 2.5 mmol/L CaCl2, 25 mmol/L NaHCO3, and 11 mmol/L glucose, pH 7.4), maintained at 37°C, and constantly bubbled with a premixed gas consisting of 20% O2, 5% CO2, and balance N2. Uterine horns were equilibrated at 0.5g of tension for approximately 30 minutes until a spontaneous contraction pattern was established.
A total of 60 mice were utilized to assess the effect of the selected treatments on uterine contractility, 30 in the spontaneous contraction group and 30 in the oxytocin-induced group. Six mice were in each progestin treatment group during both spontaneous and oxytocin-induced contractility arms of the study. To determine the nongenomic (acute) effect of the progestin treatments on spontaneous uterine contractility, a segment of uterine horn from each mouse was exposed to a single progestin (progesterone, 17-OHPC, 2α-OHP, 6β-OHP, or 16α-OHP), while a segment of the contralateral horn was exposed to a matching dose of ethanol vehicle. After a spontaneous contraction pattern was achieved, the progestin treatment was added to the tissue organ bath in successively increasing concentrations from 10−9 to 10−6 mol/L in 20-minute intervals. At the end of the treatments, the tissue was washed in modified Krebs buffer and then exposed to KCl (60 mmol/L) to elicit a tetanic response and confirm tissue viability.
To determine the nongenomic effect of progestin treatments on oxytocin-induced uterine contractility, we pretreated the uterine horns with a single progestin at 10−6 mol/L for 10 minutes after a spontaneous contractile pattern was established. The contralateral horn from the same mouse was treated with ethanol vehicle at a matching concentration. The uterine horns were subsequently exposed to increasing concentrations of oxytocin (1, 10, 50, and 100 nmol/L) in 10-minute intervals. At the end of the oxytocin treatments, the tissue was washed and then exposed to KCl (60 mmol/L) to elicit a tetanic response and confirm tissue viability.
Statistical Analysis
Uterine contraction responses were recorded using LabChart Pro (ADInstruments, Colorado Springs, Colorado) software as the force generated with time (g × min). Baseline uterine contractility was calculated as the area under the curve (AUC) for a 10-minute period of spontaneous contractions. The AUC of the uterine contractile response to each treatment (progestin or oxytocin) was determined and expressed as a percentage of the baseline AUC observed during spontaneous contraction. Spontaneous contractions in murine uterine muscle strips have previously been shown to have a linear correlation (r 2 = .90) with the AUC following treatment with 1 nmol/L oxytocin.12 To account for repeated measurements for individual muscle strips at increasing treatment doses, a repeated measures 2-way analysis of variance model was used to compare the dose–response curves. Each dose–response curve was fit by nonlinear regression and compared using an extra sum of squares F test. The mean max-fit response was compared between each progestin and vehicle control to determine maximum treatment efficacy. Half maximal inhibitory concentration (IC50; spontaneous contractility) and half maximal effective concentration (EC50; oxytocin-induced contractility) values were compared between each progestin and vehicle control to determine treatment potency. All analyses were performed using GraphPad Prism Version 6.0b for Macintosh (GraphPad Software, La Jolla, California). A P value <.05 was considered significant.
Confirmation of Progesterone Metabolites
Production of putative progestin metabolites by CYP3A was confirmed by in vitro metabolism studies. Baculovirus-inset cell-expressed human P450s (CYP3A4, CYP3A5, and CYP3A7) were purchased from Corning Inc (Woburn, Massachusetts). All chemicals were of high performance liquid chromatography (HPLC) grade. Progesterone (100 µmol/L) was reconstituted with sodium phosphate buffer (100 mmol/L, pH 7.4), MgSO4 (5 mmol/L), and recombinant human P450s (25 pmol). The reactions were initiated by adding NADPH (10 mmol/L), incubated for 30 minutes, and terminated with an identical volume of ice-cold acetonitrile (ACN). The reaction mixtures were centrifuged (3000 rpm × 5 min), and the supernatants were separated. An internal standard (6β-hydroxytestosterone [6β-OHT]) and citric acid (0.1 mol/L, pH 3.2) were added to each sample tube. Progesterone metabolites were extracted by mixing with 3 mL of methyl tert-butyl ether and centrifugation (3000 rpm × 5 min). The organic phase was removed and evaporated prior reconstitution in 100 µL of mobile phase A, from which 70 µL was injected onto the HPLC/uV/visible system as described subsequently.
Serial dilutions of 16α-OHP and 6β-OHP were prepared (1 mg/mL, methanol) for a standard curve. The total volume of the standards was the same as the incubation reaction volume in sodium phosphate buffer (100 mmol/L, pH 7.4). Extraction and separation of progestin standards were performed as described earlier. The 0 μg/mL standard contained only the internal standard, and the highest concentrations were based on the estimated metabolite formation. The quality controls were performed in triplicate along with standards.
A profile of metabolites produced by progesterone was determined by HPLC/uV detection (254 nm). An agilent column Luna 5u C18 (2) 100A (250 × 4.6 mm) was used throughout the experiments. Samples and standards were run on a gradient (mobile phase B: 0 min 40%; 1 min 40%; 28 min 95%; 28.1 min 40%, and 30 min 40%) with mobile phase A 0.25%/10%/90% (acetic acid/ACN/H2O) and mobile phase B 0.25%/90%/10% (acetic acid/ACN/H2O) at a flow rate 1.0 mL/min. Chromatographic peaks and retention times were confirmed by comparison with the standard curve. The AUCs with corresponding retention times (6β-OHT: 5.74 minutes, 16α-OHP: 8.74minutes, and 6β-OHP: 11.44 minutes) were collected.
Results
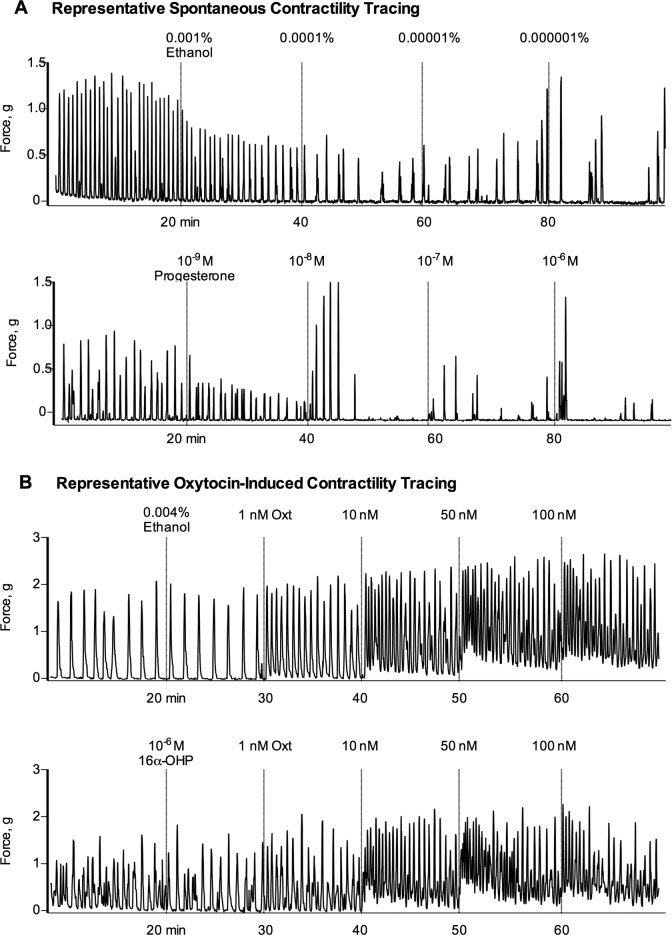
A representative tracing of an isolated uterine horn response to progesterone and vehicle control from the spontaneous uterine contractility experiment is shown in Figure 1A. Horns treated with progesterone demonstrated a reduced spontaneous contractile force compared to vehicle control (43.9% vs 64.1% of baseline AUC, P = .02) at maximal treatment dose. No significant difference in spontaneous contractile force was seen at maximal treatment dose in horns treated with 17-OHPC (P = .16), 2α-OHP (P = .78), 6β-OHP (P = .53), or 16α-OHP (P = .27) compared with the vehicle control. A summary of spontaneous contractile force findings is provided in Table 1.
Figure 1.
Representative contraction tracings of uterine horns from wild-type (WT) C57BL/6J female mice. A, Uterine horns were isolated and suspended in a tissue organ bath at 0.5g of tension. Once equilibrated, selected progestin treatments were added in successively increasing concentrations from 10−9 to 10−6 mol/L in 20-minute intervals to assess effect on spontaneous contractile activity (progesterone treatment, bottom panel). The contralateral horn from each mouse was treated with a matching ethanol vehicle (top panel). All contraction responses were measured and reported as the area under the contraction curve (AUC), normalized to the AUC of the spontaneous contraction pattern that preceded treatment. B, Treatment effect on oxytocin-induced contractility was assessed by exposing horns to selected progestin treatments (10−6 mol/L), followed by increasing doses of oxytocin (1, 10, 50, and 100 nmol/L) in 10-minute intervals (bottom panel). The contralateral horn from each mouse was treated with a matching ethanol vehicle (top panel). Contraction responses were measured and reported as described earlier.
Table 1.
Spontaneous and Oxytocin-Induced Contractility in Response to Progestin and Vehicle Treatments at Maximal Dose (10−6 mol/L).a
| Treatment | Contractile Force (% of Baseline) | Contractile Frequency/10 min (% of Baseline) | ||||
|---|---|---|---|---|---|---|
| Drug, % | Vehicle, % | P Value | Drug, % | Vehicle, % | P Value | |
| Spontaneous | ||||||
| Progesterone | 44 | 64 | .02 | 48 | 48 | .99 |
| 17-OHPC | 50 | 40 | .16 | 58 | 55 | .68 |
| 2α-OHP | 38 | 39 | .78 | 54 | 56 | .77 |
| 6β-OHP | 45 | 50 | .53 | 46 | 56 | .27 |
| 16α-OHP | 37 | 29 | .27 | 57 | 53 | .70 |
| Oxytocin induced | ||||||
| Progesterone | 267 | 498 | .02 | 141 | 258 | .02 |
| 17-OHPC | 218 | 416 | .04 | 130 | 219 | .03 |
| 2α-OHP | 330 | 391 | .34 | 169 | 238 | .12 |
| 6β-OHP | 268 | 263 | .91 | 196 | 152 | .05 |
| 16α-OHP | 302 | 442 | .07 | 217 | 365 | .03 |
Abbreviations:17-OHPC, 17-hydroxyprogesterone caproate; 2α-OHP, 2α-hydroxyprogesterone; 6β-OHP, 6β-hydroxyprogesterone; 16α-OHP, 16α-hydroxyprogesterone.
a P < .05 is significant.
A representative tracing of an isolated uterine horn response to 16α-OHP and vehicle control from the oxytocin-induced uterine contractility experiment is shown in Figure 1B. Horns pretreated with progesterone demonstrated a significant decrease in contractile force as measured by AUC at maximal oxytocin dose compared to vehicle control (266.8% vs 497.5%, P = .02). Uterine strips treated with 17-OHPC also demonstrated a significant decrease in contractile force compared to vehicle control (218.4% vs 415.7%, P = .04). There were no differences in oxytocin-induced contractile force at maximal dose (100 nmol/L) in horns pretreated with 2α-OHP (P = .34), 6β-OHP (P = .91), or 16α-OHP (P = .07) compared to vehicle control. A summary of oxytocin-induced contractile force findings is provided in Table 1.
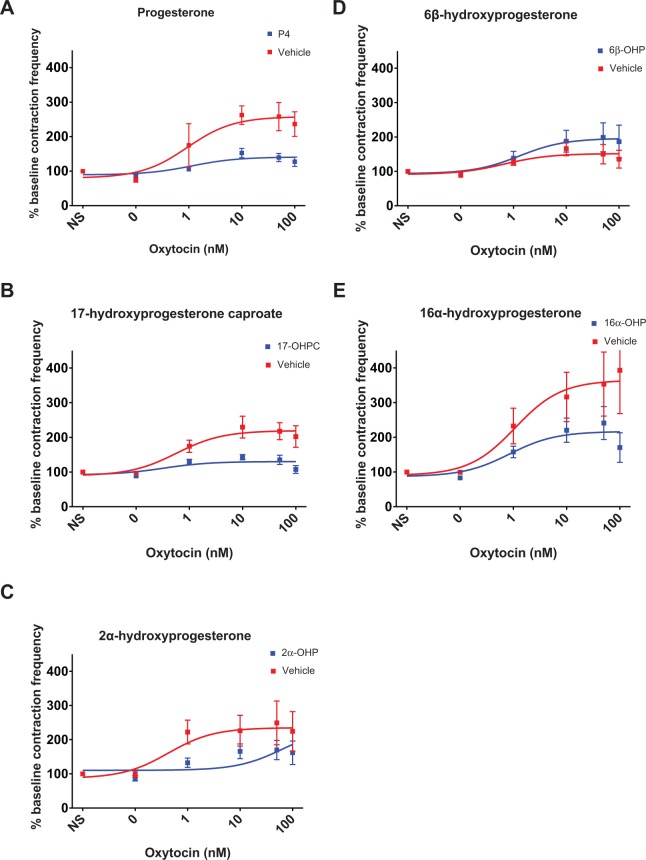
Spontaneous uterine contraction frequency was not significantly decreased after treatment with progesterone (P = .99), 17-OHPC (P = .68), 2α-OHP (P = .77), 6β-OHP (P = .27), or 16α-OHP (P = .70) at maximal treatment dose compared to vehicle control. However, oxytocin-induced contraction frequency decreased significantly after pretreatment with progesterone (141% vs 258% of baseline contraction frequency, P = .02), 17-OHPC (130% vs 219%, P = .03), or 16α-OHP (217% vs 365%, P = .03) but not 2α-OHP (P = .12) compared to control (Figure 2). 6β-Hydroxyprogesterone demonstrated an increase in oxytocin-induced contraction frequency (196% vs 152%, P = .05) compared to control. Summaries of spontaneous contraction frequency findings and oxytocin-induced contraction frequency findings are provided in Table 1.
Figure 2.
Dose–response curves of oxytocin-induced uterine contractile frequency following progestin pretreatment. Contraction frequency decreased significantly at maximal oxytocin dose (100 nmol/L) after pretreatment of uterine horns with progesterone (A, P = .02), 17-OHPC (B, P = .03), 6β-hydroxyprogesterone (D, P = .05), or 16α-hydroxyprogesterone (E, P = .03) but not 2α-hydroxyprogesterone (C, P = .12) compared to control. Error bars depict standard error (SE).
The potency of each progestin treatment and matched vehicle control was compared through IC50 and EC50 values, as listed in Table 2. All progestin treatments had IC50 values <1 nmol/L in spontaneous contractile force dose–response curves and <1 to 10 nmol/L in spontaneous contractile frequency curves. A greater range existed for EC50 values of progestin treatments in oxytocin-induced experiments. The EC50 values for oxytocin-induced contractile strength curves ranged from 5 to 150 nmol/L and from 3 to 650 nmol/L for frequency curves. None of the IC50 or EC50 values of progestin treatments significantly differed from matched vehicle controls in dose–response curves.
Table 2.
Comparison of Potency of Progestin Treatments and Matched Vehicle Controls by IC50 and EC50 Values in Dose–Response Curves.a
| Treatment | Contractile Force | Contractile Frequency | ||||
|---|---|---|---|---|---|---|
| Spontaneous | IC50, nmol/L | 95% CI | P Value | IC50, nmol/L | 95% CI | P Value |
| Progesterone | 0.31 | 0.05-1.90 | .54 | 6.58 | 0.78-55.82 | .52 |
| Vehicle | 0.90 | 0.04-18.26 | 1.46 | 0.21-9.89 | ||
| 17-OHPC | 0.78 | 0.15-3.92 | .96 | 0.31 | 0.01-8.77 | .68 |
| Vehicle | 0.28 | 0.03-2.29 | 0.55 | 0.04-7.86 | ||
| 2α-OHP | 0.32 | 0.10-1.04 | .35 | 0.55 | 0.05-5.84 | .8 |
| Vehicle | 0.71 | 0.27-1.86 | 0.99 | 0.12-8.46 | ||
| 6β-OHP | 0.48 | 0.08-2.97 | .59 | 4.10 | 0.65-25.80 | .68 |
| Vehicle | 0.32 | 0.04-2.79 | 6.76 | 0.99-46.40 | ||
| 16α-OHP | 0.77 | 0.23-2.60 | .84 | 0.74 | 0.06-9.57 | .94 |
| Vehicle | 0.38 | 0.07-2.19 | 0.66 | 0.04-11.74 | ||
| Oxytocin induced | EC50, nmol/L | 95% CI | P Value | EC50, nmol/L | 95% CI | P Value |
| Progesterone | 9.76 | 0.76-125.4 | .97 | 11.34 | 0.49-263.6 | .91 |
| Vehicle | 10.65 | 0.72-158.4 | 9.42 | 1.10-81.04 | ||
| 17-OHPC | 8.43 | 2.9-24.52 | .89 | 3.26 | 0.18-58.09 | .72 |
| Vehicle | 10.80 | 1.22-96.00 | 5.73 | 1.03-32.05 | ||
| 2α-OHP | 68.24 | 11.68-398.7 | .92 | 645.7 | 103.6-4024 | .74 |
| Vehicle | 32.88 | 4.99-216.8 | 4.29 | 0.18-102.0 | ||
| 6β-OHP | 20.94 | 2.16-203.0 | .93 | 12.85 | 0.75-220.1 | .73 |
| Vehicle | 27.26 | 2.77-267.9 | 6.71 | 0.32-142.6 | ||
| 16α-OHP | 142.50 | 28.13-721.7 | .99 | 8.46 | 0.83-86.46 | .89 |
| Vehicle | 11.65 | 0.49-277.2 | 10.81 | 1.11-105.7 | ||
Abbreviations: 17-OHPC, 17-hydroxyprogesterone caproate; 2α-OHP, 2α-hydroxyprogesterone; 6β-OHP, 6β-hydroxyprogesterone; 16α-OHP, 16α-hydroxyprogesterone; CI, confidence interval; IC50Half maximal inhibitory concentration; EC50, half maximal effective concentration.
a P < .05 is significant.
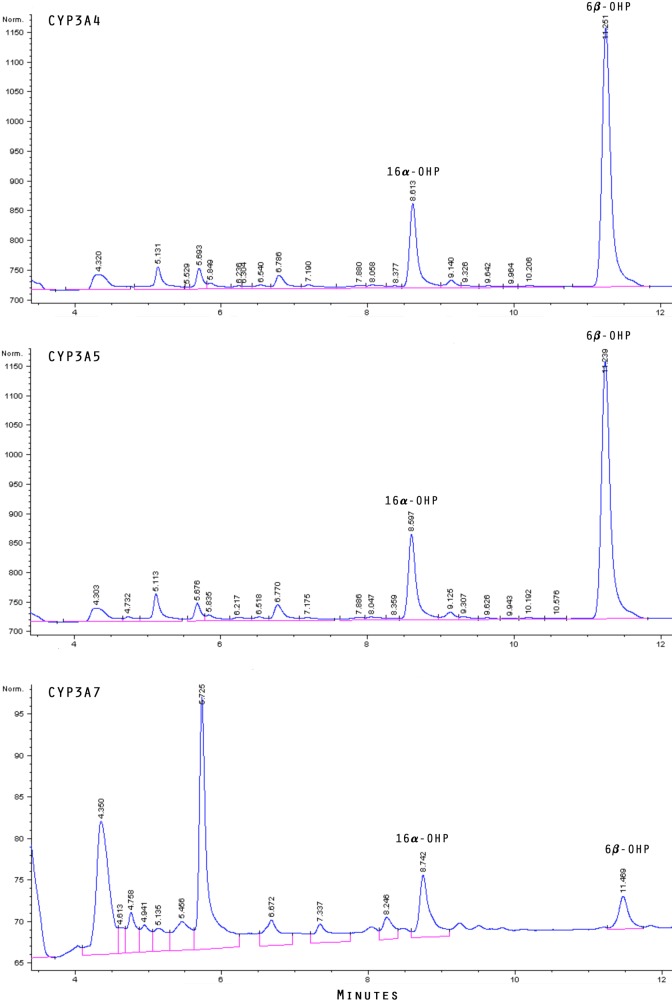
Endogenous production of the progesterone metabolites we tested was confirmed in vitro. The chromatographic profile of metabolites formed from incubation of progesterone with recombinant CYP3A4, CYP3A5, and CYP3A7 is shown in Figure 3. A peak for 16α-OHP appeared at approximately 8.6 minutes, consistent with commercial standards. Likewise, 6β-OHP appeared at approximately 11.3 minutes. Both 16α-OHP and 6β-OHP were identified as products of each CYP3A isoform tested. Overlap was seen between the peaks for 2α-OHP and 17α-hydroxyprogesterone at approximately 14-minute region. Progesterone was seen to elute at 20.7 minutes.
Figure 3.
Chromatograms of metabolites formed from incubation of progesterone with CYP3A4 (top panel), CYP3A5 (middle), and CYP3A7 (bottom). Peaks corresponding to 6β-hydroxyprogesterone and 16α-hydroxyprogesterone are labeled.
Comment
Progesterone and its esterified derivative 17-OHPC are utilized clinically in women at risk of preterm delivery. The pathway by which these progestins provide benefit is not known, but direct tocolytic effects may be partially responsible. In this study, we explored 3 putative metabolites of progesterone, 2α-OHP, 6β-OHP, and 16α-OHP, for effects on uterine contractility in a murine model. None of the progesterone derivatives significantly diminished spontaneous contractile force. Spontaneous contractile frequency was not affected by the progesterone derivatives, although 16α-OHP did significantly decrease oxytocin-induced contraction frequency. In contrast, treatment with 6β-OHP led to a significant increase in oxytocin-induced contractile frequency but not force at maximal treatment dose. None of the progesterone metabolites demonstrated dose–response findings (IC50 or EC50) different from matched vehicle controls. Recombinant human microsome studies confirmed the production of 6β-OHP and 16α-OHP by both maternal and fetal CYP3A isoforms.
Although progesterone and 17-OHPC have closely related molecular structures, there are conflicting reports of their actions on myometrial tissue. Progesterone, but not 17-OHPC, has been shown to decrease contraction amplitude in ex vivo human myometrial strips.7 We included progesterone and 17-OHPC as treatment groups in this study as a comparison to the progesterone derivatives and to confirm earlier findings. Progesterone significantly decreased the force of spontaneous and oxytocin-induced contractions, while 17-OHPC only decreased the force of oxytocin-induced contractions. Spontaneous contraction frequency was not affected by progesterone or 17-OHPC though both significantly decreased oxytocin-induced contraction frequency. However, the dose–response characteristics (IC50 or EC50) of progesterone and 17-OHPC did not differ from matched vehicle controls. Despite these mixed results, progesterone and 17-OHPC have documented clinical efficacy in reduction of preterm birth4,5,8,13,14 and contraction frequency.8
The discrepancy between dose–response characteristics (IC50 or EC50), which represent potency of the drugs tested, and maximum dose response, which represents efficacy of the drugs, is notable in our experiments. The ability of progesterone to inhibit uterine contractions has been well established. In our experiments, the maximal dose response of progesterone, but not the dose–response curve IC50 or EC50 value, differs from the vehicle control. Similarly, none of the progestins tested demonstrated potency different from the vehicle control though several modulated efficacy at maximal dose. These findings suggest that the ethanol vehicle may be masking the potency, but not the efficacy, of the tested progestins. We feel that the maximal dose response of the progestins better reflects their ability to affect uterine contractility in our experimental model.
The search for alternate therapies to prevent preterm birth has included exploration of other progesterone derivatives. Modifications to the basic ring structure of progesterone can yield molecules with variable potency. The 5α/5β derivatives have been systematically compared to progesterone to determine which molecules have the greatest uterorelaxant effect in ex vivo rat myometrial strips. Reduction in bonds at the 3α, 5α positions results in a 6-fold increased potency for uterorelaxant effect in comparison to progesterone. In comparison, reduction in bonds at the 3β, 5α positions results in <1/100th potency relative to progesterone.15 The uterorelaxant effect of the 5α/5β derivatives was observed in the micromolar concentration range,16 similar to the concentration required to see an effect in our experiments. As our study focused on CYP3A metabolites of progesterone, we did not test the 5α/5β derivatives in this study.
Cytochrome P450 3A is a major metabolic enzyme family in the maternal and fetal livers. The progesterone metabolites tested were selected based on the oxidation pattern of CYP3A and a prior study of in vitro metabolism of CYP3A4.10,11 The recombinant CYP3A microsome experiments performed in the second part of our study confirmed the production of 6β-OHP and 16α-OHP by CYP3A4, as well as the other CYP3A isoforms tested (CYP3A5 and CYP3A7). Notably, 6β-OHP and 16α-OHP demonstrated opposing effects on oxytocin-induced uterine contractility, suggesting that relative amounts of each progestin within the hormonal milieu of pregnancy may alter the likelihood of contractile activity. A recently published study on steroid metabolomics in the human fetus demonstrated 16α-OHP in significantly lower concentrations in umbilical cord blood from preterm deliveries compared to term deliveries.17 Progesterone concentrations were found to be in the micromolar range, while concentrations of 16α-OHP were similar to 17α-hydroxyprogesterone in the nanomolar range. Our finding that these progesterone metabolites are produced by the maternal CYP3A homologs (CYP3A4/5) increases the biologic plausibility that they can reach myometrial tissues to modulate contractility.
Progestins may exert progestational effects via genomic or nongenomic pathways. This study was limited to investigation of immediate, nongenomic effects of the proposed metabolites due to the short treatment period. Progesterone has been shown to rapidly inhibit oxytocin-induced contractions in vitro by uncoupling the excitation–contraction process.18 Furthermore, a metabolite of progesterone (5β-dihydroprogesterone) has been shown to act as a direct oxytocin receptor antagonist, further demonstrating the potential for nongenomic effects.19 Our study confirmed the rapid reduction in contractile force and frequency after progesterone treatment. Of the progesterone metabolites tested, 16α-OHP demonstrated a rapid reduction in oxytocin-induced contraction frequency, while 6β-OHP increased oxytocin-induced contraction frequency. Although our experiments did not explore the mechanism underlying this difference in action, it is likely that the position of monohydroxylation on the progesterone structure alters the chemical properties of the metabolites. It is possible that these structural changes may alter the binding affinity of the progesterone metabolites, specifically for the oxytocin receptor or generally for a range of ion channels associated with smooth muscle contractility. We did not test oxytocin receptor binding of these metabolites in the current study though future research efforts may investigate this as a potential mechanism for the observed effects on uterine contractility.
There are several limitations to our research. First, we utilized nonpregnant murine uterine horns in our experimentation. Although we were able to discern novel effects of the progesterone metabolite treatments, use of pregnant myometrium would have better simulated the target environment at the time of labor. Changes to the transcriptome of human myometrium have been demonstrated at the time of spontaneous labor, signaling a specific environment different from nonlaboring myometrium.20 Our use of wild-type mice to assess uterine contractility minimized any genetic variation that may have affected human myometrial contractility in our exploratory study. The variability in estrous phase of the mice at the time of contractility testing is a potential confounder. Each mouse served as its own control (1 uterine horn treated with progestin, the contralateral horn treated with vehicle), which minimized the impact of estrous phase variations and allowed assessment of the impact of progestin treatment. Although our use of uterine horns from nonpregnant mice is an accepted approach to the study of uterine contractility, progesterone metabolites should be tested in term human myometrium in future studies to confirm our findings in a pregnancy environment.
Next, ethanol, which has inherent tocolytic activity, was used as a solvent for the lipophilic progestins. We controlled for this confounder by treating the contralateral uterine horn in each mouse with a matching concentration of ethanol to distinguish drug effect from vehicle effect. The uterine horns demonstrated an appropriate decrease in spontaneous contractility at the higher ethanol concentration of 0.004% (2α-OHP and 16α-OHP) compared to 0.001% (progesterone, 17-OHPC, and 6β-OHP) though no effect was seen on oxytocin-induced contractility (Figure 4). However, dose–response curve characteristics (IC50 or EC50) of the progestin treatments were not distinguishable from the matched ethanol vehicles, limiting our ability to characterize the potency of the studied progestins. Uterine horns treated with ethanol vehicle also demonstrated greater variance in contractile response compared to progestin treatments. Future studies may be improved through the use of alternate lipophilic solvents (dimethyl sulfoxide) or complexing with cyclodextrin to enhance aqueous solubility. Additionally, progesterone is present in maternal plasma at higher concentrations than its metabolites (micromolar vs nanomolar ranges, respectively), calling into question their potential importance. However, 17-hydroxyprogesterone is an example of a metabolite that has shown clinical efficacy from supplementation during pregnancy, suggesting that other progesterone metabolites may also have the potential for clinical effect. Finally, the large number of analyses performed makes it possible that some statistically significant findings may be due to chance. Repetition of these experiments in human myometrial tissue in pregnancy will be needed to confirm our findings.
Figure 4.
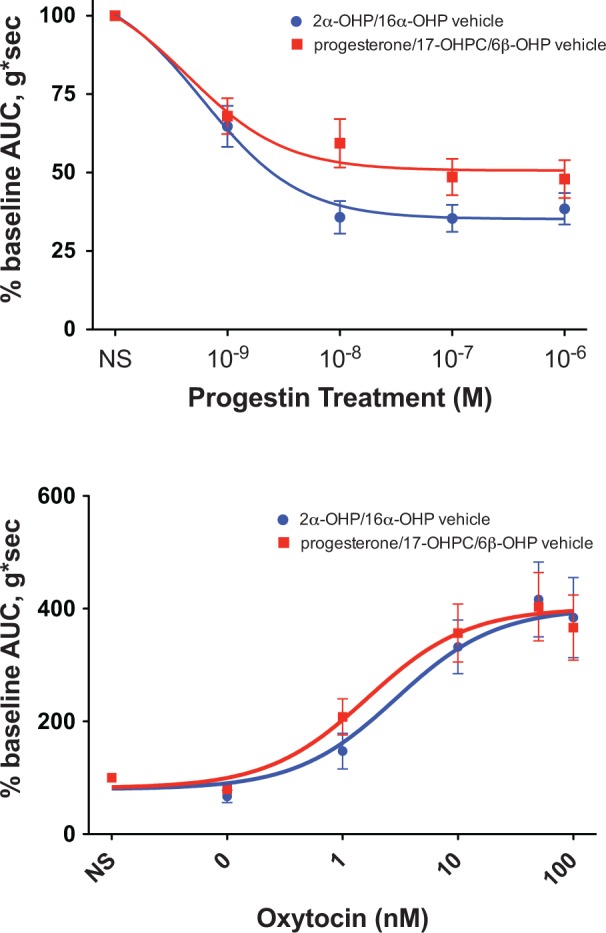
Dose–response curves of spontaneous and oxytocin-induced uterine contractile force for a composite of the vehicle controls. Nonlinear regression curves were fit to describe the spontaneous contraction dose response (top panel) seen from each vehicle treatment at successively increasing concentrations of 0.000001% to 0.001% (progesterone, 17-hydroxyprogesterone caproate [17-OHPC], and 6β-hydroxyprogesterone [6β-OHP]) and 0.000004% to 0.004% (2α-hydroxyprogesterone [2α-OHP] and 16α-hydroxyprogesterone [16α-OHP]). Dose–response curves of oxytocin-induced uterine contractions (bottom) after pretreatment with vehicle at maximal concentrations of 0.001% (progesterone, 17-OHPC, and 6β-OHP) and 0.004% (2α-OHP and 16α-OHP).
Our results suggest that CYP3A-derived progesterone metabolites may be part of a progestin milieu that influences uterine contractility. An implication of this research is that individuals with CYP3A polymorphisms may have altered progestin profiles that may increase their propensity toward uterine contractility. Future studies may include confirmation of our findings in human pregnancy myometrial tissue, characterization of the progestin profile in term and preterm pregnancies, and investigation of genomic effects of the progesterone metabolites on uterine contractility. Continued investigation of progesterone metabolites may yield new insights into the physiologic mechanisms underlying variability in preterm labor.
Acknowledgment
We thank Sara Quinney, PharmD, PhD, for her assistance with development of the HPLC protocol for separation of the progesterone metabolites.
Footnotes
Authors’ Note: This research was conducted at Duke University Medical Center and Indiana University School of Medicine.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ASP received support from T32 GM 86330-1 A1 (NIH-NIGMS) for his work in clinical pharmacology.
References
- 1. Martin JA, Hamilton BE, Osterman MJ. Births in the United States, 2013. NCHS Data Brief. 2014;175:1–8. [PubMed] [Google Scholar]
- 2. Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135(1):e59–e65. [DOI] [PubMed] [Google Scholar]
- 3. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567–572. [DOI] [PubMed] [Google Scholar]
- 4. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. [DOI] [PubMed] [Google Scholar]
- 5. Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruddock NK, Shi SQ, Jain S, et al. Progesterone, but not 17-alpha-hydroxyprogesterone caproate, inhibits human myometrial contractions. Am J Obstet Gynecol. 2008;199(4):391. e1–e7. [DOI] [PubMed] [Google Scholar]
- 7. Anderson L, Martin W, Higgins C, Nelson SM, Norman JE. The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy. Reprod Sci. 2009;16(11):1052–1061. [DOI] [PubMed] [Google Scholar]
- 8. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419–424. [DOI] [PubMed] [Google Scholar]
- 9. Rodríguez-Antona C, Jande M, Rane A, Ingelman-Sundberg M. Identification and phenotype characterization of two CYP3A haplotypes causing different enzymatic capacity in fetal livers. Clin Pharmacol Ther. 2005;77(4):259–270. [DOI] [PubMed] [Google Scholar]
- 10. Lee AJ, Conney AH, Zhu BT. Human cytochrome P450 3A7 has a distinct high catalytic activity for the 16alpha-hydroxylation of estrone but not 17beta-estradiol. Cancer Res. 2003;63(19):6532–6536. [PubMed] [Google Scholar]
- 11. Yamazaki H, Shimada T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997;346(1):161–169. [DOI] [PubMed] [Google Scholar]
- 12. Grotegut CA, Feng L, Mao L, Heine RP, Murtha AP, Rockman HA. β-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am J Physiol Endocrinol Metab. 2011;300(3):E468–E477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH, Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469. [DOI] [PubMed] [Google Scholar]
- 14. Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293(14):675–680. [DOI] [PubMed] [Google Scholar]
- 15. Kubli-Garfias C, Medrano-Conde L, Beyer C, Bondani A. In vitro inhibition of rat uterine contractility induced by 5 alpha and 5 beta progestins. Steroids. 1979;34(6 spec no):609–617. [DOI] [PubMed] [Google Scholar]
- 16. Cabral R, Gutierrez M, Fernandez AI, Cantabrana B, Hidalgo A. Progesterone and pregnanolone derivatives relaxing effect on smooth muscle. Gen Pharmacol. 1994;25(1):173–178. [DOI] [PubMed] [Google Scholar]
- 17. Hill M, Pašková A, Kančeva R, et al. Steroid profiling in pregnancy: a focus on the human fetus. J Steroid Biochem Mol Biol. 2014;139:201–222. [DOI] [PubMed] [Google Scholar]
- 18. Perusquia M. Nongenomic action of steroids in myometrial contractility. Endocrine. 2001;15(1):63–72. [DOI] [PubMed] [Google Scholar]
- 19. Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392(6675):509–512. [DOI] [PubMed] [Google Scholar]
- 20. Romero R, Tarca AL, Chaemsaithong P, et al. Transcriptome interrogation of human myometrium identifies differentially expressed sense-antisense pairs of protein-coding and long non-coding RNA genes in spontaneous labor at term. J Matern Fetal Neonatal Med. 2014;27(14):1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]