Abstract
Endometriosis is an inflammatory gynecological disorder caused by the growth of endometrial tissue outside the uterus. Endometriosis produces chemokines, including CXCL12, that attract bone marrow cells to the lesions. In this study, we describe the expression, localization, and chemotactic activity of CXCL12 in endometriotic lesions. Biopsies were collected both from women with endometriosis undergoing laparoscopy and control endometrium from women without endometriosis. Expression of CXCl12 and CXCR4 messenger RNA was increased approximately 4- and 6-fold, respectively, in endometriosis compared to eutopic endometrium. Immunohistochemistry of lesions revealed that CXCR4 was expressed in the stroma and epithelium in both endometriosis and control eutopic endometrium. The level of CXCR4 protein expression was significantly higher in all cellular compartments of the endometriotic lesions compared to control endometrium. CXCL12 protein expression was also higher in endometriotic lesions and was greatest in the epithelial compartment. CXCL12 was increased more in the condition media of cultured endometriosis than in controls as measured by enzyme-linked immunosorbent assay. Transwell chamber migration was used to demonstrate 2-fold increased chemoattraction of mouse bone marrow stem cells toward CXCL12 in the endometriotic-conditioned medium compared with eutopic endometrium. Our results indicate that a preferential recruitment of stem cells to endometriosis can explain how endometriosis outcompetes eutopic endometrium in recruiting the limited supply of circulating stem cells. The CXCL12/CXCR4 signaling axis is a potential target for the treatment of endometriosis and its associated disorders.
Keywords: CXCL12, CXCR4, chemotactic activity, endometriosis, stem cells, bone marrow
Introduction
Endometriosis is a benign, estrogen (E2)-dependent, and invasive disease that is defined as the ectopic growth of endometrial glands and stroma outside the uterus. The prevalence of this disease is approximately 5% to 10% of reproductive-age women.1 The ectopic growth of this tissue causes inflammation, fibrosis, ovarian cysts, and adhesions, which result in dysmenorrheal, chronic pelvic pain, dyspareunia, and infertility. Although the etiology is not fully characterized, endometriosis is a multifactorial disorder, resulting from retrograde menstruation, ectopic differentiation of stem cells, dysregulated immune/inflammatory responses, aberrant expression of multiple genes, and exposure to environmental factors.2–8 Various cellular mechanisms are activated, which allow survival, adhesion, proliferation, and invasion of endometrial cells that reach the peritoneal cavity.9,10 The development of endometriosis involves altered activation of immune factors that induce a permissive environment leading to the survival of endometrial cells ectopically.11 In fact, there is a strong evidence to support the role of immune and inflammatory mechanisms in endometriosis.12,13
Chemokines are small (8-12 kDa) polypeptides, which are characterized by their proinflammatory mediators, and play a role in cancer as regulators of angiogenesis, invasion, and metastases, cell growth, proliferation, and tissue remodeling.14,15 The best-known function of the chemokines is the regulation of migration of various cells in the body; more than 40 chemokines and approximately 20 receptors have been implicated in the migration of cells endogenously. Chemokine receptors are 7-transmembrane G protein-coupled receptors that are usually linked to G proteins.16,17 The chemokine receptor 4 (CXCR4) and its specific ligand, CXCL12 (also known as stromal-derived factor 1), are reported to act in a paracrine fashion in cancer, promoting tumor growth and development, angiogenesis, and metastasis to tissues where CXCL12 is expressed.18 CXCL12 and CXCR4 also have a role in placental function.19,20 Both the receptor and its ligand are also expressed by trophoblasts in the first trimester that penetrate maternal decidual blood vessels.21 CXCL12 may also be produced by decidualized stromal cells.22 CXCL12/CXCR4 signaling in trophoblast cell cultures has been shown to stimulate antiapoptotic pathways and promote cell survival.21,23,24
The interaction of CXCR4 with CXCL12 plays a key role in the mobilization and homing of stem cells.25 We have previously reported that toxicant exposure, medications, and ischemia–reperfusion can alter the migration of BM-derived stem cells (BMDSCs) to the uterus; however, the molecular mechanism responsible for the recruitment and engraftment of these cells is unknown.26–28 Recent work from our laboratory demonstrated that CXCL12 and its receptor have an essential role in the migration of bone marrow cells (BMCs) toward endometrial cells.29 Based on these data, we hypothesized that the CXCR4–CXCL12 axis is involved in endometriosis by promoting the invasion and engraftment of stem cells at ectopic sites. In this study, we report the increased expression of CXCR4 and CXCL12 in endometriosis and the chemoattractant function of CXCl12 in these lesions.
Materials and Methods
Sample Collection
Biopsies of endometriosis were obtained from 11 patients undergoing laparoscopy. Control endometrium was obtained from 11 patients without endometriosis. Endometriosis was confirmed histologically. All of the women were between 20 and 40 years of age and had regular menstrual cycles lasting between 25 and 35 days. No patients received hormonal treatments, including gonadotropin-releasing hormone agonist or sex steroids, nor did they use intrauterine contraception for at least 6 months prior to surgery. Approval for the collection of specimens was obtained from Yale University Human Investigations Committee.
RNA Isolation
Tissue samples collected from endometriosis and controls were processed immediately or frozen at −80°C in RNALater solution. The tissue was homogenized in TRIzol (100 mg/1 mL) reagent (Invitrogen, Carlsbad, California), and homogenates were kept on ice for 5 minutes, then 0.2 mL of chloroform (per 1 mL TRIzol) was added to each tube separately, and then homogenates were vortexed for 15 seconds. Samples were then incubated at room temperature (RT) for 3 minutes and centrifuged at 12 000 rpm at 4°C for 15 minutes. Next, the aqueous layer from each sample was transferred to a fresh tube, and the RNA was precipitated by adding 0.5 mL of isopropyl alcohol (per 1 mL TRIzol) to each sample and incubated at RT for 10 minutes. All tubes were centrifuged at 10 000 rpm to form the RNA pellets, which were then collected, washed with 75% ethanol, and dissolved in RNase-free water. The total RNA was purified using the Qiagen RNeasy Cleanup Kit (Qiagen, Valencia, California), according to the manufacturer’s protocol and quantified by a NanoDrop spectrophotometer (ThermoFicher Scientific, MA, USA). Purified RNA was immediately used for cDNA synthesis or stored at −80°C until use.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Purified RNA (25 ng) was reverse transcribed in 10 μL reaction mixture using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Green (Bio-Rad) and optimized in the MyQ Single Color Real-Time PCR Detection System (Bio-Rad). The specificity of the amplified transcript and the absence of primer dimers were confirmed by a melting curve analysis. All products yielded the predicted melting temperature. Gene expression was normalized to the expression of β-actin. Relative messenger RNA (mRNA) expression of each gene was calculated using the comparative cycle threshold method (2−ΔΔCT).30,31 The primers used are CXCL12: forward-AACACTCCAAACTGTGCCCT, reverse-CTCTCACATCTTGAACCTCTTGTT; CXCR4: forward-GCAGAGGAGTTAGCCAAGATGT, reverse-CATTGGGGTAGAAGCGGTCA; and β-actin: forward-ATCAAGGAGAAGCTCTGCTACATC, reverse, TCAGACTCGGCTGGAAGAGA. All experiments were carried out twice in triplicate. Negative controls were run at the same time without reverse transcriptase in the reaction.
Immunohistochemistry
The deidentified formalin-fixed, paraffin-embedded endometrium and endometriosis tissues were used to make tissue sections. Tissue sections from biopsies (each category containing at least 3 samples in the normal proliferative phase and 3 samples in the secretory phase) were deparaffinized followed by dehydration with xylene and ethanol, stained with hematoxylin and eosin. The presence of endometriosis and the menstrual cycle phase were confirmed by histology. For immunohistochemistry (IHC) analysis, sections were rinsed for 5 minutes in water, and an antigen retrieval was carried out in 0.01 mol/L sodium citrate (pH 6) buffer for 12 minutes, followed by 20-minute cooling and rinsing in phosphate-buffered saline with 0.1% Tween (PBST) for 5 minutes. Later, sections were incubated in 3% hydrogen peroxide for 10 minutes, followed by three 5-minute PBST washes for 3 times and blocked with 2% normal goat serum. Sections were incubated with primary antibodies anti-CXCL12 (Millipore, Temecula, California) or anti-CXCR4 (Thermo Fisher Scientific, MA, USA) in a humidified chamber for overnight at 4°C followed by washings and then incubated with biotinylated secondary antibody for 1 hour at RT. Sections were washed and then incubated with avidin-biotinylated enzyme complex for 30 minutes at RT and washed twice before staining for 2 minutes for CXCL12 and 1 minute for CXCR4 with diaminobenzidine tetrachloride. Sections were washed in water for 5 minutes and counterstained for 20 seconds with hematoxylin (20% in tap water) and dehydrated through alcohol, cleared in xylene, and mounted in DePeX. Negative control sections were processed in the same way but substituting primary antibodies with normal goat immunoglobulin G. Analysis of CXCL12 and CXCR4 expression was evaluated in each endometrial compartment (epithelium, glands, and stroma). The number of stained cells and the intensity of staining were evaluated in 5 high-power fields on each slide by 3 evaluators without knowledge of the specimen source. Staining was quantified using the H-score method.32,33 The Mann-Whitney rank test was used for statistical analysis.
Cell Culture
Primary cell cultures were prepared by chopping the tissue collected from 5 normal endometrium and 5 endometriosis lesions. The finely minced tissue was incubated in Hanks balanced salt solution containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; 25 mm), 1% penicillin/streptomycin, collagenase (1 mg/mL, 15 U/mg), and DNase (0.1 mg/mL, 1500 U/mg) for 45 minutes at 37°C with agitation. During and at the end of the incubation, the tissue was pipetted gently to disperse the cells every 15 minutes. Endometrial cells were pelleted, washed, and suspended in Ham’s dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12) (1:1) containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 1% amphotericin B. A mixture of endometrial cells (epithelial and stromal) was passed through a 40-µm sieve, which allowed stromal cells to pass through while epithelial cells are retained on the sieve (Millipore, Billerica, Massachusetts). The stromal cells were cultured for an additional 48 hours before carrying out the experiments.
CXCL12 Protein Assay of Conditioned Medium by Enzyme-Linked Immunosorbent Assay
The CXCL12 protein concentration in the conditioned medium from primary cell cultures was measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, Minnesota), according to the manufacturer’s instructions. Endometrial cells from endometriotic lesions and normal endometrium were cultured in DMEM/F12 supplemented with 10% FBS and 1% penicillin and streptomycin in a 6-well plate (2 × 105 cells/well). The supernatants or conditioned media were collected from 48-hour old cell cultures and were used immediately or frozen at −80°C until use.
Cell Migration Assay
The migration of mouse bone marrow cells (mBMCs) was carried out by transwell migration assay using 8-μm pore size polycarbonate membrane (Millipore, Billerica, Massachusetts). The conditioned medium (600 μL) collected from cultures of both endometriosis and normal endometrial cells after 48 hours was added into the lower chamber, and 200 μL of cells (1 × 106 cells) was placed into the upper insert. The cells in the upper insert were serum starved for 24 hours. After 16 hours, in a humidified CO2 incubator at 37°C, the nonmigrating cells were scraped with a cotton swab from the top of the membrane. The cells migrating across the membrane were fixed, stained, and lysed according to the manufacturer’s instructions. Optical densities were read in triplicate at 560 nm using a Bio-Rad Laboratories (Hercules, California) plate reader. To determine the relative percentage of migration, results were compared to the control with 100% migration. Each experiment was performed 3 times in triplicate using specimens from 5 subjects without endometriosis and 5 subjects with endometriosis. Results are reported as a chemotactic index, defined as cells migrating in response to the conditioned supernatants divided by cells response to the serum-free DMEM/F12 medium. The Mann-Whitney U test was used to assess the significance of the difference in the acquired data.
Statistical Analysis
Results from qRT-PCR and ELISA were analyzed by 1-way analysis of variance with SPSS 11.0. The Mann-Whitney rank test was used to compare migration assay data and H-scores. A P value (<.05) was considered significant.
Results
Increased Expression of CXCL12 and CXCR4 in Women With Endometriosis
The qRT-PCR results showed increased mRNA expression of CXCL12 and CXCR4 in women with endometriosis compared to women without endometriosis. As shown in Figure 1A and B, CXCR4 expression was increased nearly 4-fold, whereas CXCL12 was increased 6.5-fold in endometriosis compared to normal endometrium. In order to confirm that the increased mRNA expression of CXCL12 resulted in an increase in secretion of the cytokine, its protein levels were determined in the conditioned media from primary cell cultures obtained from endometriotic lesions and normal endometrium using the ELISA. As shown in Figure 1C, CXCL12 protein levels were increased by 2.3-fold in the conditioned media from primary stromal cell cultures of endometriotic lesions (3144 ± 324 ng/mL) compared with the conditioned media of normal endometrium stromal cells (1348 ± 251 ng/mL).
Figure 1.
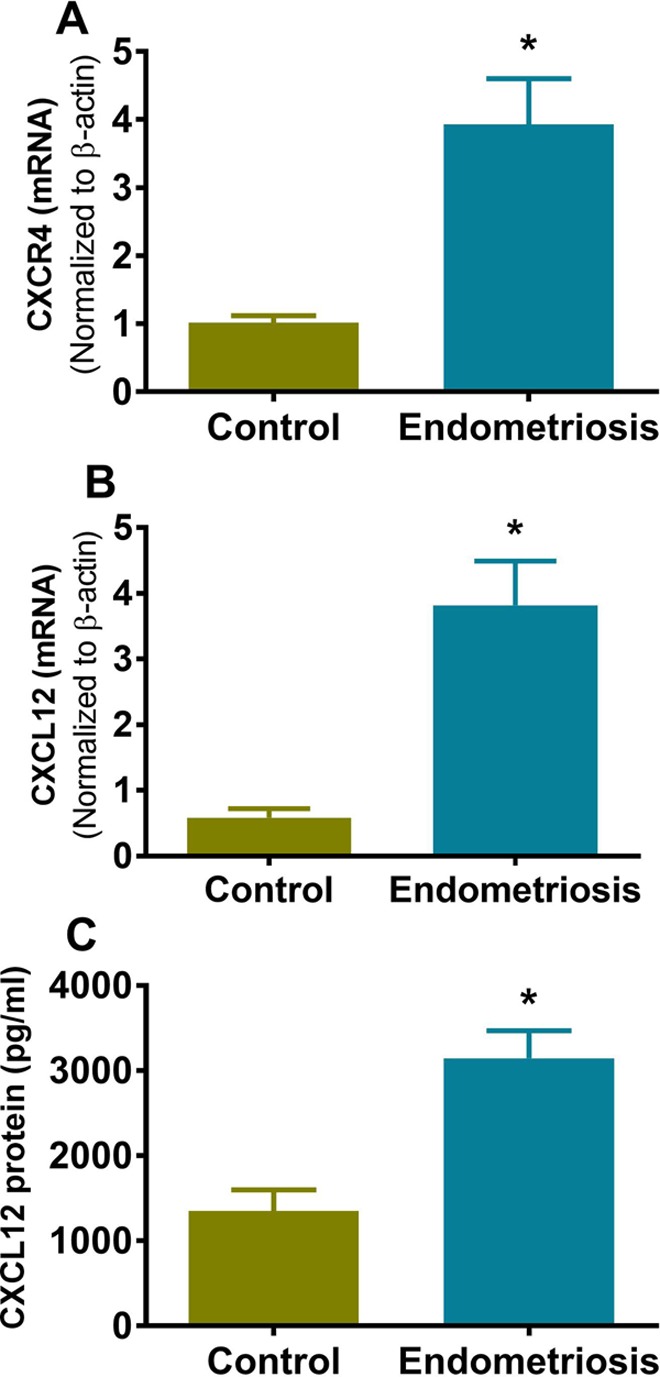
Increased expression of CXCR4 and CXCL12 in women with endometriosis. A and B, Quantitative real-time polymerase chain reaction (qRT-PCR) of CXCR4 and CXCL12, respectively. The messenger RNA (mRNA) levels of both genes were increased significantly (CXCR4, P < .02; CXCL12, P < .0001) in women with endometriosis compared to women without endometriosis. Bars represent the mean ± standard error of the mean (SEM) of 10 controls and 9 endometriosis samples from distinct subjects. C, Quantification of secreted CXCL12 protein by enzyme-linked immunosorbent assay (ELISA) tested in conditioned media of primary cell culture. The protein content was significantly higher in the conditioned medium of cells from endometriosis compared to normal endometrium (P < .001). Each bar represents the mean ± SEM of 4 independent samples from unique subjects. Each experiment was performed in triplicate. *Statistically significant difference (P < .05) versus normal endometrium.
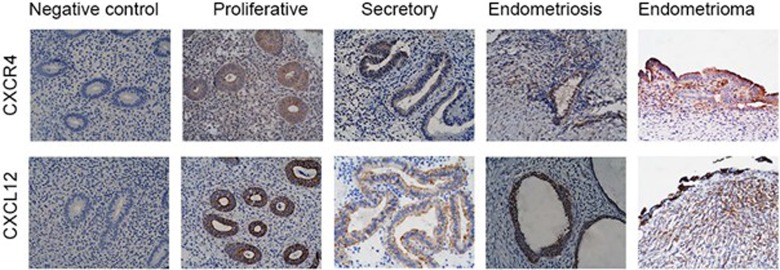
Increased CXCR4 and CXCL12 Protein in Endometriotic Epithelium and Stroma
The localization and protein expression of CXCR4 and CXCL12 were studied in both endometriotic lesions and normal endometrium using IHC. The CXCR4 was expressed in the stroma, glands, and epithelium in both endometriosis and normal endometrial tissue; however, the intensity was significantly higher in all cellular compartments of the endometriotic lesions when compared to normal endometrium (Figure 2). CXCL12 expression was also higher in endometriotic lesions and was greatest in the epithelial compartment as shown in Figure 2. The increased protein levels of CXCR4 and CXCL12 in endometriotic lesions compared to normal endometrium were confirmed by H-score on the stained sections. Table 1 shows the H-score for CXCR4 was 243 for endometriosis versus 142 for normal endometrium (P < .01), whereas the H-score for CXCL12 was 260 versus 160 (P < .02) in endometriosis and control endometrium, respectively. In glandular epithelium, CXCR4 immunostaining was predominantly localized in the cytoplasm, toward the lumen. The staining for CXCL12 was positive in the epithelial compartment and appeared to be stronger in luminal epithelium compared with the glands. The intensity was higher in endometriosis compared to normal endometrium. The CXCL12 and CXCR4 staining showed no significant difference between the proliferative and secretory phases.
Figure 2.
Detection of CXCR4 and CXCL12 by immunohistochemical staining. A, The immunostaining of CXCR4 and CXCL12 of negative control, proliferative phase, secretory phase, endometriosis, and endometrioma tissue sections, using anti-CXCR4 and anti-CXCL12 antibodies. The intensity of staining of CXCR4 and CXCL12 was greater in endometriosis and endometrioma compared to normal endometrium.
Table 1.
Quantification of CXCR4 and CXCL12 of immunostained sections by H-score.
| H-Score | ||||
|---|---|---|---|---|
| Negative Control | Normal Endometrium | Endometriosis | P-value | |
| CXCR4 | 100 | 142 | 243 | P < 0.01 |
| CXCL12 | 100 | 160 | 260 | P < 0.02 |
| Proliferative | Secretory | P-value | ||
| CXCR4 | 152 | 130 | NS | |
| CXCL12 | 160 | 163 | NS |
The average H-score is significantly higher in endometriosis than normal endometrium. Statistical analysis was performed using Mann-Whitney rank sum test, and P < .05 was considered statistically significant.
Effect of CXCl12 on Migration of BMSCs
A migration assay was carried out to determine the chemoattractant activity of CXCL12 on mBMCs, using the conditioned media from primary cultures of both endometriotic lesions and normal endometrium. Previously, we have shown that CXCL12 was necessary and sufficient to attract BMSCs to conditioned media from normal endometrial stromal cells.29 In this study, we show that conditioned medium from endometriotic cells also significantly induced the migration of mBMCs. The migration of BMSCs was greater toward conditioned medium from endometriotic cells than toward the conditioned medium from normal endometrium cells as shown in Figure 3 (2.37-fold; P < .0001).
Figure 3.
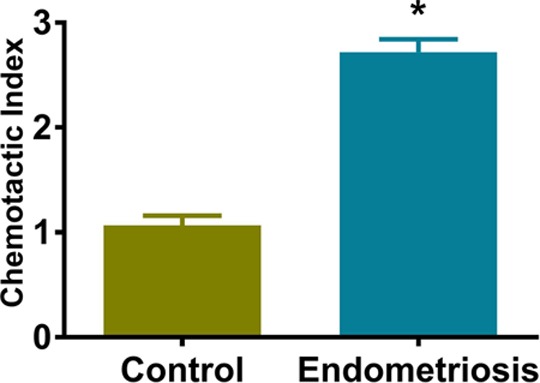
The chemotactic activity of CXCL12 in conditioned medium by migration assay. The chemoattractant activity of mouse bone marrow cells (mBMCs) was determined by migration assay. The migration of mBMCs toward hESC-conditioned medium obtained from primary cell culture of endometriotic lesions or from normal endometrium was determined by seeding cells (5 × 104/well) on inserts and measuring the migration toward serum-free medium alone (negative control) or 48-hour hESCs serum-free supernatant. Data are shown as a chemotactic index (CI): cells migrating in response to the conditioned supernatants divided by cells responding to the serum-free medium. Bars represent the mean ± standard error of the mean (SEM) of 4 samples from controls and endometriosis, each performed in triplicate. The CI is significantly greater for endometriosis than normal endometrium. *Statistically significant difference (P < .0001) versus normal endometrium.
Discussion
In previous studies, we have shown that the chemoattractant activity of the chemokine receptor CXCR4 is due to its specific ligand CXCL12 in the conditioned media of primary cultures of normal endometrium from women without endometriosis.29 Here, we demonstrate that the expression and secretion of CXCL12 is elevated in endometriosis compared to normal endometrium. We demonstrated that the expression of both CXCR4 and CXCL12 is upregulated in endometriotic lesions relative to normal endometrium. These results are in agreement with the previous reports,11,24,34,35 which described the upregulation of CXCR4 in both ectopic and eutopic endometrium of patients with endometriosis, as well as in endometriotic lesions in a rat model of endometriosis34 and the peritoneal fluid from endometriosis patients.36–38 More recently, it is been reported that the activation of the CXCR4–CXCL12 signaling axis is involved in the pathophysiology of endometriosis by promoting invasion and growth of endometrial cells ectopically39,40 and is responsible for the survival of human secretory phase endometrial stromal cells by the inhibition of autophagy.41 We demonstrated previously that E2 treatment at physiological levels induced the expression of both CXCR4 and CXCL12 in mBMCs and in human endometrial stromal cells (hESCs), respectively, and enhanced the chemoattraction activity of CXCR4.29 Similarly, Ruiz et al reported that progesterone treatment results in the downregulation of CXCR4 and CXCL12 in endometrial cell lines and estradiol treatment results in an increase in epithelial CXCL12 expression (although nonsignificant in their study).40 Microarray studies that show the increased mRNA expression of both CXCR4 and CXCL12 in endometriotic lesions further support our results.1,35
Bone marrow (BM) contains multiple cell types including mesenchymal stem cells, which have the capacity to differentiate into a number of numerous types of cells. Bone marrow–derived stem cells are involved in the regeneration of the endometrium and contribute to the remarkable regenerative capacity of the endometrium.28,42,43 Bone marrow–derived stem cells have been shown to engraft to normal endometrium as well as endometriosis.4 Previously, our laboratory described the engraftment of BMDSCs into the uterus of women who received single-antigen human leukocyte antigen–mismatched BM transplants42 and the engraftment of stem cells into the endometrium of mice.4 We have also demonstrated the increased recruitment of BMDSCs to the site of uterine injury in female mice when the BM of male mice was transplanted into female mice.4,44 The functional importance of stem cell flux to the uterus was demonstrated by experiments showing that BM cell delivery to mice after uterine injury improved the reproductive performance.45 We have recently demonstrated that endometriosis can arise from the endometrium or from an exogenous sources including BM as a result of ectopic differentiation of stem cells.4 In fact, endometriosis is more effective than endometrium in recruiting circulating BMDSCs from the circulation.26 Endometriosis competes with normal endometrium for a limited supply of stem cells, depriving the eutopic endometrium of stem cells needed for optimal regeneration. One of the mechanisms that explains this engraftment is chemokine signaling. Chemokines are small cytokine molecules that attract various cell types by their chemotactic function and regulate the mobilization and homing of stem cells.28,45,46 The chemokine receptor CXCR4 is commonly expressed on the surface of stem cells that are attracted toward its ligand CXCL12. CXCL12 is produced by many cell types, including the stroma and epithelium of the endometrium and is generally expressed at sites of inflammation and injury.47–49 The interaction between CXCR4 and CXCL12 allows the CXCR4–CXCL12 signaling axis to be involved in angiogenesis, tissue repair, migration, and invasion.50 Bone marrow–derived stem cells that express the CXCR4 receptor migrate toward hESCs that express and secrete the CXCL12 ligand; this migration was further increased by estradiol treatment, which induced CXCL12 expression in endometrial cells.29 Our present data demonstrate that BMDSCs were attracted to endometrial stromal cell–conditioned medium, and the migration was increased toward endometriosis-conditioned media that contain high concentrations of CXCL12. Variations in CXCL12 concentration create a chemical gradient that directs the migration of stem cells.47 Our study is in agreement with other studies that show higher levels of CXCR4 and CXCL12 in epithelium of ovarian endometriosis and ovarian cancer tissue when compared to normal ovary.51,52 Moreover, endometriotic lesions have been shown to contain populations of mesenchymal stem cells that contribute in the development and progression of endometriosis.53–58 CXCL12 has been previously identified as an E2-regulated gene in E2 receptor-positive ovarian and breast cancer cells.59 Previously, we have shown that in the endometrium, E2 significantly increased CXCL12 expression, suggesting a mechanism by which stem cells are recruited to the uterus in reproductive-age women.59 It is likely that this recruitment decreases after menopause when the uterus is no longer needed for reproduction. Premenopausal E2s provide a favorable environment for inducing CXCL12 and enhancing BM-derived stem cell migration to both normal and ectopic endometrium. The inflammatory environment of endometriosis may program higher CXCL12 expression that favors preferential stem cell recruitment to endometriosis.
In conclusion, the activation of the CXCR4–CXCL12 signaling axis plays a critical role in stem cell migration that results in both the formation of new endometriotic lesions, as well as the inappropriate incorporation of stem cells into the eutopic endometrium. The high CXCL12 production in endometriosis causes increased incorporation of stem cells into endometriosis. Furthermore, the elevated CXCL12 production by endometriosis relative to normal endometrium provides a mechanism by which endometriosis preferentially recruits the limited supply of circulating stem cells. Future treatments for endometriosis may alter the inappropriate migration of stem cells, specifically due to the activation of the CXCR4–CXCL12 signaling pathway. Inhibition of this signaling axis may have potential therapeutic value in the treatment of endometriosis and its associated disorders.
Acknowledgments
The authors thank Joshua Huttler for editing the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH U54 HD052668 and R01 HD076422.
References
- 1. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 2. Starzinski-Powitz A, Gaetje R, Zeitvogel A, et al. Tracing cellular and molecular mechanisms involved in endometriosis. Hum Reprod Update. 1998;4(5):724–729. [DOI] [PubMed] [Google Scholar]
- 3. Montgomery GW, Nyholt DR, Zhao ZZ, et al. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14(5):447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–2086. [DOI] [PubMed] [Google Scholar]
- 5. Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med. 2015;33(5):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Figueira PG, Abrao MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 10. Witz CA, Thomas MR, Montoya-Rodriguez IA, Nair AS, Centonze VE, Schenken RS. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Steril. 2001;75(2):385–390. [DOI] [PubMed] [Google Scholar]
- 11. Chand AL, Murray AS, Jones RL, Hannan NJ, Salamonsen LA, Rombauts L. Laser capture microdissection and cDNA array analysis of endometrium identify CCL16 and CCL21 as epithelial-derived inflammatory mediators associated with endometriosis. Reprod Biol Endocrinol. 2007;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chand AL, kConFab, Simpson ER, Clyne CD. Aromatase expression is increased in BRCA1 mutation carriers. BMC Cancer. 2009;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62(3):139–147. [DOI] [PubMed] [Google Scholar]
- 14. Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7(12):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 267(2):226–244. [DOI] [PubMed] [Google Scholar]
- 16. Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. [DOI] [PubMed] [Google Scholar]
- 17. Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768(4):952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. [DOI] [PubMed] [Google Scholar]
- 19. Hanna J, Wald O, Goldman-Wohl D, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16–human natural killer cells. Blood. 2003;102(5):1569–1577. [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16− NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. 2005;175(1):61–68. [DOI] [PubMed] [Google Scholar]
- 21. Wu X, Li DJ, Yuan MM, Zhu Y, Wang MY. The expression of CXCR4/CXCL12 in first-trimester human trophoblast cells. Biol Reprod. 2004;70(6):1877–1885. [DOI] [PubMed] [Google Scholar]
- 22. Park DW, Lee HJ, Park CW, Hong SR, Kwak-Kim J, Yang KM. Peripheral blood nk cells reflect changes in decidual nk cells in women with recurrent miscarriages. Am J Reprod Immunol. 2010;63(2):173–180. [DOI] [PubMed] [Google Scholar]
- 23. Jaleel MA, Tsai AC, Sarkar S, Freedman PV, Rubin LP. Stromal cell-derived factor-1 (SDF-1) signalling regulates human placental trophoblast cell survival. Mol Hum Reprod. 2004;10(12):901–909. [DOI] [PubMed] [Google Scholar]
- 24. Furuya M, Suyama T, Usui H, et al. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38(11):1676–1687. [DOI] [PubMed] [Google Scholar]
- 25. Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014;155(4):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou S, Zilberman Y, Wassermann K, Bain SD, Sadovsky Y, Gazit D. Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem Suppl. 2001;(suppl 36):144–155. [DOI] [PubMed] [Google Scholar]
- 28. Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 2001;938:83–95. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barr A, Manning D. G Proteins Techniques of Analysis. Boca Raton, FL: CRC Press, Inc; 1999:227–245. [Google Scholar]
- 31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 32. Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential expression and localization of de-novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Hum Reprod. 2000;15(10):2180–2185. [DOI] [PubMed] [Google Scholar]
- 33. Lessey BA, Castelbaum AJ, Sawin SW, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79(2):643–649. [DOI] [PubMed] [Google Scholar]
- 34. Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril. 2007;87(5):1180–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuzaki S, Canis M, Vaurs-Barriere C, et al. DNA microarray analysis of gene expression profiles in deep endometriosis using laser capture microdissection. Mol Hum Reprod. 2004;10(10):719–728. [DOI] [PubMed] [Google Scholar]
- 36. Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996;2(1):40–45. [DOI] [PubMed] [Google Scholar]
- 37. Akoum A, Lemay A, McColl S, Turcot-Lemay L, Maheux R. Elevated concentration and biologic activity of monocyte chemotactic protein-1 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1996;66(1):17–23. [PubMed] [Google Scholar]
- 38. Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN. Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol Hum Reprod. 2001;7(2):163–168. [DOI] [PubMed] [Google Scholar]
- 39. Leconte M, Chouzenoux S, Nicco C, et al. Role of the CXCL12-CXCR4 axis in the development of deep rectal endometriosis. J Reprod Immunol. 2014;103:45–52. [DOI] [PubMed] [Google Scholar]
- 40. Ruiz A, Salvo VA, Ruiz LA, Baez P, Garcia M, Flores I. Basal and steroid hormone-regulated expression of CXCR4 in human endometrium and endometriosis. Reprod Sci. 2010;17(10):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mei J, Zhu XY, Jin LP, Duan ZL, Li DJ, Li MQ. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum Reprod. 2015;30(7):1677–1689. [DOI] [PubMed] [Google Scholar]
- 42. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. [DOI] [PubMed] [Google Scholar]
- 43. Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16(2):126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21(18):3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-beta, and copaxone. J Interferon Cytokine Res. 2007;27(1):53–64. [DOI] [PubMed] [Google Scholar]
- 46. Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014;32(9):483–492. [DOI] [PubMed] [Google Scholar]
- 47. Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44(4):575–582. [DOI] [PubMed] [Google Scholar]
- 48. Dotan I, Werner L, Vigodman S, et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16(4):583–592. [DOI] [PubMed] [Google Scholar]
- 49. Jiang HW, Ling JQ, Gong QM. The expression of stromal cell-derived factor 1 (SDF-1) in inflamed human dental pulp. J Endod. 2008;34(11):1351–1354. [DOI] [PubMed] [Google Scholar]
- 50. Liekens S, Schols D, Hatse S. CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr Pharm Des. 2010;16(35):3903–3920. [DOI] [PubMed] [Google Scholar]
- 51. Laird SM, Widdowson R, El-Sheikhi M, Hall AJ, Li TC. Expression of CXCL12 and CXCR4 in human endometrium; effects of CXCL12 on MMP production by human endometrial cells. Hum Reprod. 2011;26(5):1144–1152. [DOI] [PubMed] [Google Scholar]
- 52. Ikoma T, Kyo S, Maida Y, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201(6):608.e601-e608. [DOI] [PubMed] [Google Scholar]
- 53. Gargett CE, Gurung S. Endometrial mesenchymal stem/stromal cells, their fibroblast progeny in endometriosis, and more. Biol Reprod. 2016;94(6):129. [DOI] [PubMed] [Google Scholar]
- 54. Barragan F, Irwin JC, Balayan S, et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod. 2016;94(5):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Santamaria X, Massasa EE, Taylor HS. Migration of cells from experimental endometriosis to the uterine endometrium. Endocrinology. 2012;153(11):5566–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wolff EF, Mutlu L, Massasa EE, Elsworth JD, Eugene Redmond D, Jr, Taylor HS. Endometrial stem cell transplantation in MPTP-exposed primates: an alternative cell source for treatment of Parkinson’s disease. J Cell Mol Med. 2015;19(1):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wolff EF, Gao XB, Yao KV, et al. Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J Cell Mol Med. 2011;15(4):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wolff EF, Wolff AB, Hongling D, Taylor HS. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14(6):524–533. [DOI] [PubMed] [Google Scholar]
- 59. Jaerve A, Schira J, Müller HW. Concise Review: the potential of stromal cell-derived factor 1 and its receptors to promote stem cell functions in spinal cord repair. Stem Cells Transl Med. 2012;1(10):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]