Abstract
Background
A prospective clinical study was conducted to assess different regimens of steroid therapy and preservation of hearing following cochlear implantation.
Material/Methods
Study participants were ≥18 years-of-age, with a cochlear duct length ≥27.1 mm measured by computed tomography (CT), with hearing sound levels in the range of 10–120 decibels (dB) and sound frequencies of 125–250 hertz (Hz); sound levels of 35–120 dB and frequencies of 500–1,000 Hz; sound levels of 75–120 dB and frequencies of 2,000–8,000 Hz. Study exclusion criteria included diseases with contraindications for steroid therapy or medications that increased the effects of steroids. Patients had cochlear implantation and were divided into three treatment groups: intravenous (IV) steroid therapy (standard steroid therapy): combined oral and IV steroid therapy (prolonged steroid therapy); and a control group (cochlear implantation without steroid therapy). Hearing preservation was established by pure tone audiometry based on the pre-operative and postoperative average hearing thresholds according to the formula developed by the HEARRING Network.
Results
There were 36 patients included in the study. In all cases, the cochlear implant electrode was inserted via the round window approach with a straight electrode length of 28 mm. Patients with combined oral and IV steroid therapy (prolonged steroid therapy) had better results when compared with patients with intravenous (IV) steroid therapy (standard steroid therapy) and the control group.
Conclusions
Prolonged steroid therapy using combined oral and IV steroids stabilized hearing thresholds and preserved hearing in adult patients following cochlear implantation.
MeSH Keywords: Cochlear Implants, Correction of Hearing Impairment, Drug Therapy, Tissue Preservation
Background
Cochlear implantation is now considered to be the ‘gold standard’ treatment method for hearing impairment [1,2]. Early treatment options were dedicated exclusively for patients who were totally deaf, but during the last 15 years, there has been an increase in the availability of cochlear implantation for different types of hearing impairment. Following expert discussions concerning hearing preservation, the introduction of standardized residual hearing protection procedures have been introduced in the leading centers around the world [3–6]. Regardless of the surgical technique for cochlear implantation, whether via an approach through the round window (RW) or cochleostomy, specialized centers have now introduced the comprehensive analysis of the non-surgical factors contributing to improved rates of hearing preservation following surgery [7].
One of the approaches to improving hearing function following cochlear implantation has been the use of corticosteroids, such as dexamethasone [8,9]. Dexamethasone is a synthetic glucocorticoid, or corticosteroid, that differs from prednisolone only by the positioning of a fluorine atom in the compound [10]. Dexamethasone has many beneficial anti-inflammatory and immunosuppressive effects and is used in the treatment of diseases including arthritis, asthma, chronic obstructive pulmonary disease (COPD), dermatitis, allergies and adrenocortical insufficiency [10,11]. In otorhinolaryngology, dexamethasone is also widely used in the treatment of severe or chronic conditions such as sudden sensorineural hearing loss (SSNHL), partial deafness, Ménière’s disease, autoimmune disorders, and following surgical procedures [11,12]. The mechanism of the anti-inflammatory activity of dexamethasone involves the prevention of adhesion of leukocytes to the vascular endothelium and phagocytic clearance of apoptotic leukocytes. However, the adverse effects of this corticosteroid may be considered to be a limitation, when treatment duration exceeds two weeks. Long-term corticosteroid use can be associated with diabetes, obesity, hemorrhage from the digestive system, osteoporosis, hypertension, glaucoma, cataract, depression, skin irritation, disorders of acid-base balance, metabolic and hormonal disorders.
The fluid of the inner ear maintains its homeostasis by a variety of regulatory mechanisms for the ion transport system, and the blood supply, and includes a blood-labyrinth barrier, which is major physical and biochemical barrier separating the inner ear from the systemic circulation. A highly regulated system of ion transport into and out of the cells of the inner ear leads to maintenance of inner ear fluid and provides optimal auditory transduction [13–15]. Any disturbance of ionic, metabolic, or osmotic balance may cause a disorder of homeostasis of the inner ear. There are now known to be many external and internal factors may induce long-term or short-term imbalance in the inner ear; the most important are noise exposure, inflammation, free radicals, hormones, stress, and drug toxicity [16]. Efflux pump systems, such as P-glycoprotein (P-gp) and multidrug resistance-related protein-1 (MRP-1) further protect the inner ear [17].
However, current knowledge of the drug transport process via the blood-labyrinth barrier is limited, but it is known that this tight junction barrier permits the delivery of only small lipid-soluble chemical and biological molecules [18]. Glycerol, which is an osmotic agent, has been shown to increase the concentration of steroids and vasodilators in the lymph of the inner ear after systemic administration It is possible to deliver drugs into the inner ear, but only a few of these drugs may reach a therapeutic concentration due to the presence of the blood-labyrinth barrier. Although the systemic route of drug administration, via the oral, intravenous (IV), and intramuscular (IM) routes, might cause serious adverse effects due to the high drug doses required, these routes are considered to be the most convenient and reliable methods of first-line drug administration for the treatment of the inner ear disorders.
The aim of the study was to assess how different models of steroid therapy influence hearing preservation following cochlear implantation. Three treatment groups were studied, including patients with IV steroid therapy (standard steroid therapy), combined oral and IV steroid therapy (prolonged steroid therapy), and a control group (cochlear implantation without steroid therapy).
Material and Methods
Ethical approval and patients studied
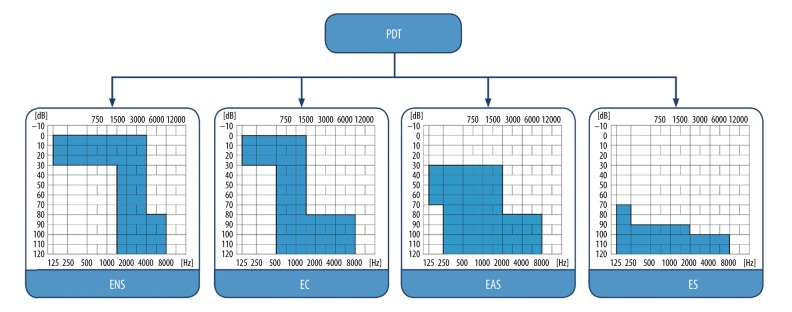
The protocol of this prospective clinical study was approved by the Bioethical Committee of the World Hearing Center of the Institute of Physiology and Pathology of Hearing and complied with the Declaration of Helsinki. In the longitudinal study, all subjects underwent audiometric testing in four intervals: pre-operatively, on cochlear implant activation, at one-month follow-up, and at a six-month follow-up. All patients suffered from severe to profound hearing loss, classified according to the Skarzynski partial deafness treatment (PDT) classification, as follows: PDT-electrical complement (PDT-EC) or PDT-electro-acoustic stimulation (PDT-EAS) (Figure 1) [19,20].
Figure 1.
Partial deafness treatment groups for cochlear implantation. ENS – electro-natural stimulation; EC – electrical complement; EAS – electrical-acoustic stimulation; ES – electrical stimulation.
Study eligibility criteria included patients ≥18 years-of-age, with a cochlear duct length ≥27.1 mm measured by computed tomography (CT) imaging, with hearing sound levels in the range of 10–120 decibels (dB) and sound frequencies of 125–250 hertz (Hz); sound levels of 35–120 dB and frequencies of 500–1,000 Hz; sound levels of 75–120 dB and frequencies of 2,000–8,000 Hz.
Study exclusion criteria included the presence of diseases with contraindications for steroid therapy, or patients on medications that increased the effects of steroids. These criteria were in accordance with the consensus of the international HEARRING group on hearing preservation in cochlear implant users [20].
Patients treatment groups
The study was prospective in design and included 36 patients who had undergone cochlear implantation and who were divided into three groups, according to their steroid treatment regimen. The first group of nine patients (six men and three women) included patients treated with intravenous (IV) steroid therapy (standard steroid therapy) following cochlear implantation, aged between 27–64 years (mean, 43±13.83 years). The second group of five patients (three men and two women) included patients treated with combined oral and IV steroid therapy (prolonged steroid therapy) following cochlear implantation, aged between 25–68 years (mean, 45±20.37 years). The control group included 22 patients (12 men and 10 women) who had undergone cochlear implantation without steroid therapy, aged between 18–74 years (mean, 52.18±16.42 years).
Steroid treatment regimens
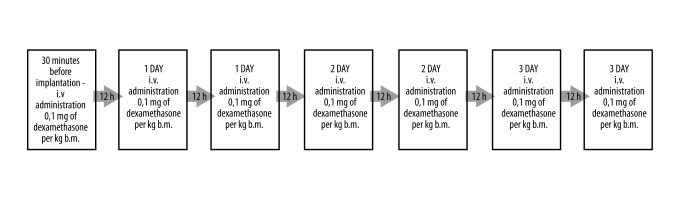
In the first group consisting of patients treated with intravenous (IV) steroid therapy (standard steroid therapy) (Figure 2), patients were treated with dexamethasone with the dose of 0.1 mg per kg of body mass, which was administered IV for 30 minutes before the cochlear implantation surgery. The same dose was administered intravenously every 12 hours for three consecutive days. One pack of dexamethasone contained ten ampoules of a 2 ml solution (4 mg/ml). Just before the injection, the contents of the ampoule were diluted with isotonic sodium chloride solution. The IV route of administration was chosen to check the effects of standard steroid administration.
Figure 2.
Study design for the first group, treated with intravenous steroid only. IV – intravenous; bm – body mass.
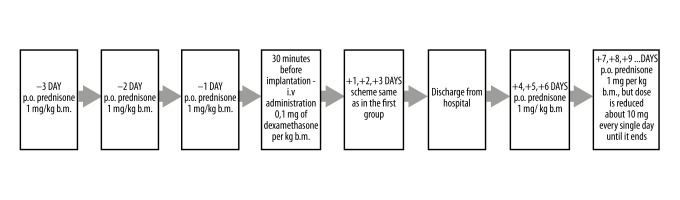
In the second group consisting of patients treated with combined oral and IV steroid therapy (prolonged steroid therapy) following cochlear implantation (Figure 3), patients were treated with prednisone at a dose of 1 mg per kg of body mass, which was administered orally three days prior to surgery. There are three types of enteric-coated tablets available on the market, which were individually suited to the patients’ medical needs: 100 tablets of 5mg each, 20 tablets of 10 mg each, and 20 tablets of 20 mg each. Then, 30 minutes before the cochlear implantation surgery, dexamethasone at a dose of 0.1 mg per kg body mass was administered IV. As in the first group, the same dose was administered IV every 12 hours during three consecutive days spent on the hospital ward. Following discharge from the hospital, during the next three days the patients were treated with the oral prednisone 1 mg/kg body mass. After this time, the dose was tapered to about 10 mg per day. The IV and oral administration routes were chosen to investigate the effects of prolonged steroid administration.
Figure 3.
Study design for the second group, treated with combined oral and intravenous (IV) steroid therapy (prolonged steroid therapy). p.o. – per oral; IV – intravenous; b.m. – body mass.
The control group of patients underwent a standard cochlear implantation procedure without steroid administration.
Audiological assessment and preservation of hearing
Hearing preservation (HP) was established based on pure tone audiometry (11 frequencies ranging from 125–8000 Hz, using both octaves and semi-octaves) according to ISO 8253-1: 2010. All measurements were conducted in the same soundproof cabin by an experienced technician, using the same diagnostic audiometer, the Madsen Itera II (GN Otometrics, Denmark) with the calibrated audiometer earphones (TDH-39P) (Telephonics, NY, USA). The audiometric testing was performed before surgery, during activation (one month after surgery), and after one month and after six months post activation. The hearing preservation rate was established by international expert consensus and was calculated according to the HP rate formula [21]:
In this equation, PTApre is pure tone average measured pre-operatively, PTApost is pure tone average measured postoperatively, and PTAmax is the maximal sound intensity generated by a standard audiometer, usually 120 dB hearing level (HL). The results were divided into three groups: minimal HP (range 0–25%); partial HP (range 26–75%); and complete HP (>75%).
Statistical analysis
Non-parametric tests were used due to the discrepancies in size between each of the compared groups. The Kruskal–Wallis test was used to compare hearing thresholds in the three patient groups. To determine possible differences in hearing thresholds obtained by patients from each group in selected time intervals, the Friedman non-parametric test was used. In both tests, p<0.05 was considered to be statistically significant. The adjustment for multiple tests, the Bonferroni correction, was applied. Statistical analysis was performed using the IBM SPSS statistics software version 24.0.
Results
Table 1 shows the pure tone average (PTA) results before cochlear implant surgery, during activation (one month after surgery), and after one month and six months post-activation for each group, separately. Preoperative hearing threshold levels of patients from all of the three groups were similar, and the difference between groups was not statistically significant (χ2 (2)=0.13; p=0.935).
Table 1.
Hearing threshold levels of patients from the three groups obtained during the study time intervals.
| Minimum (Min) | Maximum (Max) | Mean (M) | Standard deviation (SD) | Median (Md) | ||
|---|---|---|---|---|---|---|
| Intravenous (IV) group | Preoperation | 75.00 | 100.45 | 91.06 | 9.92 | 97.73 |
| Activation | 81.82 | 110.00 | 99.90 | 9.34 | 102.27 | |
| One-month follow-up | 87.27 | 108.64 | 98.69 | 8.41 | 96.36 | |
| Six-month follow-up | 86.82 | 110.00 | 97.83 | 9.25 | 94.54 | |
| Oral and IV group | Preoperation | 79.55 | 102.73 | 93.91 | 8.78 | 96.82 |
| Activation | 94.09 | 102.73 | 98.27 | 3.32 | 98.18 | |
| One-month follow-up | 92.27 | 102.73 | 95.64 | 4.44 | 93.18 | |
| Six-month follow-up | 92.27 | 102.73 | 96.73 | 4.20 | 97.23 | |
| Control group | Preoperation | 75.45 | 107.95 | 91.94 | 10.27 | 91.82 |
| Activation | 87.73 | 110.00 | 105.31 | 6.23 | 109.32 | |
| One-month follow-up | 75.00 | 110.00 | 104.48 | 9.17 | 109.55 | |
| Six-month follow-up | 75.91 | 110.00 | 105.65 | 7.91 | 110.00 |
At activation, the average hearing thresholds started to differ between treated patients according to the particular steroid administration model (χ2 (2)=7.54; p=0.023). A significant difference was observed between the patients receiving combined oral and IV steroid therapy (prolonged steroid therapy), having a better PTA in low frequencies in comparison with patients from the control group (U=17.50; p=0.016).
Also, a significant difference was found between these groups at one month after activation (χ2 (2)=9.93; p=0.007). Again, patients receiving combined oral and IV steroid therapy (prolonged steroid therapy) had, on average, improved hearing thresholds than patients from the control group (U=41.50; p=0.014), and a similar relationship was noted in patients given IV treatment (U=15; p=0.012). The two steroid-treated groups showed no significant difference in hearing thresholds (U=16; p=0.386).
In the six-month post-activation period, the observed trend continued, and significant differences between groups were again found (χ2 (2)=11.90; p=0.003). Patients treated with combined oral and IV steroid therapy (prolonged steroid therapy) had mean lower hearing thresholds compared with patients from the control group (U=10.0; p=0.003); also patients treated with IV steroid therapy (standard steroid therapy) had lower mean hearing thresholds than patients from the control group (U=41.50; p=0.009). Again, there was no significant difference between the two steroid-treated groups (U=21.00; p=0.898).
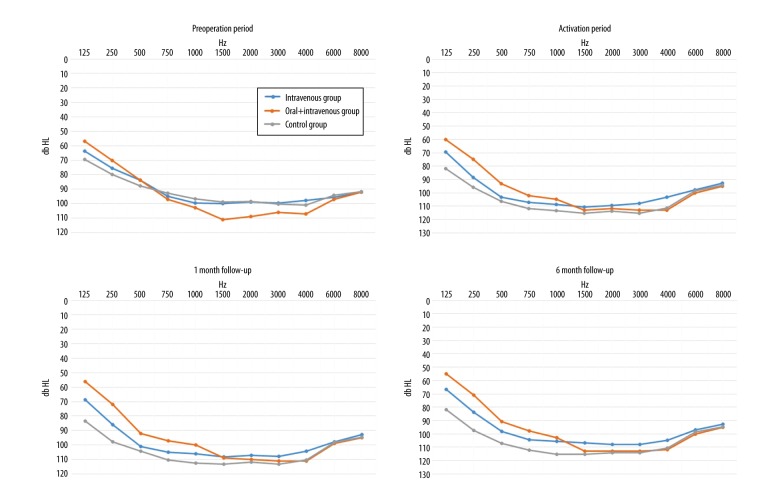
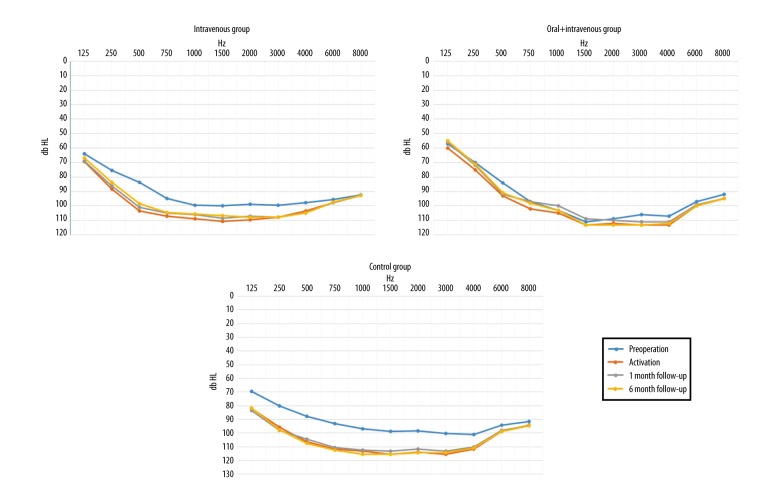
Although patients from the three patient groups appeared to have similar pre-operative hearing threshold levels, their responses began to differ over the course of their treatment (Figure 4). At the beginning of activation, results obtained by the combined oral and IV steroid therapy (prolonged steroid therapy) group began to be distinguished, as these patients had better hearing parameters when compared with the control group. In the one-month and six-month post-operative periods, patients from both steroid-treated groups had improved responses when compared with those from the control group.
Figure 4.
Average hearing threshold levels of the intravenous (IV), combined oral and IV steroid therapy (prolonged steroid therapy), and control group of patients in the pre-operative period, upon the activation of the cochlear implantation, at one-month follow-up and six-month follow-up.
Changes in the hearing thresholds were also analyzed separately for each of the patient groups (Figure 5). The study hypothesis was that the greatest changes in the hearing thresholds would be seen in patients undergoing a standard medical procedure (hearing thresholds would be lower), while steroids would stabilize hearing thresholds. Therefore, changes in the hearing parameters of the two steroid-treated groups were not expected to be significant.
Figure 5.
Average hearing threshold levels of the intravenous (IV), combined oral and IV steroid therapy (prolonged steroid therapy), and control group patients in the pre-operative period, upon activation of the cochlear implantation, at one-month follow-up and at six-month follow-up
The results of the study analysis confirmed the study hypothesis, but with some differences. In the control group, a significant difference was found between the four subsequent hearing threshold measurements was seen (χ2 (3)=37.58; p<0.001). However, a significant difference was also seen in the IV group (χ2 (3)=16.07; p=0.001). Only in the combined oral and IV steroid therapy (prolonged steroid therapy) group did hearing remain stable during the four follow-up periods and did not vary significantly (χ2 (3)=5.62; p=0.132).
The hearing preservation (HP) rate was calculated by comparing hearing thresholds in the six-month post-operative period with the preoperative hearing thresholds, which were divided, according to the HP formula, into minimal HP, partial HP, and complete HP.
Based on the HP rate, in patients who received the combined oral and IV steroid therapy, the smallest variability of results was observed, as well as the highest overall HP (Figure 6). The HP range was greatest for patients from the IV group and the control group, as shown in Figure 6, with a statistically significant difference between all groups (χ2 (2)=12.38; p=0.002).
Figure 6.
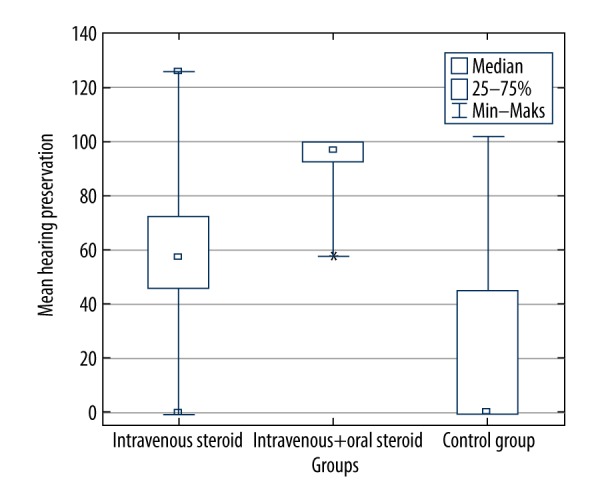
Hearing preservation (HP) rate in the study groups [21].
However, only in the combined oral and IV steroid therapy (prolonged steroid therapy) group, was the HP rate significantly greater compared with the control group (U=9.00; p=0.003), and the difference in HP between the IV and control group was insignificant after applying the adjustment for multiple tests (U=47; p=0.019). Therefore, the analysis of HP rate resulted in findings that showed that combined oral and IV steroid therapy (prolonged steroid therapy) administration had a positive impact and was more beneficial than the traditional cochlear implantation procedure.
Table 2 shows the percentage of patients who qualified for different HP groups, six months after surgery, according to treatment type. The majority of patients from the control group had minimal HP range, whereas in the iV group frequently showed partial HP. Almost all patients from the combined oral and IV steroid therapy (prolonged steroid therapy) group had complete HP, and none of them had the minimal HP range.
Table 2.
The percentage of patients who qualified for different hearing preservation (HP) groups, six months after surgery, according to treatment type.
| Minimal HP | Partial HP | Complete HP | |
|---|---|---|---|
| Intravenous (IV) group | 22.2% | 55.6% | 22.2% |
| Oral and IV group | 0.0% | 20.0% | 80.0% |
| Control group | 68.2% | 18.2% | 13.6% |
Discussion
Previously published studies have shown that, in animal models, prolonged elution of steroid treatment could significantly improve hearing preservation rate, with morphologic and pharmacokinetic analysis, demonstrating that cochlear implants with incorporated dexamethasone were able to release steroid continuously into the inner ear [22]. This previous finding has significance for the long-term recovery and preservation of auditory function after cochlear implantation. However, Honeder et al. did not confirm that steroids could have a positive impact on residual hearing in a guinea pig model [23]. These different preclinical findings may relate to the different type of steroid therapy used in these studies, which included dexamethasone and triamcinolone, the latter having a short therapeutic duration [22–24]. Douchement et al. investigated the effects of steroids using a gerbil animal model, in which the animals were implanted with an eluting electrode loaded with dexamethasone (1% and 10%) on one side and a conventional passive electrode on the contralateral side [24]. Hearing levels were established based on the tone bursts on auditory brainstem responses at 4–6 weeks post-implantation and at one-year post-implantation for older gerbils [24]. After a one-year observation period, significantly improved results were obtained for the high auditory frequencies, but the results for the low frequencies were ambiguous [24].
There are two main ways of delivering corticosteroids to the inner ear; the first is by intratympanic (IT) injection, and the second is by systemic delivery, by oral use or intravenous (IV) injection. Delivery of IT steroids is increasingly used both for research and clinical purposes [25]. However, in the present study, a decision was made to use oral or IV steroid treatment, based on promising clinical results from these methods. Also, taking oral steroids can be done away from the hospital and results in a reduction in treatment costs and saving of clinical time and other resources.
Bioavailability of dexamethasone when administered orally, is 78%; following IV treatment, the maximum concentration is achieved after 10–30 minutes. The level of protein binding for dexamethasone is 68%, and the half-life of 190 minutes. Pharmacokinetic factors influence the concentration of corticosteroids in the inner ear including differing total body volume, variability in their ability to cross the blood-labyrinth barrier, different drug metabolic pathways, and routes of excretion [26].
Another steroid used in the field of otolaryngology is prednisone, which is known to have immunosuppressive activity. Prednisolone is the metabolically active form of prednisone. Due to the short half-life of prednisolone, it is difficult to achieve the steady therapeutic state [27]. The initial dose of oral prednisone is usually between 5–60 mg per day. Dexamethasone and prednisone are transported after absorption, via the cell membrane, to the cytoplasm where specific receptors for corticosteroids are located. There then follows a complex process of transporting the drug and receptor to the cell nucleus where activation of protein transcription and translation occurs. Bioavailability of prednisone is between 70–90% and its half-life are 216 minutes. Both dexamethasone and prednisone are metabolized by the liver and then eliminated by excretion via the bile and the urine.
In routine hearing preservation surgery, when there is significant residual hearing, we prefer to apply shorter electrode insertion (20, 24, or 25 mm) than was used in the present study (28 mm). In the partial deafness treatment electro-acoustic stimulation (PDT-EAS) cases [4,20], where hearing levels are higher in the low frequencies, we routinely perform deeper insertion up to 25 mm [4,20]. In many centers, 28 mm electrodes are used more commonly for the treatment of deaf patients, replacing the standard full-length electrode (31 mm) [28].
Cho et al. analyzed the efficacy of preoperative and intraoperative steroid administration for hearing preservation after cochlear implantation [29]. The authors used dexamethasone (5 mg/ml) that was systemically administered before the operation and topically applied during cochlear implantation surgery [29]. In contrast to our study, where the whole range of audiometric frequencies was evaluated, the authors calculated the pure tone average (PTA) only from four frequencies (250, 500, 1,000, and 2,000 Hz) [29]. Although these authors did not analyze the prolonged application of steroids, statistically significant differences were observed between the steroid and control group, supporting the beneficial impact of steroid treatment, even a year after surgery [29].
The findings of this study have shown that prolonged steroid treatment can be even more beneficial for patients with cochlear implantation. Only in the second treatment group in this study, treated with combined oral and IV steroid therapy (prolonged steroid therapy), did the hearing of the study participants remain stable during the four follow-up periods, where they did not vary significantly.
Sweeney et al. analyzed the influence of oral prednisone on postoperative hearing preservation rate and degree of hearing preservation [8]. The drug was applied three days before surgery and subsequently tapered during two weeks [8]. Patients were implanted with a standard length of the electrode [8]. These authors also mainly analyzed the hearing threshold for low frequencies, providing no data on high-frequency hearing [8]. The results obtained in a relatively short period (during cochlear implantation activation) showed a positive impact on hearing preservation rate and hearing degree in the steroid-treated group when compared with controls [8].
Previously published studies have shown that there have been new directions in the development and use of electrodes and cochlear implant surgery in recent years. Currently, research, clinical practice, and commercial companies are working on developing modern steroid-eluting electrodes. Our preliminary analysis suggests that prolonged steroid administration (combined oral and IV steroid therapy) is beneficial in preserving and stabilizing hearing thresholds in patients undergoing cochlear implantation surgery. The findings of this study are supported by the results of similar studies [8,29]. However, the present study adds to the findings of previous studies by having a relatively long follow-up period, of six months, with study analysis conducted during four different follow-up periods.
Conclusions
To our knowledge, this study is the first to report the findings of two different methods of steroid administration (standard and prolonged) in human subjects who underwent cochlear implantation. The findings of this study have shown that steroid therapy stabilizes hearing thresholds and preserves hearing ability in adult patients, with the combination of IV and oral steroid therapy being the optimal treatment regimen.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Brown CJ, Abbas PJ, Bertschy M, et al. Longitudinal assessment of physiological and psychophysical measures in cochlear implant users. Ear Hear. 1995;16:439–49. doi: 10.1097/00003446-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Teschner M, Polite C, Lenarz T, Lustig L. Cochlear implantation in different health-care systems: Disparities between Germany and the United States. Otol Neurotol. 2013;34:66–74. doi: 10.1097/MAO.0b013e318278bf58. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen S, Cloutier F, Philippon D, et al. Outcomes review of modern hearing preservation technique in cochlear implant. Auris Nasus Larynx. 2016;43:485–88. doi: 10.1016/j.anl.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Skarzynski H, Lorens A, Piotrowska A, Skarzynski PH. Hearing preservation in partial deafness treatment. Med Sci Monit. 2010;16:CR555–62. [PubMed] [Google Scholar]
- 5.Skarzynski H, Matusiak M, Lorens A, et al. Preservation of cochlear structures and hearing when using the Nucleus Slim Straight (CI422) electrode in children. J Laryngol Otol. 2016;130:332–39. doi: 10.1017/S0022215115003436. [DOI] [PubMed] [Google Scholar]
- 6.Van Abel KM, Dunn CC, Sladen DP, et al. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015;36:416–21. doi: 10.1097/MAO.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skarzynski H, Matusiak M, Piotrowska A, Skarzynski PH. Surgical techniques in partial deafness. J Hear Sci. 2012;2(3):9–13. [Google Scholar]
- 8.Sweeney AD, Carlson ML, Zuniga MG, et al. Impact of perioperative oral steroid use on low-frequency hearing preservation after cochlear implantation. Otol Neurotol. 2015;36:1480–85. doi: 10.1097/MAO.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 9.Rah YC, Lee MY, Kim SH, et al. Extended use of systemic steroid is beneficial in preserving hearing in guinea pigs after cochlear implant. Acta Otolaryngol (Stockh) 2016;136:1213–19. doi: 10.1080/00016489.2016.1206965. [DOI] [PubMed] [Google Scholar]
- 10.The American Society of Health-System Pharmacists. Dexamethasone Monograph for Professionals [Internet] [cited 2017 Mar 30]. Available from: https://www.drugs.com/monograph/dexamethasone.html.
- 11.Chrousos GP. Basic Clin Pharmacol. The McGraw-Hill Companies Inc.; 2007. Adrenocorticosteroids and adrenocortical antagonists. [Google Scholar]
- 12.Alles MJRC, der Gaag MA, Stokroos RJ. Intratympanic steroid therapy for inner ear diseases, a review of the literature. Eur Arch Otorhinolaryngol. 2006;263:791–97. doi: 10.1007/s00405-006-0065-3. [DOI] [PubMed] [Google Scholar]
- 13.Juhn SK, Hunter BA, Odland RM. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int Tinnitus J. 2001;7:72–83. [PubMed] [Google Scholar]
- 14.Jahnke K. The blood-perilymph barrier. Arch Otorhinolaryngol. 1980;228:29–34. doi: 10.1007/BF00455891. [DOI] [PubMed] [Google Scholar]
- 15.Jahnke K. Permeability barriers of the inner ear. Fine structure and function. Fortschr Med. 1980;98:330–36. [PubMed] [Google Scholar]
- 16.Kastenbauer S, Klein M, Koedel U, Pfister HW. Reactive nitrogen species contribute to blood-labyrinth barrier disruption in suppurative labyrinthitis complicating experimental pneumococcal meningitis in the rat. Brain Res. 2001;904:208–17. doi: 10.1016/s0006-8993(01)02164-3. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Zhang ZJ, Tokuriki M, et al. Expression of p-glycoprotein is associated with that of multidrug resistance protein 1 (MRP1) in the vestibular labyrinth and endolymphatic sac of the guinea pig. Neurosci Lett. 2001;303:189–92. doi: 10.1016/s0304-3940(01)01738-4. [DOI] [PubMed] [Google Scholar]
- 18.Swan EEL, Mescher MJ, Sewell WF, et al. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60:1583–99. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skarzynski H, Lorens A, Dziendziel B, Skarzynski PH. Expanding pediatric cochlear implant candidacy: A case study of electro-natural stimulation (ENS) in partial deafness treatment. Int J Pediatr Otorhinolaryngol. 2015;79:1896–900. doi: 10.1016/j.ijporl.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Skarzynski H, van de Heyning P, Agrawal S, et al. Towards a consensus on a hearing preservation classification system. Acta Oto-Laryngol Suppl. 2013;564:3–13. doi: 10.3109/00016489.2013.869059. [DOI] [PubMed] [Google Scholar]
- 21.Skarżyński H, Lorens A, Skarżyński PH. Electro-natural stimulation (ENS) in partial deafness treatment: A case study. J Hear Sci. 2015;4(4):67–71. [Google Scholar]
- 22.Liu H, Hao J, Li KS. Current strategies for drug delivery to the inner ear. Acta Pharm Sin B. 2013;3:86–96. [Google Scholar]
- 23.Honeder C, Landegger LD, Engleder E, et al. Effects of intraoperatively applied glucocorticoid hydrogels on residual hearing and foreign body reaction in a guinea pig model of cochlear implantation. Acta Otolaryngol (Stockh) 2015;135:313–19. doi: 10.3109/00016489.2014.986758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douchement D, Terranti A, Lamblin J, et al. Dexamethasone eluting electrodes for cochlear implantation: Effect on residual hearing. Cochlear Implants Int. 2015;16:195–200. doi: 10.1179/1754762813Y.0000000053. [DOI] [PubMed] [Google Scholar]
- 25.Hu A, Parnes LS. Intratympanic steroids for inner ear disorders: A review. Audiol Neurotol. 2009;14:373–82. doi: 10.1159/000241894. [DOI] [PubMed] [Google Scholar]
- 26.Paulson DP, Abuzeid W, Jiang H, et al. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008;118:706–11. doi: 10.1097/MLG.0b013e31815f8e41. [DOI] [PubMed] [Google Scholar]
- 27.Frey BM, Frey FJ. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1990;19:126–46. doi: 10.2165/00003088-199019020-00003. [DOI] [PubMed] [Google Scholar]
- 28.O’Connell BP, Hunter JB, Haynes DS, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 2017;127(10):2352–57. doi: 10.1002/lary.26467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho HS, Lee K-Y, Choi H, et al. Dexamethasone is one of the factors minimizing the inner ear damage from electrode insertion in cochlear implantation. Audiol Neurootol. 2016;21:178–86. doi: 10.1159/000445099. [DOI] [PubMed] [Google Scholar]