Abstract
Experimental results obtained from research using only one sex are sometimes extrapolated to both sexes without thorough justification. However, this might cause enormous economic loss and unintended fatalities. Between years 1997 and 2000, the US Food and Drug Administration suspended ten prescription drugs producing severe adverse effects on the market. Eight of the ten drugs caused greater health risks in women. Serious male biases in basic, preclinical, and clinical research were the main reason for the problem. This mini-review will describe why and how funding organizations such as the European Commission, the Canadian Institutes of Health Research, and the US National Institutes of Health have tried to influence researchers to integrate sex/gender not only in clinical research, but also in basic and preclinical research. Editorial policies of prominent journals for sex-specific reporting will also be introduced, and some considerations in integrating sex as a biological variable will be pointed out. To produce precise and reproducible results applicable for both men and women, sex should be considered as an important biological variable from basic and preclinical research.
Keywords: Animal, Biological variable, Cell, Female, Funding, Gender, Journal, Male, Preclinical research, Sex
INTRODUCTION
Even though we know that males and females are not the same, experiments have sometimes been carried out without considering sex in scientific research. Scientists have often used only one sex (generally male) for experiments and applied the findings to both sexes, without solid grounds. These kinds of inadvertent extrapolations might cause unintentionally harmful results to the neglected sex and economic loss.
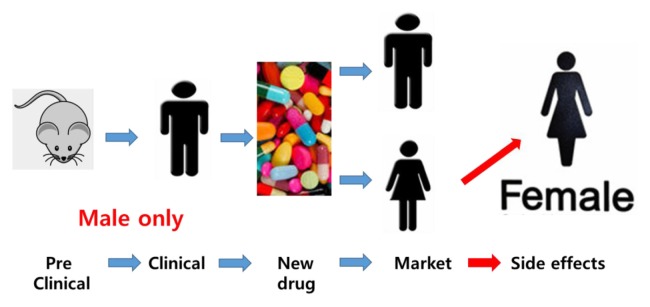
During the time period from 1997 to 2000, ten prescription drugs were withdrawn from the market by the US Food and Drug Administration (FDA). Eight of the withdrawn drugs caused greater health risks in women (1). Looking in detail, four of the drugs caused more adverse events in women because they were prescribed more often to women than to men. However, the other four drugs had more detrimental effects in women, even though they were equally prescribed to both women and men, suggesting that physiological differences between males and females predispose women to some adverse drug-related health risks (1). Deleterious effects of these drugs on females only became evident as a result of post-marketing reports, mainly because preclinical studies were undertaken using mainly male subjects (2) and, even during clinical studies, females were under-represented (Fig. 1).
Fig. 1.
Schematic drawing shows male-biased preclinical and clinical research can leave detrimental side effects for women undetected till marketing.
In 1992, the FDA released a report on the practices for approving prescription drugs (3). The report showed that women were generally under-represented in drug trials and, even when women were included in large numbers, data were not analyzed to determine sex-related differences in drug responses. After decades of clinical research, mostly excluding women, researchers began to realize that men and women have large differences beyond their reproductive systems (4). As a result, the FDA cleared restraint for the inclusion of women with childbearing potential in clinical trials and established guidelines regarding the analysis of data by sex. The US Congress codified this amendment to NIH policies into public law, through a section within the 1993 National Institutes of Health (NIH) Revitalization Act (available at https://orwh.od.nih.gov/resources/pdf/NIH-Revitalization-Act-1993.pdf). Under this law, NIH made certain that women and minorities are included in all clinical research, and Phase III clinical trials include women and minorities in sufficient numbers to enable valid analyses of differences among groups.
In 1999, the Institute of Medicine (IOM) of the National Academy of Sciences formed the ‘Committee on Understanding the Biology of Sex and Gender Differences.’ The committee, consisting of experts from a wide range of disciplines, evaluated and considered a contemporary understanding of sex differences and determinants at the biological level. As a result, IOM published a report in 2001 (5), concluding that “Sex matters” and “Being male or female is an important basic human variable that should be considered when designing and analyzing studies in all areas and at all levels of … health-related research.”
Based on human biology research over the past decade, it is now widely accepted that normal physiological functions and many pathological functions are influenced by sex-based differences (5, 6). Thanks to all these efforts, women are now better represented in clinical trials.
Much of our understanding of disease processes and treatment measures are based on the results obtained from basic and preclinical studies that use nonhuman animals and cell cultures. Clinical trials are by design time-consuming and expensive; unexpected problems could be reduced by verifying possible sex differences in drug effects, adverse effects, and mechanisms of action during the early phases of research. Thus, it is very important to integrate sex as a biological variable for preclinical research. However, the realization that sex influences biology and pathology has been slow in coming for preclinical studies (7, 8). Furthermore, instructions or guidance to consider the effect of sex on basic and preclinical research were rare, until recent years.
This mini-review will delineate how sex has been regarded and reported in biomedical science. Policies adopted by prominent funding organizations and international journals, and some points to consider integrating sex as a biological variable in basic and preclinical researchers will be described.
DEFINITION OF ‘SEX’ AND ‘GENDER’
Sex and gender are occasionally used in an interchangeable manner. Both sex and gender affect research results, but they have different meanings. Thus, it is important to know the correct meanings of them and to avoid interchangeable use. According to the US Institute of Medicine (IOM) (5), sex is “the classification of living things, generally as male or female, according to their reproductive organs and functions assigned by chromosomal complement”, while gender is “a person’s self-representation as male or female or how that person is responded to by social institutions on the basis of the individual’s gender presentation. Gender is shaped by environment and experience.” Thus, sex is related to reproductive organs, sex hormone, gene expression, anatomy, and physiology. Gender refers to socio-culturally constructed roles, norms, identities, and power relations (9) that, together, shape ‘feminine’ and ‘masculine’ behaviors (10). Sex can be used for both human and animals as whole organisms or materials derived from them such as cells and tissues, while gender is in general used only for humans. Importantly, sex and gender affect each other, as gender is rooted in biology and can influence biological outcomes.
NEGLECTED AND BIASED SEX IN BASIC AND PRECLINICAL STUDIES
Animal experiments
A literature review was conducted to grasp the sex bias in experiments (11). Among articles that reported non-human animal studies in the Journal of Pharmacology and Experimental Therapeutics and the Journal of Physiology in 1909, 79% failed to report the sex of the animal. The percentage of articles with an unspecified animal sex decreased steadily from 79% to 50% through 1969. There was a sudden drop in those values, reaching 20% in 1979, and then stabilized to around 20–30% during the period from 1979 to 2009. The ratios of papers reporting male-animal-only reports were around 5–20% between 1909 and 1969. It jumped up to 70% in 1979 and then stabilized at around 50% up until 2009. Studies that enrolled both female and male animals remained low, reaching only 15% during 1909–2009. To make matters worse, among the studies using both sexes, only 34% analyzed data separately by sex.
Journal articles published in 2009 across 10 major biological disciplines (pharmacology, endocrinology, behavior, behavioral physiology, neuroscience, general biology, zoology, physiology, reproduction, and immunology) were then analyzed to compare sex bias status among research fields (11). The articles were classified according to species studied and the sex of the subjects. Survey results showed that over 50% of articles in general biology and immunology fields did not specify the sex of the animals used in the study. For the articles that defined the sex of the animal, a male bias was observed in 8 of the 10 fields. A male skew was especially conspicuous in neuroscience (5.5: 1), pharmacology (5: 1), and physiology (3.7: 1) fields. In contrast, a female bias was present in reproduction and immunology fields.
The ‘Thomson Reuters Web of Science’ database for 2009 was also examined to investigate the use of female animals in studies for particular diseases such as anxiety, depression, epilepsy, thyroiditis, hypertension & stroke, multiple sclerosis, obesity, and pain (12). The results showed that the percentages of females in rat and mouse models of the diseases under investigation were not in proportion, but that female animals were severely under-represented, given the prevalence of corresponding diseases in women worldwide. For example, women are twice as likely to be diagnosed with anxiety and depression than men, but fewer than 45% of animal studies used females to investigate these disorders (12). Regrettably, the situation has not improved much until recent times (13, 14).
Cell experiments
Cells do have sex and the sex of cells influences experimental results by affecting cellular behaviors such as proliferation, differentiation, response to stress, and apoptosis (15–17). However, most scientists do not give any thought to the sex of the cell and the effect of sex at the cellular level. Consequently, sex of cell is not properly reported in articles. Only 45 (23.6%) out of 191 articles published in top cardiovascular journals reported cell sex in 2010 (18). Among these 45 studies, most (68.9%) used only male cells and none exclusively used female cells. Omitting the sex of cells is not limited to any specific research field. Shah et al. (19) reported that the sex of cells was described in only 25 of 100 randomly selected articles from the American Journal of Physiology-Cell Physiology published in 2013.
The sex of cells is also ignored by commercial cell vendors. Approximately 15.5% of human cell lines were sold without sex identification as of the year 2014, by three prominent cell providers: American Type Culture Collection, European Collection of Cell Cultures, and Japanese Collection of Research Bioresources (20). Sex identification was even scarce for animal cell lines compared to human cell lines. In addition, the majority of primary cells and stem cells were sold without defined sex (20).
SEX/GENDER ANALYSIS POLICIES OF MAJOR GRANTING ORGANIZATIONS
Recently, funding organizations including the European Commission (EC), Canadian Institutes of Health Research (CIHR), and the US National Institutes of Health (NIH) put efforts into influencing researchers to integrate sex and gender in the whole study processes from hypothesis to publication.
EC (EU)
The EC is an institution of the European Union (EU). EC has emphasized “questioning systematically whether and in what sense, sex and gender are relevant in the objectives and in the methodology of projects” since 2003 (European Commission, 2003). Likewise, gender has been supported as the main theme in Horizon 2020 which is the largest ever EU Research and Innovation program and the EC’s current funding framework. To propose new ways for integrating the gender dimension into all aspects of research and innovation contexts, Horizon 2020 Advisory Group for Gender issued a position paper in Dec. 2016 (21). The position paper argues that the gender dimension is an essential aspect of research excellence and the quality and accountability of research are negatively affected by not taking into account sex and gender. The paper also emphasizes that “Addressing the gender dimension in research and innovation entails accounting for sex and gender in the whole research process, when developing concepts and theories, formulating research questions, collecting and analyzing data, and using the analytical tools that are specific to each scientific area.”
CIHR
CIHR is the Government of Canada’s health research investment agency. CIHR is using four approaches to improve sex and gender integration in health research (22). 1) CIHR requires all research applicants to report sex and gender integration in their proposal. 2) CIHR mandates research teams to include a person showing sex and gender expertise (sex and gender champion) for the research topic under investigation. Sex and gender champions ensure that sex and gender are essential ingredients of the research principle, study design, experimental methods, data analysis, and knowledge interpretation. 3) CIHR asks a cross-cutting sex and gender platform is included within large research consortia. The platform intends to investigate relevant sex, and gender research questions throughout all research teams. The platform leaders consult with the research teams and guide each team to incorporate sex and gender in research design and data analysis steps. Fourth, CIHR ensures that all grant applicants complete sex and gender online training programs (available at http://www.cihr-irsc.gc.ca/igh-competency.html) which CIHR developed in September 2015. Grant applicants should submit proof of completion of at least one of three online training modules (22). Furthermore, the CIHR provides a detailed checklist for reviewers who evaluate biomedical and translational research proposal (available at http://www.cihr-irsc.gc.ca/e/49337.html).
NIH
Even after decades of efforts to integrate sex/gender in biomedical research, the change has been slow. To rectify this situation, NIH announced a policy aimed at integrating sex as a biological variable (SABV) into biomedical research in May 2014 (23). The policy requires that “applicants to report their plans for the balance of male and female cells and animals in preclinical studies in all future applications, unless sex-specific inclusion is unwarranted, based on rigorously defined exceptions.” NIH then declared NIH Guide Notice NOT-OD-15-102 in 2015 (available at http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html). A document serves as a companion reference to NOT-OD-15-102 says that “In particular, sex is a biological variable (SABV) that is frequently ignored in animal study designs and analyses, leading to an incomplete understanding of potential sex-based differences in basic biological function, disease processes, and treatment response. NIH expects that sex as a biological variable will be factored into research designs, analyses, and reporting in vertebrate animal and human studies. Strong justification from the scientific literature, preliminary data or other relevant considerations must be provided for applications proposing to study only one sex.” (available at https://orwh.od.nih.gov/resources/pdf/NOT-OD-15-102_Guidance.pdf). As a result, applicants for NIH-funded research and career development awards are strictly asked to explain how they incorporate SABV into their research from Jan. 25, 2016. Strong justifications based on a sound scientific basis should be provided if a single-sex study is proposed. In addition, NIH also prepared guidelines to help grant reviewer (available at https://grants.nih.gov/grants/peer/guidelines_general/SABV_Decision_Tree_for_Reviewers.pdf).
NIH and CIHR adopted a consensus list of 13 evaluation criteria as a minimal standard for reviewers (24). Key questions peer reviewers should ask when evaluating the overall score of a grant application include: Quality and appropriateness of SABV; Justification for a single-sex study; Evidence that the research question incorporates SABV; Potential for the research to add value to the current state of knowledge on a given topic that has potential to, but has not yet fully elucidated the impact of sex on biological mechanisms, pathophysiology, or translational science; Impact of research incorporating SABV; Potential for a significant contribution to the improvement of women and men’s health, the health of boys and girls, or the health of gender-diverse persons.
Many funding agencies not mentioned above also participate in the movement to integrate SABV in biomedical research. More information can be found at the Stanford University’s Gendered Innovations home page (available at http://genderedinnovations.stanford.edu/sex-and-gender-analysis-policies-major-granting-agencies.html).
EDITORIAL POLICIES OF SCIENTIFIC JOURNALS FOR SEX/GENDER ANALYSIS
Journal editors can facilitate innovation through their journal policies by making decisions regarding what type of research meets the standards for publication and by recommending how studies will be published in the literature. For example, approval of the institutional review board is now a universal requirement for human and animal research, at least in part because of journal policies. Thus, it is very important to set the right guidance for authors and reviewers in order to shift the momentum. Recently, major scientific journals have moved to influencing authors and reviewers to clearly report the sex/gender of the research subjects (including cell, animal models, and human) and to analyze data by sex.
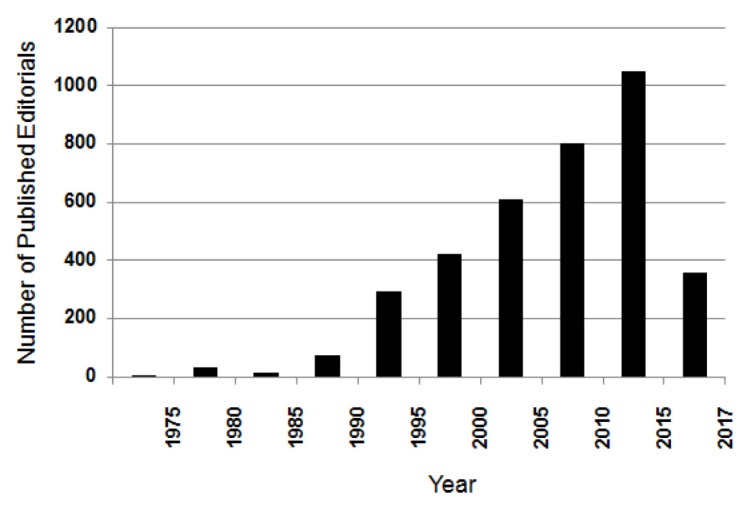
Opinions of the editors and new decisions are often expressed in editorials published in any given journal. The number of editorials published regarding sex/gender stayed low, till 1990 (Fig. 2). After 1993 when the National Institutes of Health (NIH) Revitalization Act was enacted, the number leaped rapidly and then increased steadily during 1994–2013. After the NIH announced SABV policy in 2014, around 200 editorials commenting on the sex/gender issue are published every year reflecting on the strong interest for embedding SABV among publishers.
Fig. 2.
Number of published editorials regarding sex/gender. Editorials and comments mentioning ‘sex’ or ‘gender’ in title were searched in PubMed. Article numbers published during every five year are plotted except the last column which shows number of editorials published for two years, from 2016 to 2017.
The Sex and Gender Equity in Research (SAGER) guidelines
The European Association of Science Editors (EASE) tries to improve the global standard and quality of science editing. Recognizing the importance of reporting sex and gender in research, the EASE established the Gender Policy Committee (GPC) in 2012. The GPC reviewed existing guidelines and worked to propose applicable standards for sex and gender equity in research. In 2016, GPC published the Sex and Gender Equity in Research (SAGER) guidelines to encourage a more systematic approach to the reporting of sex and gender in research across disciplines (25).
The SAGER guidelines emphasize the proper use of sex vs. gender terminology throughout the paper to avoid confusion. The guidelines accentuate needs to distinguish between research subjects by sex/gender, analyze results according to sex/gender, and reveal meaningful differences whenever possible, even if not initially expected. The guidelines also include a set of questions (available at http://www.ease.org.uk/publications/sex-and-gender). It helps authors to facilitate manuscript preparation and allow them to check whether sex/gender issues are properly addressed in their manuscripts.
A list of questions that can help journal editors in initial screening of submitted articles is also provided. It allows editors to consider the topic of the study (are sex/gender relevant?), reporting of data (are data reported disaggregated by sex/gender?), design of the study (are sex/gender considered, or is it explained? why they are not?), and discussion/limitation (are sex/gender analyses or lack thereof mentioned and discussed?). The guidelines encourage editors to contact authors to improve the reporting of sex/gender prior to peer-review if authors have not followed the guidelines. The guidelines also help peer reviewers to consider the above-mentioned issues during the review process (25).
ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines
The ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (available at https://www.elsevier.com/__data/promis_misc/622936arrive_guidelines.pdf) are intended to improve the reporting of research using animals for maximizing information published and minimizing unnecessary studies. The article appeared in a 2010 issue of PLOS Biology (26) and provided a checklist for those preparing or reviewing a manuscript intended for publication (available at https://static-content.springer.com/esm/art%3A10.1186%2Fs12863-016-0341-1/MediaObjects/12863_2016_341_MOESM2_ESM.pdf). The ARRIVE Guidelines advise to provide details of the animals used, including species, strain, sex, developmental stage, and weight.
American Physiological Society (APS) journals
In 2012, American Physiological Society (APS) journals pioneered by declaring a new editorial policy which requires reporting sex or gender where appropriate for cells, tissues, and experimental animals, and humans. In addition, APS published an editorial to explain the background for the declaration of the new editorial policy and to emphasize the importance of reporting sex of the experimental materials (10). However, this editorial policies have been poorly accepted by researchers and reviewers, judging from subsequent articles published in AJP journals (19).
To promote transparency in reporting relevant experimental information, APS journals updated several guidelines as of August 2016 (available at http://www.physiology.org/author-info.experimental-details-to-report). The updated guidelines include the addition of a new section entitled “Experimental Details to Report in Your Manuscript”. The guidelines require to strictly include sex of the animals used in the study for all animal experiments, while encourage to include sex of the source for cell experiments.
International Committee of Medical Journal Editors (ICMJE)
The ICMJE updated the “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals” in Dec. 2017 to help authors, editors, reviewers, and others involved in biomedical publishing for accurate, clear, reproducible, unbiased medical journal articles (available at www.icmje.org/icmje-recommendations.pdf). To promote SABV in biomedical research, ICMJE recommends as shown below. 1) When describe study subjects, ensure correct use of the terms sex (when reporting biological factors) and gender (when reporting identity, psychosocial, or cultural factors), and report the sex/gender of study participants, the sex of animals or cells, and describe the methods used to determine the sex and gender. If the study was done involving only one sex, authors should justify why. 2) In results, separate reporting of data by age and sex unless there are compelling reasons not to stratify reporting, which should be explained. 3) Discuss the influence or association of sex/gender on your findings where appropriate, and the limitations of the data.
Journal of Neuroscience Research (JNR)
In Jan. 2017, the JNR declared policy requiring all authors to ensure proper consideration of sex as a biological variable, and devoted an issue entirely to sex differences at all levels of nervous system (27). Now, authors for JNR are asked to complete the “Transparent Science Questionnaire” (available at http://onlinelibrary.wiley.com/journal/10.1002/%28ISSN%291097-4547/homepage/ForAuthors.html) and to submit it with their manuscript. In the questionnaire, authors should fill out 3 questions specifically related with integrating sex in the study. 1) In each study design, is sex considered as a biological variable? For details about proper reporting, authors are advised to refer to the published editorial (27). 2) Specify the total number of subjects in each experiment, including the number of animals, sex and age in each group. 3) Comment on study limitations including the inability for any reason to study possible sex influences where they may exist.
Many other journals go hand in hand for embedding SABV and editorial policies of some of those journals can be found at the Stanford University’s Gendered Innovations home page (available at http://genderedinnovations.stanford.edu/sex-andgender-analysis-policies-peer-reviewed-journals.html).
CONSIDERATIONS FOR INTEGRATING SABV IN BASIC AND PRECLINICAL RESEARCH
Double the work and money?
More money and labor will be needed to study both sexes instead of one. The doubling of cells and animals will increase not only the expenses for supplies, but also the workload for research, which might slow down research progress. Some may argue that requiring investigators to study both sexes in basic and preclinical research would be hardly practical, affordable, or scientific (28, 29). However, we cannot ignore sizable evidences showing that sex is a critical biological variable affecting experimental results, as well as physiology and pathology.
Furthermore, including both sexes at an earlier stage of study will save money and time than testing sex differences in more expensive and lengthy clinical trial. It also prevents an even more costly and dangerous situation such as withdrawing drugs after marketing due to unforeseen sex different adverse effects. Thus, analyzing sex as a variable in basic and preclinical research is likely to save money in the long run by increasing reproducibility of research and by minimizing the failure of clinical trials (30, 31).
In using female animals
Researchers want clear results. Worries for less clear results due to reproductive cycle have shunned researchers from using female animals (32). However, a meta-analysis of 293 articles which compared various traits of male mice with those of female mice at random stages of the estrous cycle revealed that for most traits, the variability of each sex was equivalent regardless of the stage of the estrous cycle in females (33). In fact, the greatest variability in both males and females was caused by casing condition (single casing vs. group casing). Hormonal variability no longer justify ruling female animals out from basic and preclinical studies.
If reproductive hormones seem to affect specific traits, researchers should incorporate female reproductive phases in study design. In that case, researchers may need four times more female animals than males as female rodents have a 4-stage ovarian cycle (34).
Negative results
Finding no sex difference is as significant as the presence of a sex difference. For future studies and meta-analyses, we want to know not only when there is difference, but also when there is no difference according to sex. For data either positive or negative to be valuable, the experimental conditions should be reported clearly. To improve the value and reproducibility of animal experiments, sex-balanced experiments are required, but careful reporting for key information such as sex, age, strain, source, casing condition, and health status of animals is also imperative (33).
CONCLUSIONS
Validating results by replication is a prerequisite for excellent science. Detailed information about materials and methods used in the experiment should be provided for other researchers to replicate published studies. Sex is an important biological variable affecting experimental outcomes as well as health and disease. However, the sex of experimental subjects such as cells, tissues, experimental animals, and humans have not been balanced in experiments or faithfully reported in the scientific literature. This oversight causes an avoidable waste of resources, and limits the generalizability of research findings and their applicability to clinical practice in particular for women but also for men as well (35).
Recently, prominent funding organizations and scientific journals began to take more strictly enforced measures to integrate SABV in preclinical and clinical studies. However, accepting SABV in the study design, data analyses, interpretation of findings, and reporting is still largely insufficient among scientists. As researchers, manuscript- and grantreviewers, we cannot continue to ignore sex differences. After all, considering SABV is fundamentally an issue of doing good science.
ACKNOWLEDGEMENTS
This study was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP(2015M3A9B6073827), and by Support Program for Women in Science, Engineering and Technology through the NRF funded by the Ministry of Science and ICT(No. 2016H1C3A1903202).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.GAO-01-286R. (available at https://www.gao.gov/new.items/d01286r.pdf)
- 2.Hughes RN. Sex does matter: comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behav Pharmacol. 2007;18:583–589. doi: 10.1097/FBP.0b013e3282eff0e8. [DOI] [PubMed] [Google Scholar]
- 3.GAO, Women’s Health-FDA Needs to Ensure More Study of Gender Differences in Prescription Drug Testing. (available at http://archive.gao.gov/d35t11/147861.pdf)
- 4.Gochfeld M. Sex Differences in Human and Animal Toxicology. Toxicol Pathol. 2017;45:172–189. doi: 10.1177/0192623316677327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wizemann TM, Pardue ML, editors. Institute of Medicine. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington (DC): 2001. [PubMed] [Google Scholar]
- 6.Regitz-Zagrosek V. Sex and gender differences in pharmacology. Springer; Berlin; New York: 2012. [DOI] [Google Scholar]
- 7.Yoon DY, Mansukhani NA, Stubbs VC, Helenowski IB, Woodruff TK, Kibbe MR. Sex bias exists in basic science and translational surgical research. Surgery. 2014;156:508–516. doi: 10.1016/j.surg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Sechzer JA, Rabinowitz VC, Denmark FL, McGinn MF, Weeks BM, Wilkens CL. Sex and gender bias in animal research and in clinical studies of cancer, cardiovascular disease, and depression. Ann N Y Acad Sci. 1994;736:21–48. doi: 10.1111/j.1749-6632.1994.tb12816.x. [DOI] [PubMed] [Google Scholar]
- 9.Clayton JA, Tannenbaum C. Reporting Sex, Gender, or Both in Clinical Research? JAMA. 2016;316:1863–1864. doi: 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
- 10.Miller VM. In pursuit of scientific excellence: sex matters. Am J Physiol Gastrointest Liver Physiol. 2012;302:G907–908. doi: 10.1152/ajpgi.00101.2012. [DOI] [PubMed] [Google Scholar]
- 11.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]
- 13.Kong BY, Haugh IM, Schlosser BJ, Getsios S, Paller AS. Mind the Gap: Sex Bias in Basic Skin Research. J Invest Dermatol. 2016;136:12–14. doi: 10.1038/JID.2015.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakiniaeiz Y, Cosgrove KP, Potenza MN, Mazure CM. Balance of the Sexes: Addressing Sex Differences in Preclinical Research. Yale J Biol Med. 2016;89:255–259. [PMC free article] [PubMed] [Google Scholar]
- 15.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–2550. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deasy BM, Lu A, Tebbets JC, et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penaloza C, Estevez B, Orlanski S, et al. Sex of the cell dictates its response: differential gene expression and sensitivity to cell death inducing stress in male and female cells. FASEB J. 2009;23:1869–1879. doi: 10.1096/fj.08-119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor KE, Vallejo-Giraldo C, Schaible NS, Zakeri R, Miller VM. Reporting of sex as a variable in cardiovascular studies using cultured cells. Biol Sex Differ. 2011;2:11. doi: 10.1186/2042-6410-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am J Physiol Cell Physiol. 2014;306:C3–18. doi: 10.1152/ajpcell.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MN, Park JH, Paik HY, Lee SK. Insufficient sex description of cells supplied by commercial vendors. Am J Physiol Cell Physiol. 2015;308:C578–580. doi: 10.1152/ajpcell.00396.2014. [DOI] [PubMed] [Google Scholar]
- 21.AG Gender position paper 2018–2020. (availabe at http://ec.europa.eu/transparency/regexpert/index.cfm?do=groupDetail.groupDetailDoc&id=28824&no=1.pdf)
- 22.Duchesne A, Tannenbaum C, Einstein G. Funding agency mechanisms to increase sex and gender analysis. The Lancet. 2017;389:699. doi: 10.1016/S0140-6736(17)30343-4. [DOI] [PubMed] [Google Scholar]
- 23.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tannenbaum C, Schwarz JM, Clayton JA, de Vries GJ, Sullivan C. Evaluating sex as a biological variable in preclinical research: the devil in the details. Biol Sex Differ. 2016;7:13. doi: 10.1186/s13293-016-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Castro P, Heidari S, Babor TF. Sex And Gender Equity in Research (SAGER): reporting guidelines as a framework of innovation for an equitable approach to gender medicine. Commentary Ann Ist Super Sanita. 2016;52:154–157. doi: 10.4415/ANN_16_02_05. [DOI] [PubMed] [Google Scholar]
- 26.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prager EM. Addressing sex as a biological variable. J Neurosci Res. 2017;95:11. doi: 10.1002/jnr.23979. [DOI] [PubMed] [Google Scholar]
- 28.Fields RD. NIH policy: mandate goes too far. Nature. 2014;510:340. doi: 10.1038/510340a. [DOI] [PubMed] [Google Scholar]
- 29.Sandberg K, Verbalis JG, Yosten GL, Samson WK. Sex and basic science. A Title IX position. Am J Physiol Regul Integr Comp Physiol. 2014;307:R361–365. doi: 10.1152/ajpregu.00251.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ. 2014;5:15. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandberg K, Umans JG Georgetown Consensus Conference Work Group. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 2015;29:1646–1652. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wald C, Wu C. Biomedical research. Of mice and women: the bias in animal models. Science. 2010;327:1571–1572. doi: 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- 33.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Becker JB, Arnold AP, Berkley KJ, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 35.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]