Abstract
Patient: Female, 44
Final Diagnosis: Subarachnoid hemorrhage
Symptoms: Sudden severe onset of headache • decreased level of consciousness • right-sided weakness over a period of 1 week
Medication: —
Clinical Procedure: MRI
Specialty: Neurology
Objective:
Rare co-existance of disease or pathology
Background:
Subarachnoid hemorrhage is rarely the first presentation of cerebral venous sinus thrombosis. This case study emphasizes the presentation of perimesencephalic subarachnoid hemorrhage due to cerebral venous sinus thrombosis and the importance of neurovascular imaging for reliable diagnosis of nonaneurysmal perimesencephalic subarachnoid hemorrhage due to cerebral venous sinus thrombosis.
Case Report:
We describe a case of cerebral venous sinus thrombosis manifesting initially as subarachnoid hemorrhage. Non-contrast computed tomography showed evidence of subarachnoid hemorrhage involving the prepontine and suprasellar cisterns. Cerebral convexities were totally spared while parenchymal microbleeding was observed in the midbrain. The diagnosis was confirmed by magnetic resonance arteriography and venography. Treatment included low molecular weight heparin and warfarin therapy to restore the international normalization ratio of the patient to 2.5, followed by oral warfarin therapy for 3 months.
Conclusions:
Cerebral venous sinus thrombosis manifesting initially as subarachnoid hemorrhage is rare. Subarachnoid hemorrhage caused by cerebral venous sinus thrombosis has been reported previously to be confined to the cerebral convexities, sparing the basal cistern. However, this is not always the case where the radiological confirmation suggests the occurrence of nonaneurysmal perimesencephalic subarachnoid hemorrhage.
MeSH Keywords: Cerebral Hemorrhage; Headache; Neuroimaging; Sinus Thrombosis, Intracranial; Subarachnoid Hemorrhage; Thrombosis
Background
Cerebral venous sinus thrombosis (CVST) is an uncommon disease with a high morbidity rate that has been increasing in frequency with multiple nonspecific symptoms and presentations. Due to the involvement of multiple sinuses, a vast range of symptoms is observed in patients with CVST. These include headaches, seizures, lethargy, focal neurological deficits, and confusion [1]. Acute spontaneous subarachnoid hemorrhage (SAH) signifies a vascular origin. The most common cause of spontaneous SAH is ruptured cerebral arterial aneurysm [2,3]. Alternatively, nonaneurysmal perimesencephalic subarachnoid hemorrhage (PNSAH) presents as a distinct etiology from aneurysmal rupture wherein the hemorrhage is centered anterior to the midbrain and leading towards prepontine and suprasellar cisterns. Therefore, PNSAH is most likely associated with CVST due to close proximity [4,5]. However, patients with CVST and clinical symptoms of PNSAH are rarely reported [6,7].
Likewise, subarachnoid hemorrhage is rarely the first presentation of cerebral venous sinus thrombosis [2,3]. The diagnosis of CVST manifesting with SAH demands a high index of clinical suspicion along with radiological confirmation to initiate treatment as early as possible to avoid the resultant morbidity. Therefore, other differential diagnoses such as the location of hemorrhage, the presence of an aneurysm, and the basilar artery dissection need to be verified. Hence, neuroim-aging via radiological confirmation and differentiation are the cornerstones in CVST diagnosis and treatment.
Here, we describe a case of CVST manifesting initially as PNSAH. The main objective of this report is to further highlight the fact of PNSAH manifestation in the context of CVST, and the role of neuroimaging in confirming the diagnosis.
Case Report
A 44-year-old woman developed sudden onset of a severe headache and presented at our Emergency Department with no history of trauma, coagulation disorders, or allergies. She reported no history of oral contraceptive use, or misuse of alcohol, cigarettes, and drugs. Upon examination, the patient was noted to have papilledema, impaired pupillary light reflex, neck rigidity with decreased range of motion, and marked weakness of the right upper and lower limbs. The patient was alert, with a blood pressure of 130/70, heart rate of 61, respiratory rate of 15, temperature of 36.8°C, and 99% oxygen saturation on room air. Pulmonary examination and EKG were recorded as normal. The initial Glasgow coma scale 11/15 upon presentation and the level of consciousness deteriorated to 9/15 in a few hours.
All initial laboratory results were normal, including complete blood count test results and coagulation profile. Comprehensive hypercoagulability tests of proteins C and S, fibrinogen, antithrombin III, antiphospholipid antibody, anticardiolipin antibody, and homocysteine were performed, and all findings were within normal limits.
The magnetic resonance arteriogram and venogram were done first, as CT angiography was not available at the time. The diagnosis presented a reassessment of the cerebral venous sinuses, which showed evidence of a filling defect in the left transverse sinus and an internal jugular vein, in addition to hyperdensity in the right prepontine and suprasellar cisterns (Figures 1–4). Moreover, the brain magnetic resonance imaging scan indicated the presence of parenchymal microbleeding in the midbrain (Figure 5). Accordingly, the patient was immediately started on a dose-adjusted intravenous anticoagulation therapy. Low molecular weight heparin (Nadroparin, 6150AXaIU, subcutaneous injection, twice daily) in combination with intravenous warfarin 3 mg/day to maintain a target international normalization ratio of 2.5. The clinical symptoms of the patient gradually improved over the next week.
Figure 1.
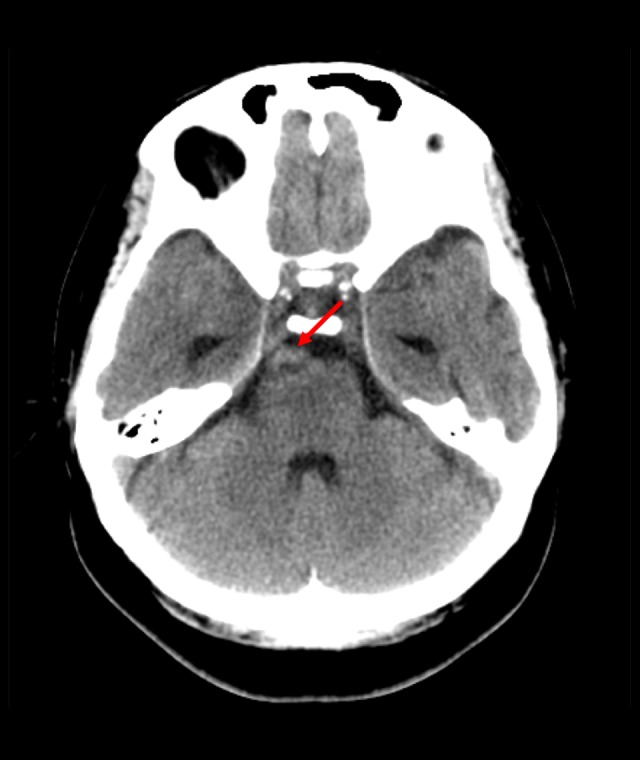
Axial fluid attenuation inversion recovery image showing hyperintensity in the right prepontine lesion, signifying subacute bleeding (red arrow).
Figure 2.
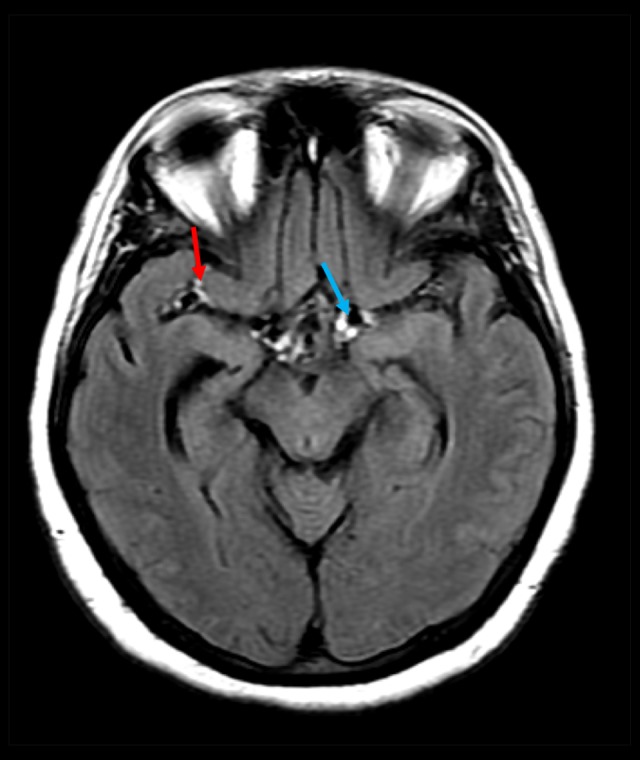
Axial fluid attenuation inversion recovery image showing a hyperintense signal in the suprasellar cistern (blue arrow) and the right Sylvian fissure (red arrow).
Figure 3.
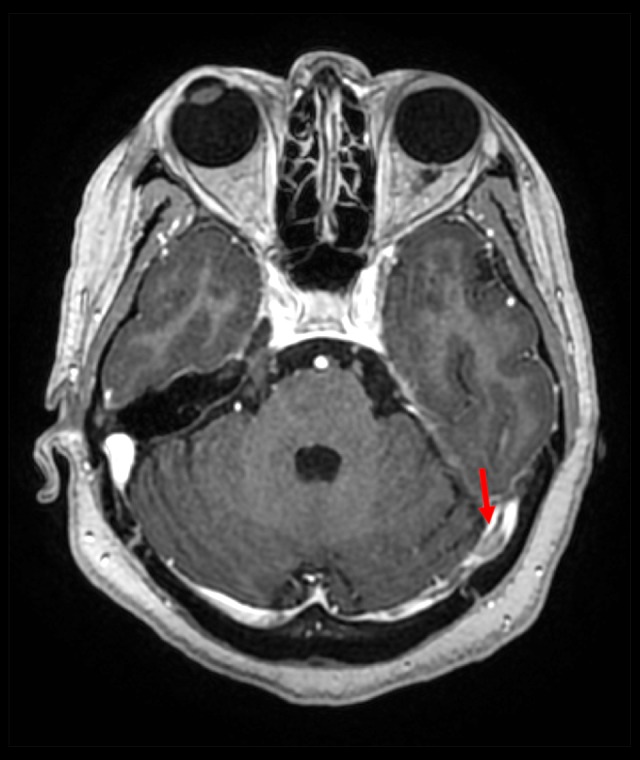
Axial enhanced 3-dimensional spoiled gradient echo image showing a linear intraluminal non-enhancing area indicating a thrombus in the left transverse sinus (red arrow).
Figure 4.
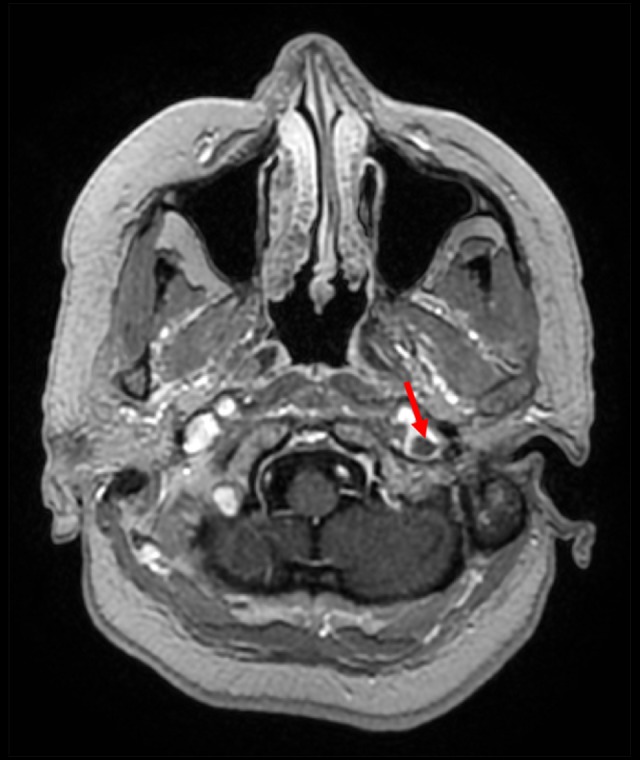
Axial enhanced 3-dimensional spoiled gradient echo image showing a linear intraluminal non-enhancing area indicating thrombus in the left internal jugular vein (red arrow).
Figure 5.
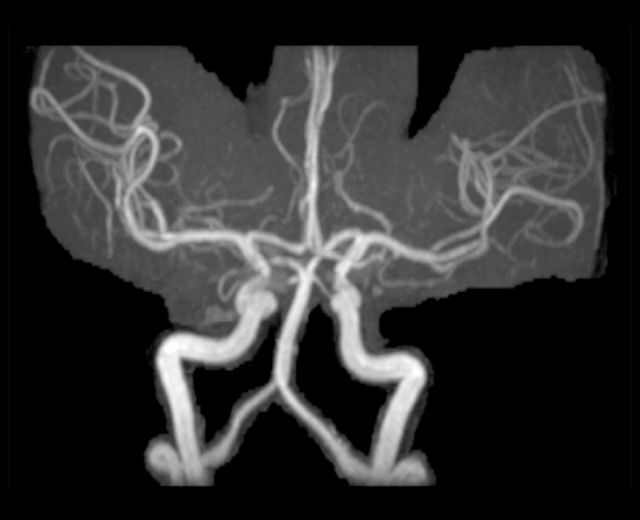
Three-dimensional time of flight magnetic resonance arteriogram showing a normally appearing basilar artery, and confirming the absence of basilar artery dissection and arterial aneurysms.
Three days after the patient was admitted, a non-contrast brain computed tomography (CT) scan was performed, which showed an ill-defined hyperdense area in the prepontine cistern signifying SAH (Figure 6). The results obtained in the initial and follow-up CT confirmed the exclusion of basilar artery dissection and arterial aneurysm as a cause of SAH (Figure 7); therefore, a lumbar puncture was not performed. The cerebral angiogram showed a moderate filling defect along the left transverse and sigmoid sinuses, verifying the diagnosis of CVST made by the radiologist.
Figure 6.
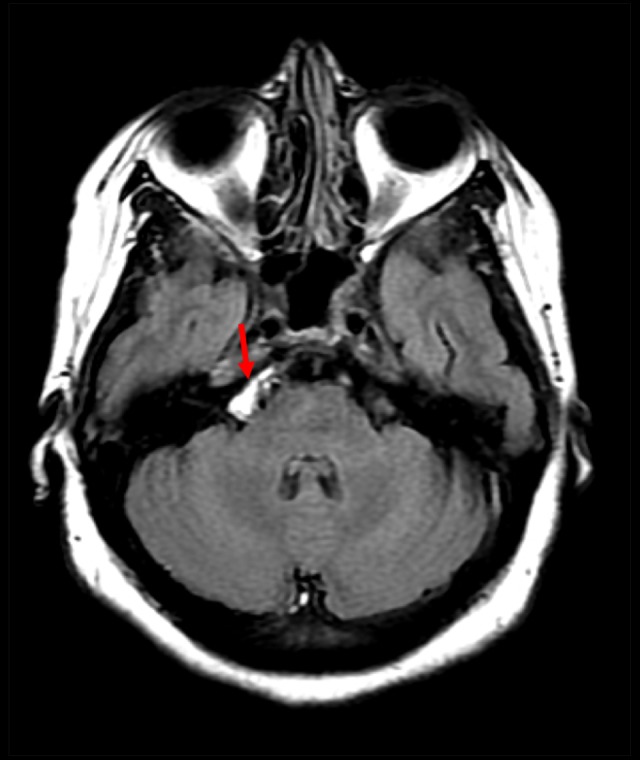
Axial non-enhanced computed tomography scan of the brain showing hyperdensity in the right prepontine cistern (red arrow).
Figure 7.
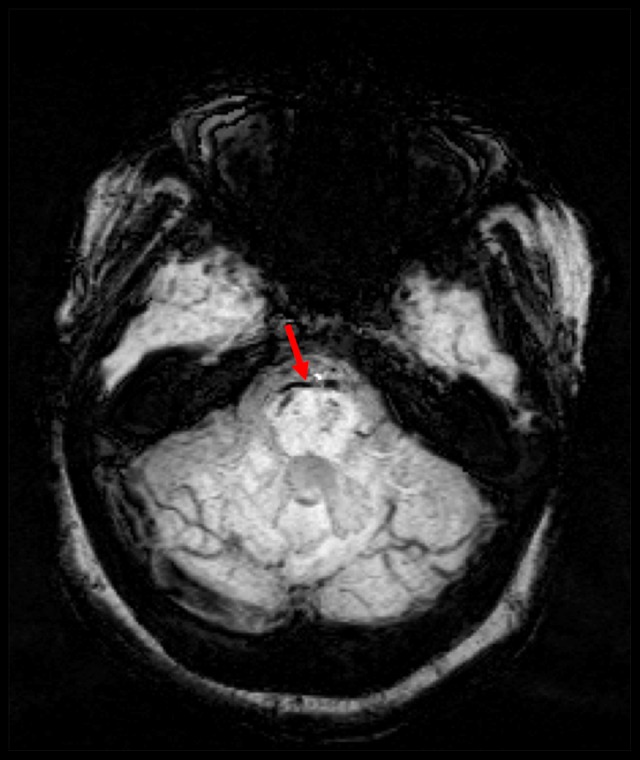
Axial susceptibility weighted imaging sequence showing a blooming effect in the prepontine cistern due to bleeding, with evidence of microbleeding in the right side of the midbrain (red arrow).
The patient continued to improve over the next 2 weeks with the resolution of her neurological symptoms. She was discharged without any neurological deficit after 2 weeks of hospital stay. She was switched to an oral warfarin with an INR target of 2–3 for 3 months while following up as an outpatient.
Further laboratory testing was commenced to determine the underlying etiology, and all tests were negative, including thrombophilia workup. Magnetic resonance arteriography and venography were repeated at 1 month after discharge, and the images showed complete recanalization of both the left transverse sinus and internal jugular vein. Currently, the patient is no longer taking anticoagulants and is free of any neurological symptoms.
Discussion
Acute spontaneous non-traumatic SAH is mainly caused by a ruptured arterial aneurysm in more than 85% of cases, while 10% of cases are associated with nonaneurysmal hemorrhage presenting a negative angiogram for a saccular aneurysm [8]. Accordingly, nonaneurysmal perimesencephalic hemorrhage portrays a distinct radiographic pattern confined to hemorrhage at the anterior of the midbrain or pons where blood may extend to the brainstem, proximal Sylvian fissures, or into a suprasellar cistern [5]. Since the CT imaging of blood patterns represented by nonaneurysmal subarachnoid are distributed in the perimesencephalic region, they can be described as a distinct clinical entity with an excellent prognosis, a benign clinical course, and minimal risk of rebleeding [9,10]. Because aneurysmal ruptures are rarely associated with PNSAH like bleeding patterns, full cerebral angiography is recommended by radiologists to avoid missing any vascular malformations or vertebrobasilar aneurysms. If the angiography is negative, hemorrhage bleeding in the initial CT scan is essential to establish a perimesencephalic pattern [11].
PNSAH caused by CVST presentation is still considered rare, and not all emergency physicians and neuroradiologists are familiar with it [2]. CVST is a difficult clinical diagnosis to make and requires radiological evidence to confirm due to its wide range of clinical presentations. The most commonly associated symptoms are usually progressive and indolent headaches, a decrease in the level of consciousness, focal seizures, and focal motor and sensory deficits [12]. The most commonly affected cerebral venous sinuses are the lateral, superior sagittal, transverse, and straight sinuses [1]. Even though the incidence of CVST manifesting as isolated SAH is very low, reaching 6.4% [13], researchers have previously reported the association of sinus thrombosis with dilation of the cortical veins that may rupture and cause bleeding in the subarachnoid space, resulting in SAH [14]. The most accepted hypothesis is that the origin of PNSAH is a venous pathology of straight sinus stenosis, in which jugular vein occlusion may present as a contributing factor to PNSAH. These arguments provide further support for CVST as a venous source of PNSAH [15,16].
The case we described here had typical symptoms of SAH, including a severe headache, neck stiffness, and motor weakness, and imaging evidence of SAH simulating a ruptured arterial aneurysm. Thus, establishing the diagnosis of CVST can be more difficult in the presence of SAH clinically.
The precise underlying pathophysiology of SAH caused by CVST remains undetermined, but multiple theories have been presented by clinicians in accordance with their correlation. For instance, an increase in the vascular permeability due to the local inflammatory response may lead to blood extravasation to the subarachnoid space [17]. Another theory indicates the development of venous parenchymal hemorrhagic infarcts leading to rupture of veins in the subarachnoid space [17]. Finally, sinus thrombosis extension into the superficial cortical veins may have induced venous hypertension and eventual rupture into the subarachnoid space [17].
In this case study, the most suitable pathophysiology is the first and second theory, in which radiological findings were consistent with the presence of blood in multiple subarachnoid cisterns, along with evidence of microbleeding in the midbrain, which suggests the diagnosis of PNSAH. The parenchymal microbleeding in the midbrain spreading towards a suprasellar cistern and the right Sylvian fissures also explain the manifestation of motor weakness in the patient (Figures 2, 5, 7). On the other hand, the final theory implies the presence of SAH confined to the cerebral convexities only, without the involvement of the subarachnoid cisterns, which does not relate to our case. Appropriately, most case reports support the second and third theories, as most cases of SAH caused by CVST are found to have bleeding confined to the cerebral convexities, sparing the basal cistern.
In addition, basilar artery dissection is a very important differential diagnosis in cases of PNSAH with involvement of the prepontine cistern, as in this case. It is a life-threatening condition that needs to be excluded whenever there is a nonaneurysmal SAH because it requires prompt neurosurgical interventions [18]. Accordingly, Figure 7 presents evidence towards the absence of an arterial aneurysm and PNSAH-like bleeding patterns.
Moreover, the difference in the radiological appearance of CVST and SAH from the aneurysmal arterial origin is the most important differentiating factor between these 2 conditions [12]. Localized SAH is usually confined to the dorsolateral and para-sagittal cerebral convexities, sparing the basal cistern [12]. These findings should prompt physicians to search for nonaneurysmal causes for PNSAH such as CVST, which requires additional vascular imaging, including magnetic resonance arteriography and venography, to characterize the patterns of extravasated blood. As in our case, CT arteriography, magnetic resonance arteriography, magnetic resonance venography, and conventional cerebral angiography were performed rapidly, and imaging studies confirmed the absence of arterial aneurysms and the presence of CVST. Due to this pathological assumption of PNSAH, anticoagulation therapy is most likely to be effective to prevent rebleeding and vasospasm. However, standard anticoagulation dosing parameters, initiation time, and monitoring protocols are not well established, which makes the intervention for PNSAH induced by CVST slightly more complicated [19]. This allowed us to commence the administration of dose-adjusted anticoagulation therapy early during the treatment course and improve the patients’ prognosis of PNSAH, even though the morbidity rate of CVST is considered high.
Conclusions
CVST can be difficult to diagnose due to a wide spectrum of clinical symptoms, especially in the presence of acute SAH. Although SAH caused by CVST has been reported to be confined to cerebral convexities, the involvement of subarachnoid cisterns, including the basal cistern, as well as midbrain, should raise the index of suspicion for PNSAH to CVST after excluding arterial cerebral aneurysms and basilar artery dissection via neuroradiological characteristics. The present case report, combined with the underlying radiological distinction of PNSAH induced by CVST, which is a rare presentation of CVST, provides a better understanding of the differential diagnosis and prompt management of PNSAH in the context of CVST.
References:
- 1.Kajtazi NI, Zimmerman VA, Arulneyam JC, et al. Cerebral venous thrombosis in Saudi Arabia. Clinical variables, response to treatment, and outcome. Neurosciences (Riyadh) 2009;14:349–54. [PubMed] [Google Scholar]
- 2.Oppenheim C, Domigo V, Gauvrit J-Y, et al. Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. Am J Neuroradiol. 2005;26:614–17. [PMC free article] [PubMed] [Google Scholar]
- 3.Chang R, Friedman DP. Isolated cortical venous thrombosis presenting as subarachnoid hemorrhage: A report of three cases. Am J Neuroradiol. 2004;25:1676–79. [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty ML, Haverbusch M, Kissela B, et al. Perimesencephalic subarachnoid hemorrhage: Incidence, risk factors, and outcome. J Stroke Cerebrovasc Dis. 2005;14:267–71. doi: 10.1016/j.jstrokecerebrovasdis.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz TH, Solomon RA. Perimesencephalic nonaneurysmal subarachnoid hemorrhage: Review of the literature. Neurosurgery. 1996;39:433–40. doi: 10.1097/00006123-199609000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Cuvinciuc V, Viguier A, Calviere L, et al. Isolated acute nontraumatic cortical subarachnoid hemorrhage. Am J Neuroradiol. 2010;31:1355–62. doi: 10.3174/ajnr.A1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spitzer C, Mull M, Rohde V, Kosinski C. Non-traumatic cortical subarachnoid haemorrhage: Diagnostic work-up and aetiological background. Neuroradiology. 2005;47:525–31. doi: 10.1007/s00234-005-1384-6. [DOI] [PubMed] [Google Scholar]
- 8.Almalki YE. Acute subarachnoid hemorrhage as the initial presentation of extensive cortical and dural sinus venous thrombosis. International Journal of Science and Research. 2014;3:2319–7064. [Google Scholar]
- 9.Van Gijn J, Van Dongen K, Vermeulen M, Hijdra A. Perimesencephalic hemorrhage a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35:493–97. doi: 10.1212/wnl.35.4.493. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SK, Gupta R, Khosla VK, et al. Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: is it a benign entity? Surg Neurol. 2009;71:566–71. doi: 10.1016/j.surneu.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Coelho LGBSA, Costa JMD, Silva EIPA. Non-aneurysmal spontaneous subarachnoid hemorrhage: Perimesencephalic versus non-perimesencephalic. Rev Bras Ter Intensiva. 2016;28(2):141–46. doi: 10.5935/0103-507X.20160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan A, Ahmad B, Ahmed Z, Al-Quliti KW. Acute subarachnoid hemorrhage: An unusual clinical presentation of cerebral venous sinus thrombosis. Neurosciences. 2015;20:61–64. [PMC free article] [PubMed] [Google Scholar]
- 13.Boukobza M, Crassard I, Bousser M-G, Chabriat H. Radiological findings in cerebral venous thrombosis presenting as subarachnoid hemorrhage: A series of 22 cases. Neuroradiology. 2016;58:11–16. doi: 10.1007/s00234-015-1594-5. [DOI] [PubMed] [Google Scholar]
- 14.De Bruijn S, Budde M, Teunisse S, et al. Cerebral Venous Sinus Thrombosis Study Group Long-term outcome of cognition and functional health after cerebral venous sinus thrombosis. Neurology. 2000;54:1687–89. doi: 10.1212/wnl.54.8.1687. [DOI] [PubMed] [Google Scholar]
- 15.Fu F-W, Rao J, Zheng Y-Y, et al. Perimesencephalic nonaneurysmal subarachnoid hemorrhage caused by transverse sinus thrombosis: A case report and review of literature. Medicine (Baltimore) 2017;96(33):e7374. doi: 10.1097/MD.0000000000007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shad A, Rourke TJ, Jahromi AH, Green AL. Straight sinus stenosis as a proposed cause of perimesencephalic non-aneurysmal haemorrhage. J Clin Neurosci. 2008;15:839–41. doi: 10.1016/j.jocn.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Takeda H, Furuya D, et al. Subarachnoid hemorrhage as the initial presentation of cerebral venous thrombosis. Intern Med. 2010;49:467–70. doi: 10.2169/internalmedicine.49.2789. [DOI] [PubMed] [Google Scholar]
- 18.Shin LM, Dougherty DD, Orr SP, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 19.Hegazi MO, Ahmed S, Sakr MG, Hassanien OA. Anticoagulation for cerebral venous thrombosis with subarachnoid hemorrhage: A case report. Med Princ Pract. 2010;19:73–75. doi: 10.1159/000252839. [DOI] [PubMed] [Google Scholar]


