Abstract
Engineered tissues are being used clinically for tissue repair and replacement, and are being developed as tools for drug screening and human disease modeling. Self-assembled tissues offer advantages over scaffold-based tissue engineering, such as enhanced matrix deposition, strength, and function. However, there are few available methods for fabricating 3D tissues without seeding cells on or within a supporting scaffold. Previously, we developed a system for fabricating self-assembled tissue rings by seeding cells into non-adhesive agarose wells. A polydimethylsiloxane (PDMS) negative was first cast in a machined polycarbonate mold, and then agarose was gelled in the PDMS negative to create ring-shaped cell seeding wells. However, the versatility of this approach was limited by the resolution of the tools available for machining the polycarbonate mold. Here, we demonstrate that 3D-printed plastic can be used as an alternative to machined polycarbonate for fabricating PDMS negatives. The 3D-printed mold and revised mold design is simpler to use, inexpensive to produce, and requires significantly less agarose and PDMS per cell seeding well. We have demonstrated that the resulting agarose wells can be used to create self-assembled tissue rings with customized diameters from a variety of different cell types. Rings can then be used for mechanical, functional, and histological analysis, or for fabricating larger and more complex tubular tissues.
Keywords: Bioengineering, Issue 134, 3D printing, self-assembled tissue, vascular tissue engineering, agarose, custom mold, tissue engineering
Introduction
Cellular self-assembly approaches to fabricating tissue engineered blood vessels are an alternative to scaffold-based approaches. Self-assembled, scaffold-free tissues may have greater cell density, enhanced matrix deposition and strength, and improved biological function compared to scaffold-based tissues1,2,3,4. However, forming 3D tissues without the use of exogenous scaffold support with specific sizes and shapes remains a challenge. Some methods fuse together layers of cell sheets to form thicker constructs, although this process can be time consuming and labor intensive5. Alternatively, cells can be seeded into non-adhesive molds and allowed to aggregate into spheroids, rings, and other tissue shapes6,7,8.
Self-assembled tissue rings require fewer cells, shorter culture times, and less reagents than larger tubular engineered tissues, but can still be mechanically tested, examined histologically, or used for contractility and other functional testing7,9,10,11. Because they can be rapidly fabricated and easily tested, tissue rings are ideal for screening large numbers of culture parameters, and have potential for use as disease models11 or tools for drug screening12. Additionally, rings can be fused into more complex tissue structures such as blood vessels or trachea7,13, and rings may fuse more completely than other shapes such as spheroids14,15.
Agarose is widely used as a mold material for fabricating self-assembled tissues due to its biocompatibility, permeability, and non-cell adhesive properties. For example, Norotte et al. fabricated agarose molds from extruded rods, which enabled limited control over mold shape and required specialized equipment15. Tan et al. deposited alginate droplets as building units to fabricate custom hydrogel molds in various shapes (pyramid, square)16. However, the large diameter of the alginate spheroids (300 µm) resulted in features with low resolution. Such a low resolution may result in uneven mold surfaces that can adversely affect cell aggregation consistency. Alternatively, agarose can be cast into polymer negatives to create non-adhesive molds with smooth features and specific dimensions6,7,17.
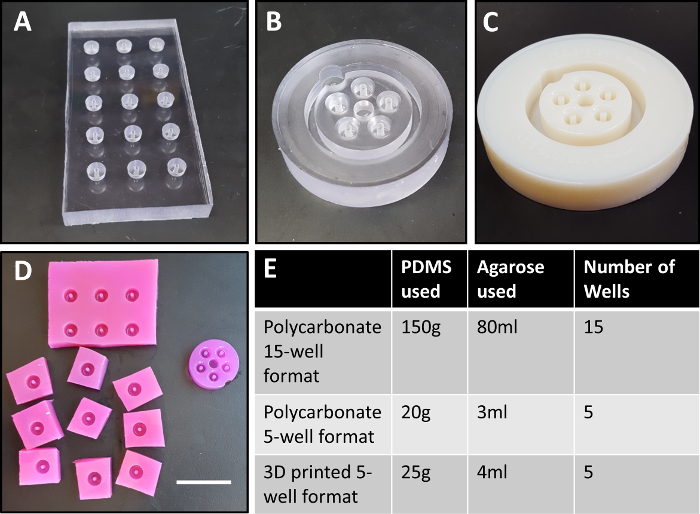
We previously reported a system for fabricating custom annular agarose cell-seeding wells from a PDMS negative cast in a milled polycarbonate mold7,18. Agarose was poured into the PDMS negative and allowed to set7,18. Cells were then seeded into agarose wells, where they aggregated to form self-assembled, scaffold-free tissue rings in less than 24 h7,18. PDMS negatives are autoclavable, can be reused many times, and are soft and flexible, making it easy to remove the solidified agarose wells. When this system was initially reported in Gwyther et al.7, PDMS negatives were cast from milled polycarbonate molds (Figure 1A). After agarose casting, the cell seeding wells were individually cut out and placed into wells of a 12-well plate7,18. The design was more recently modified such that a single agarose mold produces 5 rings and fits in a well of a 6-well plate, eliminating the need to cut out individual wells and reducing the amount of PDMS and agarose required to produce each ring (Figure 1B). A smaller cell seeding trough width was used to reduce the number of seeded cells required to achieve ring formation. Despite these changes, the resolution and customization of molds were restricted to available standard endmill dimensions, and micromilling can be prohibitively expensive. Additionally, computer numerical control (CNC) machining can be time consuming and cumbersome due to the need to reserve time on heavily utilized custom equipment, additional computer-aided manufacturing (CAM) software to convert the computer-aided design (CAD) file to a programmable tool path, and reliable fixturing of the polycarbonate part during machining.
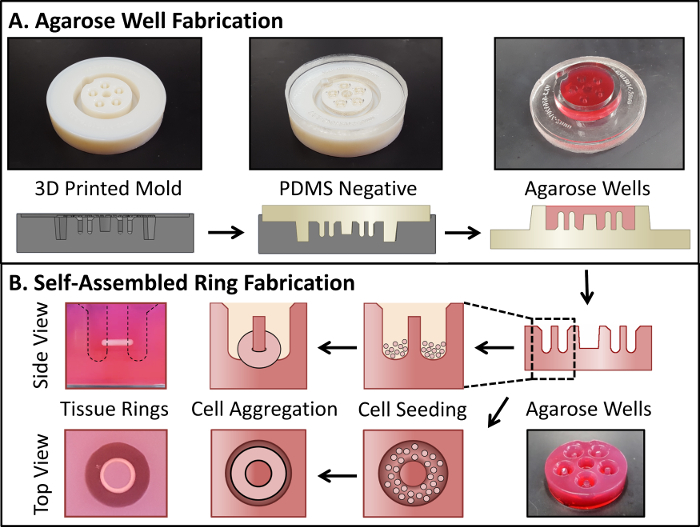
In the present study, we examined the use of 3D printing as an alternative to CNC machining. 3D printing is widely used for engineering custom implants, fabricating scaffold materials, and for direct printing of cells and tissue spheroids15,19,20. We used a high-resolution 3D printer, and specialized 3D printing material that enabled us to print a rigid mold with a smooth, glossy surface finish (see Table of Materials). Our technique allows for fabrication of highly customizable, high-resolution plastic molds that can be used for casting PDMS negatives and agarose wells. Design iterations are summarized in Figure 1. The mold design was further modified in the 3D-printed mold version to include tapered outer walls and a center hole in order to ease removal of both the PDMS negatives from 3D-printed molds and the agarose wells from PDMS negatives. These tapered features cannot be achieved with standard machining processes. The distance from the bottom of the wells to the bottom of the mold was increased in this iteration, resulting in a thicker agarose base below the posts to reduce the risk of posts breaking during agarose well removal. The mold and ring fabrication procedure is shown schematically in Figure 2.
Protocol
1. Preparing the 3D-printed Mold
Prepare a CAD drawing of the mold in the desired dimensions.
Send the CAD file to a high-resolution 3D printer. Select the appropriate plastic printing material, with glossy finish.
After printing, thoroughly wash the mold with detergent and water.
2. Casting PDMS Negatives
Measure the desired amount of PDMS base on a balance. For a mold with a 60 mm diameter and 2 mm well posts, use 25 g.
Add curing agent at a 1:10 (w/w) ratio to the PDMS base.
Stir vigorously until the two components are thoroughly combined. Insufficient mixing may result in incomplete curing of PDMS, resulting in a "tacky" surface.
Place a piece of laboratory tape around the border of 3D-printed molds, to enable the formation of a PDMS base layer above the printed wells.
Pour the PDMS mixture into the mold and place the mold into a vacuum chamber to de-gas until all bubbles are released.
Cure the PDMS at 50 °C for 2–4 h, or until solidified enough to remove.
Remove the lab tape and carefully extract the PDMS negative.
Incubate the PDMS at 60 °C for an additional 1 h (after removal from the 3D-printed mold) to ensure that mold is fully cured.
Wash the PDMS thoroughly with detergent and water to remove any residue. Insufficient washing may result in poor ring formation for the first uses of the PDMS negative.
3. Fabricating Agarose Wells
Prepare agarose wells 1 day prior to seeding cells.
Make a 2% (w/v) agarose solution in DMEM and autoclave.
Pipet 4 mL molten agarose into an autoclaved PDMS negative. Then pipet agarose directly into the posts of PDMS negatives. Remove any air bubbles with a pipet tip, as bubbles may cause inhomogeneities in the well dimensions.
NOTE: Do not overfill molds; the top surface of the agarose must be flat, so that agarose wells will be level upon removal from PDMS and placement into 6-well culture plates. Leftover agarose may be re-autoclaved and used again, although not more than once.
After the agarose cools (approximately 10 min for 4 mL agarose; larger molds may need longer cooling times), carefully separate the agarose wells from the PDMS negatives using blunt forceps and transfer into a well of a 6-well plate.
Submerge the agarose wells in complete culture medium, and equilibrate overnight in a 37 °C incubator prior to use.
4. Seeding Tissue Rings
Culture rat aortic smooth muscle cells21 (RaSMCs) in DMEM containing 10% fetal bovine serum (FBS), 1% L-glutamine, 1% non-essential amino acids, 1% sodium pyruvate, and 1% penicillin-streptomycin until they reach 70% confluence. NOTE: Cells should be cultured at 5% CO2 and 37 °C in 15 cm tissue culture plates.
After molds are equilibrated (section 3), prepare RaSMCs for seeding.
Rinse plates twice with 5 mL per plate of phosphate buffered saline (PBS).
Add 3 mL 0.25% trypsin per 15 cm Petri dish. Move the plates to a 37 °C incubator for 2–3 min, or until cells have lifted off the plate.
Neutralize trypsin with an equal volume (3 mL per plate) of complete culture medium. Resuspend the cells thoroughly to break up clumps.
Dilute an aliquot of cell suspension 1:1 with trypan blue dye, and count the cells with a hemocytometer.
Centrifuge the total volume of cell suspension for 5 min at 200 x g to pellet cells.
Resuspend cells at a concentration of 10 million cells per mL; this will result in 500,000 cells per 50 µL. NOTE: Different cell types may require different cell concentrations.
Aspirate all medium from the agarose mold. Be careful to remove all medium from individual wells, but to not puncture the bottoms of the wells.
Pipet 50 µL of suspension into each well.
Carefully add 2 mL of fresh medium around the outside of the agarose mold. Be careful not to let medium overflow into the wells of the agarose. Place plates in the incubator overnight (approximately 16 h).
After overnight incubation, aspirate the medium from outside of the molds, and add 4.5 mL fresh medium to each well of the 6-well plate so that molds and rings are completely submerged. Change medium daily.
Representative Results
This system allows for the simple, customized fabrication of agarose cell seeding wells that enables cell self-assembly of ring-shaped 3D engineered tissues. 3D printing allows better resolution and greater flexibility in mold design than machining polycarbonate, where dimensions are constrained by the available tool sizes. With a high-resolution 3D printer, walls as thin as 0.254 mm can be printed, and trough dimensions are limited only by the resolution of the printer (15.2 µm resolution for these studies). Typically, CNC endmills less than 0.3 mm are not commercially available, so it is not possible to create troughs of smaller widths. Micro-milling can produce smaller features, although the equipment required may be prohibitively expensive or inaccessible to many biomedical research labs. Trough dimensions and curvature are also limited by the endmill tip shape. Additionally, features such as angled or tapered walls are not possible, and small features may break during the machining process.
CAD drawings can be easily modified to produce 3D-printed molds and PDMS negatives of customizable dimensions. Figure 3 describes the dimensions of our current 2 mm post 3D-printed molds, compared to previous design iterations. The process is inexpensive; the 2 mm, 5-ring 3D-printed mold cost 44.67 USD to 3D print, and each part can be used to create multiple PDMS negatives. To date, we have created more than 30 PDMS negatives from a single 3D-printed mold. Each negative requires 25 g of PDMS at 0.11 USD per g (2.75 USD per PDMS negative). Each PDMS negative can be cleaned with detergent, autoclaved, and re-used for up to several years, depending on frequency of use. Compared to the original design18, the compact 3D-printed mold uses considerably less PDMS and agarose per 2 mm tissue ring (Figure 1E).
Cells seeded in the equilibrated agarose wells aggregate to form tissue rings in less than 24 h. Ring dimensions depend on the dimensions of the agarose wells. Here, we demonstrated that self-assembled rat smooth muscle cell rings can be fabricated in wells with 2, 4, or 12 mm diameter posts (Figure 4). While rings in this study were only cultured for 3 days, we have previously cultured hSMC rings for up to 2 weeks22, and hMSC-cartilage rings for up to 3 weeks13. As reported previously, rings can function as 3D in vitro models of human tissues for quantitative assessment of tissue function11 and mechanical strength13,22, and can also serve as modular building units to generate tube-shaped tissue constructs7,13. Cell seeding conditions and functional attributes of tissue rings engineered from these cell types are summarized in Table 1.
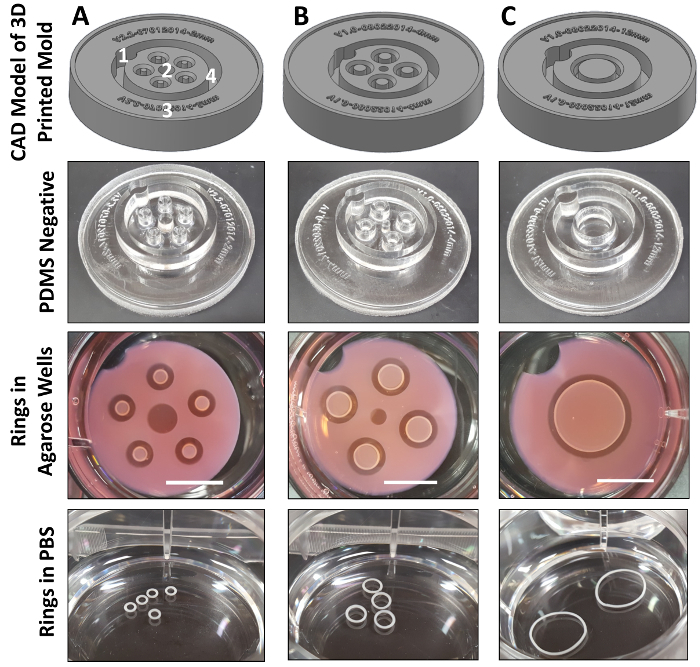
Figure 1: Polycarbonate mold designs. Initially, 15-well molds were machined in polycarbonate (A). Later versions featured a more compact design (B), with 5 wells designed to fit in a single polycarbonate mold, and within one well of a 6-well plate. Here, we modified this design to use 3D-printed plastic as a more customizable alternative to machined polycarbonate (C). Shown in (D) are agarose wells fabricated from the initial design18 (left) compared to the current compact design (right), which requires significantly less agarose, and does not require manual separation of wells. Quantities of PDMS and agarose required for each design iteration are shown in (E). Center posts are 2 mm in diameter. Mold in (A) is 6 cm x 12 cm, (B) has a 5 cm diameter, and (C) has a 6 cm diameter. Scale bar in (D) = 3 cm. Please click here to view a larger version of this figure.
Figure 2: Fabrication of self-assembled tissue rings. A 3D-printed mold is used to cast a PDMS negative, which is then used to cast the agarose wells (A). Cells are then seeded directly into the agarose wells, where they aggregate in less than 24 h to form tissue rings (B). Dashed lines in (B) show the well outline. Please click here to view a larger version of this figure.
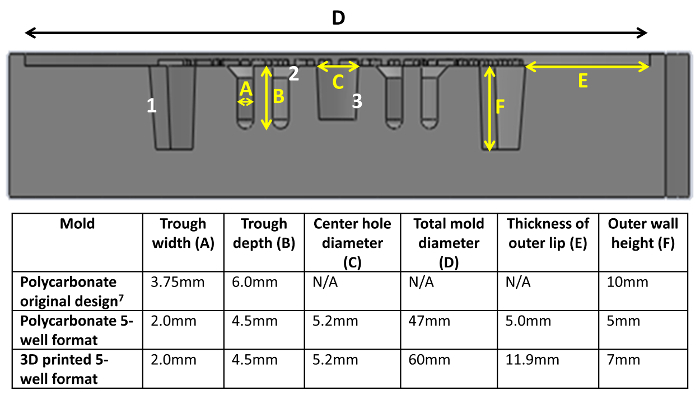
Figure 3: Cross-sectional view of 3D-printed mold CAD drawing. Dimensions for trough width (A), trough height (B), center hole (C), total diameter (D), outer lip (E), and outer wall height (F) are shown. The outer walls (1), upper portion of the wells (2), and center hole (3) are tapered (at a 5 ° angle for 1 and 3, 45 ° for 2) to improve ease of PDMS negative removal. Please click here to view a larger version of this figure.
Figure 4: CAD drawings with corresponding PDMS negatives and self-assembled SMC rings with 2 (A), 4 (B), and 12 (C) mm post diameters. In (A), (1) indicates a notch for providing orientation, (2) indicates a central hole to improve perfusion, (3) is a file number, which imprints directly onto the PDMS negatives, and (4) shows the outer wall, which is tapered to ease PDMS negative removal. Scale bars in agarose wells = 1 cm. Please click here to view a larger version of this figure.
| Cell type | Cells seeded per ring | Ring diameter | Culture Duration | Functional analysis performed |
| Rat SMC | 0.5, 1, or 3 x 106 | 2, 4, or 12 mm | 3 days | Current study; N/A |
| iPSC derived SMC 11 | 0.6 x 106 | 2 mm | 17 days | Contractility testing, uniaxial tensile test, histological analysis |
| Human SMC 22 | 0.4 x 106 | 2 mm | 2, 7, or 14 days | Uniaxial tensile test, histological analysis |
| Human MSC 14 | 0.4 x 106 | 2 mm | 21 days | Uniaxial tensile test, histological analysis, ring fusion and tube compression testing, differentiation into cartilage tissue |
Table 1: Cell numbers required for ring fabrication from different cell types.
Discussion
Here we have presented a versatile method for the rapid fabrication of self-assembled tissue rings with easily customized dimensions using 3D printing. Our method is similar to that reported in Svoronos et al.6, where 3D-printed honeycomb and dog-bone shaped wax molds were used to cast PDMS negatives. However, the molds have been modified to contain several unique design features. A notch (Figure 4A(1)) provides orientation of the mold to allow each ring to be labeled and monitored individually. The central hole (Figure 4A(2)) helps improve diffusion of medium into the wells. CAD file numbers are printed directly on the mold; therefore, PDMS negatives are each labeled with version number and post diameter (Figure 4A3). The tapered outer walls (Figure 3(1), 5 °), at the top of the well troughs (Figure 3(2), 45 °), and central hole (Figure 3(3), 5 °) make it easier to remove PDMS negatives from the 3D-printed molds, and agarose wells are easier to remove from the PDMS negatives (Figure 4A(2), A(4)).
We have demonstrated the versatility of this system by fabricating self-assembled ring-shaped tissues of a variety of diameters and cell types, including primary human smooth muscle cells (SMCs)18,22, rat aortic SMCs7,23, human mesenchymal stem cells (hMSCs)13, and SMCs derived from induced pluripotent stem cells (iPSCs)11 (Table 1). In ongoing work, we are evaluating the formation of rings from additional cell types such as endothelial cells, and fusing cartilage rings of varying sizes for potential applications in tracheal replacement. In addition to completely cell-derived constructs, we have also used this system to fabricate rings with incorporated cross-linked gelatin microspheres13,22. Microspheres can be incorporated within tissue rings during self-assembly to provide additional mechanical strength, or for localized delivery of growth factors13,22.
When fabricating tissue rings, optimization of cell number may be required for different cell types. Minimum cell numbers may vary based on the size and type of cells. For example, hSMCs derived from iPSCs are seeded at 600,000 cells/ring11, hMSCs and primary hSMCs are seeded at 400,000 cells/ring13,22, and rat aortic SMCs are seeded at 500,000 cells/ring18. Trough dimensions may also affect ring formation and the minimum number of cells required for ring formation24. For studies with human cells and 3D-printed molds, a trough width of 2 mm was used. The original polycarbonate molds had a trough width of 3.75 mm, which required 750,000 hSMCs to form a 2 mm cell ring18. With the reduced trough width, we were able to reduce the number of cells necessary for ring formation by 46%, to 400,000 cells per ring25. Quantities of cells seeded per ring are summarized in Table 1.
When choosing a 3D-printed material, many factors need to be considered. Because PDMS is typically cured at 60 °C, the 3D-printed material must have a high enough melting temperature to avoid damage during PDMS curing. The melting temperature of the material used in this study (a proprietary material, see Table of Materials) is not available. However, when baked at 60 °C for 1 h, we observed that the material began to produce an odor. Thus, we decided to lower the curing temperature to 50 °C and increase the curing time in order to bake the PDMS without damaging the 3D-printed material. Adjustments in curing time may be necessary if molds are modified to form larger PDMS negatives. An additional curing period at 60 °C after PDMS removal from the 3D-printed molds prevents the final PDMS negative from remaining tacky, while limiting the temperature the 3D-printed mold is exposed to. Note that some materials inhibit curing of PDMS, so ensure that the selected material is compatible with PDMS. Finally, the mold material toxicity must also be considered. While the 3D-printed mold will not be in direct contact with cells, it is possible that some residue from the mold may be transferred to the PDMS negative during the curing procedure. We found that very thorough washing with detergent was sufficient to remove any residue from the PDMS negative. However, we previously observed that inadequate washing led to poor ring formation in agarose wells for the first few uses of the PDMS negative. The use of PDMS cast from other 3D-printed materials may require additional investigation to verify that detergent is sufficient to remove mold residues, including any potential leachates. Periodic testing may also be necessary, as it possible that repeated heating cycles (even to 50 °C) may damage the mold over time, and cause increases in residue after repeated use. To date, we have used a single 3D-printed mold to produce more than 30 PDMS negatives that have been used to successfully generate tissue rings.
Overall, 3D printing allows greater versatility for the fabrication of agarose molds than machining of polycarbonate. It provides a higher resolution than is possible with tooling, and mold design is not limited by the dimensions of the tools available. This allows for greater customization, and the addition of features such as tapering that may not be possible with machining. This system may be applied to fabricating self-assembled tissues in other shapes as well, in addition to rings6,17. Using the ring fabrication method, we have developed tissue rings from a variety of cell types and sizes for potential applications in tracheal tissue engineering13, engineered blood vessels7, and modeling vascular diseases11.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We gratefully acknowledge Dr. Erica Stults (Academic Research & Application Scientist, WPI Information Technology Services) for her assistance with 3D printing, Amanda Zoë Reidinger, Ph.D., Chris Nycz, and Karen Levi, M.E., for their input on mold design, Kathy Suqui and Jennifer Mann for their assistance testing mold designs, and Michael O’Keefe for his assistance with filming. This work was supported by NSF IGERT DGE 1144804 (MWR, HAS), NIH R15 HL097332 (MWR, TAH), NSF REU EEC0754996 (BA), NIH 1R01 EB023907 (MWR, HAS) and NIH R15 HL137197 (MWR, HAS).
References
- L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- Adebayo O, Hookway TA, Hu JZ, Billiar KL, Rolle MW. Self-assembled smooth muscle cell tissue rings exhibit greater tensile strength than cell-seeded fibrin or collagen gel rings. J Biomed Mater Res A. 2013;101(2):428–437. doi: 10.1002/jbm.a.34341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7(7):2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Kelm JM, et al. A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol. 2010;148(1):46–55. doi: 10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- McAllister TN, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- Svoronos AA, Tejavibulya N, Schell JY, Shenoy VB, Morgan JR. Micro-mold design controls the 3D morphological evolution of self-assembling multicellular microtissues. Tissue Eng Part A. 2014;20(7-8):1134–1144. doi: 10.1089/ten.tea.2013.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwyther TA, et al. Engineered vascular tissue fabricated from aggregated smooth muscle cells. Cells Tissues Organs. 2011;194(1):13–24. doi: 10.1159/000322554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehesz AN, et al. Scalable robotic biofabrication of tissue spheroids. Biofabrication. 2011;3(2):025002. doi: 10.1088/1758-5082/3/2/025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterreur V, et al. Comparison of the direct burst pressure and the ring tensile test methods for mechanical characterization of tissue-engineered vascular substitutes. J Mech Behav Biomed Mater. 2014;34:253–263. doi: 10.1016/j.jmbbm.2014.02.017. [DOI] [PubMed] [Google Scholar]
- L'Heureux N, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash BC, et al. Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells. Stem Cell Reports. 2016;7(1):19–28. doi: 10.1016/j.stemcr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heureux N, et al. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J. 2001;15:515–524. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]
- Dikina AD, Strobel HA, Lai BP, Rolle MW, Alsberg E. Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs. Biomaterials. 2015;52:452–462. doi: 10.1016/j.biomaterials.2015.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twal WO, et al. Cellularized microcarriers as adhesive building blocks for fabrication of tubular tissue constructs. Ann Biomed Eng. 2014;42(7):1470–1481. doi: 10.1007/s10439-013-0883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30):5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, et al. 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication. 2014;6(2):1–11. doi: 10.1088/1758-5082/6/2/024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DM, Napolitano AP, Youssef J, Morgan JR. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. The FASEB Journal. 2007;21(14):4005–4012. doi: 10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- Gwyther TA, Hu JZ, Billiar KL, Rolle MW. Directed cellular self-assembly to fabricate cell-derived tissue rings for biomechanical analysis and tissue engineering. J Vis Exp. 2011. p. e3366. [DOI] [PMC free article] [PubMed]
- Zhu W, et al. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 2016;40:103–112. doi: 10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Jakab K, et al. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2(2):022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire JM, Potter-Perigo S, Hall KL, Wight TN, Schwartz SM. Distinct Rat Aortic Smooth Muscle Cells Differ in Versican/PG-M Expression. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16(6):821–829. doi: 10.1161/01.atv.16.6.821. [DOI] [PubMed] [Google Scholar]
- Strobel HA, et al. Cellular self-assembly with microsphere incorporation for growth factor delivery within engineered vascular tissue rings. Tissue Eng Part A. 2017;23(3-4):143–155. doi: 10.1089/ten.tea.2016.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel HA, Calamari EL, Beliveau A, Jain A, Rolle MW. Fabrication and characterization of electrospun polycaprolactone and gelatin composite cuffs for tissue engineered blood vessels. JBMR Part B. 2017. In Press. [DOI] [PubMed]
- Napolitano AP, Chai P, Dean DM, Morgan JR. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;13(8):2087–2094. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- Gwyther T. Engineered Vascular Tissue Generated by Cellular Self-Assembly. Worcester Polytechnic Institute; 2012. [Google Scholar]


