Summary:
Perfluorooctanoic acid (PFOA) is a persistent environmental contaminant that has been detected in public water systems in the United States alone. A variety of epidemiological investigations of PFOA have been published, mostly using measured or modeled serum PFOA concentrations as the exposure metric. Comparison of drinking water PFOA concentrations to those study findings or to typical serum concentrations requires pharmacokinetic modeling. This brief communication describes an online Javascript calculator that easily plots the expected serum PFOA concentration over time and at steady state for adults after starting or stopping consumption of PFOA-contaminated water. Two examples of usage are provided, including increasing serum PFOA after ongoing consumption of contaminated water at the federal limit, and decreasing serum PFOA after carbon filtration began in a contaminated water system. https://doi.org/10.1289/EHP2820
Introduction
Perfluorooctanoic acid (PFOA) is a synthetic hydrocarbon and a common environmental contaminant as a result of the long use of its ammonium salt in the manufacturing and processing of fluoropolymers used in cookware, waterproof fabrics, food packaging, and other applications (IARC 2016). Recent reviews of the toxicological and epidemiological literature have concluded that PFOA is known to be toxic to human reproduction and development (Lam et al. 2014) and a presumed immune hazard to humans (NTP 2016), possibly carcinogenic to humans (Benbrahim-Tallaa et al. 2014; IARC 2016), and probably linked to kidney cancer, testicular cancer, ulcerative colitis, thyroid disease, hypercholesterolemia, and pregnancy-induced hypertension in a highly exposed community in the eastern United States (C8 Science Panel 2012).
PFOA has been found in air samples and water supplies around the world (IARC 2016) and was detected in of blood samples in the U.S. 2011–2012 National Health and Nutrition Examination Survey at a median concentration of (CDC 2017). A national water testing program recently revealed (Hu et al. 2016) that the toxicant is present in public water systems and that about 6 million U.S. residents are supplied with drinking water at concentrations exceeding the U.S. Environmental Protection Agency (EPA) health advisory limit of (U.S. EPA 2016) for the sum of PFOA and perfluorooctanesulfonic acid (PFOS).
By how much do we expect a person’s serum PFOA concentration to increase from drinking PFOA-contaminated water, how quickly does it increase, and how long will it take to return to “normal” serum levels after switching to filtered or bottled water? A modified one-compartment exponential decay model with adjustment for background exposures adequately describes the relationship between PFOA intake and serum concentrations in adults (Olsen et al. 2007; Bartell et al. 2010; Bartell 2012). But this calculation may be unfamiliar or beyond the reach of some researchers, physicians, and journalists and the millions of people who have consumed PFOA-contaminated water.
This article describes an online Javascript serum PFOA calculator, available at http://www.ics.uci.edu/~sbartell/pfoacalc.html, that makes this pharmacokinetic model accessible and easy to use by anyone who wants to understand the relationship between consumption of contaminated drinking water and the resulting changes in blood serum concentration over time. In brief, users enter a water PFOA concentration and an initial serum PFOA concentration, and the web calculator returns results based on the modified one-compartment model for adults. Advanced users can select alternative estimates for the pharmacokinetic parameters and background contribution to serum PFOA.
Discussion
The mathematical solution for a one-compartment pharmacokinetic model with a constant exposure rate is well known (Thuresson et al. 2006;Bartell 2012):
where is the serum toxicant concentration at time t, is the serum toxicant concentration at steady state (i.e., after enough time has passed for the serum concentration to stabilize after continuous exposure), is the initial serum toxicant concentration, and k is the elimination rate constant. k is related to the biological half-life through the expression , where is the half-life. Although any consistent set of units can be applied, the web calculator uses units of nanograms per milliliter for serum PFOA concentrations, per year for the elimination rate constant, and years for time. For the web calculator, users enter in the first field, labeled “Starting serum PFOA concentration” (Figure 1). The other values are entered or calculated as described below.
Figure 1.
Input form for online serum PFOA calculator.
Average biological half-lives for PFOA reported in previous human studies range from 2.1 to 10.1 y, but most estimates are (ATSDR 2015; Russell et al. 2015). The calculator uses a default value of 2.3 y based on a prospective subcohort of 200 participants from the C8 Science Panel studies (Bartell et al. 2010). If desired, a user can input an alternative value for the half-life by clicking on the “Advanced options” button and changing the value in the text box for “Half-life of PFOA in serum” (Figure 1).
For individuals consuming PFOA-contaminated water, the steady-state serum PFOA concentration can be written as follows:
where W is the water PFOA concentration (ng/L), S is the steady-state ratio of serum:water PFOA concentrations (unitless), and B is the background serum PFOA concentration(ng/mL) contributed by sources other than local drinking water. For the web calculator, the user enters W in the second field, labeled “Water PFOA concentration for ongoing consumption” (Figure 1). The default values of S and B are 114 (unitless) and , respectively, using published values from pharmacokinetic regression for private water consumers in the C8 Health Project (Hoffman et al. 2011) and the median serum PFOA concentration from a nationally representative sample (CDC 2017). Users can input alternative values for S and/or B in the “Advanced options” section.
Once the user presses the “Submit” button, an interactive plot is displayed showing predicted serum concentrations over time, and the predicted steady-state serum PFOA concentration is reported below the plot along with all of the input values. Users can move the cursor over any point on the curve to display the predicted serum PFOA concentration at that particular time (Figure 2).
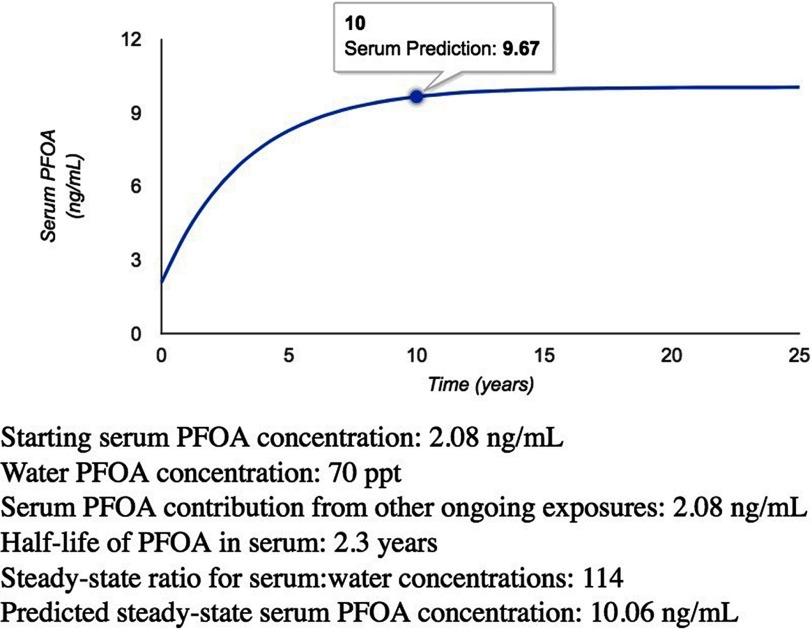
Figure 2.
Predicted serum concentration over time from online serum PFOA calculator.
The web calculator is implemented in HTML and JavaScript, both of which are supported by a wide variety of computer operating systems and web browsers. All source code for the web calculator is in a single file, which can be viewed directly by any user (e.g., in the Chrome browser by navigating to the web calculator and pressing ).
Two examples are provided to illustrate usage: predictions for an unidentified highly exposed individual in Pease Tradeport, New Hampshire, and predictions for a hypothetical person consuming PFOA-contaminated drinking water at the U.S. EPA–recommended limit.
Pease Tradeport
After being notified by the U.S. Air Force of contamination of several wells supplying Pease Tradeport, the New Hampshire Department of Environmental Services began a blood testing program in 2015. The program has measured PFOA and other perfluoroalkyl substances in serum samples from people who were potentially exposed via consumption of contaminated drinking water (New Hampshire Department of Health and Human Services 2016). The highest reported PFOA measurement was (Bagenstose 2017). The most contaminated wells were shut down or began treatment with granular activated carbon by late 2016, so presumably the Pease Tradeport water supply now has a PFOA concentration near 0.
The web calculator can be used to obtain future serum PFOA predictions for the individual in Pease Tradeport with the highest serum PFOA measurement by entering 32 for the starting serum PFOA concentration and 0 for the water PFOA concentration for ongoing consumption. Using the defaults for the pharmacokinetic parameters and background exposure level (i.e., assuming that this individual is now only exposed to PFOA through diet, air, dust, and other sources at typical levels), the web calculator shows a predicted serum PFOA concentration of after 5 y, after 10 y, with a subsequent decline approaching a steady-state serum PFOA concentration of . These values are substantially higher than estimates from the traditional exponential decay formula, which ignores the background contribution (7.09, 1.57, and , respectively).
Consumption at the U.S. EPA—Recommended Limit
The U.S. EPA recommends that the sum of PFOA and PFOS concentrations in drinking water not exceed in the short term (i.e., weeks or months) or in the long term, based on a reference dose derived from developmental toxicity in mice and consideration of toxicity data from a variety of studies in animals and humans (U.S. EPA 2016). By entering the starting serum concentration and the water PFOA concentration for ongoing consumption, the calculator can be used to predict serum concentrations each year over 25 y for a typical adult consuming PFOA at the recommended limit.
Entering 2.08 as the starting serum concentration is reasonable for an individual who had typical background exposures to PFOA before recently beginning to drink contaminated water. Entering 70 for the water PFOA concentration and using the defaults for the other parameters, the calculator shows a predicted serum concentration of after 1 y of exposure, after 5 y of exposure, and at steady state after many years of exposure. For comparison, the 95th percentile of serum PFOA concentrations in the United States was in 2011–2012 (CDC 2017), and the highest measured maternal serum PFOA concentration in the Odense Child Cohort in Denmark was (Dalsager et al. 2016).
The web calculator predicts average values for adults; individual results may vary based on tap water consumption, PFOA excretion, and exposures from other sources. Hypothesized or known differences can be accounted for by clicking on the “Advanced options” button and adjusting the parameters accordingly. For example, the default value of 114 for the steady-state ratio S is based on empirical population measurements and best predicts the consequences of the average water consumption rate. Individual-specific values of S are directly proportional to individual water consumption rates, and average direct and indirect community water consumption is about for adults (U.S. EPA 2011). An individual-specific value of S can be computed as , where I is the individual rate of community water consumption. Thus, a steady-state ratio of 219 would be appropriate for an individual who consumes of community water.
Individuals may also differ in their capacity for bodily storage and excretion of PFOA. For example, there is some evidence of small effects of age and sex on PFOA retention (Brede et al. 2010; Zhang et al. 2013; Gribble et al. 2015), potentially affecting individual-specific values for the half-life and steady-state ratio. At present these effects are not very well characterized, but their potential impacts can be explored by modifying those parameters in the “Advanced options” section.
The calculator also assumes that the background contribution to serum PFOA is constant over time. Although this is a reasonable approximation for highly contaminated water or for slowly changing background concentrations, in which case small variations in background exposure will have a negligible impact on the serum concentration, it may not be accurate for predictions over decades with lower water concentrations. Geometric mean serum PFOA concentrations in the National Health and Nutrition Examination Survey were , , , , , and in 1999–2000, 2003–2004, 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (CDC 2017). Although average serum PFOA concentrations were relatively stable over the period 1999–2012, they decreased in the last two sampling rounds and may continue to decline as manufacturers transition to replacement compounds, and as more PFOA-contaminated water supplies are identified and treated.
Conclusions
Pharmacokinetic models are useful for understanding the relationship between exposure and measured biomarkers and are increasingly being applied in environmental health research and regulation. However, these models are complex enough to be a potential barrier for some researchers, exposed individuals, and other stakeholders. Online calculators using appropriate literature-based default parameters can facilitate research translation for such models.
Acknowledgments
The author thanks J. Wagner of the Bucks County Courier Times for asking thoughtful questions that inspired the calculator.
References
- ATSDR (Agency for Toxic Substances and Disease Registry). 2015. Draft Toxicological Profile for Perfluoroalkyls. Atlanta, GA:Centers for Disease Control and Prevention. https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf [accessed 3 September 2017].
- Bagenstose K. 2017. Ivyland woman sues Navy after finding high PFOA blood level. The Intelligencer. Doylestown, PA; 12 January 2017 http://www.theintell.com/news/horsham-pfos/ivyland-woman-sues-navy-after-finding-high-pfoa-blood-level/article_83b1fada-d8e8-11e6-b2c4-7f62537e561a.html [accessed 3 September 2017].
- Bartell SM. 2012. Bias in half-life estimates using log concentration regression in the presence of background exposures, and potential solutions. J Expo Sci Environ Epidemiol 22(3):299–303, PMID: 22333729, 10.1038/jes.2012.2. [DOI] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect 118(2):222–228, PMID: 20123620, 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Lauby-Secretan B, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, et al. 2014. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol 15(9):924–925, PMID: 25225686, 10.1016/S1470-2045(14)70316-X. [DOI] [PubMed] [Google Scholar]
- Brede E, Wilhelm M, Göen T, Müller J, Rauchfuss K, Kraft M, et al. 2010. Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int J Hyg Environ Health 213(3):217–223, PMID: 20488749, 10.1016/j.ijheh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- C8 Science Panel. 2012. C8 Probable Link Reports. http://www.c8sciencepanel.org/prob_link.html [accessed 3 September 2017].
- CDC (Centers for Disease Control and Prevention). 2017. “Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables.” Atlanta, GA:CDC; https://www.cdc.gov/exposurereport/ [accessed 3 September 2017]. [Google Scholar]
- Dalsager L, Christensen N, Husby S, Kyhl H, Nielsen F, Høst A, et al. 2016. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1–4 years among 359 children in the Odense Child Cohort. Environ Int 96:58–64, PMID: 27608427, 10.1016/j.envint.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Gribble MO, Bartell SM, Kannan K, Wu Q, Fair PA, Kamen DL. 2015. Longitudinal measures of perfluoroalkyl substances (PFAS) in serum of Gullah African Americans in South Carolina: 2003–2013. Environ Res 143(pt B):82–88, PMID: 25819541, 10.1016/j.envres.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM. 2011. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ Health Perspect 119:92–97, PMID: 20920951, 10.1289/ehp.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3(10):344–350, PMID: 27752509, 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2016. Perfluorooctanoic acid. IARC Monogr Eval Carcinog Risk Hum 110:37–110, http://monographs.iarc.fr/ENG/Monographs/vol110/mono110.pdf [accessed 11 October 2017]. [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, et al. 2014. The Navigation Guide—evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect 122(10):1040–1051, PMID: 24968389, 10.1289/ehp.1307923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Hampshire Department of Health and Human Services. 2016. Pease Tradeport Water System Investigation. https://www.dhhs.nh.gov/dphs/investigation-pease.htm [accessed 3 September 2017].
- NTP (National Toxicology Program). 2016. NTP Monograph: Immunotoxicity Associated with Exposure to Perfluorooctanoic acid or Perfluorooctane Sulfonate. Research Triangle Park, NC:NTP. http://ntp.niehs.nih.gov/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf [accessed 3 September 2017].
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, Waterland RL, Wong F. 2015. Calculation of chemical elimination half-life from blood with an ongoing exposure source: the example of perfluorooctanoic acid (PFOA). Chemosphere 129:210–216, PMID: 25149361, 10.1016/j.chemosphere.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman A, Jakobsson K. 2006. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect 114(2):176–181, PMID: 16451851, 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2011. Exposure Factors Handbook 2011 Edition (Final Report), EPA/600/R-09/052F. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 [accessed 11 October 2017].
- U.S. EPA. 2016. Drinking Water Health Advisories for PFOA and PFOS (Overviews and Factsheets). https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos [accessed 3 September 2017].
- Zhang Y, Beesoon S, Zhu L, Martin JW. 2013. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47(18):10619–10627, PMID: 23980546, 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]