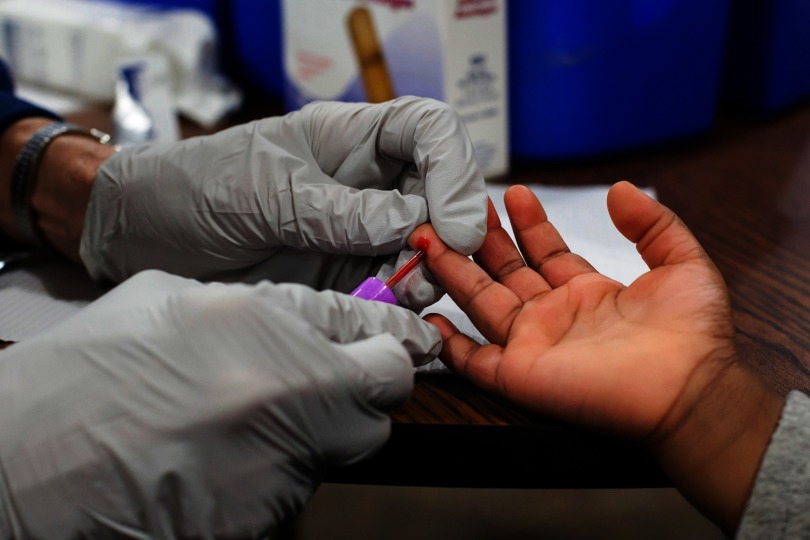
For perspective on the tenacious problem with childhood lead exposure in the United States, one can look to the Environmental Health and Lead Clinic at Cincinnati Children’s Hospital Medical Center (CCHMC). Approximately half a million U.S. children have elevated blood lead levels (BLLs),1 and the busy staff at CCHMC cares for hundreds of them every year, many of them arriving from impoverished inner-city neighborhoods where flaking lead paint is still pervasive.
BLLs in American children have fallen in the decades since lead was removed from gasoline and paint in the United States. But many children are still exposed to the metal in old paint chips, contaminated products imported from abroad, and drinking water flowing through leaded pipes. Because lead is a potent neurotoxicant, early exposure to it may have lifelong consequences for cognitive function and behavior.2 More than anything else, caregivers and parents “want to know if there’s anything they can do to help a kid who has been exposed to lead,” says Nicholas Newman, a pediatrician and medical director of the CCHMC.
Newman says he is routinely asked about interventions that might reduce lead’s impacts on the brain, or whether it is possible to ameliorate the consequences of exposure so that exposed children can still thrive as they grow. The answer to these questions, Newman says, is a qualified yes, given evidence that effects of lead on cognition and behavior may be modified by nutrition and neurodevelopmental supports at home and at school.3,4 Newman says he has treated some children with very high BLLs who “wound up doing quite well” and others with similar levels who did poorly by comparison.
By focusing on factors that might reduce the potential effects of lead on cognition, Newman and his colleagues try to put lead-exposed children in the best position possible to succeed. “Lead affects each child differently, and the exposures happen in a context that may or may not allow for optimal development,” he says. “And when there’s a strong family structure to support our efforts, I find the outcomes are better.”
State and federal screening programs are intended to catch high blood lead levels (BLLs) early. Most states at least recommend that children be tested at ages 1 and 2 years. Some states require that all 1- and 2-year-olds be tested. Image: © freemixer/iStockphoto.
Managing the Threat
In the period 1976–1980, while leaded paint and gasoline were still being phased out, BLLs among U.S. children aged 1–5 years averaged .5 As noted in one study from that time, levels as high as were considered “elevated but nontoxic.”6 Since then, repeated studies have been unable to identify a BLL that is not associated with a reduction in neurodevelopmental performance at the population level (although individual children may not show symptoms).7
“Whatever cell system you put it into, lead will perturb and ultimately damage it,” says Kim Cecil, a professor at CCHMC. Lead interferes with normal development of the brain’s synaptic architecture, with effects on attention, language, memory, and visual–motor integration.2
Since 2012, the Centers for Disease Control and Prevention (CDC) has recommended that a BLL over in children aged 1–5 years trigger actions to reduce exposure. That reference level, as the CDC calls it, is a statistical measure based on the 97.5th percentile of BLLs in U.S. children as of 2010 (meaning that at that time, only 2.5% of children had a BLL of or higher).8 But as a health indicator, this reference level “is irrelevant since we still do not know what specific blood level to associate with toxicity,” says Morri Markowitz, a pediatrician and director of the Lead Poisoning Prevention Program at Children’s Hospital at Montefiore.
One of the first steps in managing an elevated BLL is to identify and, to the extent possible, eliminate sources of lead. Paint, soil, drinking water, and imported goods such as glazed pottery and candy can all contribute to exposure. Image: © Jamie Hooper/Alamy Stock Photo.
Guidelines for managing lead-exposed children call for increasingly aggressive measures as the child’s BLL rises.9 Severely poisoned children may experience symptoms that include abdominal pain, seizures, anemia, and evidence of renal failure. In the worst cases, brain swelling can lead to coma and death; survivors may be left intellectually disabled, blind, or unable to walk.10
Chelation is a life-saving treatment for cases of severe lead poisoning. This treatment relies on chemical agents that attach to heavy metals and pull them from the bloodstream so they can be excreted in urine. Early treatment employed two agents in tandem: calcium disodium ethylenediaminetetraacetic acid (EDTA), which is given intravenously, and dimercaprol, which is injected into muscle tissue. That regimen saved many lives before the U.S. Food and Drug Administration in 1991 approved an oral chelator called dimercaptosuccinic acid, or succimer. Today, succimer is used most often for chelation, although calcium disodium EDTA and dimercaprol are still used in some cases.11
The standard course of succimer treatment lasts 19 days: 5 days at a high dose given in the hospital followed by 2 weeks at a lower dose that can be given at home if sources of lead exposure have been identified.12 Most of the lead will be eliminated in the first week of treatment, after which excretion rates will decline.11
The CDC, the American Academy of Pediatrics, and state health departments all recommend that doctors consider chelating children with a BLL of or higher, regardless of whether they show severe symptoms.9 “It is not unusual for two children with the same BLL—say —to act very differently,” says Markowitz. “One might complain of stomach aches and constipation, and the other seems fine. [But] both should be chelated.”
Newman adds that it is imperative that doctors chelate children at the hospital if their levels exceed . Otherwise, they risk brain swelling, coma, severe neurological damage, and death.10 But while chelation does improve neurological status for highly exposed children with severe encephalopathy, Markowitz says there are no data to show chelation improves IQ.
Reversing Low-Level Effects
Evidence that chelation also has no cognitive benefits for moderately exposed children came from an investigation by the National Institute of Environmental Health Sciences (NIEHS) that was launched in the early 1990s. Called the Treatment of Lead-Exposed Children (TLC) study, this randomized, double-blind clinical trial enrolled 780 children aged 12–33 months who had a BLL of .13 The children were randomly assigned to either succimer treatment or placebo.
Within 6 months, BLLs were lower on average in the succimer-treated group than they were in the placebo-treated controls. Even so, the authors concluded there was no evidence of either a benefit or adverse effect of chelation. A 3-year follow-up of the TLC cohort published in 2004 also reported no cognitive benefit from chelation, and guidelines ever since have indicated that the treatment need not be used for BLLs lower than .14 However, according to Newman, such guidelines are not legally binding, and doctors can prescribe at their discretion.
Still, Markowitz emphasizes that although IQ measures in the TLC cohort did not appear to benefit from chelation, brain function may yet improve as BLLs decline naturally in the absence of further exposure. He says that BLLs from acute exposures ordinarily have a half-life of 5 weeks, although with chronic exposure, some lead will sequester in the skeleton for decades, and then be remobilized as bone density declines with age or during pregnancy.2,15
The longer a child is exposed to lead, the longer the decline in BLL will take; Markowitz explains that lead that has accumulated in bone is released slowly over the years, and can result in persistent elevations in BLLs, even in the absence of further exposure. Poor nutrition will also influence excretion rates, since children with iron and calcium deficiencies absorb more lead from their gut into their blood.16
Besides its other nutritional benefits, eating plenty of iron helps children absorb less of any lead they may ingest. Image: © GMVozd/iStockphoto.
Conversely, Markowitz says protecting children from further exposure while improving their diets may allow them to excrete more lead than they ingest. Furthermore, during a 1993 study, Markowitz found that reductions in BLL were accompanied by corresponding improvements in tests of cognitive functioning.17 He and his colleagues spent months working with participating families, educating them on how lead gets into their children’s bodies, how to prevent further exposure, and how to manage a lead-exposed child’s nutritional needs.
“We analyzed the data from our intervention study and were unable to identify single components that explained the successful outcomes,” Markowitz says. “It was a package deal, or perhaps the kids got better in spite of our efforts to help them. But the takeaway point is that reductions in BLLs were associated with improved cognitive scores.”
Kim Dietrich, a professor of epidemiology and environmental health at the University of Cincinnati College of Medicine, says the likelihood of reversing cognitive damage as BLLs fall is influenced in no small part by the socioeconomic status of the child’s family. Effects of lead among children from the poorest families appear to be more difficult to reverse, he says. This is partly because the effects may be compounded by other stressors that affect neurodevelopment, including not only nutritional deficiencies, but also lower parental IQ, poorer educational infrastructures, and a lack of intellectual stimulation at home.
Management Priorities
In Newman’s experience, children whose BLLs stay elevated for prolonged periods of time do worse than those whose levels come down quickly. Experts interviewed for this story emphasized a number of key priorities in managing children with low to moderate lead exposure: a) identify and eliminate lead sources in a child’s environment, b) address the behaviors that expose children to lead, particularly the persistent eating of substances such as soil and paint, and c) ensure that children consume enough calcium and iron, which compete with lead for absorption and retention.
“A very high percentage of the kids I see are low in iron,” Newman says. “So we like to shift that dietary balance so that kids are not so likely to absorb lead. And this also gives families something tangible they can do to engage in their child’s improvement.” A recent review of dietary approaches for preventing or treating the effects of lead exposure found that supporting evidence in favor of a positive benefit for lead-exposed children was strongest for iron.18 Weaker evidence suggested calcium, vitamin C, and zinc may have similar benefits.
Another critical strategy is to boost cognitive stimulation in the child’s environment. Kim Cecil’s research suggests that lead damages neural circuits in the brain’s left hemisphere that ordinarily support language acquisition. However, upon scanning the brains of lead-exposed individuals, she discovered evidence suggesting that the brain compensated by recruiting alternative language circuits in the right hemisphere.19 In Cecil’s view, that finding supplies an evidence-based incentive for providing educational interventions that could counter the effects of lead.
A study of children living in a Mexican lead smelter community explored the potential neurodevelopmental benefits of cognitive stimulation at home. In this case, a mother’s own education and support of schoolwork and extracurricular activities were positively associated with improvements in her child’s cognition and behavior, suggesting that attentive home environments may lessen lead’s effects and improve educational outcomes. However, the authors also point out that “mothers may be more supportive of children with lower BLLs, who potentially have lower ratings of behavior problems and who perform better in school.”20
Newman points out that speech deficits raise frequent concerns for the parents of lead-exposed children. If a child’s language skills are not age appropriate, Newman says he might recommend speech therapy while encouraging parents to read, talk, and sing to their children. “And if it is feasible, I will encourage parents to enroll their kids in preschool or Early Head Start programs,” he says.
Reading, talking, and singing are important for helping children catch up language skills that may not have developed as expected. Image: © Jim West/Alamy Stock Photo.
Mary Jean Brown, an adjunct professor at the Harvard T.H. Chan School of Public Health and former chief of the CDC’s Healthy Homes and Lead Poisoning Prevention Program, is frustrated by the lack of educational interventions that have been tested and found to be effective in children with elevated BLLs. A 2015 CDC report that she co-authored recommended that interventions should be initiated at young ages, when they are more likely to be effective.21 She says that interventions geared for 2- to 3-year-old children that emphasize language development, executive functioning, and impulse control should be further evaluated, adding that “there are many good reasons to educate well, and lead exposure is one of them.”
Screening Children for Exposure
As it stands now, state and federal screening programs attempt to evaluate children at high risk for elevated BLLs.22 For instance, the Centers for Medicaid and Medicare Services requires that all children enrolled in Medicaid be tested for lead at ages 1 and 2 years.23 New York law is one of the most stringent: It requires that all children living in the state be tested annually at 1 and 2 years of age, and possibly up to age 6 years, for high-risk children, says Deborah Nagin, director of the Healthy Homes Program at the New York City Department of Health and Mental Hygiene. During these assessments, doctors ask caregivers about exposure risks from peeling paint, potentially contaminated consumer products in the child’s environment, and recent travel.
Nagin says that each year, the program receives roughly 300,000–400,000 blood test results from physicians, and that 1.7% of children tested in 2015 were found to have a BLL of or greater. Between 2005 and 2015, there was an 86% decline in the prevalence of BLLs of or greater, “which to us is a tremendous public health success story,” she says.
New York City is mandated to investigate lead hazards in the homes of children up to 18 years of age who have a BLL of or above. In addition, the Healthy Homes Program offers home inspections to families with children up to age 6 years with a BLL of and families with children up to age 15 months with a BLL of . If lead-paint hazards are identified, the building owner is ordered to safely correct the problem within a specified time frame. If that does not occur, the case is referred to the housing department’s Emergency Repair Program, which completes the work and puts a lien on the property until the landlord pays the bill, Nagin says.
When lead screening turns up a BLL of , Nagin’s department sends a cautionary letter to the family and the child’s physician, alerting them of the need for a follow-up blood test. The letter also counsels the family on ways to prevent exposure.
At the same time, Brown cautions against stigmatizing children with low to moderate BLLs that would have been typical for their parents’ and grandparents’ generations. “The idea of self-fulfilling prophecies is very powerful,” she says. “You do not want parents or teachers saying, ‘Well, this is a lead-exposed kid, so what do you expect?’”
Martha Stanbury, manager of the Environmental Health Surveillance Section in the Michigan Department of Health and Human Services, says stigma is a particular concern for the 99,000 residents of Flint, given the publicity surrounding lead-contaminated municipal drinking water in that city. Although the range of BLLs has never been publicly disclosed, the CDC estimated that Flint children were 46% more likely to have a BLL greater than after the switch than before.24
Stanbury says her department averts stigmatization by making lead abatement services available to families that qualify based on income and other factors, regardless of a child’s BLL (although families whose child has an elevated BLL are given higher priority for these resources). “A BLL of zero does not mean you were not exposed to lead in the past,” she says. “For us, the primary emphasis is on prevention, because if you wait for BLLs to become elevated, then the horse has already left the barn.”
It is important not to stigmatize or expect failure from children for whom testing reveals a high BLL. “The idea of self-fulfilling prophecies is very powerful,” says Mary Jean Brown, former chief of the CDC's Healthy Homes and Lead Poisoning Prevention Program. Image: © Jim West/Alamy Stock Photo.
Setting Goals
In December 2016, the CDC announced it was considering whether to lower the reference level from to for consistency with the current 97.5th percentile of BLLs in U.S. children.25 The move is expected to increase the overall number of children whose BLLs are considered elevated. But Markowitz points out that the difference between 3.5 and 5 is within laboratory error for individual measurements, and that most commercial laboratories cannot measure lead accurately enough to the decimal place.
The proposed measure is controversial for other reasons. Dietrich explicitly opposes the change, saying that public health officials and clinics have a hard-enough time as it is managing the population of children with a BLL in the range of . “My view is that proposal is statistically rather than health based and motivated to eliminate lead exposure entirely—not really an achievable goal,” he says.
He also questions how this reduction would translate to public health practice. “I cannot say that a child with a BLL of is lead poisoned and predict neurodevelopmental impacts with any confidence. But that’s not how the new BLL will be interpreted by the media. And now you have these remarkable pronouncements coming out of Flint that kids with these low levels are permanently injured, which is complete nonsense,” he says. “The best we can do when we encounter such a low BLL is to alert parents of a potential exposure source in the child’s environment.”
In Newman’s opinion, “what we really need to do is stop lead exposure, and insofar as lowering the reference level raises awareness and helps us accomplish that goal, I agree [it should be done].” He adds that lead screening means clinical interactions with children who, in many cases, are confronted by many neurodevelopmental risk factors: multigenerational poverty, nutritional problems, drug use, and more. “It provides an opportunity to help these kids and the rest of the family, too.”
Biography
Charles W. Schmidt, MS, an award-winning science writer from Portland, Maine, writes for Scientific American, Science, various Nature publications, and many other magazines, research journals, and websites.
References
- 1.CDC (Centers for Disease Control and Prevention). 2013. Childhood Lead Poisoning [fact sheet]. Atlanta, GA: Centers for Disease Control and Prevention; https://www.cdc.gov/nceh/lead/factsheets/lead_fact_sheet.pdf [accessed 4 September 2017]. [Google Scholar]
- 2. Lidsky TI, Schneider JS. 2013. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126(Pt 1):5–19, PMID: 12477693, 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 3. Chiodo LM, Jacobson SW, Jacobson JL. 2004. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol 26(3):359–371, PMID: 15113598, 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 4.U.S. EPA (U.S. Environmental Protection Agency). 2001. Fight Lead Poisoning with a Healthy Diet: Lead Poisoning Prevention Tips for Families. EPA-747-F-01-004. https://www.epa.gov/sites/production/files/2014-02/documents/fight_lead_poisoning_with_a_healthy_diet.pdf [accessed 4 September 2017].
- 5. Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. 1994. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Survey (NHANES). JAMA 272(4):284–291, PMID: 8028141, 10.1001/jama.1994.03520040046039. [DOI] [PubMed] [Google Scholar]
- 6. David OJ, Hoffman SP, Sverd J, Clark J, Voeller K. 1976. Lead and hyperactivity. Behavioral response to chelation: a pilot study. Am J Psychiatry 133(10):1155–1158, PMID: 823826, 10.1176/ajp.133.10.1155. [DOI] [PubMed] [Google Scholar]
- 7. Budtz-Jørgensen E, Bellinger D, Lanphear B, Grandjean P. 2013. An international pooled analysis for obtaining a benchmark dose for environmental lead exposure in children. Risk Anal 33(3):450–461, PMID: 22924487, 10.1111/j.1539-6924.2012.01882.x. [DOI] [PubMed] [Google Scholar]
- 8.CDC. 2017. What Do Parents Need to Know to Protect Their Children? Atlanta, GA: Centers for Disease Control and Prevention; https://www.cdc.gov/nceh/lead/acclpp/blood_lead_levels.htm [accessed 4 September 2017]. [Google Scholar]
- 9.PEHSU (Pediatric Environmental Health Specialty Units). 2013. Recommendations on Medical Management of Childhood Lead Exposure and Poisoning. Elk Grove Village, IL, and Phoenix, AZ: Pediatric Environmental Health Specialty Units; http://www.pehsu.net/_Library/facts/medical-mgmnt-childhood-lead-exposure-June-2013.pdf [accessed 4 September 2017]. [Google Scholar]
- 10.CDC. 1991. Preventing Lead Poisoning in Young Children: Chapter 7. Atlanta, GA: Centers for Disease Control and Prevention; https://www.cdc.gov/nceh/lead/publications/books/plpyc/chapter7.htm [accessed 4 September 2017]. [Google Scholar]
- 11. Nolans GN, Karalliedde L, Wiseman HM. 2010. Review of Succimer for Treatment of Lead Poisoning. Geneva, Switzerland:World Health Organization. http://www.who.int/selection_medicines/committees/expert/18/applications/succimer.pdf [accessed 4 September 2017].
- 12.PDR (Prescribers’ Digital Reference). 2017. Chemet (Succimer)—Full Prescribing Information. http://www.pdr.net/full-prescribing-information?druglabelid=2041#ID_03de32c4-b8ec-20bc-0780-49a70536e1f8 [accessed 4 September 2017].
- 13. Rogan WJ, Dietrich KN, Ware JH, Dockery DW, Salganik M, Radcliffe J, et al. 2001. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med 344(19):1421–1426, PMID: 11346806, 10.1056/NEJM200105103441902. [DOI] [PubMed] [Google Scholar]
- 14.CDC. 2012. “Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention. Report of the Advisory Committee on Childhood Lead Poisoning Prevention of the Centers for Disease Control and Prevention.” Atlanta, GA:Centers for Disease Control and Prevention; https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf [accessed 4 September 2017]. [Google Scholar]
- 15. Silbergeld EK. 1991. Lead in bone: implications for toxicology during pregnancy and lactation. Environ Health Perspect 91(2):63–70, PMID: 2040252 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1519355/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyer RA. 1995. Nutrition and metal toxicity. Am J Clin Nutr 61(suppl 3):646S–650S, PMID: 7879732. [DOI] [PubMed] [Google Scholar]
- 17. Ruff HA, Bijur PE, Markowitz M, Ma YC, Rosen JF. 1993. Declining blood lead levels and cognitive changes in moderately lead-poisoned children. JAMA 269(13):1641–1646, PMID: 8455297, 10.1001/jama.1993.03500130055032. [DOI] [PubMed] [Google Scholar]
- 18. Kordas K. 2017. The “lead diet”: can dietary approaches prevent or treat lead exposure? J Pediatr 185:224–231.e1, PMID: 28283259, 10.1016/j.jpeds.2017.01.069. [DOI] [PubMed] [Google Scholar]
- 19. Yuan W, Holland SK, Cecil KM, Dietrich KN, Wessel SD, Altaye M, et al. 2006. The impact of early childhood lead exposure on brain organization: a functional magnetic resonance imaging study of language function. Pediatrics 118(3):971–977, PMID: 16950987, 10.1542/peds.2006-0467. [DOI] [PubMed] [Google Scholar]
- 20. Moodie S, Ialongo N, López P, Rosado J, Garcia-Vargas G, Ronquillo D, et al. 2013. The conjoint influence of home enriched environment and lead exposure on children's cognition and behaviour in a Mexican lead smelter community. Neurotoxicology 34:33–41, PMID: 23110976, 10.1016/j.neuro.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 21.CDC. 2015. Educational Interventions for Children Affected by Lead. Atlanta, GA:Centers for Disease Control and Prevention; https://www.cdc.gov/nceh/lead/publications/educational_interventions_children_affected_by_lead.pdf [accessed 4 September 2017]. [Google Scholar]
- 22. Dickman J. 2017. “Children at Risk: Gaps in State Lead Screening Policies. Washington, DC:Safer Chemicals Healthy Families.” http://saferchemicals.org/sc/wp-content/uploads/2017/01/saferchemicals.org_children-at-risk-report.pdf [accessed 4 September 2017]
- 23.Centers for Medicare & Medicaid Services. 2014. Lead Screening. Baltimore, MD:Centers for Medicare & Medicaid Services; https://www.medicaid.gov/medicaid/benefits/epsdt/lead-screening/index.html [accessed 4 September 2017]. [Google Scholar]
- 24. Kennedy C, Yard E, Dignam T, Buchanan S, Condon S, Brown MJ, et al. 2016. Blood lead levels among children aged <6 years—Flint, Michigan, 2013–2016. MMWR 65( 25):650–654, PMID: 27359350, 10.15585/mmwr.mm6525e1. [DOI] [PubMed] [Google Scholar]
- 25. Robbins R. 2017. CDC panel urges new guidelines for diagnosing high lead levels in children. STAT, Health section, online edition. 20 January 2017. https://www.statnews.com/2017/01/20/cdc-lead-children/ [accessed 4 September 2017].