Abstract
Background:
Oxidative potential (OP) has been proposed as a measure of toxicity of ambient particulate matter (PM).
Objectives:
Our goal was to address an important research gap by using daily OP measurements to conduct population-level analysis of the health effects of measured ambient OP.
Methods:
A semi-automated dithiothreitol (DTT) analytical system was used to measure daily average OP () in water-soluble fine PM at a central monitor site in Atlanta, Georgia, over eight sampling periods (a total of 196 d) during June 2012–April 2013. Data on emergency department (ED) visits for selected cardiorespiratory outcomes were obtained for the five-county Atlanta metropolitan area. Poisson log-linear regression models controlling for temporal confounders were used to conduct time-series analyses of the relationship between daily counts of ED visits and either the 3-d moving average (lag 0–2) of or same-day . Bipollutant regression models were run to estimate the health associations of while controlling for other pollutants.
Results:
was measured for 196 d (, ). Lag 0–2 was associated with ED visits for respiratory disease (, 95% confidence interval (CI): 1.00, 1.05 per interquartile range increase in ), asthma (, 95% CI: 1.03, 1.22), and ischemic heart disease (, 95% CI: 1.03, 1.38). Same-day was not associated with ED visits for any outcome. Lag 0–2 remained a significant predictor of asthma and ischemic heart disease in most bipollutant models.
Conclusions:
Lag 0–2 was associated with ED visits for multiple cardiorespiratory outcomes, providing support for the utility of as a measure of fine particle toxicity. https://doi.org/10.1289/EHP1545
Introduction
Fine particulate matter (PM with aerodynamic diameter , or ) has been associated with hospital admissions and emergency department (ED) visits for several respiratory outcomes (e.g., asthma, chronic obstructive pulmonary disease, and bronchitis) and cardiovascular outcomes (e.g., myocardial infarction, coronary heart disease, and stroke) (Brook et al. 2010; Dockery and Pope 1994; Kim et al. 2015; Rückerl et al. 2011). Given that is a heterogeneous mixture and that distinct particulate components could have different health effects (Bell et al. 2009; Zanobetti et al. 2009), measurement of mass concentration may not be the optimal way to quantify risk to human health. One commonly proposed mechanism for the toxicity of is through oxidative stress-driven pathways.
can contain a variety of species that contribute to its oxidative potential (OP), including transition metals (e.g., copper, iron), quinones, polycyclic aromatic hydrocarbons (PAHs), and elemental carbon (Cho et al. 2005; González-Flecha 2004; Tao et al. 2003). Several assays have been developed to attempt to measure the OP of ambient fine PM. The electron spin resistance (ESR) assay measures the capacity of PM to convert hydrogen peroxide to hydroxyl radicals (Shi et al. 2003). Assays for ascorbic acid (AA) and glutathione (GSH), two antioxidants, measure the level of depletion of these compounds when added to PM sample extract (Godri et al. 2011). The dithiothreitol (DTT) assay mimics the in vivo generation of superoxide radicals by particles transferring electrons from nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADPH) to oxygen (Kumagai et al. 2002; Verma et al. 2014). Cellular assays, such as those using rat alveolar macrophage (NR8383) cells, can directly measure the oxidation of intracellular probes (Hopke 2015). For this study, a semi-automated system was used to measure DTT activity as a measure of OP () of water-soluble fine PM in order to generate a time-series of daily measurements for a central site in Atlanta, Georgia.
Exposure to high levels of diesel exhaust and other sources of particulate matter repeatedly has been shown to be associated with measureable amounts of oxidative stress (Møller and Loft 2010; Xiao et al. 2003). Additionally, exposure to diesel exhaust can result in acute oxidative stress and release of pro-inflammatory cytokines in airway tissues (Pourazar et al. 2005; Salvi et al. 1999). Inhalation of particulate matter is associated with the release of cytokines, activated immune cells, and other mediators of inflammation in the upper and lower airways (Ghio and Devlin 2001; Nel 2005). This respiratory inflammation can lead to exacerbation of asthma symptoms, chronic bronchitis, and decreased gas exchange. The release of pro-inflammatory mediators into the bloodstream results in elevated levels of white blood cells, platelets, and the enzyme myeloperoxidase; these changes are linked to vasoconstriction, atherosclerosis, and endothelial dysfunction, all major risk factors for future cardiac outcomes (Brook et al. 2010; Sun et al. 2010). These inflammatory pathways are hypothesized to be driven or mediated by oxidative stress caused by the in vivo generation of reactive oxygen species (Gurgueira et al. 2002; Xiao et al. 2003).
Although there is growing evidence linking OP to adverse health outcomes (González-Flecha 2004; Hopke 2015; Øvrevik et al. 2015; Qu et al. 2017; Yang and Omaye 2009), the only study to date assessing population-level impact of measured daily ambient OP did not reveal significant health effects (Atkinson et al. 2016). Additional studies are necessary to a) determine whether OP is a major mechanism of harm for PM; b) assess alternate measures of OP; c) determine health outcomes for people exposed to ambient levels of OP, not just experimental doses; and d) quantify health effects at the population level. In our study, we use time-series methodology to estimate associations between daily measured ambient and cardiorespiratory ED visits, helping to fill these critical research gaps.
Methods
Air sampling took place from June 2012 through April 2013 at a mixed industrial/residential location in Atlanta, Georgia (Jefferson Street), roughly northwest of downtown Atlanta and about from a major interstate highway. Daily samples were taken over eight distinct sampling periods each lasting roughly a month in order to obtain sufficient measurements over each season. During each of the sampling periods, measurements were also conducted at one of three additional locations (roadside, near-road, and rural) to characterize spatial variation in , though only the central-site observations are used in the health analysis. To measure oxidative potential, we used a semi-automated system that measures the capacity of water-soluble to generate reactive oxygen species using the DTT assay. Our method and this Atlanta sampling campaign have been described extensively in previous publications (Fang et al. 2014; Verma et al. 2009, 2012, 2014). Particles were collected with a high-volume sampler (HiVol, Thermo Anderson, nondenuded, nominal flow rate , cut size by impactor) onto prebaked quartz filters to collect over 23-h periods (1200–1100 hours daily). After sampling, filters were immediately wrapped in prebaked aluminum foil and stored in a freezer. Analysis of filters for and other pollutant measures started in March 2013. A fraction of the high-volume filter was extracted in water, the extract was filtered and then was determined with the automated analytical system, which allowed for consistent analysis on a large number of filter samples with less effort compared with manual analysis. was measured in nanomoles per minute per cubic meter, which corresponds to the loss rate of DTT when exposed to an aerosol sample per volume of air from which the sample was collected. The coefficient of variation for standards was 12% (Fang et al. 2014). Daily measurements on additional particulate and gaseous pollutants were also taken at this location; methods for their collection have been previously described (Edgerton et al. 2005; Hansen et al. 2003, 2006). Meteorological data collected at Hartsfield-Jackson airport, about south of downtown Atlanta, were also acquired.
Computerized billing records on ED visits were acquired from the Georgia Hospital Association for all 38 nonfederal acute care hospitals with emergency departments in the 20-county Atlanta metropolitan area for the study period (O’Lenick et al. 2017; Sarnat et al. 2010). Patient variables included date of admission, all recorded International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes, date of birth, sex, race, and five-digit residential ZIP code. ED visits were included in the study if the patient residential ZIP code was located wholly or partially within the five primary urban counties of metropolitan Atlanta (Fulton, DeKalb, Gwinnett, Cobb, Clayton). Daily counts of ED visits were calculated for the following outcome categories based on primary ICD-9 codes: asthma (ICD-9 codes 493, 786.07), chronic obstructive pulmonary disease (COPD) (491, 492, 496), pneumonia (480–486), upper respiratory infection (URI) (460–465, 466.0, 477), congestive heart failure (CHF) (428), and ischemic heart disease (IHD) (410–414). In addition, daily counts were determined for combined categories of respiratory diseases (RD) (460–465, 466.0, 466.1, 466.11, 466.19, 477, 480–486, 491–493, 496, 786.07) and cardiovascular diseases (CVD) (410–414, 427, 428, 433–437, 440, 443–445, 451–453). The combined RD and CVD categories represent multiple respiratory and cardiovascular subcategories that have been shown in our previous studies to be linked to air pollution (Metzger et al. 2004; Peel et al. 2005, 2007; Winquist et al. 2012).
We estimated associations between and daily counts of ED visits for the selected cardiorespiratory outcomes using Poisson log-linear models accounting for overdispersion. We used the 3-d moving average of (the average of on the same day as the ED visit, 1 d previous, and 2 d previous, or lag 0–2) as the exposure of interest because our prior studies have shown consistent associations of multiday elevated pollutant levels (Metzger et al. 2004; Peel et al. 2005, 2007; Strickland et al. 2010). Observations without three consecutive daily measurements were excluded from this analysis. Some of our prior studies had also shown evidence of associations between ED visits and same-day (lag 0) ambient pollutant levels (Strickland et al. 2016; Winquist et al. 2016; Ye et al. 2017), so we ran separate analyses using same-day as the exposure of interest.
Information from our previous studies was used to construct time-series models with optimal confounder control. To control for seasonal trends, the models included cubic splines with monthly knots. The models also controlled for weekdays and federal holidays, as well as temperature (cubic polynomial of the lag 0–2 moving average of daily maximum temperature) and dew point (cubic polynomial of the lag 0–2 moving average of daily mean dew point). Models included indicator variables for periods of hospital data contribution (to control for hospitals opening or closing, or days for which data from individual hospitals were unavailable): Indicators for each hospital had the value 1 if the hospital contributed data on a given day and 0 if the hospital did not contribute. To determine the utility of as a measure of ambient air toxicity independent of other pollutant measures, we ran bipollutant models that included and one of several common pollutant measures for which daily values were available over this time period and were hypothesized to be potential indicators of air quality. These pollutant measures were: total mass, carbon monoxide (), nitrogen dioxide (), ozone (), and sulfur dioxide (), as well as the following components: sulfate (), elemental carbon (), organic carbon (), ammonium (), nitrate (), water-soluble manganese (), water-soluble iron (Fe), and water-soluble copper (). Health associations were measured as risk ratio (RR) per interquartile range (IQR) of daily or other pollutants.
All analyses were performed using SAS version 9.3 (SAS Institute, Inc.).
Results
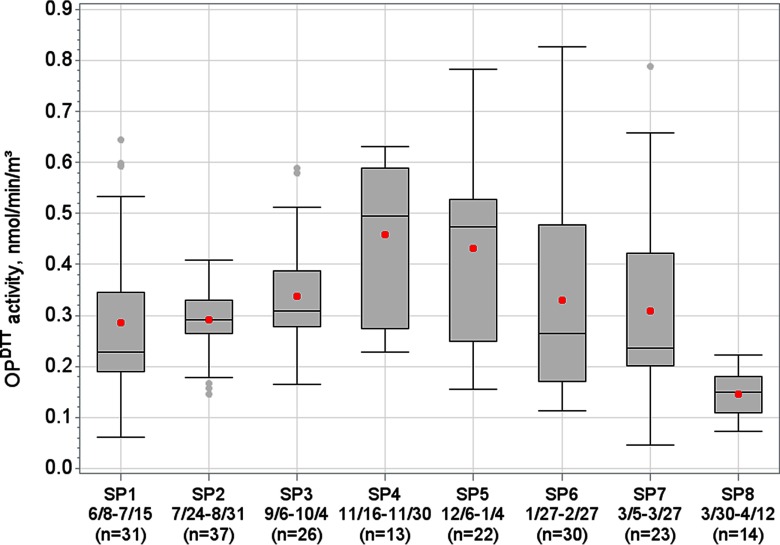
There were 196 d of daily levels recorded from 8 June 2012 through 12 April 2013 in eight separate sampling periods (Figure 1). Mean daily was (range: 0.05–0.83, IQR: 0.21). tended to be highest in Sampling Period 4 (16 November 2012–30 November 2012) and Sampling Period 5 (6 December 2012–4 January 2013). was generally higher from Friday through Sunday compared with the other days of week (see Figure S1). was most correlated with EC (), (), and OC () (Table 1). For the days with measurements, there were over 730,000 total ED visits; on average there were 391 daily ED visits per day for the combined respiratory disease group, of which an average of 85 visits were for asthma, 20 visits for COPD, 227 visits for URI, and 45 visits for pneumonia. There was an average of 99 ED visits per day for the combined cardiovascular disease group, of which an average of 25 visits were for CHF and 20 visits were for IHD. For the time-series analyses using the 3-d moving average of , excluding data for which the full 3-d moving average was unavailable left 156 d of observations. Daily values for and cardiorespiratory ED visit categories did not significantly differ between these 156 d and the remaining 40 d without a full 3-d moving average of (for all pooled t-tests, ).
Figure 1.
Distribution of the oxidative potential of water-soluble as measured by the DTT assay (), for eight different sampling periods (SPs), June 2012–April 2013, Atlanta, Georgia. Boxes encompass 25th through 75th percentiles, middle horizontal line represents the median, dots within boxes represent the mean, whiskers extend to the most extreme point within 1.5 interquartile ranges (IQRs) of the box, dots outside boxes indicate outliers. Dates and number of measurements for each sampling period are displayed on the x-axis.
Table 1.
Pearson correlation coefficients (upper right) between daily values for the oxidative potential of water-soluble as measured by the DTT assay () and daily mean values for other air quality variables, June 2012–April 2013, Atlanta, Georgia.
| — | 0.55 | 0.46 | 0.56 | 0.26 | 0.27 | 0.24 | 0.01 | 0.51 | 0.28 | 0.14 | 0.42 | 0.43 | 0.41 | |
| 196 | — | 0.44 | 0.65 | 0.63 | 0.36 | 0.14 | 0.41 | 0.86 | 0.24 | 0.66 | 0.38 | 0.62 | 0.48 | |
| 196 | 196 | — | 0.78 | 0.11 | 0.72 | 0.25 | 0.02 | 0.46 | 0.46 | 0.08 | 0.29 | 0.40 | 0.45 | |
| 191 | 191 | 191 | — | 0.23 | 0.59 | 0.22 | 0.07 | 0.70 | 0.45 | 0.21 | 0.40 | 0.51 | 0.50 | |
| 193 | 193 | 193 | 188 | — | 0.10 | 0.42 | 0.08 | 0.33 | 0.83 | 0.07 | 0.31 | 0.22 | ||
| 196 | 196 | 196 | 191 | 193 | — | 0.26 | 0.15 | 0.42 | 0.27 | 0.09 | 0.24 | 0.33 | 0.39 | |
| 194 | 194 | 194 | 189 | 191 | 194 | — | 0.07 | 0.06 | 0.08 | |||||
| 196 | 196 | 196 | 191 | 193 | 196 | 194 | — | 0.43 | 0.31 | 0.19 | 0.37 | 0.28 | ||
| 190 | 190 | 190 | 185 | 187 | 190 | 188 | 190 | — | 0.19 | 0.35 | 0.39 | 0.60 | 0.43 | |
| 196 | 196 | 196 | 191 | 193 | 196 | 194 | 196 | 190 | — | 0.01 | 0.22 | 0.17 | 0.19 | |
| 196 | 196 | 196 | 191 | 193 | 196 | 194 | 196 | 190 | 196 | — | 0.13 | 0.41 | 0.27 | |
| 158 | 158 | 158 | 155 | 157 | 158 | 156 | 158 | 152 | 158 | 158 | — | 0.63 | 0.38 | |
| 157 | 157 | 157 | 154 | 156 | 157 | 155 | 157 | 151 | 157 | 157 | 156 | — | 0.70 | |
| 152 | 152 | 152 | 149 | 151 | 152 | 150 | 152 | 147 | 152 | 152 | 151 | 150 | — |
Note: Only days with measurements were used for these correlations; the lower left section of the table shows the number of days included in each correlation. , carbon monoxide; , copper; , elemental carbon; , iron; , manganese; , ammonium; , nitrogen dioxide; , nitrate; , organic carbon; , ozone; , fine particulate matter; , sulfur dioxide; , sulfate.
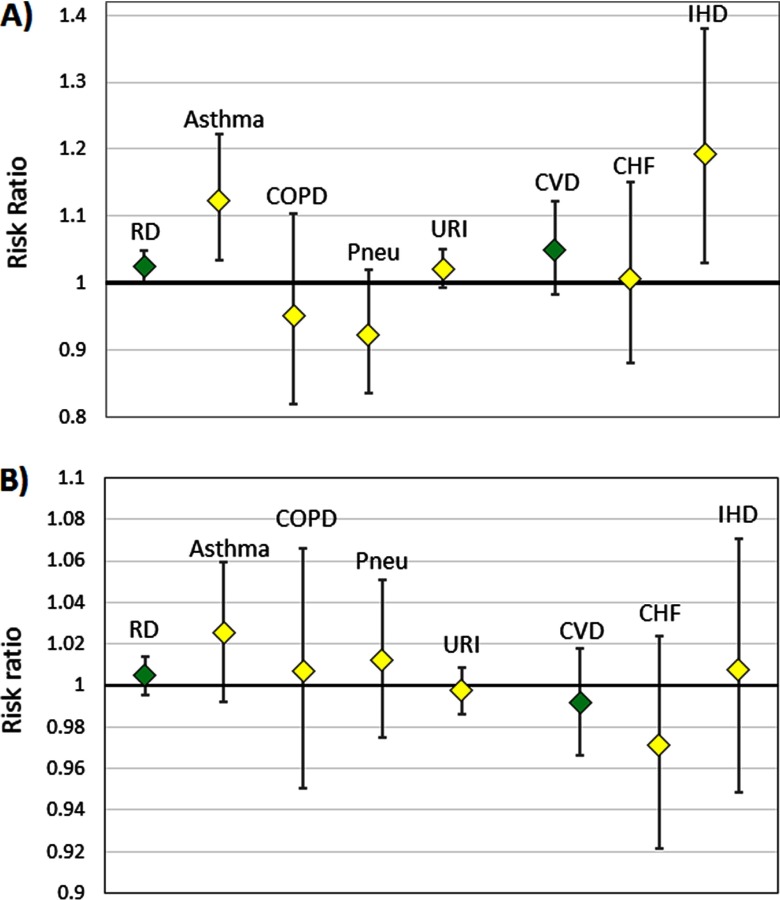
Lag 0–2 was significantly positively associated with the combined respiratory disease group (, 95% CI: 1.00, 1.05) and positively associated, but not significantly, with the combined cardiovascular disease group (, 95% CI: 0.98, 1.12) (Figure 2A). Within more specific outcome categories, lag 0–2 was significantly positively associated with asthma (, 95% CI: 1.03, 1.22) and IHD (, 95% CI: 1.03, 1.38). Point estimates for these risk ratios were all somewhat greater than associations using lag 0–2 (combined respiratory disease group: , 95% CI: 1.01, 1.04; combined cardiovascular disease group: , 95% CI: 0.97, 1.07; asthma: , 95% CI: 1.04, 1.17; and IHD: , 95% CI: 0.97, 1.21). Lag 0–2 was not significantly associated with CHF, COPD, pneumonia, or URI (although the association with URI was suggestive). Lag 0 was not significantly associated with ED visits for any outcome (Figure 2B).
Figure 2.
Risk ratio for emergency department (ED) visit outcomes per interquartile range (IQR) of the oxidative potential of water-soluble as measured by the DTT assay (), for (A) lag 0–2 and (B) lag 0 , June 2012–April 2013, Atlanta, Georgia. The IQR of is . Note: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, all cardiovascular diseases (combined); IHD, ischemic heart disease; Pneu, pneumonia; RD, all respiratory diseases (combined); URI, upper respiratory infection.
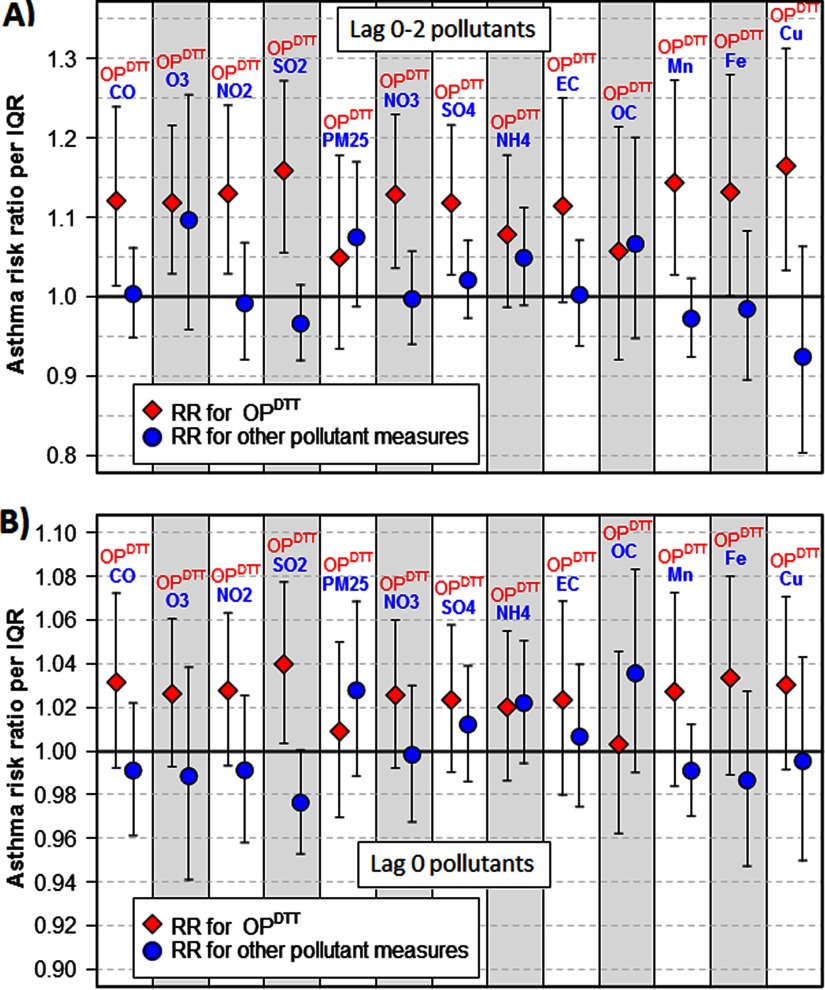
Given that lag 0–2 was strongly associated with asthma ED visits, we examined 13 separate bipollutant models that included the 3-d moving averages of both and another pollutant to assess whether the observed health associations with might be explained by a copollutant. In each bipollutant model, the risk ratio point estimate for lag 0–2 was above 1 (Figure 3A). In 11 of the 13 models, the risk ratio point estimate for lag 0–2 was greater than the risk ratio for the other lag 0–2 pollutant included; the only exceptions were models that included or . was significantly associated with asthma ED visits in bipollutant models that included , , , , , , , , and . In bipollutant analyses using same-day values for and other pollutants, similar trends were observed: The risk ratio point estimate for lag 0 was greater than the risk ratio for the other lag 0 pollutant included for all models except those including , , and (Figure 3B). Although all risk ratios for lag 0 in bipollutant models were above 1, these associations with asthma ED visits were not statistically significant except for the model that included .
Figure 3.
Asthma risk ratios for the oxidative potential of water-soluble as measured by the DTT assay () and other pollutant measures in bipollutant models, for (A) lag 0–2 pollutants and (B) lag 0 pollutants, June 2012–April 2013, Atlanta, Georgia. Risk ratio for (diamond markers) are per interquartile range (IQR) of (); risk ratio for all other pollutant measures (circular markers) are per IQR of that particular pollutant. Note: , carbon monoxide; , copper; , elemental carbon; , iron; , manganese; , ammonium; , nitrogen dioxide; , nitrate; , organic carbon; , ozone; , fine particulate matter; , sulfur dioxide; , sulfate. , , , , , , , and are components of .
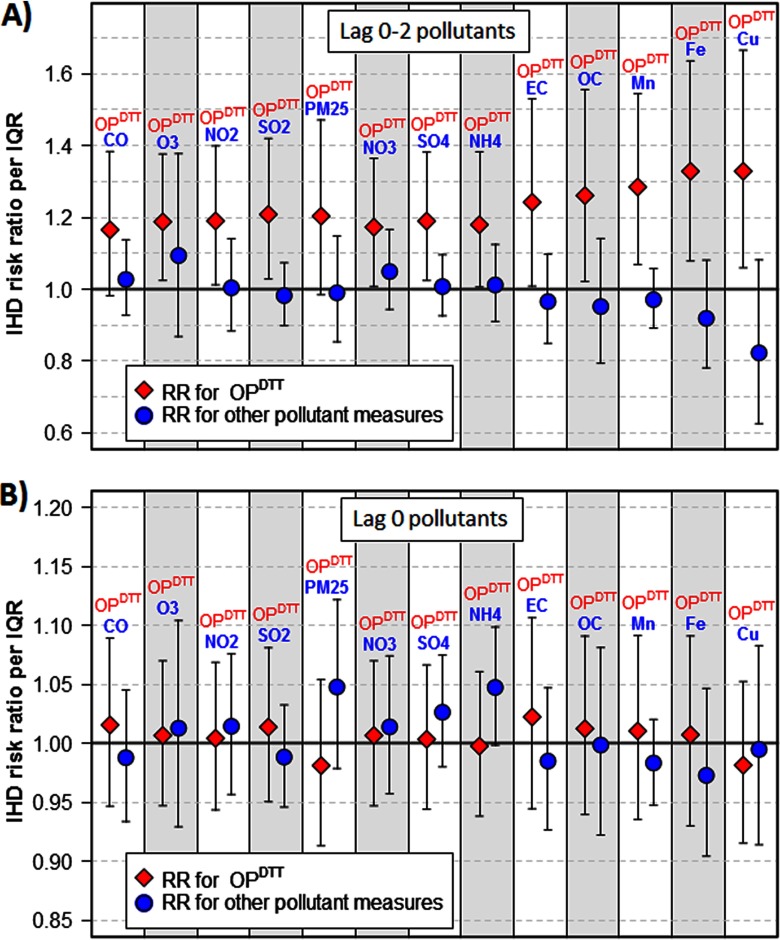
In bipollutant models with IHD as the outcome, the estimated health associations for lag 0–2 were even stronger. In every bipollutant model, the risk ratio point estimate for lag 0–2 was above 1 and lag 0–2 had a higher risk ratio point estimate than the other pollutant included (Figure 4A). In all but two models, lag 0–2 was significantly and positively associated with IHD; the exceptions, which were also suggestive of positive associations, were models that included (, 95% CI: 0.98, 1.47) and CO (, 95% CI: 0.98, 1.38). In bipollutant analyses using same-day values for and other pollutants, lag 0 was not associated with IHD in any model (Figure 4B).
Figure 4.
Ischemic heart disease (IHD) risk ratios for the oxidative potential of water-soluble as measured by the DTT assay () and other pollutant measures in bipollutant models, for (A) lag 0–2 pollutants and (B) lag 0 pollutants. Risk ratio for (diamond markers) are per interquartile range (IQR) of (); risk ratio for all other pollutant measures (circular markers) are per IQR of that particular pollutant. Note: , carbon monoxide; , copper; , elemental carbon; iron; , manganese; , ammonium; , nitrogen dioxide; , nitrate; , organic carbon; , ozone; , fine particulate matter; , sulfur dioxide; , sulfate. , , , , , , , and are components of .
Bipollutant models were also conducted for all other outcomes. For the RD and CVD categories, point estimates for lag 0–2 risk ratios remained positive in all models, and these point estimates were generally higher than point estimates for the other pollutant included; however, most lag 0–2 risk ratios confidence intervals included the null. Lag 0 was not associated with RD or CVD in bipollutant models. In bipollutant models for COPD, pneumonia, URI, and CHF, associations for lag 0 and lag 0–2 were almost entirely nonsignificant.
Discussion
This study represents an important report of population-level health associations for directly measured OP, with a focus on OP measured using the DTT assay. The study draws upon a comprehensive hospital database consisting of data from all nonfederal acute care hospitals with emergency departments serving an area with over 3.3 million residents (U.S. Census Bureau 2010). Daily measurements of collocated air quality data for and a large number of other pollutants, as well as meteorological variables, allowed for assessment of correlations and control of potential confounders. The Poisson log-linear regression models build upon our previous analyses in the Atlanta metropolitan area and use the strengths of established quantitative methodologies.
Because many methods for measuring OP are labor intensive, prior studies of the health effects of OP have typically been over relatively short time periods and have compared relatively minor clinical outcomes within small study groups. In each of two studies that exposed volunteers to PM mixtures of similar concentration but different composition, exposure to a mixture high in metals with considerable OP such as zinc, copper, and iron produced significantly higher inflammatory responses (Ghio and Devlin 2001; Schaumann et al. 2004). OP measured by cellular rat macrophage was linked to decreased lung function in children with asthma and markers of inflammation in elderly subjects (Delfino et al. 2010, 2013). Markers of inflammation were associated with three separate acellular measures of particulate OP (Janssen et al. 2015). However, ascorbate-related OP () and glutathione-related OP () have not been consistently associated with markers of respiratory or cardiovascular toxicity (Steenhof et al. 2013; Strak et al. 2012, 2013a, 2013b), and a small case-crossover study showed no association between three acellular measures of particulate OP and hospital admissions for asthma/chronic obstructive pulmonary disorder (Canova et al. 2014).
In large-scale case-crossover studies in Ontario, Canada, using city-level estimates of long-term OP, was found to modify the association between and respiratory disease, as well as the association between and myocardial infarction, but did not modify these associations (Weichenthal et al. 2016a, 2016b). had previously been found to be well correlated with but not (Mudway et al. 2009). A recent study assessed population-level health impacts of directly measured OP, in which daily and measurements were taken in central London and associations were estimated with daily hospital admissions and deaths (Atkinson et al. 2016). In that study, neither nor were associated with daily mortality or cardiovascular hospital admissions, but there were trends toward positive associations with respiratory hospital admissions among children. Different OP measurement assays may be sensitive to dissimilar sets of particulate compounds (Fang et al. 2016; Godri et al. 2011; Janssen et al. 2014) that may be linked to different cardiorespiratory health effects (Sarnat et al. 2016), highlighting the need to assess as a potential measure of particulate toxicity.
A previous study by our group utilizing modeled OP estimates and the same Atlanta ED visits database found that was associated with ED visits for asthma and CHF (Bates et al. 2015), and a related study exploring additional models showed that these associations held for but not (Fang et al. 2016). Another study had found that residential estimates were associated with prevalence of asthma and rhinitis and with measured lung capacity, whereas similar estimates were not (Yang et al. 2016). These studies all increased sample size by utilizing modeled values; although these results were informative, modeled ambient OP may be prone to substantial measurement errors. The results of our current study, which used directly measured , lend additional support to the usefulness of as an indicator of air pollution toxicity.
Importantly, this study only assessed health associations with modeled water-soluble . Various prior analyses of these data indicate that the main sources of aerosol include biomass burning and vehicle emissions through tail pipe and tire and brake wear and that atmospheric processing following emissions plays a key role in the observed DTT activities. Water-soluble measurements have been shown to capture organic components (e.g., quinones) as well as transition metal ions (e.g., soluble forms of copper and manganese) but not DTT-active species associated with solid particle surfaces of such as soot or EC (Fang et al. 2017a, 2017b; Verma et al. 2012, 2014, 2015). We chose to use water-soluble because the measurement of total can depend on how the solid aerosol components are brought into contact with the assay. Differences between measurements due to assay preparation are currently being investigated (Gao et al. 2017). Further epidemiological analysis of OP using different assays, measured over different time frames and geographic areas, using alternate outcomes, or focused on specific population subgroups would be useful to determine variability in adverse health effects.
In our study, a lagged 0–2 moving average of was a significant predictor of ED visits for respiratory disease, asthma, and IHD. We ran multiple bipollutant models to assess whether was a proxy for another pollutant. Lag 0–2 was more strongly associated with asthma ED visits than the other pollutant measure in 11 of 13 bipollutant models. The exceptions included , which had a slightly higher risk ratio for asthma than lag 0–2 in a bipollutant model (1.07 compared with 1.05 per IQR), though these risk ratios were both lower than the corresponding RRs from single-pollutant models; the same was also true for lag 0–2 OC and lag 0–2 . This finding is consistent with water-soluble OP explaining part of the respiratory toxicity of and OC; these mixtures may also cause adverse effects either through oxidative stress mediated by water-insoluble particles (Fang et al. 2017b) or through pathways unrelated to OP. For IHD visits, lag 0–2 was more strongly predictive than the other pollutant measure in every bipollutant model. These results provide evidence that OP may offer additional information about health risks of air pollution beyond the risks captured by other pollutant measures.
In analyses of ED visits and same-day , there were no statistically significant associations, suggesting that many adverse health outcomes of oxidative stress may not be immediately fully realized. However, in bipollutant models for asthma ED visits, lag-0 point estimates were consistently positive and generally greater than the point estimate for the other pollutant in the model, which could be suggestive of some immediate toxic effect of OP on asthma exacerbation.
observations were available for 196 d from June 2012 through April 2013. Although this represents a larger sample size than available for most prior studies of OP, this group of observations comprises still relatively few observations compared with other time-series analyses of acute effects of air pollution (Atkinson et al. 2014). The time-series analyses included numerous covariates (39 additional model parameters) to control for potential temporal confounders; consequently, the risk ratio estimates had relatively large confidence intervals. Given the limited sample size, the fact that this study showed statistically significant effects of on cardiorespiratory ED visits indicates that may be a relatively strong predictor of health outcomes. Furthermore, other results were suggestive for certain outcomes (such as URI and the combined CVD ED visits), but the sample size was not sufficient to detect a significant effect. We tested more parsimonious models (e.g., without temperature and dew point control, without cubic splines and weekdays) and results were not substantially dissimilar, with estimated associations for lag 0–2 remaining strongest for respiratory disease, asthma, URI, and IHD. The results of this study should provide a strong impetus to produce longer time series of measurements of and other characterizations of OP in order to produce more stable risk estimates for multiple outcome groups and further elucidate particulate matter toxicity.
The use of pollutant measurements at a single location to predict health outcomes over a large metropolitan area is not ideal. However, other studies showed that different urban locations in Atlanta had similar daily measurements (Fang et al. 2014; Verma et al. 2014); comparison of measurements from separate locations are presented in Table S1. In addition, a previous analysis of exposure measurement error in Atlanta demonstrated that the use of measurements from urban monitors [within of the city center] that were located different distances from geographic subpopulations produced similar associations between pollutants and health outcomes, particularly for secondary pollutants (Sarnat et al. 2010). Because water-soluble is strongly linked to secondary organic particles (Verma et al. 2009, 2014), this suggests the viability of using a single central monitor as a surrogate for ambient experienced by a population spread out over a sizable metropolitan area. Zeger et al. (2000) suggest that if pollutant measurements in a time-series analysis are close to the average pollutant exposure levels for the population of interest (i.e., Berkson-type error), then the associations between pollutants and health outcomes should have minimal bias. On the other hand, if the measurements differ meaningfully from population average exposures, bias can be created with the direction most likely toward the null (Zeger et al. 2000). Our group previously investigated the effects of measurement error on associations between air pollutants and health outcomes using Poisson log-linear models similar to those used in this study; the associations were all biased toward the null, though less so for Berkson-type errors (Goldman et al. 2011).
False health associations could be estimated for an air quality variable with no true causal effect if it were correlated with a toxic pollutant; this may be observed even in bipollutant models with the toxic pollutant if the variable with no effect were better measured than the variable with the true effect (Dionisio et al. 2016). If had substantially lower measurement error than the other air quality variables in this study, this potential bias would be a valid concern. However, daily values for other pollutants considered in this study were also measured at the same central location. Furthermore, instrument measurement error for is expected to be similar to all other filter-based measurements used in this study. Therefore, the significant health associations for in bipollutant models, especially in models with copollutants that were secondary pollutants or had otherwise comparable spatial variability, are not readily explained by differences in measurement error between pollutants.
Conclusions
The health effects of OP have been previously explored in panel studies assessing markers of toxicity, small cohort studies assessing health outcomes in subjects with differing levels of exposure, and case-crossover studies analyzing relationships between OP and health outcomes over time; however, additional research is needed to assess population-level impacts of ambient OP. In this study, we present support for the measurement of as a predictor of acute cardiorespiratory outcomes in a time-series study of the population of a large metropolitan area. These results provide key evidence for OP as an important and useful integrated indicator of particulate matter toxicity for future air pollution studies.
Supplemental Material
Acknowledgments
This work was supported by a Clean Air Research Center grant to Emory University and the Georgia Institute of Technology from the U.S. Environmental Protection Agency (EPA; R834799) and by a training grant from the National Institute for Occupational Safety and Health (5T03OH8609-9). The contents of the publication are solely the responsibility of the grantee and do not necessarily represent the official view of the U.S. EPA. Furthermore, the U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
References
- Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. 2014. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 69(7):660–665, PMID: 24706041, 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RW, Samoli E, Analitis A, Fuller GW, Green DC, Anderson HR, et al. 2016. Short-term associations between particle oxidative potential and daily mortality and hospital admissions in London. Int J Hyg Environ Health 219(6):566–572, PMID: 27350257, 10.1016/j.ijheh.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Bates JT, Weber RJ, Abrams J, Verma V, Fang T, Klein M, et al. 2015. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ Sci Technol 49(22):13605–13612, PMID: 26457347, 10.1021/acs.est.5b02967. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. 2009. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med 179(12):1115–1120, PMID: 19299499, 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Canova C, Minelli C, Dunster C, Kelly F, Shah PL, Caneja C, et al. 2014. PM10 oxidative properties and asthma and COPD. Epidemiology 25(3):467–468, PMID: 24713885, 10.1097/EDE.0000000000000084. [DOI] [PubMed] [Google Scholar]
- Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, et al. 2005. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res 99(1):40–47, PMID: 16053926, 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, Gillen DL, et al. 2010. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology 21(3):892–902, PMID: 20811287, 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM. 2013. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J Expo Sci Environ Epidemiol 23(5):466–473, PMID: 23673461, 10.1038/jes.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio KL, Chang HH, Baxter LK. 2016. A simulation study to quantify the impacts of exposure measurement error on air pollution health risk estimates in copollutant time-series models. Environ Health 15(1):114, PMID: 27884187, 10.1186/s12940-016-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA III. 1994. Acute respiratory effects of particulate air pollution. Annu Rev Public Health 15:107–132, PMID: 8054077, 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- Edgerton ES, Hartsell BE, Saylor RD, Jansen JJ, Hansen DA, Hidy GM. 2005. The Southeastern Aerosol Research and Characterization Study: part II. filter-based measurements of fine and coarse particulate matter mass and composition. J Air Waste Manag Assoc 55(10):1527–1542, PMID: 16295278, 10.1080/10473289.2005.10464744. [DOI] [PubMed] [Google Scholar]
- Fang T, Guo H, Zeng L, Verma V, Nenes A, Weber RJ. 2017a. Highly acidic ambient particles, soluble metals, and oxidative potential: a link between sulfate and aerosol toxicity. Environ Sci Technol 51(5):2611–2620, PMID: 28141928, 10.1021/acs.est.6b06151. [DOI] [PubMed] [Google Scholar]
- Fang T, Verma V, Bates JT, Abrams J, Klein M, Strickland MJ, et al. 2016. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos Chem Phys 16(6):3865–3879, 10.5194/acp-16-3865-2016. [DOI] [Google Scholar]
- Fang T, Verma V, Guo H, King LE, Edgerton ES, Weber RJ. 2014. A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE). Atmos Meas Tech Discuss 7(7):7245–7279, 10.5194/amtd-7-7245-2014. [DOI] [Google Scholar]
- Fang T, Zeng L, Gao D, Verma V, Stefaniak AB, Weber RJ. 2017b. Ambient size distributions and lung deposition of aerosol dithiothreitol-measured oxidative potential: contrast between soluble and insoluble particles. Environ Sci Technol 51(12):6802–6811, PMID: 23673461, 10.1021/acs.est.7b01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Fang T, Verma V, Zeng L, Weber RJ. 2017. A method for measuring total aerosol oxidative potential (OP) with the dithiothreitol assay and comparisons between an urban and roadside site of water-soluble and total OP. Atmos Meas Tech Discuss, 10.5194/amt-2017-70. [DOI] [Google Scholar]
- Ghio AJ, Devlin RB. 2001. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med 164(4):704–708, PMID: 11520740, 10.1164/ajrccm.164.4.2011089. [DOI] [PubMed] [Google Scholar]
- Godri KJ, Harrison RM, Evans T, Baker T, Dunster C, Mudway IS, et al. 2011. Increased oxidative burden associated with traffic component of ambient particulate matter at roadside and urban background schools sites in London. PLoS One 6(7):e21961, PMID: 21818283, 10.1371/journal.pone.0021961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman GT, Mulholland JA, Russell AG, Strickland MJ, Klein M, Waller LA, et al. 2011. Impact of exposure measurement error in air pollution epidemiology: effect of error type in time-series studies. Environ Health 10:61, PMID: 21696612, 10.1186/1476-069X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Flecha B. 2004. Oxidant mechanisms in response to ambient air particles. Mol Aspects Med 25(1–2):169–182, PMID: 15051325, 10.1016/j.mam.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Murthy GG, González-Flecha B. 2002. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect 110(8):749–755, PMID: 12153754, 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DA, Edgerton E, Hartsell B, Jansen J, Burge H, Koutrakis P, et al. 2006. Air quality measurements for the Aerosol Research and Inhalation Epidemiology Study. J Air Waste Manag Assoc 56(10):1445–1458, PMID: 17063867, 10.1080/10473289.2006.10464549. [DOI] [PubMed] [Google Scholar]
- Hansen DA, Edgerton ES, Hartsell BE, Jansen JJ, Kandasamy N, Hidy GM, et al. 2003. The Southeastern Aerosol Research and Characterization Study: part 1—overview. J Air Waste Manag Assoc 53(12):1460–1471, PMID: 14700133, 10.1080/10473289.2003.10466318. [DOI] [PubMed] [Google Scholar]
- Hopke PK. 2015. “ Reactive ambient particles.” In: Air Pollution and Health Effects, Molecular and Integrative Toxicology. Nadadur SS, Hollingsworth JW, eds. London, UK:Springer-Verlag, 1–24. [Google Scholar]
- Janssen NAH, Strak M, Yang A, Hellack B, Kelly FJ, Kuhlbusch TAJ, et al. 2015. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup Environ Med 72(1):49–56, PMID: 25104428, 10.1136/oemed-2014-102303. [DOI] [PubMed] [Google Scholar]
- Janssen NAH, Yang AL, Strak M, Steenhof M, Hellack B, Gerlofs-Nijland ME, et al. 2014. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci Total Environ 472:572–581, PMID: 24317165, 10.1016/j.scitotenv.2013.11.099. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kabir E, Kabir S. 2015. A review on the human health impact of airborne particulate matter. Environ Int 74:136–143, PMID: 25454230, 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Koide S, Taguchi K, Endo A, Nakai Y, Yoshikawa T, et al. 2002. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem Res Toxicol 15(4):483–489, PMID: 11952333, 10.1021/tx0100993. [DOI] [PubMed] [Google Scholar]
- Metzger KB, Tolbert PE, Klein M, Peel JL, Flanders WD, Todd K, et al. 2004. Ambient air pollution and cardiovascular emergency department visits. Epidemiology 15(1):46–56, PMID: 14712146, 10.1097/01.EDE.0000101748.28283.97. [DOI] [PubMed] [Google Scholar]
- Møller P, Loft S. 2010. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect 118(8):1126–1136, PMID: 20423813, 10.1289/ehp.0901725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudway IS, Fuller G, Green D, Dunster C, Kelly FJ. 2009. “Report: Quantifying the London Specific Component of PM10 Oxidative Activity.” London, UK:Department for Environment, Food and Rural Affairs (DEFRA), the Scottish Executive, the Welsh Assembly Government and the DoE in Northern Ireland. [Google Scholar]
- Nel A. 2005. Atmosphere. Air pollution-related illness: effects of particles. Science 308(5723):804–806, PMID: 15879201, 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- O’Lenick CR, Winquist A, Chang HH, Kramer MR, Mulholland JA, Grundstein A, et al. 2017. Evaluation of individual and area-level factors as modifiers of the association between warm-season temperature and pediatric asthma morbidity in Atlanta, GA. Environ Res 156:132–144, PMID: 28342349, 10.1016/j.envres.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øvrevik J, Refsnes M, Låg M, Holme JA, Schwarze PE. 2015. Activation of proinflammatory responses in cells of the airway mucosa by particulate matter: oxidant- and non-oxidant-mediated triggering mechanisms. Biomolecules 5(3):1399–1440, PMID: 26147224, 10.3390/biom5031399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel JL, Metzger KB, Klein M, Flanders WD, Mulholland JA, Tolbert PE. 2007. Ambient air pollution and cardiovascular emergency department visits in potentially sensitive groups. Am J Epidemiol 165(6):625–633, PMID: 17194748, 10.1093/aje/kwk051. [DOI] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. 2005. Ambient air pollution and respiratory emergency department visits. Epidemiology 16(2):164–174, PMID: 15703530. [DOI] [PubMed] [Google Scholar]
- Pourazar J, Mudway IS, Samet JM, Helleday R, Blomberg A, Wilson SJ, et al. 2005. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol 289(5):L724–L730, 10.1152/ajplung.00055.2005. [DOI] [PubMed] [Google Scholar]
- Qu JJ, Li YY, Zhong W, Gao PS, Hu CP. 2017. Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis 9(1):E32–E43, PMID: 28203435, 10.21037/jtd.2017.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückerl R, Schneider A, Breitner S, Cyrys J, Peters A. 2011. Health effects of particulate air pollution: a review of epidemiological evidence. Inhal Toxicol 23(10):555–592, PMID: 21864219, 10.3109/08958378.2011.593587. [DOI] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandström T, Holgate ST, et al. 1999. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 159(3):702–709, PMID: 10051240, 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Chang HH, Weber RJ. 2016. Ambient PM2.5 and health: does PM2.5 oxidative potential play a role? Am J Respir Crit Care Med 194(5):530–531, PMID: 27585377, 10.1164/rccm.201603-0589ED. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Klein M, Sarnat JA, Flanders WD, Waller LA, Mulholland JA, et al. 2010. An examination of exposure measurement error from air pollutant spatial variability in time-series studies. J Expo Sci Environ Epidemiol 20(2):135–146, PMID: 19277071, 10.1038/jes.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumann F, Borm PJA, Herbrich A, Knoch J, Pitz M, Schins RPF, et al. 2004. Metal-rich ambient particles (particulate matter2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med 170(8):898–903, PMID: 15229099, 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- Shi T, Knaapen AM, Begerow J, Birmili W, Borm PJ, Schins RP. 2003. Temporal variation of hydroxyl radical generation and 8-hydroxy-2′-deoxyguanosine formation by coarse and fine particulate matter. Occup Environ Med 60(5):315–321, PMID: 12709515, 10.1136/oem.60.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhof M, Mudway IS, Gosens I, Hoek G, Godri KJ, Kelly FJ, et al. 2013. Acute nasal pro-inflammatory response to air pollution depends on characteristics other than particle mass concentration or oxidative potential: the RAPTES project. Occup Environ Med 70(5):341–348, PMID: 23428835, 10.1136/oemed-2012-100993. [DOI] [PubMed] [Google Scholar]
- Strak M, Hoek G, Godri KJ, Gosens I, Mudway IS, van Oerle R, et al. 2013a. Composition of PM affects acute vascular inflammatory and coagulative markers—the RAPTES project. PloS One 8(3):e58944, PMID: 23516583, 10.1371/journal.pone.0058944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strak M, Hoek G, Steenhof M, Kilinc E, Godri KJ, Gosens I, et al. 2013b. Components of ambient air pollution affect thrombin generation in healthy humans: the RAPTES project. Occup Environ Med 70(5):332–340, PMID: 23378445, 10.1136/oemed-2012-100992. [DOI] [PubMed] [Google Scholar]
- Strak M, Janssen NAH, Godri KJ, Gosens I, Mudway IS, Cassee FR, et al. 2012. Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential—the RAPTES project. Environ Health Perspect 120(8):1183–1189, PMID: 22552951, 10.1289/ehp.1104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. 2010. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med 182(3):307–316, PMID: 20378732, 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland MJ, Hao H, Hu XF, Chang HH, Darrow LA, Liu Y. 2016. Pediatric emergency visits and short-term changes in PM2.5 concentrations in the U.S. state of Georgia. Environ Health Perspect 124(5):690–696, PMID: 26452298, 10.1289/ehp.1509856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Hong X, Wold LE. 2010. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 121(25):2755–2765, PMID: 20585020, 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, González-Flecha B, Kobzik L. 2003. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic Biol Med 35(4):327–340, PMID: 12899936, 10.1016/S0891-5849(03)00280-6. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2010. Population, Housing Units, Area, and Density: 2010 - State – County/County Equivalent. American FactFinder. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk [accessed 21 August 2017].
- Verma V, Fang T, Guo H, King L, Bates JT, Peltier RE, et al. 2014. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: spatiotemporal trends and source apportionment. Atmos Chem Phys 14(23):12915–12930, 10.5194/acp-14-12915-2014. [DOI] [Google Scholar]
- Verma V, Fang T, Xu L, Peltier RE, Russell AG, Ng NL, et al. 2015. Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5. Environ Sci Technol 49(7):4646–4656, PMID: 25748105, 10.1021/es505577w. [DOI] [PubMed] [Google Scholar]
- Verma V, Ning Z, Cho AK, Schauer JJ, Shafer MM, Sioutas C. 2009. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmos Environ 43(40):6360–6368, 10.1016/j.atmosenv.2009.09.019. [DOI] [Google Scholar]
- Verma V, Rico-Martinez R, Kotra N, King L, Liu J, Snell TW, et al. 2012. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols. Environ Sci Technol 46(20):11384–11392, PMID: 22974103, 10.1021/es302484r. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Lavigne E, Evans G, Pollitt K, Burnett RT. 2016a. Ambient PM2.5 and risk of emergency room visits for myocardial infarction: impact of regional PM2.5 oxidative potential: a case-crossover study. Environ Health 15:46, PMID: 27012244, 10.1186/s12940-016-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal SA, Lavigne E, Evans GJ, Godri Pollitt KJ, Burnett RT. 2016b. Fine particulate matter and emergency room visits for respiratory illness. Effect modification by oxidative potential. Am J Respir Crit Care Med 194(5):577–586, PMID: 26963193, 10.1164/rccm.201512-2434OC. [DOI] [PubMed] [Google Scholar]
- Winquist A, Grundstein A, Chang HH, Hess J, Sarnat SE. 2016. Warm season temperatures and emergency department visits in Atlanta, Georgia. Environ Res 147:314–323, PMID: 26922412, 10.1016/j.envres.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Klein M, Tolbert P, Flanders WD, Hess J, Sarnat SE. 2012. Comparison of emergency department and hospital admissions data for air pollution time-series studies. Environ Health 11:70, PMID: 22998927, 10.1186/1476-069X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GG, Wang M, Li N, Loo JA, Nel AE. 2003. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem 278(50):50781–50790, PMID: 14522998, 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- Yang A, Janssen NA, Brunekreef B, Cassee FR, Hoek G, Gehring U. 2016. Children’s respiratory health and oxidative potential of PM2.5: the PIAMA birth cohort study. Occup Environ Med 73(3):154–160, PMID: 26755634, 10.1136/oemed-2015-103175. [DOI] [PubMed] [Google Scholar]
- Yang W, Omaye ST. 2009. Air pollutants, oxidative stress and human health. Mutat Res 674(1–2):45–54, PMID: 19013537, 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Ye DN, Klein M, Chang HH, Sarnat JA, Mulholland JA, Edgerton ES, et al. 2017. Estimating acute cardiorespiratory effects of ambient volatile organic compounds. Epidemiology 28(2):197–206, PMID: 27984424, 10.1097/EDE.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Franklin M, Koutrakis P, Schwartz J. 2009. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health 8:58, PMID: 20025755, 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. 2000. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect 108(5):419–426, PMID: 10811568, 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.