Key Points
Question
Is inheritance of the HSD3B1 (1245C) genotype that encodes for a gain-of-function in 3β-hydroxysteroid dehydrogenase isoenzyme 1 (3βHSD1) and an increase in potent androgen synthesis from extragonadal precursor steroids associated with more favorable treatment outcomes with nonsteroidal 17α-hydroxylase/17,20-lyase (CYP17A1) inhibition among men with metastatic castration-resistant prostate cancer (CRPC)?
Findings
In this study of 90 men with metastatic CRPC, the presence of the HSD3B1 (1245C) variant allele was associated with increased duration of therapy and increased progression-free survival with ketoconazole treatment.
Meaning
HSD3B1 (1245C) inheritance, which is a known predictive biomarker of resistance to castration, is also predictive of response to nonsteroidal CYP17A1 inhibition, identifying a subset of tumors that are clinically more dependent on extragonadal precursor steroids.
This study compared response to ketoconazole treatment among patients with castration-resistant prostate cancer who had 0, 1, or 2 HSD3B1 variant alleles.
Abstract
Importance
The HSD3B1 (1245C) germline variant encodes for a gain-of-function missense in 3β-hydroxysteroid dehydrogenase isoenzyme 1 (3βHSD1) that results in increased dihydrotestosterone synthesis from extragonadal precursors and is predictive of more rapid progression to castration-resistant prostate cancer (CRPC).
Objective
To determine whether the HSD3B1 (1245C) genotype is predictive of clinical response to extragonadal androgen ablation with nonsteroidal 17α-hydroxylase/17,20-lyase (CYP17A1) inhibition in men with metastatic CRPC.
Design, Setting, and Participants
An observational study of men with metastatic CRPC treated with ketoconazole between June 1998 and December 2012 was conducted at the University of California, San Francisco.
Exposures
Extragonadal androgen ablation with the nonsteroidal CYP17A1 inhibitor ketoconazole among men with metastatic CRPC.
Main Outcomes and Measures
The primary end points of analysis were duration of ketoconazole therapy and time to disease progression stratified by HSD3B1 genotype. Disease progression was defined as either biochemical or radiographic progression, using the Prostate Cancer Working Group 3 and Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 definitions, respectively. Kaplan-Meier analysis was used to estimate time on therapy and time to disease progression. A log-rank test for trend was used to compare outcomes by HSD3B1 genotype.
Results
A total of 90 men (median [interquartile range] age, 61.5 [55.3-67.0] years) with metastatic CRPC were included in the analysis, with sufficient data to determine duration of ketoconazole therapy and time to disease progression in 88 and 81 patients, respectively. The median duration of therapy increased with the number of inherited HSD3B1 (1245C) variant alleles: 5.0 months (95% CI, 3.4-10.4) for 0 variant alleles; 7.5 months (95% CI, 4.9-19.2) for 1; and 12.3 months (95% CI, 1.8-not reached) for 2 (overall comparison for trend, P = .01). Median progression-free survival also increased with number of HSD3B1 (1245C) variant alleles inherited: 5.4 months (95% CI, 3.7-7.5) for 0 variant alleles; 9.7 months (95% CI, 5.6-32.9) for 1; and 15.2 months (95% CI, 7.8-not reached) for 2 (overall comparison for trend, P = .03).
Conclusions and Relevance
Inheritance of the HSD3B1 (1245C) variant allele, which is a predictive biomarker of resistance to castration, is also a predictive biomarker of sensitivity to extragonadal androgen ablation with a nonsteroidal CYP17A1 inhibitor. These findings signal a possible pathway of treatment stratification for patients with prostate cancer.
Introduction
Two major sources of androgens supply prostate cancer: (1) gonadal testosterone and (2) extragonadal androgens that mainly originate from the human adrenal reticularis but may also arise in part from de novo steroidogenesis from cholesterol within tumors. The requirement for gonadal androgens and efficacy of androgen deprivation therapy (ADT) by medical or surgical castration in men with metastatic prostate cancer has been well established since the initial description by Huggins and Hodges. Androgen deprivation therapy remains a cornerstone treatment for men with metastatic disease and has demonstrated benefit in earlier-stage disease, such as when used concurrently with radiotherapy for select men with localized prostate cancer.
Despite the initial efficacy of ADT, men with advanced disease invariably develop castration-resistant prostate cancer (CRPC), which is driven by synthesis of the potent androgens, testosterone and/or dihydrotestosterone, and stimulation of the androgen receptor (AR) by extragonadal precursor steroids. The enzyme 3β-hydroxysteroid dehydrogenase isoenzyme 1 (3βHSD1, encoded by the gene HSD3B1) is required for intratumoral synthesis of potent androgens from extragonadal precursors. The common germline variant HSD3B1 (1245C) encodes for a gain-of-function in 3βHSD1, increasing what is otherwise the rate-limiting step for intratumoral androgen synthesis from extragonadal precursor steroids. In 3 cohorts of patients with advanced prostate cancer, HSD3B1 (1245C) inheritance is associated with more rapid progression from initiation of ADT to development of CRPC. These findings have been independently validated in a fourth cohort and further support variant HSD3B1 (1245C) inheritance as a predictive biomarker of ADT resistance.
We hypothesized that patients with variant HSD3B1 (1245C) inheritance develop CRPC because they have tumors that are more dependent on extragonadal precursor steroids and thus would have more durable responses to pharmacologic inhibition of 17α-hydroxylase/17,20-lyase (CYP17A1), which is required for extragonadal androgen synthesis. Testing this hypothesis with abiraterone is problematic, because this steroidal CYP17A1 inhibitor is also converted by 3βHSD1 to multiple downstream steroidal metabolites, including an AR agonist that may oppose its effects downstream of blocking endogenous androgen synthesis. Therefore, we chose to study the association between HSD3B1 inheritance and duration of CRPC response to ketoconazole, a nonsteroidal CYP17A1 inhibitor that is not clouded by the same issues.
Methods
Men with metastatic CRPC who were treated with ketoconazole and did not receive abiraterone before or during ketoconazole treatment were identified from a prospectively maintained database at the University of California, San Francisco, and made up the cohort of this observational study. Clinical data and biological samples were obtained using informed consent with a protocol approved by the institutional review board of the University of California, San Francisco. HSD3B1 germline genotype was determined from DNA extracted from peripheral blood mononuclear cells using a polymerase chain reaction–based melting curve assay, described previously. The primary end points of analysis were duration of ketoconazole therapy and time to disease progression stratified by HSD3B1 genotype. Clinical progression was defined as the time of first occurrence of either biochemical progression, using the Prostate Cancer Working Group 3 definition, or radiographic progression, using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria. Kaplan-Meier analysis was used to estimate time on ketoconazole therapy and time to disease progression. A log-rank test for trend was used to compare outcomes among HSD3B1 homozygous wild type, heterozygous, and homozygous variant genotypes. The Cox proportional hazards model was used to estimate hazard ratio of the primary outcomes between genotypes.
Results
Ninety men met inclusion criteria and were included in the study. Forty-four patients (49%) were HSD3B1 homozygous wild type; 34 (38%), heterozygous; and 12 (13%), homozygous variant (Table), for a total variant allelic frequency of 32%, which is consistent with prior cohorts. Sufficient data were available to determine duration of therapy and time to progression for 88 and 81 patients, respectively.
Table. Patient Characteristics.
| Characteristic | No. (%) |
|---|---|
| Age at diagnosis in years, median (IQR), y | 61.5 (55.3-67.0) |
| Grade group at diagnosis | |
| I | 10 (11.1) |
| II | 13 (14.4) |
| III | 10 (11.1) |
| IV | 17 (18.9) |
| V | 27 (30.1) |
| Unknown | 13 (14.4) |
| PSA at diagnosis, median (IQR) | 15.2 (7.0-84.1) |
| Primary local therapy | |
| Radical prostatectomy | 24 (26.7) |
| External beam radiation therapy | 24 (26.7) |
| Brachytherapy | 10 (11.1) |
| None | 32 (35.5) |
| Ketoconazole dose | |
| 400 mg every 8 h | 75 (83.3) |
| 200 mg every 8 h | 8 (8.9) |
| Unknown | 7 (7.8) |
| HSD3B1 Genotype | |
| Homozygous wild type | 44 (49.0) |
| Heterozygous | 34 (38.0) |
| Homozygous variant | 12 (13.0) |
Abbreviations: IQR, interquartile range; PSA, prostate-specific antigen.
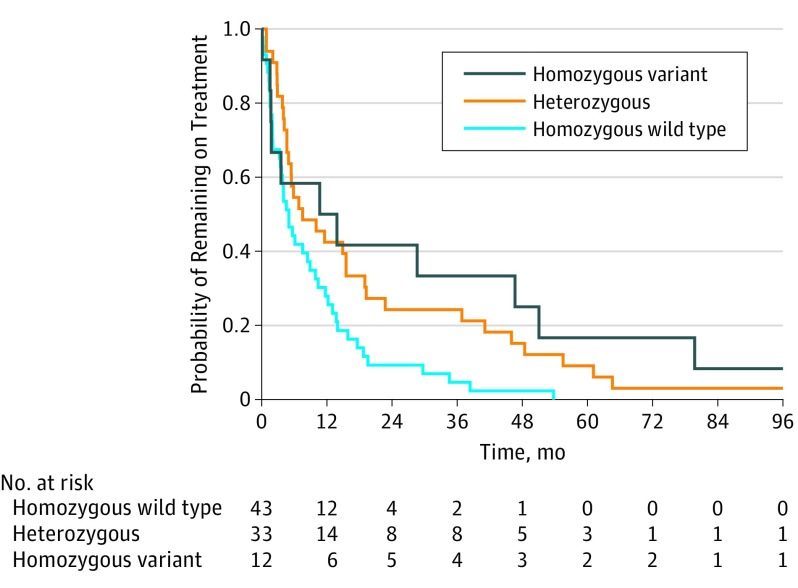
Median duration of therapy increased with the number of variant HSD3B1 (1245C) alleles inherited: 5.0 months (95% CI, 3.4-10.4) for 0 variant alleles; 7.5 months (95% CI, 4.9-19.2) for 1; and 12.3 months (95% CI, 1.8-not reached) for 2 (overall comparison for trend, P = .01; pairwise comparison of 0 and 1 variant alleles, P = .01; pairwise comparison of 0 and 2 variant alleles, P = .03) (Figure 1). Compared with homozygous wild type, the hazard ratio for remaining on treatment was 1.8 (95% CI, 1.1-2.9; P = .01) for the heterozygous group and 2.2 (95% CI, 1.1-4.4; P = .02) for the homozygous variant group.
Figure 1. Duration of Ketoconazole Therapy Stratified by HSD3B1 Genotype.
Sufficient data were available to determine the duration of therapy for 88 patients. Forty-three patients were HSD3B1 homozygous wild type; 33, heterozygous; and 12, homozygous. Median duration of therapy increased with the number of variant HSD3B1 alleles inherited.
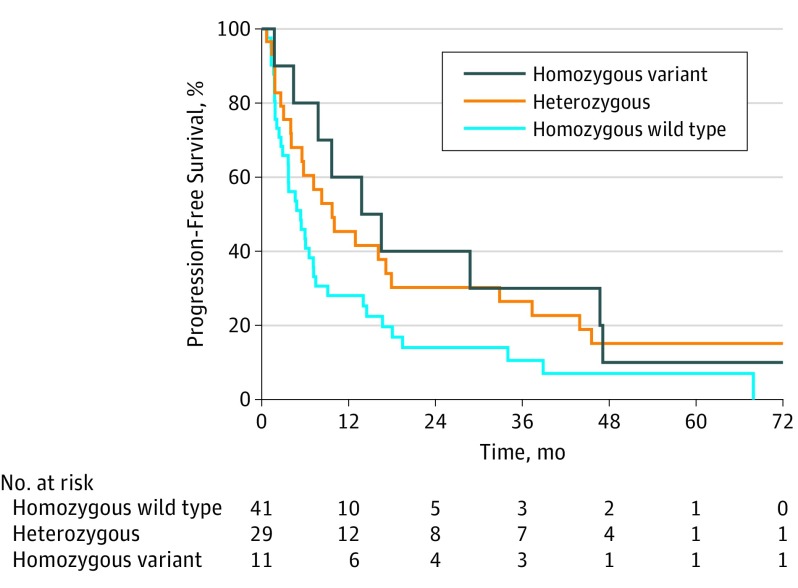
Median time to disease progression similarly increased with the number of variant HSD3B1 (1245C) alleles inherited: 5.4 months (95% CI, 3.7-7.5) for 0 variant alleles; 9.7 months (95% CI, 5.6-32.9) for 1; and 15.2 months (95% CI, 7.8-not reached) for 2 (overall comparison for trend, P = .03; pairwise comparison of 0 and 1 variant alleles, P = .07; pairwise comparison of 0 and 2 variant alleles, P = .07) (Figure 2). Compared with the homozygous wild-type group, the hazard ratio for disease progression was 0.6 (95% CI, 0.4-1.0; P = .06) for the heterozygous group and 0.5 (95% CI, 0.3-1.1; P = .08) for the homozygous variant group.
Figure 2. Progression-Free Survival Stratified by HSD3B1 Genotype.
Sufficient data were available to determine time to progression for 81 patients. Forty-one patients were HSD3B1 homozygous wild type; 29, heterozygous; and 11 homozygous. Median time to disease progression increased with the number of variant HSD3B1 alleles inherited.
Discussion
Inheritance of the HSD3B1 (1245C) germline variant that encodes a missense in 3βHSD1 and increases synthesis of potent androgens from extragonadal precursor steroids enables prostate cancer to use this alternative androgen supply in the absence of gonadal testosterone, thus facilitating more rapid development of CRPC. Our data indicate that the same mechanism that enables earlier CRPC by engaging extragonadal steroids more effectively may make them more dependent on these steroids. Therefore, this tumor resistance mechanism may also be exploited clinically as a tumor vulnerability to drugs that block the synthesis of potent androgens from extragonadal steroids, or possibly to AR antagonists that prevent these potent androgens from activating downstream pathways. As only a proportion of patients treated with potent CYP17A1 inhibitors or AR antagonists respond clinically, a predictive biomarker for the identification of patients who benefit would undoubtedly have clinical value. Although this study specifically addressed HSD3B1 (1245C) as a biomarker of response to a nonsteroidal CYP17A1 inhibitor, it is possible it may also identify responders to steroidal inhibitors; however, steroidal metabolites of the latter agents make evaluation more complex.
Limitations
The present study has limitations that warrant consideration. This observational study is subject to selection biases inherent to its retrospective design. Importantly, we excluded patients who were administered abiraterone before ketoconazole therapy to eliminate prior exposure to CYP17A1 inhibition as a possible confounder on our end points of analysis. Finally, the clinical utility of a biomarker predictive of response to ketoconazole is limited as this agent is no longer routinely used for men with metastatic CRPC in the United States and Europe. The study was specifically designed to study nonsteroidal CYP17A1 inhibition because 3βHSD mediates conversion of the steroidal CYP17A1 inhibitor abiraterone acetate to several steroidal metabolites, including an AR agonist, which would be expected to partially or wholly obscure the hypothesized impact of HSD3B1 genotype on treatment outcomes.
Conclusions
The HSD3B1 (1245C) variant allele is associated with prolonged time to disease progression among men with metastatic CRPC treated with nonsteroidal CYP17A1 inhibition. These findings suggest that the variant allele, which increases the synthesis of potent androgens from extragonadal precursor steroids and hastens the development of CRPC, may be a predictive biomarker of tumor vulnerability to pharmacologic CYP17A1 inhibition with a nonsteroidal drug.
References
- 1.Sharifi N. Minireview: androgen metabolism in castration-resistant prostate cancer. Mol Endocrinol. 2013;27(5):708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostate cancer: I, the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1(4):293-297. [DOI] [PubMed] [Google Scholar]
- 3.Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387(10013):70-82. [DOI] [PubMed] [Google Scholar]
- 4.Yuan X, Cai C, Chen S, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2014;33(22):2815-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253-8261. [DOI] [PubMed] [Google Scholar]
- 6.Evaul K, Li R, Papari-Zareei M, Auchus RJ, Sharifi N. 3β-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology. 2010;151(8):3514-3520. [DOI] [PubMed] [Google Scholar]
- 7.Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hearn JWD, AbuAli G, Reichard CA, et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 2016;17(10):1435-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal N, Hahn AW, Gill DM, Farnham JM, Poole AI, Cannon-Albright L. Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 2017;3(6):856-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Bishop AC, Alyamani M, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523(7560):347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Alyamani M, Li J, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533(7604):547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, Morris MJ, Stadler WM, et al. ; Prostate Cancer Clinical Trials Working Group 3 . Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 14.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23228172&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer TM, Armstrong AJ, Rathkopf DE, et al. ; PREVAIL Investigators . Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433. [DOI] [PMC free article] [PubMed] [Google Scholar]