Abstract
Background:
Circulating metals from both the natural environment and pollution have been linked to cardiovascular disease. However, few prospective studies have investigated the associations between exposure to multiple metals and incident coronary heart disease (CHD).
Objectives:
We conducted a nested case–control study in the prospective Dongfeng-Tongji cohort, to investigate the prospective association between plasma metal concentrations and incident CHD.
Methods:
A total of 1,621 incident CHD cases and 1,621 controls free of major cardiovascular disease at baseline and follow-up visits were matched on age () and sex. We measured baseline fasting plasma concentrations of 23 metals and used conditional logistic regression models to estimate odds ratios (ORs) of CHD for metal concentrations categorized according to quartiles in controls.
Results:
Five metals (titanium, arsenic, selenium, aluminum, and barium) were significantly associated with CHD based on trend tests from single-metal multivariable models adjusted for established cardiovascular risk factors. When all five were included in the same model, adjusted ORs for barium and aluminum were close to the null, whereas associations with titanium, arsenic, and selenium were similar to estimates from single-metal models, and ORs comparing extreme quartiles were 1.32 (95% CI: 1.03, 1.69; ), 1.78 (95% CI: 1.29, 2.46; ), and 0.67 (95% CI: 0.52, 0.85; ), respectively.
Conclusions:
Our study suggested that incident CHD was positively associated with plasma levels of titanium and arsenic, and inversely associated with selenium. Additional research is needed to confirm these findings in other populations. https://doi.org/10.1289/EHP1521
Introduction
Exposure to metals from both the natural environment and pollution may influence the development of chronic diseases, including cardiovascular disease (CVD). As ubiquitous components of the natural environment as well as of pollutants, multiple metals coexist in the ecosystem and reach the public through ambient air, drinking water, food, medications, and consumer products (Nordberg et al. 2014; Saper et al. 2008). Several prospective studies have evaluated associations between cardiovascular outcomes and exposures to single metals, such as arsenic (Argos et al. 2010; Moon et al. 2013; Wu et al. 2015), lead (Menke et al. 2006; Weisskopf et al. 2009), and selenium (Rayman 2012; Zhang et al. 2016). In a prospective case–control study nested in the Health Effects of Arsenic Longitudinal Study (HEALS) cohort of Bangladeshi adults exposed to high levels of arsenic via drinking water, researchers found positive associations between well-water arsenic and fatal and nonfatal CVD (Wu et al. 2015). The Strong Heart Study (SHS) further confirmed the association between urinary arsenic levels and incident CVD and coronary heart disease (CHD) among American Indians with low to moderate exposure levels (Moon et al. 2013). Baseline blood lead concentrations were significantly associated with CVD mortality during follow-up in a representative sample of U.S. adults [National Health and Nutrition Examination Survey III (NHANES III Study), USA] (Menke et al. 2006), whereas weak positive associations were reported between blood lead and CVD mortality (38 deaths) and ischemic heart disease mortality (based on 17 deaths) among men in the Normative Aging Study (Weisskopf et al. 2009). On the other hand, a meta-analysis of prospective studies showed a significant inverse association between selenium status and CVD risk within a narrow selenium range, whereas a null effect of selenium supplementation on CVD was observed in randomized controlled trials (RCTs) (Zhang et al. 2016). This finding might be due to a potential U-shaped association between selenium and CVD and differences in exposure levels among various study populations (Zhang et al. 2016). Limited information about associations between exposures to other metals and CVD is available. For example, Lind et al. (2012) reported an inverted U-shaped association between blood aluminum levels and the prevalence of carotid artery plaques in elderly residents of Uppsala, Sweden. Peters et al. (2013) reported that cardiovascular mortality was associated with the duration of aluminum dust inhalation in underground gold miners, but no difference in CVD mortality was found between miners with any vs. no aluminum dust exposure.
In addition, although humans are exposed to many metals in daily life, few studies have examined the relationships between exposure to multiple metals and cardiovascular risk, particularly in China, where air pollution (Rich et al. 2012) and water pollution (Tan et al. 2015) are major public health concerns (Zhang et al. 2010). Given that CHD remains a leading cause of death and disease both in China and globally (Murray and Lopez 2013; Wilkins et al. 2012), even a modest increase in CHD would translate into a large burden of increasing morbidity and mortality. Therefore, we applied a novel method to measure 23 plasma metals using inductively coupled plasma mass spectrometry (ICP-MS) in a cohort of Chinese adults, and estimated the associations between plasma metal levels and incident CHD.
Methods
Study Design and Participants
We used data from the Dongfeng-Tongji (DFTJ) cohort (Wang et al. 2013), an ongoing prospective study in Shiyan, China. Shiyan is an inland city located in central China. In comparison with other cities, there is no clear evidence suggesting special natural and anthropogenic sources of metals in this city. Dongfeng Motor Corporation (DMC) is one of the largest auto manufacturers in China. In 2008, the Retirement Office and the Social Insurance Center of DMC provided a list of all living retired employees (). They were invited to participate in the study, and 27,009 were enrolled (a response rate of 87%), completed questionnaires, underwent physical examinations, and provided blood specimens during September 2008–June 2010. Most of the employees who were invited but not enrolled (approximately 3,000 out of 3,991) had relocated to other cities and could not be reached (Wang et al. 2013). The participants were invited to a follow-up examination in 2013 with a follow-up rate of 96.2% ().
Ascertainment of CHD Cases
Incident CHD events were defined as first occurrence of nonfatal myocardial infarction (MI), fatal CHD, stable and unstable angina, or coronary revascularization (coronary artery bypass graft or percutaneous transluminal coronary angioplasty) during follow-up, as recommended in guidelines for observational research from the American Heart Association (Luepker et al. 2003). All participants were covered by the health-care service system of the Dongfeng Corporation and thus could to be tracked for morbidity and mortality records. Possible cases were initially identified through review of medical insurance documents, hospital records, and death certificates up to 31 December 2013 and were adjudicated by an expert panel of physicians. The medical records were available for all participants with diagnosed diseases and cover the entire follow-up period. CHD was diagnosed following the World Health Organization criteria using clinical symptoms, cardiac enzymes, and electrocardiograms or by coronary angiography (stenosis in at least one major coronary artery) (American Heart Association 1979). We included only definite or probable MIs, where definite MI was defined by diagnostic ECG or enzymes, and probable MI was defined by positive ECG findings in the presence of ischemic signs or symptoms or equivocal diagnostic enzymes (Luepker et al. 2003). Fatal CHD cases were identified by death certificates with International Classification of Diseases (ICD) codes (ICD-9 410–414 and ICD-10 I20–I25). Stable angina was defined as angina without alteration of frequency or pattern for six weeks before hospitalization; unstable angina included angina occurring at rest and prolonged, new onset of serious angina, or deteriorating angina before hospitalization (Cannon et al. 2001). Revascularization was verified through hospital records. At baseline, we excluded participants with self-reported CHD or diagnosed CHD with medical records (prevalent CHD cases, ), participants with self-reported stroke () or cancer (), and participants with abnormal results on resting ECG (). A total of 1,962 incident CHD cases were identified from the remaining participants during the follow-up until the end of 2013. Incident CHD cases were excluded if their diagnosis date was unknown, or if they were diagnosed after completing their baseline survey (). We also excluded 48 cases with missing data for covariates (38 had missing baseline blood pressure data, and 10 had missing BMI values) and cases with insufficient plasma samples for metal measurements (). Consequently, a total of 1,621 incident CHD cases were included in the analysis. Each incident case was matched to one control that was randomly selected from study participants who were CVD-free at the end of follow-up, had complete covariate data and sufficient samples for metals analysis, and had at least as much follow-up time as the matched cases. Cases and controls were matched for sex and age (within five years).
Metals Correlation Study
Previous studies have measured metal concentrations in plasma (Zhang et al. 2016), whole blood (Menke et al. 2006), or urine (Moon et al. 2013) as internal biomarkers to characterize exposures to different metals, and it is not clear whether plasma was an appropriate biological matrix for all metals that we examined. For example, it has been recommended that human exposure to chromium be characterized based on concentrations in both plasma and erythrocytes (Paustenbach et al. 1997) because concentrations in plasma represent chromium (III) only, whereas chromium (VI) is present primarily in erythrocytes (Wiegand et al. 1988). Whole blood has been proposed as the best matrix for characterizing iron exposure, because 60–70% of total body iron is present in hemoglobin in circulating erythrocytes (Nordberg et al. 2014). Similarly, cadmium in blood is mainly concentrated in the blood cells, with low levels in human plasma (Nordberg et al. 2014). Therefore, we conducted a correlation substudy to compare the concentrations of each metal in plasma, whole blood, and urine samples. Those samples were collected from a separate population of 94 healthy volunteers living in Shiyan who were free of CVD, cancer, and diabetes, and who completed questionnaires and physical examinations that were similar to questionnaires and examinations completed by the nested case–control study population. Our intention was to use the findings of this analysis to support the use of plasma concentrations to characterize exposures, or to identify metals for which plasma concentrations might not be a reliable biomarker of exposure based on low correlations with concentrations measured in whole blood or urine.
Metals Variability Study
We also performed a separate study to evaluate the inter- and intra-individual variability of plasma metals by comparing plasma metal concentrations measured at baseline (in 2008) and at a follow-up visit (in 2013) in a separate group of 138 cohort members who were free of CVD, cancer, and diabetes at the baseline and follow-up visits.
Written informed consent was obtained from each participant, including participants in the correlation and variability studies, as well as the primary case–control study, and all parts of the study were approved by the Ethics and Human Subject Committees of Tongji Medical College.
Metals Exposure
Peripheral venous EDTA blood specimens were collected after overnight fasting. The samples were centrifuged and frozen within two hours of collection, and stored at . We measured case and control specimens in random order, with laboratory personnel blinded to the case–control status. Similarly, samples from the correlation and variability studies were measured separately and in random order. Plasma concentrations of 23 metals were determined by Agilent 7700x ICP-MS with an octopole-based collision/reaction cell (Agilent Technologies), following previously reported protocols (Cesbron et al. 2013). In the metal correlation study, similar methods were used to measure metals in the whole blood (Cesbron et al. 2013) and urine (Feng et al. 2015). The urinary metals concentrations were standardized on creatinine.
For quality-control purposes, we measured metals in standard reference materials once in every 20 samples [specifically, 1640a (Trace Elements in Natural Water from the National Institute of Standards and Technology) and certified reference materials (ClinChek® human plasma controls for trace elements no. 8,883 and no. 8,884; Recipe Chemicals)] and confirmed that values measured in the reference materials were within the recommended range for each metal. For titanium, rubidium, and tungsten, which did not have certified reference samples available, we assayed a spiked pooled plasma specimen (gathered randomly from 100 specimens). The spike recovery values of the three metals were 82.9–105.8%. Intra-assay and interassay CVs for all plasma metals were (see Table S1).
Metal concentrations that were below the limit of detection (LOD) (listed in Table S1) were imputed with a value equal to the half of the detection limit. More than half of the participants [98.3%, 80.2%, and 54.0%, respectively (see Table S1)] had plasma tungsten, tin, and uranium concentrations . Therefore, we excluded these metals from further analysis. For all other metals, fewer than 12% of the samples had values below the LOD (see Table S1).
Covariate Data
Data on age, sex, education, medication use, smoking, alcohol consumption, physical activity, occupational history, and diet were collected at baseline by trained interviewers using semistructured questionnaires. Medication use was assessed by asking participants about all medications used in the previous 2 wk. Physical activity was classified as exercise for at least 20 min per week for more than half a year, based on information about activity at work and at leisure. Occupation category was measured as the most recent occupation engaged for more than 3 y before retirement. Diet was classified according to usual intakes of major food groups (meats, vegetables, fruits, beans, eggs, and dairy) in the previous 1 y using a simplified food frequency questionnaire. Participants also were asked about previous medical diagnoses and about their family history of specific diseases (limited to first-degree family members and doctor-diagnosed conditions), including CHD.
Standing height and body weight were measured by trained personnel during the baseline physical examination. In addition, each participant’s resting blood pressure [systolic (SBP) and diastolic (DBP)] was measured one time, and average SBP and DBP values were calculated. Fasting blood was drawn for laboratory assays of blood lipids, fasting glucose, and renal function. Hypertension was defined if the measured BP values were for SBP or for DBP, or if the participant self-reported physician diagnosis or use of antihypertensive medications. Hyperlipidemia was defined if total cholesterol was , or triglycerides was , or the participant self-reported physician diagnosis or use of antihyperlipidemia medications. Diabetes was defined if the fasting glucose level was , or the participant self-reported physician diagnosis or use of antidiabetic medications (insulin or oral hypoglycemic agents). We applied the Modification of Diet in Renal Disease equation based on the Chinese patients with chronic kidney disease to calculate estimated glomerular filtration rates (eGFR) (Ma et al. 2006).
Statistical Analyses
Baseline characteristics of cases and controls were compared using t-tests or Mann-Whitney U tests for continuous variables, and chi-square tests for categorical variables.
We used Spearman’s rank correlation analysis to explore correlations among the plasma metal concentrations in the case–control study population, after natural log-transformation to account for their right-skewed distributions. In addition, we used Spearman’s rank correlations to evaluation correlations between concentrations of individual metals measured in plasma, whole blood, and urine for the metals correlation study. For the metals variability study, we derived Pearson correlation coefficients and intraclass correlation coefficients (ICCs) to assess consistency between concentrations measured at baseline and follow-up.
We used separate conditional logistic regression models to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for incident CHD and individual plasma metals categorized into quartiles according to their distributions among the controls. These models were limited to metals with at least 50% of plasma concentrations , and to metals that plasma was deemed to be a reliable biospecimen matrix based on the correlation substudy and prior information. All models were adjusted for baseline values of potential confounders that were selected a priori: body mass index (BMI, continuous), smoking (current, former, and never), smoking pack-years, alcohol consumption (current, former, and never), education (, middle school, ), physical activity (yes or no), family history of CHD (any or none), hypertension (yes or no, as defined above), hyperlipidemia (yes or no), diabetes (yes or no), and eGFR. Linear trend p-values were derived by modeling the median value of each metal quartile as a continuous variable in adjusted models.
After deriving trend p-values for each individual metal, we derived corresponding q-values for a False Discovery Rate (FDR) [using software published by Pike (2011)] to identify a subset of the 20 metals for simultaneous evaluation in a multiple-metal model that included the same covariates as the single-metal models. We also performed sensitivity analyses by additionally adjusting the multiple-metal models for each participant’s most recent occupation (categorized into six groups), and for dietary consumption of meats, vegetables, fruits, beans, eggs, and dairy, with each food group modeled using a separate dichotomous variable based on consumption or .
The subset of metals that were significantly associated with CHD in the primary multiple-metals model () underwent additional evaluation. First, we estimated associations between the metals and CHD using a multiple-metal model with each ln-transformed metal modeled using restricted cubic splines with knots at the 20th, 40th, 60th, and 80th percentiles of its distribution; the reference value () set at the 10th percentile (Moon et al. 2013); and the measured concentration replaced with the mean concentration for all observations with measured concentrations above this value. We also performed a sensitivity analysis of restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles.
Stratified analyses were performed by including metals in the multiple-metal model and corresponding dichotomous variables for baseline characteristics, including age (, ), sex, BMI (, ), smoking status (never-smokers, ever-smokers), presence of hypertension, and diabetes (yes, no), as well as renal function (eGFR , per ). In addition, we evaluated the joint associations between metals that were significant in the multiple-metal model, and the individual metal was dichotomized as low () and high (), and a four-category variable was created for two metals (i.e., low/low, high/low, low/high, and high/high) with low/low group as the reference. To determine whether the pairwise interactions were significantly different from expectations for multiplicative risks, we derived likelihood ratio test p-values, comparing the fit of models with jointly categorized exposures to corresponding models with lower-order terms for each metal only. Finally, we compared the baseline characteristics according to the quartiles of titanium using linear regression for continuous variables and chi-square tests for categorical variables. The purpose of this analysis was to explore the potential correlations between titanium and participants’ characteristics because there was limited information about the sources of exposure to this metal, whereas it showed significant association with CHD (as described in the “Results” section). Analyses were conducted using SAS (version 9.3; SAS Institute Inc.) and R software (version 3.3.2; R Core Team), and a two-sided was considered statistically significant.
Results
Characteristics of the Study Population
In comparison with the controls, CHD cases were more likely to have hypertension, hyperlipidemia, and diabetes at baseline; they had slightly but significantly higher BMIs, higher pack-years among ever-smokers, and lower eGFRs; in addition, they were less likely to have never smoked or to have a high-school education (Table 1). No significant differences were found for alcohol intake status, physical activity levels, or family history of heart disease between the cases and controls. Concentrations of aluminum, titanium, manganese, copper, zinc, arsenic, strontium, barium, and lead were significantly higher in cases than controls, whereas selenium concentrations were significantly lower (Table 2). Among the 20 metals with less than 12% of samples below the LOD (see Table S1), most of them were significantly but modestly correlated with each other (Spearman’s rank correlation coefficients ; see Table S2 and Figure S1).
Table 1.
Basic characteristics of study participants at baseline.
| Variables | Controls () | Cases () | p-Valuea |
|---|---|---|---|
| Age (years) | 0.72 | ||
| Male sex, (%) | 789 (48.7) | 789 (48.7) | |
| BMI () | |||
| Smoking status, (%) | |||
| Current smoker | 305 (18.8) | 344 (21.2) | |
| Former smoker | 179 (11.0) | 203 (12.5) | 0.06 |
| Never smoker | 1137 (70.1) | 1074 (66.3) | |
| Pack-years among ever-smokersb | 0.04 | ||
| Alcohol intake status, (%) | |||
| Current drinker | 360 (22.2) | 361 (22.3) | |
| Former drinker | 78 (4.8) | 70 (4.3) | 0.80 |
| Never drinker | 1183 (73.0) | 1190 (73.4) | |
| Education level, (%) | |||
| Primary school or below | 512 (31.6) | 543 (33.5) | |
| Middle school | 563 (34.7) | 597 (36.8) | 0.05 |
| High school or beyond | 546 (33.7) | 481 (29.7) | |
| Physical activity (yes), (%)c | 1466 (90.4) | 1437 (88.6) | 0.10 |
| CHD family history, (%) | 77 (4.8) | 78 (4.8) | 0.93 |
| Hypertension, (%)d | 699 (43.1) | 1074 (66.3) | |
| Hyperlipidemia, (%)e | 711 (43.9) | 993 (61.3) | |
| Diabetes, (%)f | 171 (10.5) | 383 (23.6) | |
| eGFR () | |||
| eGFR , (%) | 648 (40.0) | 553 (34.1) | |
| Occupational categories | |||
| Raw material production | 210 (13.0) | 229 (14.1) | |
| Automobile parts manufacturing | 662 (40.8) | 700 (43.2) | |
| Automobile assembly | 129 (8.0) | 93 (5.7) | 0.07 |
| Auxiliary service and management | 32 (2.0) | 22 (1.4) | |
| Others (e.g., affiliated schools) | 564 (34.8) | 558 (34.4) | |
| Missing indicator | 24 (1.5) | 19 (1.2) | |
| Diet categories () | |||
| Meat | 467 (28.8) | 423 (26.1) | 0.08 |
| Fish or seafood | 142 (8.8) | 140 (8.6) | 0.90 |
| Milk or dairy products | 585 (36.1) | 558 (34.4) | 0.32 |
| Beans or soy foods | 420 (25.9) | 402 (24.8) | 0.47 |
| Fruits or vegetables | 1579 (97.4) | 1562 (96.4) | 0.09 |
Note: Normally distributed variables were presented as . Non-normally distributed variables were presented as median (IQR). Categorical variables were presented as numbers (percentage). Data were complete for all variables except occupation. BMI, body mass index; eGFR, estimated glomerular filtration rate.
p-Values were derived from Student’s t-tests or Mann-Whitney U tests for continuous variables according to the data distribution, and chi-square test for the category variables.
Numbers of packs smoked/, among former and current smokers.
Physical activity was defined as exercise for at least 20 min per week for more than half a year.
Hypertension was defined as measured values for SBP or for DBP, self-reported physician diagnosis, or reported use of antihypertensive medication.
Hyperlipidemia was defined as total cholesterol , triglycerides , or a self-reported physician diagnosis, or antihyperlipidemia medication use.
Diabetes was defined as fasting glucose , self-reported physician diagnosis, or antidiabetic medication use (insulin or oral hypoglycemic agents).
Table 2.
Concentrations of plasma metals among study participants.
| Variables | Controls () | Cases () | p-Valuea |
|---|---|---|---|
| Plasma metals () | |||
| Aluminum | 48.95 (31.00–97.29) | 57.41 (33.14–144.75) | |
| Antimony | 0.14 (0.09–0.22) | 0.14 (0.09–0.21) | 0.37 |
| Arsenic | 1.96 (1.27–3.49) | 2.32 (1.42–4.49) | |
| Barium | 35.47 (23.25–62.55) | 40.53 (25.98–71.05) | |
| Cadmium | 0.30 (0.19–0.53) | 0.27 (0.17–0.53) | 0.06 |
| Chromium | 3.44 (2.67–4.58) | 3.49 (2.64–4.54) | 0.82 |
| Cobalt | 0.15 (0.12–0.20) | 0.15 (0.12–0.20) | 0.56 |
| Copper | 962.14 (856.05–1072.48) | 970.93 (867.11–1097.38) | 0.02 |
| Iron | 1183.21 (958.77–1483.43) | 1202.71 (976.27–1471.85) | 0.34 |
| Lead | 13.12 (8.84–20.71) | 13.76 (9.52–23.05) | 0.01 |
| Manganese | 4.05 (2.97–5.72) | 4.21 (3.17–5.77) | 0.03 |
| Molybdenum | 1.36 (1.09–1.74) | 1.36 (1.10–1.78) | 0.51 |
| Nickel | 3.03 (2.17–4.54) | 3.07 (2.22–4.69) | 0.27 |
| Rubidium | 357.09 (318.22–399.30) | 354.91 (315.48–400.34) | 0.31 |
| Selenium | 67.48 (57.67–78.69) | 65.85 (56.88–76.87) | 0.02 |
| Strontium | 35.91 (30.12–42.31) | 36.67 (30.66–44.08) | 0.003 |
| Thallium | 0.13 (0.10–0.18) | 0.14 (0.10–0.19) | 0.08 |
| Titanium | 29.14 (24.41–35.71) | 30.32 (25.34–36.90) | |
| Vanadium | 0.67 (0.53–0.99) | 0.68 (0.55–1.00) | 0.33 |
| Zinc | 1193.53 (1014.72–2623.69) | 1245.56 (1040.06–3012.34) | 0.005 |
Note: Plasma tungsten, tin, and uranium concentrations were excluded from further analysis because of many samples (98.3%, 80.2%, and 54.0%, respectively). Plasma cadmium, chromium, and iron were excluded from further analyses because of concerns about the use of plasma concentrations as a biomarker. Metal concentrations are presented as median (IQR).
p-Values were derived from Mann-Whitney U tests.
Metals Correlation Study
In comparison with controls in the main study, the 94 participants in the metals correlation study were younger (mean age 44 vs. 66 y), more likely to be male (56.4% vs. 48.7%) and to be current alcohol consumers (42.6% vs. 22.2%), and less likely to have diabetes (3.1% vs. 10.5%) (Table S3). Plasma levels of chromium, iron, and cadmium were not significantly correlated with concentrations in whole blood or in urine (Table S4), and previous studies have suggested that plasma concentrations may not be a reliable measure of internal exposure (Nordberg et al. 2014; Paustenbach et al. 1997; Wiegand et al. 1988). Therefore, these metals were excluded from subsequent analyses.
Metals Exposure and Incident CHD
Five metals met our criterion ( in the single-metal model) for further analysis: aluminum, arsenic, barium, selenium, and titanium (Table S5). Although the single-metal model p-trend for plasma rubidium was marginally significant (), the FDR p-trend was 0.14, and it was therefore excluded from the multiple-metal models. None of the other metals evaluated in single-metal models had significant trend p-values.
In the multiple-metals model, trend tests remained significant, and adjusted ORs were similar to those from single-metal models for titanium (; 95% CI: 1.03, 1.69 for the fourth vs. first quartile; ), arsenic (; 95% CI: 1.29, 2.46; ), and selenium (; 95% CI: 0.52, 0.85; ) (Table 3). In contrast, ORs for aluminum and barium were close to the null after adjustment for the other metals. The ORs did not change appreciably for any of the metals after additional adjustment of the multiple-metals model for occupation or consumption of major food groups (Table S6). Therefore, further analyses were limited to titanium, arsenic, and selenium, unless otherwise indicated.
Table 3.
Adjusted odds ratios [95% confidence interval (CI)] for incident CHD according to quartiles of exposure for plasma metals included in the multiple-metal model.
| Plasma metals | Quartiles of plasma metals ()a | p-Trendb | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Aluminum ()c | 31.03–48.95 | 48.95–97.15 | |||
| (case/control) | 358/405 | 349/405 | 395/405 | 519/406 | |
| Model 1d | 1.00 | 0.93 (0.74, 1.16) | 1.05 (0.83, 1.32) | 1.33 (1.07, 1.65) | 0.001 |
| Model 2e | 1.00 | 0.85 (0.67, 1.08) | 0.90 (0.70, 1.15) | 0.94 (0.71, 1.25) | 0.83 |
| Arsenic () | 1.28–1.96 | 1.96–3.49 | |||
| (case/control) | 323/405 | 357/405 | 369/405 | 572/406 | |
| Model 1 | 1.00 | 1.17 (0.93, 1.48) | 1.12 (0.89, 1.40) | 1.68 (1.35, 2.09) | |
| Model 2 | 1.00 | 1.17 (0.91, 1.50) | 1.15 (0.88, 1.50) | 1.78 (1.29, 2.46) | 0.001 |
| Barium ()c | 23.26–35.48 | 35.48–62.52 | |||
| (case/control) | 328/405 | 375/405 | 424/405 | 494/406 | |
| Model 1 | 1.00 | 1.21 (0.96, 1.53) | 1.25 (1.00, 1.56) | 1.44 (1.15, 1.79) | 0.002 |
| Model 2 | 1.00 | 1.15 (0.90, 1.47) | 1.03 (0.79, 1.34) | 0.91 (0.66, 1.25) | 0.41 |
| Selenium () | 57.69–67.48 | 67.48–78.66 | |||
| (case/control) | 438/405 | 437/405 | 392/406 | 354/405 | |
| Model 1 | 1.00 | 0.97 (0.79, 1.19) | 0.88 (0.71, 1.10) | 0.72 (0.57, 0.91) | 0.007 |
| Model 2 | 1.00 | 0.92 (0.74, 1.14) | 0.80 (0.64, 1.00) | 0.67 (0.52, 0.85) | 0.001 |
| Titanium () | 24.42–29.14 | 29.14–35.70 | |||
| (case/control) | 319/405 | 396/405 | 441/405 | 465/406 | |
| Model 1 | 1.00 | 1.28 (1.02, 1.61) | 1.35 (1.07, 1.69) | 1.37 (1.09, 1.73) | 0.010 |
| Model 2 | 1.00 | 1.28 (1.01, 1.62) | 1.33 (1.05, 1.69) | 1.32 (1.03, 1.69) | 0.04 |
Plasma metal concentration was presented as raw data.
p-Trend across quartiles of metals were obtained by including the median of each quartile (natural log-transformed) as a continuous variable in logistic regression models.
For aluminum, the inclusion of barium or arsenic in the model would attenuate the association to non-significant. For barium, the inclusion of arsenic or aluminum would attenuate the association to nonsignificant. Aluminum, arsenic and barium are significantly correlated with each other (), with high correlation coefficients [aluminum–arsenic 0.58, aluminum–barium 0.62, arsenic–barium 0.71].
Model 1: Metals were included in the conditional logistic regression models separately (single-metal model) and adjusted for BMI, smoking status, pack year, alcohol intake status, education, physical activity, hypertension, hyperlipidemia, family history of coronary heart disease, diabetes, and eGFR. The results for the rest metals in the single-metal model were shown in Table S5.
Model 2: Metals that were significant in the single-metal model () were included in the conditional logistic regression model simultaneously (multiple-metals model) and adjusted for the same variables as Model 1.
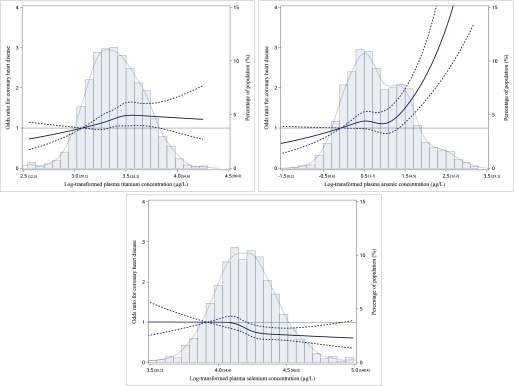
Spline regression analysis indicated significant linear associations for titanium and selenium ( and , respectively, with p-values for nonlinearity of 0.36 and 0.25) (Figure 1). In contrast, the association between arsenic and CHD was nonlinear (), with a sharp increase in slope for concentrations above . Results were similar in the sensitivity analysis when different knots were applied (Figure S2).
Figure 1.
The restricted cubic spline for the association between plasma metals and incident CHD. The lines represent adjusted odds ratios based on restricted cubic splines for the log-transformed levels of plasma titanium, arsenic, and selenium in the multiple-metals conditional regression model. Knots were placed at the 20th, 40th, 60th, and 80th percentiles of the plasma metal distribution, and the reference value was set at the 10th percentile. Adjustment factors were BMI, smoking status, pack year, alcohol intake status, education, physical activity, hypertension, hyperlipidemia, family history of CHD, diabetes, and eGFR. The bars represent histograms of plasma metal distribution among the total population. The model included barium and aluminum as well. The numbers in parentheses show the plasma metal concentrations before log-transformation.
Subgroup Analyses and Metals Interaction Analyses
There were no clear differences in associations between CHD and titanium, arsenic, or selenium according to strata of age, gender, BMI, smoking, hypertension, diabetes, and renal function (Table S7). The sample size in certain strata may be small to limit definite conclusion of effect modification by those variables (Table S7).
In the joint association analysis of two metals, although no significant p-interaction values were found, higher levels of titanium or arsenic were associated with increased risk of CHD when selenium level was low (equal to or below the median), and the association was attenuated to null when selenium level was high (above the median) (Table 4).
Table 4.
Adjusted odds ratios (95% CI) for incident CHD according to the combined categories of plasma metal levels.
| Metals | (case/control) | Odds ratio (95% CI)a | p-Interactionb |
|---|---|---|---|
| Selenium-Arsenic | |||
| 369/426 | 1.00 | 0.52 | |
| 506/384 | 1.37 (1.10, 1.69) | ||
| 311/384 | 0.86 (0.68, 1.08) | ||
| 435/427 | 1.05 (0.85, 1.32) | ||
| Selenium-Titanium | |||
| 435/474 | 1.00 | 0.29 | |
| 440/336 | 1.33 (1.07, 1.66) | ||
| 280/336 | 0.87 (0.69, 1.11) | ||
| 466/475 | 0.98 (0.79, 1.22) |
Note: Metals that were significant in the multiple-metal model () were included in the combined effect analysis. As, Arsenic; Se, Selenium; Ti, Titanium.
The multivariable-adjusted model included the combined categories of metals levels [Low Se (), high Se (). Low As (), high As (). Low Ti (), high Ti ()], BMI, smoking status, pack year, alcohol intake status, education, physical activity, hypertension, hyperlipidemia, family history of coronary heart disease, diabetes, and eGFR.
p-Interaction was the likelihood ratio test p-values comparing the fit of models with jointly categorized exposures to corresponding models with lower-order terms for each metal only.
Metals Variability Study
In general, the 138 participants in the metals variability study were similar to the control participants in the primary study, though they were slightly younger (mean age 62 vs. 66 y) and less likely to have diabetes (1.4% vs. 10.5%) at baseline (Table S8). When concentrations were compared in plasma samples collected 5 y apart, fair to good reproducibility was observed for titanium (, ) and selenium (, ); however, the reproducibility was poor for arsenic (, ) (see Table S9). Of the other metals, reproducibility was fair to good (ICC 0.45–0.74) for plasma concentrations of barium, copper, lead, rubidium, and strontium, and poor for the remaining metals.
When comparing the baseline characteristics of the participants according to quartiles of titanium (Table S10), we found that, with increasing quartiles of plasma titanium, the proportions of women, never-smokers, and participants with hypertension and hyperlipidemia were higher ( for all).
Discussion
To the best of our knowledge, this study is the first and largest study to evaluate the prospective associations between internal exposures to multiple metals and incident CHD among Chinese adults. In our study population, plasma concentrations of titanium and arsenic were positively associated with incident CHD, whereas plasma selenium concentrations were inversely associated with incident CHD.
A meta-analysis of 18 studies calculated pooled relative risks of arsenic exposure by comparing the highest versus the lowest exposure category across studies (Moon et al. 2012). The pooled relative risks (95% CI) for CVD and CHD were 1.32 (95% CI: 1.05, 1.67) and 1.89 (95% CI: 1.33, 2.69), respectively (Moon et al. 2012). Yuan et al. (2007) used group-level exposure from drinking water to explore the association with myocardial infarction mortality. Meanwhile, the HEALS cohort in the Bangladesh population used individual-level data on well water or urinary arsenic concentrations and reported a positive relation with all-cause (Argos et al. 2010) and cardiovascular mortality (Chen et al. 2011), and a nested case–control study within the HEALS cohort reported that well-water arsenic was associated with the incidence of fatal and nonfatal CVD (Wu et al. 2015). Furthermore, Moon et al. (Moon et al. 2013) found that urinary arsenic levels were positively associated with incident CHD () in a cohort of 3,575 American Indians with low-moderate arsenic exposure (median urinary arsenic concentration was creatinine, in comparison with creatinine in the Bangladeshi population). A hospital based case–control study in Inner Mongolia examined the association of water and toenail arsenic with CVD (Wade et al. 2015). The median arsenic in drinking water sampled from each participant’s home was , and the adjusted OR for a increase in water arsenic was 1.19 (95% CI: 1.03, 1.38) (Wade et al. 2015). Positive associations also were reported for toenail arsenic (Wade et al. 2015). In accordance with a study of groundwater arsenic contamination across China (Rodríguez-Lado et al. 2013), our study was conducted in a low-exposure area (below drinking water). We used plasma arsenic concentrations to estimate participants’ exposure levels, rather than urine concentrations, but we also measured plasma and urine arsenic concentrations in a separate sample of 98 healthy adult residents of Shiyan, China. In this study sample, plasma and urine arsenic concentrations were moderately correlated () with median concentrations of and creatinine (), respectively. A study conducted among 100 healthy volunteers in France reported that the median concentration of plasma and urine arsenic was and , respectively (Goullé et al. 2005). The median concentration of plasma arsenic in a study (conducted 8 y later) of a similar French population (Cesbron et al. 2013) was close to the concentration found in our study (2.19 and , respectively). Therefore, the plasma concentration of arsenic in our study (median concentration of controls ) was lower than the concentration in other studies. Our findings suggest a nonlinear association between plasma arsenic and incident CHD, with a significant positive association for the highest vs. lowest quartile of exposure ( vs. ), whereas ORs were close to the null for the second and third quartiles, and showed a positive association with concentrations , based on the spline regression model. Similar patterns were found in the HEALS study (Chen et al. 2011) and the Strong Heart Study (Moon et al. 2013). Further studies are needed to evaluate the threshold and reference dose of arsenic exposure and CVD risk. Our data provide evidence for a positive association between plasma arsenic and incident CHD in Chinese adults, even at relatively low to moderate exposure levels. The maximum arsenic concentration in our population was , with the median values for cases and controls in the highest quartile of exposure 5.39 and , respectively. The underlying biologic mechanisms may include increase of the reactive oxygen species production (Xu et al. 2016), may induce alterations in nitric oxide metabolism, and may affect endothelial function (Ellinsworth 2015).
Fish and seafood intake is known to be important source of organic arsenic species (Navarro Serrano et al. 2016; Soleo et al. 2008); however, other nutrients in fish and seafood may have beneficial effects that reduce the risk of CVD (Mozaffarian and Wu 2011). Therefore, fish and seafood consumption may confound associations between plasma arsenic and incident CVD. However, fish and seafood intake levels were low in the Shiyan study population (median of 1 serving/wk in both cases and controls), and thus the confounding could be minimal.
To the best of our knowledge, we are the first to report a significant positive association between plasma titanium and incident CHD. Titanium is the ninth most abundant element in the earth’s crust and is widely used in many products, including paints, coatings, plastics, pharmaceuticals, food, cosmetics, and toothpaste (Shi et al. 2013). Furthermore, is commonly used as a component for articulating prosthetic implants (Shi et al. 2013). The median plasma titanium concentration among control participants in the present study was . Information about titanium levels in general populations is not available, but the mean serum titanium level in 32 U.S. patients 6 wk after repair of a femoral fracture with an intramedullary titanium implant was (McGarry et al. 2008), and the mean serum titanium concentration measured in 30 U.S. adults 23–28 months after receiving titanium spinal implants was , in comparison with in 13 samples from uncharacterized controls (Richardson et al. 2008). These limited reports suggest that plasma titanium may be relatively high in our study population, but more information is needed to characterize titanium concentrations in representative populations of healthy individuals.
Although humans may be widely exposed to titanium in daily life, there is very little information on the potential health effects of chronic exposure to titanium. In our previous community-based cross-sectional study of 2,004 adult residents of Wuhan, China, who did not have a history of CVD or kidney diseases (Feng et al. 2015), urine titanium concentrations were associated with increased heart rate variability, an indicator of autonomic modulation of rhythmic heart rate. This finding suggests that titanium might affect cardiac autonomic balance. The median urine concentrations in this study were creatinine, which was similar to the median concentration of our correlation study population ( creatinine). In vitro administration of nanoparticles to bronchial epithelial cells induced oxidative stress and production of a pro-inflammatory cytokine (Hussain et al. 2009), which suggests that titanium might contribute to the etiology of CVD through pro-inflammatory effects.
Given the lack of information on titanium levels in general populations, we performed a post hoc analysis to identify participant characteristics associated with plasma titanium concentrations. The proportion of women, never-smokers, and participants with hypertension and hyperlipidemia increased from the lowest to highest quartile of plasma titanium (chi-square ); however, the potential mechanism for the association remains to be explored. Our novel findings warrant confirmation in other populations and further investigation of potential mechanisms and sources of exposure.
Our analysis also identified significant inverse associations between incident CHD and selenium. Results from previous studies have been inconsistent, and a recent meta-analysis of 16 prospective studies suggested a significant benefit of CVD incidence and mortality within a narrow selenium range of (Zhang et al. 2016). The NHANES III study in U.S. adults found no significant association between selenium (mean serum selenium concentration ) and CVD mortality overall, with a U-shaped dose–response curve, indicating an inverse association in the middle of the exposure distribution, and a positive association at the high end (Bleys et al. 2008). The participants in a small Chinese cohort study (116 CVD deaths during follow-up of 1,103 cohort participants) reporting an inverse association with CHD mortality had a mean serum concentration of (Wei et al. 2004). However, a matched case–control study of 204 pairs of metabolic syndrome patients and controls in China reported a positive association between plasma selenium and the prevalence of metabolic syndrome and elevated fasting plasma glucose (Yuan et al. 2015). The median plasma selenium concentration in the control group was . Wilhelm et al. (2004) reported a reference range for plasma or serum selenium of , according to a previous review performed by the Human Biomonitoring Commission (2002). In our study, the median concentration was in the controls, and our results were consistent with studies in populations with lower baseline selenium concentrations (Zhang et al. 2016). Our spline analysis suggested that the association continued to be negative at higher exposure levels. However, the estimates are no longer significant, which may due to sparse data for higher exposures, and it should be interpreted with great caution. Therefore, more studies are needed before firm conclusions can be made about the dose–response relationship between selenium and cardiovascular health.
With respect to the interaction between metals, we found exposure to high levels of selenium appeared to attenuate the positive associations between CVD and high-plasma titanium and arsenic. Selenium was reported to play a role in counteracting arsenic poisoning in in vivo and in vitro studies (Zwolak and Zaporowska 2012), and the antioxidant property of selenium may be involved (Zwolak and Zaporowska 2012). Xue et al. (2010) studied biomarkers of oxidative stress and antioxidant activity in residents of a region in China where high-arsenic coal is used for home heating and cooking, including 138 residents (cases) with skin lesions associated with arsenic exposure (hyperkeratosis, depigmentation, and hyperpigmentation) and 76 controls without skin lesions. In comparison with cases that had high blood arsenic and low blood selenium (), cases with high arsenic and high selenium had lower levels of serum malondialdehyde (MDA, an indicator of lipid peroxidation); higher serum levels of the antioxidant enzymes glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase; lower urinary concentrations of 8-oxo-dG; and higher expression (mRNA and protein) of 8-oxoguanine DNA glucosylase 1 (a DNA repair enzyme) in peripheral blood mononuclear cells (Xue et al. 2010). Additional research is needed to confirm whether selenium may attenuate adverse associations of arsenic and titanium with CHD, and to investigate potential underlying mechanisms and the possibility of public health interventions.
For the present study, we systematically measured 23 metals in individual plasma specimens and applied multiple-metal models to examine the independent associations between metals and incident CHD. Furthermore, our case–control study was nested within a large prospective cohort, and the plasma metals were measured years before CHD onset. The incident CHD cases were confirmed by medical and death records and adjudicated by physicians. Medical record reviews and comprehensive clinical examinations were also performed to minimize the potential for undiagnosed or preclinical CHD cases among the controls. Additionally, we performed standardized quality controls in data collection and laboratory assays to reduce the potential of measurement error and systemic bias.
Nevertheless, this study also has potential limitations. First, we did not distinguish among different arsenic species or metabolites in plasma, and our results therefore represent associations averaged over both toxic inorganic arsenic and relatively nontoxic organic arsenic exposures primarily from fish and seafood intake. Second, as each metal has unique distributions in organs and in the circulation system, plasma metals may not be suitable biomarkers of internal exposure for all metals. For example, plasma metals are useful and valid to assess the exposure status for some metals (e.g., plasma selenium), whereas urine or whole blood concentrations are commonly used for others (e.g., urine arsenic and whole blood lead). However, we found significant correlations between plasma and blood concentrations for 13 of 20 metals examined in a separate correlation study, including arsenic and selenium; we also found significant concentrations between plasma and urine concentrations for 8 of the metals, including arsenic, selenium, and titanium. Nonetheless, plasma concentrations of some metals may be subject to potential contamination and should therefore be interpreted with caution (e.g., plasma lead contamination from hemolysis) (Smith et al. 2002). Third, although we could not explore the sources of metals, circulating metals can be viewed as internal biomarkers integrating all sources of exposure. Plasma metals were measured at only one timepoint and may not reflect usual or chronic exposures for metals with large fluctuations in plasma concentrations. Unfortunately, information regarding the biological half-lives of many metals is limited. However, concentrations of 11 of 17 plasma metals, including selenium and titanium (but not arsenic), were significantly correlated between samples from a separate population of 138 DFTJ cohort members that were collected 5 y apart. We selected age- and gender-matched controls from cohort members who were free of CVD at the end of the follow-up period, instead of using incidence density sampling to generate an estimate of the incidence rate ratio. Finally, estimating associations with multiple highly correlated metals is challenging (Bobb et al. 2015), and future studies should explore the use of advanced statistical models to better account for the complexity of mixed exposures.
Conclusions
In conclusion, we observed significant associations between plasma concentrations of several metals and incident CHD in a Chinese population. Our findings require confirmation but may have important implications for public health, given the high burden of CVD, both in China and worldwide, and the possibility that low-to-moderate exposures to metals may be widespread.
Supplemental Material
Acknowledgments
The study was funded by the Natural National Scientific Foundation of China (81230069 and 81390542); the Foundation of National Key Program of Research and Development of China (2016YFC0900800); the 111 Project and the Program for Changjiang Scholars and Innovative Research Team in University; the Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology.
References
- American Heart Association. 1979. Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 59:607–609, PMID: 761341, 10.1161/01.CIR.59.3.607. [DOI] [PubMed] [Google Scholar]
- Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, et al. 2010. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (heals): a prospective cohort study. Lancet 376(9737):252–258, PMID: 20646756, 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleys J, Navas-Acien A, Guallar E. 2008. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med 168(4):404–410, PMID: 18299496, 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian Kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: 25532525, 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. 2001. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol 38(7):2114–2130, PMID: 11738323, 10.1016/S0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- Cesbron A, Saussereau E, Mahieu L, Couland I, Guerbet M, Goullé JP. 2013. Metallic profile of whole blood and plasma in a series of 106 healthy volunteers. J Anal Toxicol 37(7):401–405, PMID: 23794607, 10.1093/jat/bkt046. [DOI] [PubMed] [Google Scholar]
- Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. 2011. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 342:d2431, PMID: 21546419, 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinsworth DC. 2015. Arsenic, reactive oxygen, and endothelial dysfunction. J Pharmacol Exp Ther 353(3):458–464, PMID: 25788710, 10.1124/jpet.115.223289. [DOI] [PubMed] [Google Scholar]
- Feng W, He X, Chen M, Deng S, Qiu G, Li X, et al. 2015. Urinary metals and heart rate variability: a cross-sectional study of urban adults in Wuhan, China. Environ Health Perspect 123(3):217–222, PMID: 25356836, 10.1289/ehp.1307563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, et al. 2005. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci Int 153(1):39–44, PMID: 15979835, 10.1016/j.forsciint.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Human Biomonitoring Commission, 2002. (Kommission “Human-Biomonitoring” des Umweltbundesamtes): Selen und Human-Biomonitoring. Bundesgesundheitsbl. Gesundheitsforsch. [In German] Gesundheitsschutz 45:190–195. [DOI] [PubMed] [Google Scholar]
- Hussain S, Boland S, Baeza-Squiban A, Hamel R, Thomassen LC, Martens JA, et al. 2009. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amount. Toxicology 260(1-3):142–149, PMID: 19464580, 10.1016/j.tox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Lind PM, Olsén L, Lind L. 2012. Circulating levels of metals are related to carotid atherosclerosis in elderly. Sci Total Environ 416:80–88, PMID: 22178028, 10.1016/j.scitotenv.2011.11.064. [DOI] [PubMed] [Google Scholar]
- Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al. 2003. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: A Statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 108(20):2543–2549, PMID: 14610011, 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. 2006. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17(10):2937–2944, PMID: 16988059, 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- McGarry S, Morgan SJ, Grosskreuz RM, Williams AE, Smith WR. 2008. Serum titanium levels in individuals undergoing intramedullary femoral nailing with a titanium implant. J Trauma 64(2):430–433, PMID: 18301210, 10.1097/01.ta.0000240445.20220.54. [DOI] [PubMed] [Google Scholar]
- Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. 2006. Blood lead below 0.48 micromol/l (10 microg/dl) and mortality among US adults. Circulation 114(13):1388–1394, PMID: 16982939, 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A. 2012. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 14(6):542–555, PMID: 22968315, 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. 2013. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. a prospective cohort study. Ann Intern Med 159(10):649–659, PMID: 24061511, 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JH. 2011. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58(20):2047–2067, PMID: 22051327, 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. 2013. Measuring the global burden of disease. N Engl J Med 369(5):448–457, PMID: 23902484, 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- Navarro Serrano I, Llorente Ballesteros MT, Sánchez Fernández Pacheco S, Izquierdo Álvarez S, Lopez Colón JL. 2016. Total and speciated urinary arsenic levels in the Spanish population. Sci Total Environ 571:164–171, PMID: 27471981, 10.1016/j.scitotenv.2016.07.134. [DOI] [PubMed] [Google Scholar]
- Nordberg GF, Fowler BA, Nordberg M. 2014. Handbook on the Toxicology of Metals. 4th ed Cambridge, MA, USA:Academic Press. [Google Scholar]
- Paustenbach DJ, Panko JM, Fredrick MM, Finley BL, Proctor DM. 1997. Urinary chromium as a biological marker of environmental exposure: what are the limitations?. Regul Toxicol Pharmacol 26(1 Pt 2):S23–S34, PMID: 9380834, 10.1006/rtph.1997.1135. [DOI] [PubMed] [Google Scholar]
- Peters S, Reid A, Fritschi L, de Klerk N, Musk AW. 2013. Long-term effects of aluminium dust inhalation. Occup Environ Med 70(12):864–868, PMID: 24142983, 10.1136/oemed-2013-101487. [DOI] [PubMed] [Google Scholar]
- Pike N. 2011. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol E 2(3):278–282, 10.1111/j.2041-210X.2010.00061.x. [DOI] [Google Scholar]
- Rayman MP. 2012. Selenium and human health. Lancet 379(9822):1256–1268, PMID: 22381456, 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, et al. 2012. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 307(19):2068–2078, PMID: 22665106, 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson TD, Pineda SJ, Strenge KB, Van Fleet TA, MacGregor M, Milbrandt JC, et al. 2008. Serum titanium levels after instrumented spinal arthrodesis. Spine 33(7):792–796, PMID: 18379407, 10.1097/BRS.0b013e318169574d. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, et al. 2013. Groundwater arsenic contamination throughout China. Science 341(6148):866–868, PMID: 23970694, 10.1126/science.1237484. [DOI] [PubMed] [Google Scholar]
- Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, et al. 2008. Lead, mercury, and arsenic in US- and Indian-manufactured ayurvedic medicines sold via the internet. JAMA 300(8):915–923, PMID: 18728265, 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Magaye R, Castranova V, Zhao J. 2013. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10:15, PMID: 23587290, 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Hernandez-Avila M, Téllez-Rojo MM, Mercado A, Hu H. 2002. The relationship between lead in plasma and whole blood in women. Environ Health Perspect 110(3):263–268, PMID: 11882477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleo L, Lovreglio P, Iavicoli S, Antelmi A, Drago I, Basso A, et al. 2008. Significance of urinary arsenic speciation in assessment of seafood ingestion as the main source of organic and inorganic arsenic in a population resident near a coastal area. Chemosphere 73(3):291–299, PMID: 18657289, 10.1016/j.chemosphere.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Tan X, Xia XL, Li SY, Zhang QF. 2015. Water quality characteristics and integrated assessment based on multistep correlation analysis in the Danjiangkou Reservoir, China. J Env Inform 25(1):60–70, 10.3808/jei.201500296. [DOI] [Google Scholar]
- Wade TJ, Xia Y, Mumford J, Wu K, Le XC, Sams E, et al. 2015. Cardiovascular disease and arsenic exposure in Inner Mongolia, China: a case control study. Environ Health 14:35, PMID: 25889926, 10.1186/s12940-015-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Yao P, Li X, He M, Liu Y, et al. 2013. Cohort Profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol 42(3):731–740, PMID: 22531126, 10.1093/ije/dys053. [DOI] [PubMed] [Google Scholar]
- Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, et al. 2004. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr 79(1):80–85, PMID: 14684401. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, et al. 2009. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study. Circulation 120(12):1056–1064, PMID: 19738141, 10.1161/CIRCULATIONAHA.108.827121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand HJ, Ottenwälder H, Bolt HM. 1988. Recent advances in biological monitoring of hexavalent chromium compounds. Sci Total Environ 71(3):309–315, PMID: 3406703. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ewers U, Schulz C. 2004. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int J Hyg Environ Health 207(1):69–73, PMID: 14762976, 10.1078/1438-4639-00260. [DOI] [PubMed] [Google Scholar]
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. 2012. Lifetime risk and years lived free of total cardiovascular disease. JAMA 308(17):1795–1801, PMID: 23117780, 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Jasmine F, Kibriya MG, Liu M, Cheng X, Parvez F, et al. 2015. Interaction between arsenic exposure from drinking water and genetic polymorphisms on cardiovascular disease in Bangladesh: a prospective case-cohort study. Environ Health Perspect 123(5):451–457, PMID: 25575156, 10.1289/ehp.1307883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Rui D, Yan Y, Xu S, Niu Q, Feng G, et al. 2016. Oxidative damage induced by arsenic in mice or rats: a systematic review and meta-analysis. Biol Trace Elem Res 176(1):154–175, PMID: 27498811, 10.1007/s12011-016-0810-4. [DOI] [PubMed] [Google Scholar]
- Xue W, Wang Z, Chen Q, Chen J, Yang H, Xue S. 2010. High selenium status in individuals exposed to arsenic through coal-burning in Shaanxi (PR of China) modulates antioxidant enzymes, heme oxygenase-1 and DNA damage. Clin Chim Acta 411(17–18):1312–1318, PMID: 20478284, 10.1016/j.cca.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, et al. 2007. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol 166(12):1381–1391, PMID: 17875584, 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Xu X, Ye H, Jin L, Zhang X, Zhu Y. 2015. High levels of plasma selenium are associated with metabolic syndrome and elevated fasting plasma glucose in a Chinese population: a case–control study. J Trace Elem Med Biol 32:189–194, PMID: 26302928, 10.1016/j.jtemb.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV. 2010. Environmental health in China: progress towards clean air and safe water. Lancet 375(9720):1110–1119, PMID: 20346817, 10.1016/S0140-6736(10)60062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu C, Guo J, Song Y. 2016. Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials. Eur J Clin Nutr 70(2):162–169, PMID: 25990689, 10.1038/ejcn.2015.78. [DOI] [PubMed] [Google Scholar]
- Zwolak I, Zaporowska H. 2012. Selenium interactions and toxicity:a review. Selenium interactions and toxicity. Cell Biol Toxicol 28(1):31–46, PMID: 21913064, 10.1007/s10565-011-9203-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.