This cohort study evaluates whether overlapping surgery is associated with worsened morbidity and mortality in a large series of neurosurgical cases.
Key Points
Question
Is overlapping surgery associated with increased morbidity and mortality and worsened outcome measures compared with nonoverlapping surgery?
Findings
In this retrospective cohort study that included 2275 neurosurgical cases, no difference between overlapping and nonoverlapping surgery was identified for mortality, morbidity, or worsened functional status at discharge and follow-up.
Meaning
These data suggest that overlapping surgery can be safely done without risking patient safety in a large series of mostly complex neurosurgical cases.
Abstract
Importance
Overlapping surgery (OS) is common. However, there is a dearth of evidence to support or refute the safety of this practice.
Objective
To determine whether OS is associated with worsened morbidity and mortality in a large series of neurosurgical cases.
Design, Setting, and Participants
A retrospective cohort study was completed for patients who underwent neurosurgical procedures at Emory University Hospital, a large academic referral hospital, between January 1, 2014, and December 31, 2015. Patients were operated on for pathologies across the spectrum of neurosurgical disorders. Propensity score weighting and logistic regression models were executed to compare outcomes for patients who received nonoverlapping surgery and OS. Investigators were blinded to study cohorts during data collection and analysis.
Main Outcomes and Measures
The primary outcome measures were 90-day postoperative mortality, morbidity, and functional status.
Results
In this cohort of 2275 patients who underwent neurosurgery, 1259 (55.3%) were female, and the mean (SD) age was 52.1 (16.4) years. A total of 972 surgeries (42.7%) were nonoverlapping while 1303 (57.3%) were overlapping. The distribution of American Society of Anesthesiologists score was similar between nonoverlapping surgery and OS cohorts. Median surgical times were significantly longer for patients in the OS cohort vs the nonoverlapping surgery cohort (in-room time, 219 vs 188 minutes; skin-to-skin time, 141 vs 113 minutes; both P < .001). Overlapping surgery was more frequently elective (93% vs 87%; P < .001). Regression analysis failed to demonstrate an association between OS and complications, such as mortality, morbidity, or worsened functional status. Measures of baseline severity of illness, such as admission to the intensive care unit and increased length of stay, were associated with mortality (intensive care unit: odds ratio [OR], 25.5; 95% CI, 6.22-104.67; length of stay: OR, 1.03; 95% CI, 1.00-1.05), morbidity (intensive care unit: OR, 1.85; 95% CI, 1.43-2.40; length of stay: OR, 1.06; 95% CI, 1.04-1.08), and unfavorable functional status (length of stay: OR, 1.03; 95% CI, 1.02-1.05).
Conclusions and Relevance
These data suggest that OS can be safely performed if appropriate precautions and patient selection are followed. Data such as these will help determine health care policy to maximize patient safety.
Introduction
Surgeons routinely schedule operations for 2 patients such that portions of each procedure are conducted concomitantly. This phenomenon, known as simultaneous, concurrent, or overlapping surgery (OS), is an established practice that the press and Senate Finance Committee have recently questioned. Media and congressional criticism of OS is based on concerns about informed consent, patient safety and education, medical ethics, and hospital billing.
Misconception outside of the medical community regarding OS has driven much of the apprehension surrounding its practice. Until recently, the definition of OS was ambiguous and led to confusion among medical practitioners, patients, and the general population. The American College of Surgeons defines operations as concurrent “when the critical or key components of the procedures for which the primary attending surgeon is responsible are occurring all or in part at the same time” and as overlapping “when the key or critical elements of the first operation have been completed, and … a second operation is started in another operating room while a qualified practitioner performs noncritical components of the first operation.”
While several opinion pieces support OS as safe if appropriate precautions are followed, to our knowledge, a paucity of data exists in this area. The few recently published studies that evaluate the safety of OS stress the need for more widespread analyses from varied institutions and the importance of mid-term and long-term follow-up. Therefore, we systematically analyzed whether OS was associated with mortality, morbidity, and change in functional status in a series of 2275 neurosurgical cases. We hypothesized that OS was not associated with such negative outcomes.
Methods
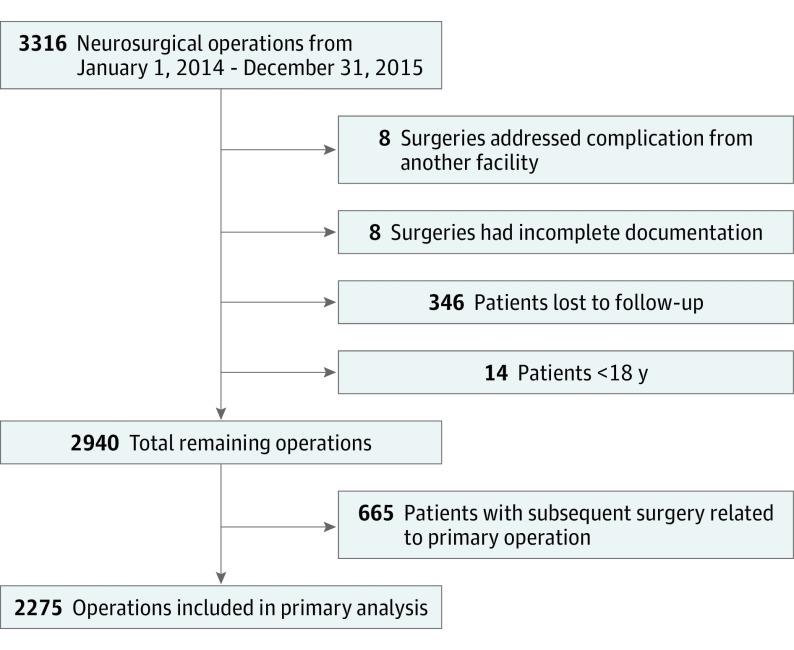
All neurosurgical operations (n = 3316) performed at Emory University Hospital from January 1, 2014, through December 31, 2015, were retrospectively identified from a centralized surgical database. Patients were excluded if surgery addressed a complication from a referring institution (n = 8), documentation was incomplete (n = 8), the patient was younger than 18 years (n = 14), or the patient was lost to follow-up at 90 days (n = 346). Of the remaining 2940 surgeries, 1103 (37.5%) were from 438 patients who underwent more than 1 operation during the study period (range, 2-12). To minimize confounding of outcomes by subsequent surgeries, only the first surgery from each unique patient was used for primary analysis. Therefore, an additional 665 surgeries were removed from the initial analysis. After exclusion, 2275 surgeries were analyzed (Figure 1). The Emory University Institutional Review Board approved this study, and informed consent was waived because data were deidentified.
Figure 1. Flow Diagram Depicting Inclusion and Exclusion Criteria for Study Cohort.
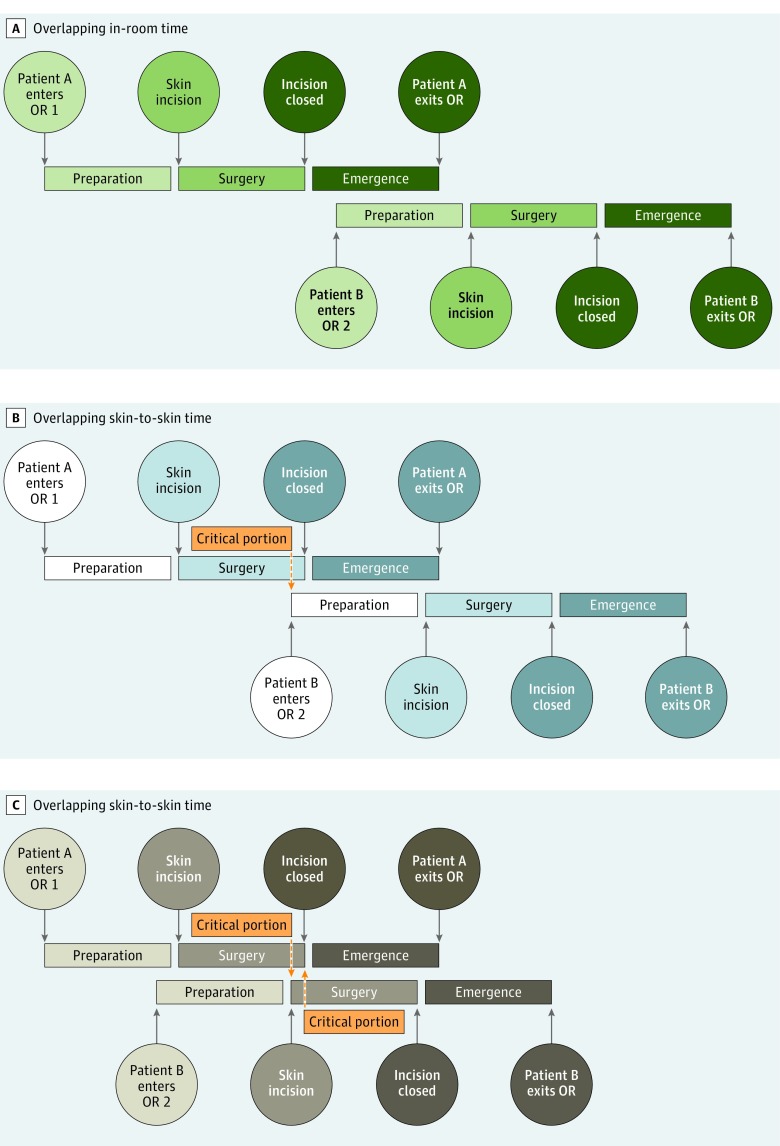
Two main study groups were defined. Nonoverlapping surgery (NOS) was defined as surgery without any portion of the operation occurring concomitantly with another operation performed by the same attending surgeon. Overlapping surgery was defined as an operation for which any portion of the surgery, from the time the patient entered until the patient exited the operating room, overlapped with another operation for which the same surgeon was responsible. Overlapping surgery was further divided into 2 subgroups: overlapping in-room time (OS-R), when the preparation phase of surgery for a second patient occurred simultaneously with the emergence phase of surgery for the first patient, and overlapping skin-to-skin time (OS-StS), when any portion of the operative time ran concomitantly with that of another patient operated on by a single surgeon. In accordance with departmental policy, the critical portion of the surgery did not overlap with that of another case performed by an individual surgeon in any of the OS-StS cases (Figure 2).
Figure 2. Schematic Representation of Various Permutations of Overlapping Surgery.
A, Two patients in the overlapping in-room time cohort, in which emergence from anesthesia for patient A is concomitant with the preparatory phase of surgery for patient B. The operative, or skin-to-skin, portion of surgery does not overlap for either patient. B, The first scenario for patients in the overlapping skin-to-skin time cohort. As the surgical portion of the case is ending for patient A, patient B enters a separate operating room (OR) for the preparatory phase of surgery. Patients similar to patient A were included in the overlapping skin-to-skin time cohort for subgroup analysis, while those like patient B were included in the overlapping in-room time group. C, The second scenario for patients in the overlapping skin-to-skin time cohort. As the surgical portion of the case is ending for patient A, patient B enters the surgical phase of surgery. Note that although the surgical portions of the operations overlap, the critical portions do not.
Demographic data and parameters of surgery were extracted from the electronic surgical management system. All relevant information regarding baseline health status, hospital course, and follow-up within the 90-day global period was collected. Indicators were created for having multiple, related surgeries, unplanned return to the operating room or need for an invasive procedure (eg, external ventricular drain placement) within 90 days, unplanned admission within 30 days after surgery, and surgical site infection. Data were extracted by 5 investigators who were blinded to study cohorts during data collection and analysis. The primary investigator (B.M.H.) independently scored the modified Rankin Scale (mRS) for 50 patients per extractor to assure consistency (concordance, 189 of 200 [94.5%]).
Primary outcomes were 90-day mortality, morbidity, and functional status as measured by the mRS at baseline and at 90-day follow-up (dichotomized as “good” for scores ≤2 or as “unchanged poor or worse” for scores ≥3 that remained unchanged from baseline or for patients whose mRS score worsened between baseline and follow-up). Clinical measures evaluated were skin-to-skin time, total operating room time, unexpected intensive care unit (ICU) admission, ICU length of stay (LOS), total LOS, discharge location (ie, home, acute rehabilitation facility, or other), baseline mRS score (dichotomized into ≤2 vs ≥3), American Society of Anesthesiologists (ASA) score (considered as an ordinal variable and dichotomized into low to standard risk [≤2] vs higher risk [≥3]), and unexpected 30-day readmission. Morbidity was graded using the Clavien-Dindo scale and dichotomized into none vs any (Clavien-Dindo score, ≥1). Patients who died were excluded from morbidity and functional status outcomes.
Data were analyzed with SAS version 9.4 (SAS Institute). Normally distributed, continuous variables are reported as mean and standard deviation. Nonparametric continuous data are presented as median and first and third quartiles (Q1, Q3). t Tests, Wilcoxon rank sum, and χ2 tests were used to compare demographic and clinical data for bivariate analyses as appropriate.
Propensity score (PS) weighting adjusted for observed significant differences between the NOS and OS groups on surgery category, primary surgeon, and other baseline factors assessed prior to the primary surgeon’s NOS/OS decision. The amount of variation in outcomes attributable to individual surgeons was assessed by intraclass correlation coefficient (0.0405, 0.0718, and 0.0975 for mortality, morbidity, and change in functional status, respectively). An intraclass correlation coefficient greater than 0.2 suggests correlation between the outcomes of interest by cluster (primary surgeon) that should be accounted for in additional analyses. Because the threshold was not met, independence between primary surgeon and outcomes was assumed.
A logistic regression model with NOS/OS as the dependent variable was fit with primary surgeon, surgery category, ASA score, dichotomized baseline mRS score, whether a cosurgeon was present, sex, planned ambulatory surgery status (outpatient vs admitted), age at surgery, and case urgency (elective vs emergent/urgent) as independent variables. The predicted probabilities of OS from the PS model (ie, the PS score) were used to create average treatment effect for treated (ATT) weights. Improvement in the distribution of baseline covariates by NOS/OS group after ATT PS weighting was confirmed (Table 1).
Table 1. Demographic and Summary Data for All Patients and by Study Group.
| Variable | No. (%) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Overall | Nonoverlapping Surgery | Overlapping Surgery | |||||
| Unweighted (n = 2275) | Weighted (n = 2607) | Unweighted (n = 972) | Weighted (n = 1306) | Unweighted (n = 1303) | Unweighted | Weighted | |
| Type of overlap | |||||||
| No overlap | 972 (42.7) | 1307 (50.1) | 972 (100) | 1306 (100) | 0 | NA | NA |
| In-room overlap | 231 (10.2) | 231 (8.9) | 0 | 0 | 231 (17.7) | NA | NA |
| Skin-to-skin overlap | 1072 (47.1) | 1072 (41.1) | 0 | 0 | 1072 (82.3) | NA | NA |
| Patient characteristics | |||||||
| Sex | .005 | .76 | |||||
| Female | 1259 (55.3) | 1518 (57.9) | 505 (52.0) | 764 (58.5) | 754 (57.9) | ||
| Male | 1016 (4.7) | 1092 (41.9) | 467 (48.0) | 543 (41.6) | 549 (42.1) | ||
| Age, mean (SD), y | 52.1 (16.4) | 52 (17.5) | 51.9 (16.6) | 51.9 (19.0) | 52.2 (52.2) | .69 | .66 |
| ASA score | .07 | .51 | |||||
| 1-2 | 685 (30.1) | 841 (32.3) | 273 (28.1) | 429 (32.9) | 412 (31.6) | ||
| ≥3 | 1590 (70.0) | 1769 (67.9) | 699 (71.9) | 878 (67.2) | 891 (68.4) | ||
| mRS score at baseline | <.001 | .95 | |||||
| 0-2 | 1768 (77.7) | 2115 (81.1) | 713 (73.4) | 1060 (81.2) | 1055 (81.0) | ||
| 3-5 | 506 (22.2) | 496 (19.0) | 258 (26.5) | 248 (19.0) | 248 (19.0) | ||
| Surgery characteristics | |||||||
| Surgical staff | |||||||
| Cosurgeon present | 451 (19.8) | 627 (24.1) | 148 (15.2) | 324 (24.8) | 303 (23.3) | <.001 | .38 |
| Fellow present | 439 (19.3) | 473 (18.1) | 225 (23.1) | 258 (19.8) | 214 (16.4) | <.001 | .03 |
| Resident present | 2080 (91.4) | 2372 (91.0) | 896 (92.2) | 1188 (91.0) | 1184 (91.0) | .27 | .96 |
| Urgency | <.001 | .97 | |||||
| Elective | 2054 (90.3) | 2427 (93.1) | 842 (86.6) | 1215 (93.0) | 1212 (93.0) | ||
| Emergent/urgent | 221 (9.7) | 182 (7.0) | 130 (13.4) | 91 (7.0) | 91 (7.0) | ||
| Admission status | .07 | .91 | |||||
| Day surgery | 350 (15.4) | 435 (16.7) | 134 (13.8) | 219 (16.8) | 216 (16.6) | ||
| Admitted | 1925 (84.6) | 2175 (83.4) | 838 (86.2) | 1088 (83.3) | 1087 (83.4) | ||
| Surgery category | <.001 | .98 | |||||
| CSF diversion | 188 (8.3) | 156 (6.0) | 109 (11.2) | 77 (6.0) | 79 (6.1) | ||
| Functional | 527 (23.2) | 601 (22.9) | 222 (22.8) | 296 (22.7) | 305 (23.4) | ||
| Other | 151 (6.6) | 144 (5.6) | 81 (8.3) | 73 (5.7) | 70 (5.4) | ||
| Peripheral nerve | 233 (10.2) | 293 (11.2) | 87 (9.0) | 147 (11.1) | 146 (11.2) | ||
| Spine | 71 (3.1) | 87 (3.3) | 27 (2.8) | 43 (3.3) | 44 (3.4) | ||
| Tumor | 813 (35.7) | 977 (37.6) | 334 (34.4) | 498 (38.4) | 479 (36.8) | ||
| Vascular | 292 (12.8) | 354 (13.5) | 112 (11.5) | 174 (13.2) | 180 (13.8) | ||
Abbreviations: ASA, American Society of Anesthesiologists; CSF, cerebrospinal fluid; mRS, modified Rankin Scale; NA, not applicable.
Bivariate analyses were computed both before and after ATT PS weighting for study group (NOS/OS) comparisons. Multivariable logistic regression models were used to evaluate the association between each outcome (mortality, morbidity, and change in function) and study group. Double robust PS methods were used so that observations were weighted by ATT PS weight, and the model also included logit(PS score) as a covariate. Backward selection (P = .20) was used to find the most parsimonious model after forcing study group and logit(PS score). Firth penalized likelihood method was implemented when independent variable cell sizes were zero or close to zero. Evaluated independent variables included total in-room time and skin-to-skin time, presence of a resident, dichotomized resident level, presence of a fellow, postoperative ICU admission, ICU LOS, overall LOS, surgical site infection, unplanned readmission within 30 days, multiple dependent surgeries, and disposition. Adjusted odds ratios (ORs) with 95% CIs are reported for each model. Model fit was assessed. Subanalyses assessed the association between subtypes of OS (OS-R vs OS-StS) with each of the 3 outcomes using the same logistic regression methods described above. A bootstrap was performed using 10 000 replicate samples of the original data set to refit the mortality model for each sample to get an estimate of the effect of OS. Significance was set at P < .05, and all P values were 2-tailed.
Results
Baseline demographic data are presented in Table 1, both before and after PS weighting was applied to the NOS group. The cohort included 1259 female patients (55.3%), and the mean (SD) age was 52.1 (16.4) years. Of 2275 operations, 972 (42.7%) were NOS while 1303 (57.3%) were OS (1072 OS-StS [82.3%]; 231 OS-R [17.7%]). Most patients underwent elective surgery (2054 [90.3%]) and had higher ASA scores (1590 [69.9%]), and there was a relatively high proportion of patients with poor baseline mRS score (≥3; 506 [22.2%]). Significant differences were observed between NOS and OS groups at baseline on sex, surgery category, case urgency, cosurgeon presence, and mRS score at baseline. After ATT PS weighting, no significant differences were observed on the baseline covariates.
Clinical data assessed during and after surgery are presented in Table 2. Residents were present in 2080 surgeries (91.4%; 1338 [58.8%] covered by upper-level residents), while fellows were present in 439 surgeries (19.3%). A cosurgeon was present in 451 surgeries (19.8%). Median in-room time was 206 minutes, and median skin-to-skin time was 130 minutes. Median overall LOS was 3 days. After surgery, 979 patients (43.0%) went to the ICU, 95 (4.2%) had a surgical site infection, 210 (9.2%) had an unplanned readmission within 30 days, 158 (6.9%) had an unexpected return to the operating room or invasive procedure, and 108 (4.7%) had subsequent related/dependent surgeries. Of the patients with more than 1 surgery, 330 (75.3%) had subsequent surgeries that were not a result of a complication from their first operation, while 108 (24.7%) had more than 1 operation related to the first (range, 2-5). Significant differences were observed between the NOS and OS groups on presence of a fellow, in-room and skin-to-skin operating times, unexpected return to the operating room, related/dependent surgeries, admission to the ICU, disposition, overall LOS, and ICU LOS. After ATT PS weighting, significant differences were still observed on in-room and skin-to-skin operating times and presence of a fellow. eTables 1 and 2 in the Supplement describe baseline characteristics and outcomes for OS subgroups (OS-R and OS-StS).
Table 2. Surgical and Outcomes Data Presented by Study Group.
| Variable | No. (%) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Overall | Nonoverlapping | Overlapping | |||||
| Unweighted (n = 2275) | Weighted (n = 2610) | Unweighted (n = 972) | Weighted (n = 1307) | Unweighted (n = 1303) | Unweighted | Weighted | |
| Operative time, median (Q1-Q3), min | |||||||
| In-room overlap | 206 (135-292) | 211 (138-291) | 188 (125-284) | 203 (129-286) | 219 (146-296) | <.001 | <.001 |
| Skin-to-skin overlap | 130 (65-196) | 135 (67-198) | 113 (57-187) | 129 (61-193) | 141 (71-202) | <.001 | .004 |
| Length of stay, median (Q1-Q3), d | |||||||
| ICU LOS | 1 (1-5) | 1 (1-4) | 2 (1-8) | 1 (1-5) | 1 (1-3) | <.001 | .05 |
| Overall LOS | 3 (1-6) | 3 (1-6) | 3 (1-8) | 3 (1-6) | 3 (1-6) | <.001 | .20 |
| Discharge location | <.001 | .47 | |||||
| Good disposition (home or AR) | 2113 (92.8) | 2486 (95.2) | 876 (90.1) | 1249 (95.6) | 1237 (95.0) | ||
| Poor disposition | 162 (7.1) | 125 (4.8) | 96 (10.0) | 58 (4.4) | 66 (5.1) | ||
| Mortality | 53 (2.3) | 38 (1.5) | 31 (3.2) | 16 (1.2) | 22 (1.7) | .02 | .25 |
| mRS score at discharge | .02 | .31 | |||||
| 0-2 | 1654 (72.7) | 1926 (73.8) | 681 (70.1) | 953 (72.9) | 973 (74.7) | ||
| 3-5 | 621 (27.3) | 684 (26.2) | 291 (29.9) | 354 (27.1) | 330 (25.3) | ||
| mRS score at follow-up vs baseline | .47 | .14 | |||||
| 0-2 | 1762 (79.3) | 2024 (78.6) | 739 (78.6) | 1001 (77.4) | 1023 (80.0) | ||
| Unchanged poor (3-5) or worse | 459 (20.7) | 550 (21.4) | 201 (21.4) | 292 (22.6) | 258 (20.0) | ||
| Morbidity within 90 d | .19 | .08 | |||||
| No | 1898 (85.4) | 2188 (85.0) | 793 (84.3) | 1083 (83.8) | 1105 (86.3) | ||
| Yes | 324 (14.6) | 386 (15.0) | 148 (15.7) | 210 (16.2) | 176 (13.7) | ||
| Severity of morbidity | .84 | .14 | |||||
| Clavien-Dindo score of 1 or 2 | 112 (34.6) | 147 (38.1) | 52 (35.1) | 87 (41.4) | 60 (34.1) | ||
| Clavien-Dindo score of 3 or 4 | 212 (65.4) | 239 (61.9) | 96 (64.9) | 123 (58.6) | 116 (65.9) | ||
| 30-d Readmission | .32 | .30 | |||||
| No | 2064 (90.7) | 2366 (91.0) | 875 (90.0) | 1177 (90.1) | 1189 (91.3) | ||
| Yes | 211 (9.3) | 244 (9.0) | 97 (10.0) | 130 (9.9) | 114 (8.7) | ||
| Unexpected return to OR or invasive procedure within 90 d | .006 | .06 | |||||
| No | 2117 (93.1) | 2437 (93.4) | 888 (91.4) | 1208 (92.4) | 1229 (94.3) | ||
| Yes | 158 (6.9) | 173 (6.6) | 84 (8.6) | 99 (7.6) | 74 (5.7) | ||
| Surgical site infection | .47 | .87 | |||||
| No | 2180 (95.8) | 2506 (96.0) | 928 (95.5) | 1254 (95.9) | 1252 (96.1) | ||
| Yes | 95 (4.2) | 104 (4.0) | 44 (4.5) | 53 (4.1) | 51 (3.9) | ||
| Multiple related surgeries | .002 | .23 | |||||
| No | 2167 (95.3) | 2505 (96.0) | 910 (93.6) | 1248 (95.5) | 1257 (96.5) | ||
| Yes | 108 (4.7) | 105 (4.0) | 62 (6.4) | 59 (4.5) | 46 (3.5) | ||
Abbreviations: AR, acute rehabilitation facility; ICU, intensive care unit; LOS, length of stay; mRS, modified Rankin Scale; OR, operating room; Q1, first quartile; Q3, third quartile.
As described in Table 2, 53 deaths were observed during the follow-up period. Of the remaining 2222 patients, 324 (14.6%) experienced morbidity and 459 (20.7%) experienced the same poor or worse mRS score at follow-up compared with baseline. Of those who suffered morbidity, 212 (65.4%) were severe (Clavien-Dindo score ≥3).
eTable 3 in the Supplement shows bivariate analysis results for mortality, morbidity, and change in function. Patients who died were almost uniformly admitted to the ICU (n = 51 [96%]), had a poor disposition (n = 52 [98%]), and had a poor mRS score at discharge (n = 52 [98%]). Compared with OS, NOS was associated with mortality (n = 31 vs 22; P = .02) but not morbidity (n = 148 vs 176; P = .19) or change in function (n = 201 vs 258; P = .47).
Table 3 depicts multivariate model results for mortality. After adjusting for relevant covariates, mortality and study group were not associated (OS vs NOS: OR, 1.62; 95% CI, 0.84-3.14). Similarly, the observed 95% CI for the OR of OS/NOS from the 10 000 bootstrap samples was 0.81 to 3.38. Longer overall LOS (OR, 1.03; 95% CI, 1.00-1.05; P = .02) and admission to the ICU (OR, 25.5; 95% CI, 6.22-104.67; P < .001) were significantly associated with increased odds of mortality. Table 3 also demonstrates multivariate model results for morbidity. After adjusting for relevant covariates, morbidity and study group were not associated (OS vs NOS: OR, 0.86; 95% CI, 0.68-1.08). Presence of morbidity was inversely associated with discharge mRS score and associated with ICU admission, unplanned readmission, multiple dependent surgeries, longer total in-room time, and overall LOS. Table 3 also describes multivariate model results for change in function. After adjusting for relevant covariates, change in function and study group were not associated (OS vs NOS: OR, 0.87; 95% CI, 0.71-1.06). Worsening change in function was associated with having a resident present, poor discharge disposition, and increasing overall LOS (Table 3).
Table 3. Multivariable Logistic Regression (MLR) for Covariates Associated With Mortality, Morbidity, and Unchanged Poor or Worse Modified Rankin Scale (mRS) Score at 90 Days vs Baselinea.
| Variable | Estimate (SE) | OR (95% CI) | P Value |
|---|---|---|---|
| MLR for covariates associated with mortality | |||
| All surgeries | |||
| OS (reference, NOS) | 0.48 (0.34) | 1.62 (0.84-3.14) | .15 |
| Admitted to ICU (reference, no) | 3.24 (0.72) | 25.5 (6.22-104.67) | <.001 |
| Overall LOS, d | 0.03 (0.01) | 1.03 (1.00-1.05) | .02 |
| OS only | |||
| OS-StS (reference, OS-R) | 0.74 (0.72) | 2.09 (0.51-8.54) | .30 |
| Admitted to ICU (reference, no) | 3.02 (0.84) | 20.55 (3.94-107.20) | <.001 |
| Overall LOS, d | 0.04 (0.01) | 1.04 (1.01-1.07) | .01 |
| MLR for covariates associated with morbidity | |||
| All surgeries | |||
| OS (reference, NOS) | −0.16 (0.12) | 0.86 (0.68-1.08) | .59 |
| Discharge mRS score 0-2 (reference, 3-5) | −0.56 (0.13) | 0.57 (0.44-0.74) | <.001 |
| Admitted to ICU (reference, no) | 0.62 (0.13) | 1.85 (1.43-2.40) | <.001 |
| 30-d Readmission (reference, no) | 4.02 (0.19) | 55.70 (38.12-81.37) | <.001 |
| Multiple related surgeries (reference, no) | 0.93 (0.26) | 2.53 (1.54-4.18) | <.001 |
| Total in-room time, min | 0.002 (0.00) | 1.00 (1.00-1.00) | <.001 |
| Overall LOS, d | 0.06 (0.01) | 1.06 (1.04-1.08) | <.001 |
| OS only | |||
| OS-StS (reference, OS-R) | 0.36 (0.32) | 1.43 (0.76-2.68) | .26 |
| Discharge mRS score 0-2 (reference, 3-5) | −1.37 (0.24) | 0.25 (0.16-0.41) | <.001 |
| Admitted to ICU (reference, no) | 0.60 (0.24) | 1.82 (1.13-2.91) | .01 |
| 30-d Readmission (reference, no) | 4.26 (0.29) | 70.80 (40.08-125.09) | <.001 |
| Multiple related surgeries (reference, no) | 1.84 (0.43) | 6.27 (2.70-14.59) | <.001 |
| Total in-room time, min | 0.00 (0.00) | 1.00 (1.00-1.00) | .04 |
| Overall LOS, d | 0.07 (0.02) | 1.07 (1.04-1.11) | <.001 |
| MLR for covariates associated with unchanged poor (3-5) or worse mRS score at 90 d vs baseline | |||
| All cases | |||
| OS (reference, NOS) | −0.15 (0.10) | 0.87 (0.71-1.06) | .15 |
| Resident present (reference, no) | 0.69 (0.22) | 2.06 (1.35-3.13) | .001 |
| Good disposition (reference, bad) | −2.16 (0.28) | 0.12 (0.07-0.20) | <.001 |
| Overall LOS, d | 0.03 (0.01) | 1.03 (1.02-1.05) | <.001 |
| OS only | |||
| OS-StS (reference, OS-R) | −0.25 (0.18) | 0.78 (0.55-1.12) | .17 |
| Resident present (reference, no) | 0.70 (0.32) | 2.02 (1.09-3.74) | .03 |
| Good disposition (reference, bad) | −2.30 (0.38) | 0.10 (0.05-0.21) | <.001 |
| SSI (reference, no) | 0.70 (0.32) | 2.01 (1.07-3.80) | .03 |
Abbreviations: ICU, intensive care unit; LOS, length of stay; NOS, nonoverlapping surgery; OR, odds ratio; OS, overlapping surgery; OS-R, overlapping in-room time; OS-StS, overlapping skin-to-skin time; SSI, surgical site infection.
Model included logit(propensity score) and used average treatment effect for treated weights.
Because outcomes among patients who underwent more than 1 surgery could be influenced by repeated operations, sensitivity analyses were also performed. The final models for each outcome were rerun among patients with only a single surgery. The adjusted results for all outcomes were similar (eTable 4 in the Supplement). Mortality was not associated with study group (OS vs NOS: OR, 1.64; 95% CI, 0.83-3.30). Morbidity was not associated with study group (OS vs NOS: OR, 0.81; 95% CI, 0.64-1.04). Change in function was not associated with study group (OS vs NOS: OR, 0.86; 95% CI, 0.71-1.06).
Table 3 describes multivariate analysis results for association of type of overlap (OS-StS vs OS-R) with each outcome. Type of overlap was not associated with mortality (OS-StS vs OS-R: OR, 2.09; 95% CI, 0.51-8.54), morbidity (OS-StS vs OS-R: OR, 1.43; 95% CI, 0.76-2.68), or change in function (OS-StS vs OS-R: OR, 0.78; 95% CI, 0.55-1.12).
Discussion
Surgeons, particularly in academic and tertiary care settings, often conduct operations on separate patients, portions of which overlap. In a 2017 high-profile malpractice suit, the overlapping nature of surgery was determined not have had a role in the patient’s complication, but the case’s details brought widespread criticism of the practice by the lay press. This case served as the basis for an inquiry by the Senate Finance Committee and, rightfully, evoked self-scrutiny by the medical community. In the wake of the media spotlight, several opinion pieces published in prominent journals defended the purported advantages of OS, but nearly all of these articles emphasized the need for systematic and widespread evaluation of safety and outcomes, given the near absolute lack of evidence to support the practice. To our knowledge, only 4 studies have been published to date in which outcomes for overlapping or concurrent surgery have been evaluated. In each, OS was not associated with increased risk of complication, but none of the studies examined outcomes beyond 30 days. In this study, complications and outcomes were compared between large cohorts of neurosurgical patients who underwent NOS and OS through the 90-day postoperative global period. Consequently, this study not only adds meaningfully to the small but growing body of literature regarding the safety of OS but also is the first to examine outcomes and complications over the protracted postoperative period. Moreover, to our knowledge, the study presented herein is the first to evaluate the subset of procedures for which the operative, or skin-to-skin, time was overlapping as a distinct study group.
The analysis included diverse metrics to assess functional outcomes, complications, and resource use for cases involving NOS and OS. Regression modeling failed to demonstrate any statistically significant difference between the OS-R and OS-StS cohorts and the NOS group for mortality, overall and severe morbidity (including major organ system failure), ICU LOS, overall LOS, discharge location, functional status at discharge and within the 90-day global period, unexpected readmission within 30 days, and unplanned return to the operating room or invasive neurosurgical procedure within 90 days. In sum, these data suggest that OS can be safely performed without risking patient safety in a large series of mostly complex neurosurgical cases.
Instead, proxy measures for baseline severity of illness, such as being admitted to the ICU and overall LOS, were associated with mortality, overall morbidity, and unfavorable functional status. Not unexpectedly, 30-day readmission was strongly associated with morbidity, as was having to undergo an unexpected subsequent operation. Age was independently assessed for association with outcomes because it was accounted for in the propensity score model. These data are generally consistent with previous reports.
A commonly cited concern is that OS may lead to increased anesthesia and operative time for patients, which, in turn, may lead to complications, such as respiratory failure and wound infections. Both OS-R and OS-StS cases were significantly longer than NOS cases in this study. Longer in-room time was associated with morbidity, but not mortality or follow-up mRS score. More importantly, skin-to-skin time was not associated with mortality, morbidity, or worsened functional status. An article published in 2016 in which outcomes were compared between NOS and OS cases demonstrated that longer surgical time was associated with complications, both minor and severe. Despite longer total in-room and skin-to-skin time in the OS-R and OS-StS cohorts, SSI and major morbidity were no different between the NOS and OS groups, consistent with a previous report.
Limitations
This study has limitations. Most prominently, the analysis was retrospective. Although PS weighting mitigated bias to the best extent possible, to completely account for unrecognized influences and associations that may affect adverse events is impossible. Propensity score weighting eliminated the differences in baseline characteristics between the NOS and OS groups for factors chosen prior to the decision to schedule surgery as overlapping or not; however, differences in presence of a fellow and operative and in-room time remained after weighting. Moreover, that surgery was completed solely at a single academic neurosurgical referral center may limit generalizability of the results. However, analyses from centers and practices of varying types are of crucial importance to develop robust and substantive literature that ultimately supports or refutes the safety of OS. Interrater reliability for post hoc mRS score extraction was not formally evaluated. In addition, 346 patients (15.2%) were lost to follow-up, and while we cannot be certain of the fate of those patients, follow-up was equally likely in each of the 3 study groups in the regression analysis, tempering bias.
Perhaps the most important partiality is that of the surgeon. The American College of Surgeons, multiple editorials, and organizational position statements emphasize that the responsibility to safely conduct and oversee OS rests entirely with the attending surgeon. Surgeons must use their experience, keen intuition, and respect for their own ability and limitations to carefully select patients for OS. Patients who underwent OS in 2 publications in which NOS and OS were compared were healthier at baseline, while patients in our OS-StS cohort were more likely to have a baseline ASA score of 1 or 2, although this was not statistically significant (P = .07). Cases of OS-R and OS-StS were more likely to involve a cosurgeon, and OS was more likely to be ambulatory surgery. Although difficult to prove, these data suggest that surgeons effectively manage their operating room schedules such that these factors combine to make OS safe and effective.
Conclusions
These data suggest that overlapping neurosurgery is safe and has the potential to benefit patients by maximizing efficiency and making highly sought-after specialists available to a greater number of patients. By carefully training the next generation of surgeons through a system of supervision and progressive autonomy, OS may also be a key factor in maintaining a competent surgical workforce for the future. Data such as these will help determine health care standards and policies that have the potential to broadly affect the delivery of surgical care in the United States. An evidence-based approach to policy surrounding OS is crucial not only to maximize patient safety but also to make highly specialized surgical care available to as many patients as possible.
eTable 1. Demographic and summary data by subgroup of overlapping surgery.
eTable 2. Surgical and outcomes data presented by subgroup of overlapping surgery.
eTable 3. Bivariate analysis for factors associated with mortality, morbidity, and functional status.
eTable 4. Multivariable logistic regression for covariates associated with mortality, morbidity, and unchanged poor (3-5) or worse mRS score at 90 days for patients who underwent only 1 operation.
References
- 1.Senate Finance Committee Concurrent and overlapping surgeries: additional measures warranted. https://www.finance.senate.gov/imo/media/doc/Concurrent%20Surgeries%20Report%20FINAL%20.pdf. Accessed January 15, 2017.
- 2.American College of Surgeons Statements on principles. https://www.facs.org/about-acs/statements/stonprin. Accessed February 9, 2017.
- 3.Abelson J, Saltzman J Surgeons urged to better govern dual bookings. Boston Globe April 14, 2016. http://edition.pagesuite.com/popovers/article_popover.aspx?guid=79b35938-3cca-42ef-b84b-d9d16b8b2364. Accessed January 15, 2017.
- 4.Abelson J, Saltzman J Surgeons must tell patients of double-booked surgeries, new guidelines say. Boston Globe April 13, 2016. https://www.bostonglobe.com/metro/2016/04/13/surgery/Jn7Lb0Hq3VUGeZGBgjiw0M/story.html. Accessed January 15, 2017.
- 5.Abelson J, Saltzman J, Kowalczyk L Clash in the name of care. Boston Globe October 25, 2015:A1, A8.
- 6.Abelson J, Saltzman J, Kowalczyk L A new wave of scrutiny for simultaneous surgeries. Boston Globe December 20, 2015:A1, A20.
- 7.Twedt S. Senate committee looks at policies on surgeons performing more than one surgery at once. Pittsburgh Post-Gazette March 28, 2016. http://www.post-gazette.com/business/healthcare-business/2016/03/28/Senate-committtee-looks-at-hospital-policies-regarding-concurrent-surgeries/stories/201603270074. Accessed January 15, 2017.
- 8.Langerman A. Concurrent surgery and informed consent. JAMA Surg. 2016;151(7):601-602. [DOI] [PubMed] [Google Scholar]
- 9.Mello MM, Livingston EH. Managing the risks of concurrent surgeries. JAMA. 2016;315(15):1563-1564. [DOI] [PubMed] [Google Scholar]
- 10.Hyder JA, Hanson KT, Storlie CB, et al. Safety of overlapping surgery at a high-volume referral center. Ann Surg. 2017;265(4):639-644. [DOI] [PubMed] [Google Scholar]
- 11.American Association of Neurological Surgeons; American Board of Neurological Surgery; Congress of Neurological Surgeons; Society of Neurological Surgeons Position statement on intraoperative responsibility of the primary neurosurgeon. http://www.aans.org/pdf/Legislative/Neurosurgery%20Position%20Statement%20on%20Overlapping%20Surgery%20FINAL.pdf. Accessed February 9, 2017.
- 12.Hoyt DB. Overlapping surgery: safety data and ongoing concerns. Ann Surg. 2017;265(4):645-646. [DOI] [PubMed] [Google Scholar]
- 13.Guan J, Brock AA, Karsy M, et al. Managing overlapping surgery: an analysis of 1018 neurosurgical and spine cases [published online December 2, 2016]. J Neurosurg. [DOI] [PubMed] [Google Scholar]
- 14.Zhang AL, Sing DC, Dang DY, et al. Overlapping surgery in the ambulatory orthopaedic setting. J Bone Joint Surg Am. 2016;98(22):1859-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zygourakis CC, Keefe M, Lee J, et al. Comparison of patient outcomes in 3725 overlapping vs 3633 nonoverlapping neurosurgical procedures using a single institution’s clinical and administrative database. Neurosurgery. 2017;80(2):257-268. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saltzman J. Double surgery did not cause man's quadriplegia, jury finds. Boston Globe January 30, 2017. https://www.bostonglobe.com/metro/2017/01/30/surgeon-failed-inform-patient-about-double-booked-surgery-jury-finds/xzWz0hrRNCDea0vYetugzK/story.html. Accessed February 9, 2017.
- 18.Saltzman J, Abelson J, Kowalczyk L MGH files sought in federal inquiry. Boston Globe November 15, 2015:A1, A15.
- 19.Beasley GM, Pappas TN, Kirk AD. Procedure delegation by attending surgeons performing concurrent operations in academic medical centers: balancing safety and efficiency. Ann Surg. 2015;261(6):1044-1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Demographic and summary data by subgroup of overlapping surgery.
eTable 2. Surgical and outcomes data presented by subgroup of overlapping surgery.
eTable 3. Bivariate analysis for factors associated with mortality, morbidity, and functional status.
eTable 4. Multivariable logistic regression for covariates associated with mortality, morbidity, and unchanged poor (3-5) or worse mRS score at 90 days for patients who underwent only 1 operation.