This longitudinal study examines the association of age, β-amyloid levels, and the apolipoprotein E ε4 allele with memory decline in a large group of cognitively healthy older adults enrolled in the Australian Imaging, Biomarkers, and Lifestyle study.
Abstract
Importance
Older age, high levels of β-amyloid (Aβ), and the presence of the apolipoprotein E (APOE) ε4 allele are risk factors for Alzheimer disease (AD). However, the extent to which increasing age, Aβ, and ε4 are associated with memory decline remains unclear, and the age at which memory decline begins for Aβ-positive ε4 carriers and noncarriers has not been determined.
Objective
To determine the association of age, Aβ level, and APOE ε4 with memory decline in a large group of cognitively healthy older adults.
Design, Setting, and Participants
This longitudinal observational study included cognitively healthy older adults (age >60 years) enrolled in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study from March 31, 2006, through March 31, 2017; of 1583 individuals enrolled, 1136 refused or were excluded owing to other criteria (eg, having mild cognitive impairment or AD). Participants underwent Aβ imaging in research clinics in Perth and Melbourne and more than 72 months of follow-up (at 18-month intervals). The association of age with memory was fitted to a quadratic model. Age was treated as a continuous, time-dependent variable.
Exposures
β-Amyloid imaging using positron emission tomography, genotyping for APOE ɛ4, and longitudinal neuropsychological assessments of episodic memory during the 72-month follow-up.
Main Outcomes and Measures
Episodic memory composite score.
Results
Of the 447 participants, 203 (45.4%) were men and 244 (54.6%) were women; mean (SD) age was 72.5 (6.6) years. Equal proportions of female participants were observed in each Aβ-ɛ4 group (24 of 51 Aβ-positive ε4 noncarriers [47.1%] ; 35 of 64 Aβ-negative ε4 carriers [54.7%]; 40 of 72 Aβ-positive ε4 carriers [55.6%]; and 145 of 260 Aβ-negative ε4 noncarriers [55.8%]). Adults with Aβ findings (mean [SD] age, 74.4 [6.8] years) were approximately 4 years older than those negative for Aβ (mean [SD] age, 69.8 [6.1] years). Memory decline diverged significantly from Aβ-negative ɛ4 noncarriers at an earlier age in Aβ-positive ɛ4 carriers (64.5 years) than in Aβ-positive ɛ4 noncarriers (76.5 years), such that by 85 years of age, Aβ-positive ε4 carriers performed approximately 1.5 SD units worse on the episodic memory composite than Aβ-negative ε4 noncarriers and approximately 0.8 SD units worse than Aβ-positive ε4 noncarriers. Memory performance of Aβ-negative ɛ4 carriers did not differ from that of the Aβ-negative ɛ4 noncarriers (estimate [SE], 0.001 [0.001]; t = 0.526; P = .77).
Conclusions and Relevance
Prior work has shown that Aβ and ε4 combine to influence memory decline in nondemented older adults. Results of this study indicate that increasing age may further exacerbate these effects. The estimates provided may be used to determine the risk of memory decline associated with Aβ and ε4 at each age.
Key Points
Question
What is the association of β-amyloid and the presence of the apolipoprotein E (APOE) ε4 with memory decline with increasing age?
Findings
In this longitudinal study of 447 cognitively healthy older adults, memory decline in β-amyloid–positive ɛ4 carriers began earlier (64.5 years of age) than in β-amyloid–positive ɛ4 noncarriers (76.5 years of age). The rate of decline was also faster, such that by 85 years of age, β-amyloid–positive ε4 carriers performed worse than β-amyloid–positive ε4 noncarriers.
Meaning
These results suggest that memory decline in β-amyloid–positive adults may accelerate with older age and that this increase in acceleration may be associated with the APOE ε4 allele.
Introduction
In cognitively healthy older adults (eg, >60 years of age), an elevated level of β-amyloid (Aβ), determined from positron emission tomography (PET) or examination of cerebrospinal fluid samples, indicates that Alzheimer disease (AD) has begun. This hypothesis is supported by observations that Aβ positivity in cognitively healthy older adults is associated with a decline in episodic memory and other aspects of cognition, greater loss of volume in medial temporal lobe areas, faster accumulation of Aβ, and greater rates of progression to clinical classification of mild cognitive impairment or AD dementia. The greatest risk for Aβ positivity in cognitively healthy adults is age, with approximately 10% of cognitively healthy adults aged 60 to 70 years, 25% aged 71 to 80 years, and 40% older than 80 years classified as being Aβ positive.
Age-related risk for AD dementia is increased further by carriage of the apolipoprotein E (APOE) ε4 allele. For example, carriage of an APOE ε4 allele lowers the age of onset of AD dementia in a gene-dose–dependent manner, with the mean age of onset of AD dementia in ε4 homozygotes at 68 years compared with 76 years for ε4 heterozygotes and 84 years for ε4 noncarriers. Consistent with these findings, a meta-analysis of clinicopathologic studies of preclinical AD showed that although cognitively healthy APOE ε4 carriers are more likely to be classified as positive for Aβ, this risk increases with age. For example, 20% of cognitively healthy ε4 carriers aged 60 years are classified as positive for Aβ (compared with <10% of ε4 noncarriers), which increases to 70% at 80 years of age (compared with 25% of ε4 noncarriers).
Although greater Aβ-associated memory decline in APOE ε4 carriers has been observed consistently in preclinical AD, whether this association also increases with age remains unknown. In cognitively healthy adults, application of nonlinear analyses showed that APOE ε4 carriage accelerated age-related memory decline in a gene-dose manner. Although Aβ levels were not determined in these studies, a substantial proportion of the age-related variance in memory decline observed in the ε4 carriers was likely owing to the presence of Aβ. However, APOE ε4 may have been associated with age-related memory decline in addition to Aβ. Both potential explanations require extension of the approach of Caselli et al to modeling interactions among APOE ε4, age, and memory decline to include Aβ levels.
The aim of this study was therefore to determine the extent to which Aβ levels (as determined by PET neuroimaging) and APOE ε4 are associated with age-related changes in memory in cognitively healthy adults. The first hypothesis was that Aβ-related memory decline would accelerate with age and that this acceleration would be greater in ε4 carriers than noncarriers. The second hypothesis was that in the absence of Aβ, APOE ε4 would not be associated with age-related memory decline.
Methods
Participants
Four hundred forty-seven cognitively healthy older adults enrolled in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study from March 31, 2006, through March 31, 2017, underwent Aβ neuroimaging and APOE genotyping (Table 1) and completed neuropsychological testing at baseline and 18-, 36-, 54-, and 72-month follow-up. Of the 1583 individuals enrolled in AIBL, 1136 refused or were excluded owing to other criteria (eg, having mild cognitive impairment or AD). Details of the recruitment process and classification of the cognitive health of the participants has been reported elsewhere. The AIBL study was approved by institutional human research and ethics committees of Austin Health, Heidelberg, Australia, St Vincent’s Health, Kew, Australia, Hollywood Private Hospital, Perth, Australia, and Edith Cowan University, Joondalup, Australia, which also ensured compliance with study protocols. Written informed consent was obtained from all participants before study participation.
Table 1. Demographic and Clinical Characteristics of the Sample.
| Characteristic | Aβ-Negative ε4 Noncarriers (n = 260) |
Aβ-Negative ε4 Carriers (n = 64) |
Aβ-Positive ε4 Noncarriers (n = 51) |
Aβ-Positive ε4 Carriers (n = 72) |
Planned Comparison, P Value | ||
|---|---|---|---|---|---|---|---|
| Aβ-Negative ε4 Carriers vs Noncarriers | Aβ-Positive ε4 Carriers vs Noncarriers | Aβ-Negative ε4 Noncarriers vs Aβ-Positive ε4 Carriers | |||||
| Female, No. (%) | 145 (55.8) | 35 (54.7) | 24 (47.1) | 40 (55.6) | .88 | .35 | .97 |
| Age, mean (SD), y | 70.2 (5.7) | 69.4 (6.0) | 75.7 (5.9) | 73.1 (6.4) | .32 | .02 | <.001 |
| Premorbid IQ, mean (SD) | 108.6 (6.8) | 107.9 (7.6) | 110.3 (7.9) | 108.2 (8.1) | .50 | .17 | .70 |
| GDS score, mean (SD)a | 0.91 (1.33) | 1.02 (1.49) | 0.85 (1.22) | 1.11 (1.26) | .56 | .26 | .25 |
| BECKET | 1.28 (0.24) | 1.33 (0.32) | 2.11 (0.37) | 1.88 (0.46) | .17 | .004 | <.001 |
| Time between PET scans, mean (SD), yb | 3.20 (1.55) | 2.69 (1.51) | 2.66 (1.73) | 1.99 (1.40) | .02 | .02 | <.001 |
| CDR score, mean (SD)c | 0.04 (0.14) | 0.06 (0.17) | 0.17 (0.26) | 0.19 (0.24) | .33 | .66 | <.001 |
| CDR sum of boxes score, mean (SD)c | 0.07 (0.25) | 0.10 (0.30) | 0.22 (0.41) | 0.43 (0.77) | .41 | .08 | <.001 |
| MMSE score, mean (SD)d | 28.91 (1.25) | 28.90 (1.16) | 28.67 (1.45) | 28.10 (1.79) | .95 | .06 | <.001 |
Abbreviations: Aβ, β-amyloid; BECKET, before the centiloid kernel transformation; CDR, Clinical Dementia Rating Scale; GDS, 15-item Geriatric Depression Scale; MMSE, Mini-Mental State Examination; PET, positron emission tomography.
Scores range from 0 to 6, with higher scores indicating higher levels of depressive symptoms.
Indicates length of the time interval from baseline neuropsychological assessment to the first Aβ positron emission tomographic scan.
Scores range from 0 to 0.5, with higher scores indicating more severe symptoms of dementia.
Scores range from 0 to 1.5, with higher scores indicating more severe symptoms of dementia.
In brief, exclusion criteria included a previous confirmed diagnosis of any of the following: schizophrenia, Parkinson disease, sleep apnea, depression (eg, Geriatric Depression Score of 6 or greater of a possible range of 0-15), cancer (except basal cell skin carcinoma) in the past 2 years, symptomatic stroke, or uncontrolled diabetes and if current alcohol use exceeded 4 standard drinks per day for men or 2 per day for women. All neuropsychological, psychiatric, and medical information for participants was reviewed by an expert clinical panel led by one of us (D.A.) to determine the cognitive health of participants. This panel was masked to outcomes of PET imaging and APOE genotyping.
Assessments
Neuroimaging
β-Amyloid PET imaging was conducted using the Pittsburgh Compound B (PiB), florbetapir F 18, or flutemetamol F 18 radioligands, with the acquisition protocol for each radioligand described previously. In brief, a 30-minute acquisition was started 40 minutes after PiB injection, and 20-minute acquisitions were performed 50 minutes after florbetapir F 18 injection and 90 minutes after flutemetamol F 18 injection. For PiB acquisition, standardized uptake value (SUV) data for key regions of interest were summed and normalized to the cerebellar cortex SUV. This process resulted in a region-to-cerebellar ratio termed the SUV ratio (SUVR). For florbetapir F 18, the SUVR is generated using the whole cerebellum as the reference region; for flutemetamol F 18, the pons is used as the reference region. A linear regression transformation was applied to the SUVRs for florbetapir F 18 and flutemetamol F 18 to transform them into a PiB-like SUVR. These SUVRs were termed BECKET (before the centiloid kernel transformation). An SUVR/BECKET threshold of at least 1.5 was used to classify Aβ positivity. Participants in the AIBL study underwent Aβ neuroimaging a mean (SD) of 2.5 (1.6) years after their baseline assessment (Table 1). This scan time difference was included as a covariate in all statistical models.
APOE Genotyping
A blood sample from each participant was forwarded for DNA extraction using commercially available kits (QIAamp DNA blood Midi or Maxi kit; Qiagen), applying the protocol given by the manufacturer. The APOE genotype was determined through genotyping assays (TaqMan; Life Technologies) for rs7412 (assay identification: C____904973_10) and rs429358 (assay identification: C___3084793_20) on a real-time polymerase chain reaction system (QuantStudio 12K-Flex; Applied Biosystems) using the genotyping master mix (TaqMan GTXpress; Life Technologies) methods according to the manufacturer’s instructions.
Neuropsychological Assessment
The rationale and validation of the AIBL episodic memory composite score has been described elsewhere. First, scores on the California Verbal Learning Test (Second Edition) delayed recall trial, the Logical Memory delayed recall trial, and the Rey Complex Figure Test 30-minute delayed recall trial were standardized using the baseline mean and SD in the cognitively healthy group. A mean (SD) score was then calculated for these standard scores for each assessment.
Statistical Analysis
All statistical analyses were conducted in SAS software (version 9.2; SAS Institute). A 1-way analysis of variance was conducted to investigate group differences at baseline. Demographic or clinical factors that differed between groups were entered as covariates in subsequent statistical models.
To test our hypotheses that acceleration of memory decline with age in Aβ-positive adults would be greater in ε4 carriers than ε4 noncarriers and that, in the absence of Aβ, APOE ε4 would not be associated with age-related memory decline, a mixed-effects model with an unstructured covariance matrix was used. The episodic memory composite was defined as the dependent variable, age as the continuous time-dependent variable, and group (Aβ-negative ɛ4 noncarriers, Aβ-negative ɛ4 carriers, Aβ-positive ɛ4 noncarriers, and Aβ-positive ɛ4 carriers) as a fixed factor. Time was modeled as a repeated measure within participants. The time from the baseline assessment to Aβ PET (ie, scan time) was also entered as a covariate. Model best fits were determined by least squares estimations of linear, quadratic, and cubic terms with the derived goodness-of-fit coefficients (ie, variance in memory scores explained) for each model compared statistically with that of the previous model.
Once the best-fit characteristic had been determined, data for the association between the change in episodic memory and increasing age were modeled separately for each group. To determine the age at which memory decline for each group became abnormal, the point at which the trajectory of each quadratic curve became different from that of the Aβ-negative ɛ4 noncarriers was estimated by computing the 95% CIs for each curve and determining the age at which the CIs did not overlap with those of the Aβ-negative ɛ4 noncarrier group. We conducted a series of planned comparisons to determine whether differences existed between (1) Aβ-negative ɛ4 noncarriers and carriers, (2) Aβ-positive ɛ4 noncarriers and carriers, and (3) Aβ-negative ɛ4 noncarriers and Aβ-positive ɛ4 carriers.
Results
Demographic and Clinical Characteristics
Of the 447 participants, 203 (45.4%) were men and 244 (54.6%) were women; mean (SD) age was 72.5 (6.6) years. Table 1 summarizes the demographic and clinical characteristics of each Aβ-ɛ4 group. A series of planned comparisons determined that no differences existed in the proportion of female participants in each group (24 of 51 Aβ-positive ε4 noncarriers [47.1%]; 35 of 64 Aβ-negative ε4 carriers [54.7%]; 40 of 72 Aβ-positive ε4 carriers [55.6%]; and 145 of 260 Aβ-negative ε4 noncarriers [55.8%]). Adults with Aβ findings (mean [SD] age, 74.4 [6.8] years) were approximately 4 years older than those negative for Aβ (mean [SD] age, 69.8 [6.1] years). Premorbid intelligence and levels of depressive symptoms were also equivalent across all groups. However, both Aβ-positive groups were on average 4 years older (mean [SD] ages, 75.7 [5.9] and 73.1 [6.4] years) than Aβ-negative groups (mean [SD] ages, 70.2 [6.0] and 69.4 [6.0] years). When we compared the mean (SD) time between PET scans with that of Aβ-negative ɛ4 noncarriers (3.20 [1.55] years), time between PET scans was shorter for Aβ-negative ɛ4 carriers (2.69 [1.51] years; P = .02), Aβ-positive ɛ4 noncarriers (2.66 [1.73] years; P = .02), and Aβ-positive ɛ4 carriers (1.99 [1.40] years; P < .001).
Association of Age With Memory Change
A quadratic model explained significantly more variance in the association between age and change in episodic memory than the linear model (−2 log likelihood ratio, 3491.8, in which a lower number indicates a better fit). Compared with the quadratic model (−2 log likelihood ratio, 3473.09; χ24 = 18.67; P < .001), the cubic model did not provide a statistically significant increase in variance explained and was not as parsimonious (−2 log likelihood ratio, 3470.1; χ24 = 2.96; P = .56). Scan time was not a significant covariate in these analyses and was therefore removed from the model.
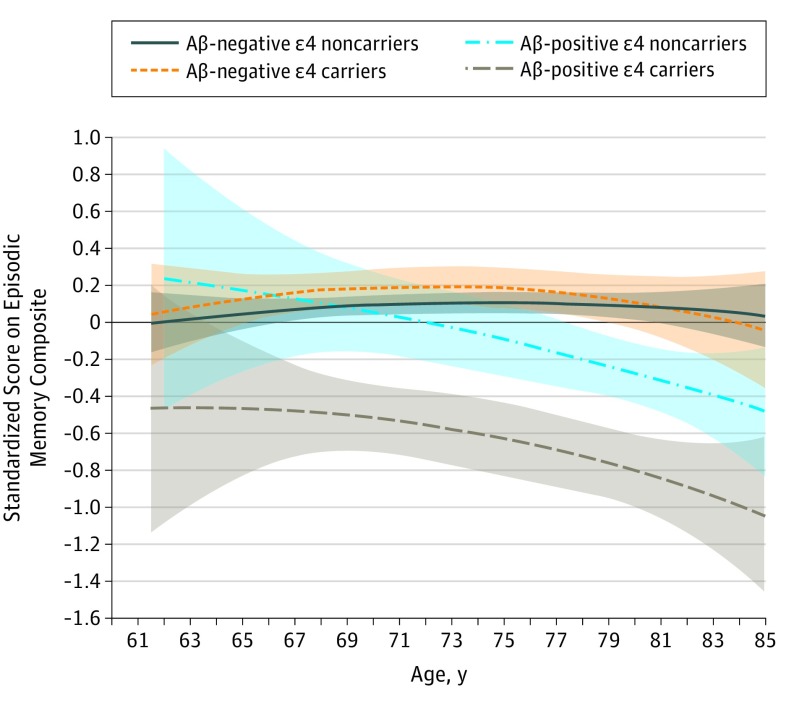
The longitudinal association of group (F3,640 = 9.42; P < .001), age (F1,559 = 10.31; P = .001), and square of age (F1,578 = 12.15; P = .001) with memory was statistically different between groups. The interaction between the square of age and group was also statistically significant (F3,767 = 16.60; P < .001). The Figure shows that this interaction occurred because the quadratic association between change in memory and increasing age was greatest in Aβ-positive ɛ4 carriers, followed by Aβ-positive ɛ4 noncarriers. The Figure also shows no statistically significant decline in memory as age increased in Aβ-negative ɛ4 carriers (estimate [SE], −0.001 [0.001]; t = −1.268; P = .21) and Aβ-negative ɛ4 noncarriers (estimate [SE], −0.001 [0.001]; t = −1.017; P = .11). In contrast, significantly greater decline in memory as age increased was found in the Aβ-positive ɛ4 noncarriers (estimate (SE), = −0.118 [0.012]; t = −0.980; P = .02) and Aβ-positive ɛ4 carriers (estimate [SE] = −0.551 [0.093]; t = −5.960; P < .001). A series of planned comparisons determined that the trajectory of memory decline of Aβ-positive ɛ4 carriers was statistically different from that of Aβ-negative ɛ4 noncarriers (estimate [SE], −0.019 [0.012]; t = −1.605; P = .049) and Aβ-negative ɛ4 noncarriers (estimate [SE], −0.033 (0.011); t = −2.869; P < .001). The estimated age at which the trajectory of memory decline became different from that of Aβ-negative ɛ4 noncarriers was 64.5 years in Aβ-positive ɛ4 carriers and 76.5 years in Aβ-positive ɛ4 noncarriers. The trajectory of memory decline in Aβ-negative ɛ4 carriers was not statistically different from that of Aβ-negative ɛ4 noncarriers (estimate [SE], 0.001 [0.001]; t = 0.526; P = .77). Thus, at no estimated age did trajectories of memory decline for Aβ-negative ɛ4 carriers differ from those of Aβ-negative ɛ4 noncarriers.
Figure. Standardized Score on the Episodic Memory Composite by Age.
Scores range from −3.51 to 1.93, with higher scores indicating better performance. Quadratic rates of change in episodic memory are shown for each β-amyloid (Aβ)–negative and Aβ-positive group by apolipoprotein E ε4 allele carrier status. Shading indicates 95% CIs of slope.
The greater acceleration of memory decline in Aβ-positive ɛ4 carriers was also revealed by the lower intercept and higher slope estimates compared with Aβ-positive ɛ4 noncarriers, Aβ-negative ɛ4 carriers, and Aβ-negative ɛ4 noncarriers (Table 2). These intercept and slope estimates can also be used to estimate an individual’s memory performance at a given age. In Table 3, three examples of estimated memory performance at ages 65, 75, and 85 for Aβ-negative ɛ4 noncarriers, Aβ-negative ɛ4 carriers, Aβ-positive ɛ4 noncarriers, and Aβ-positive ɛ4 carriers are given. Estimation of memory performance at any age throughout the range studied in each of the groups can also be calculated using the following formula: c + (β1 × age) + [β2(age × age)].
Table 2. Estimates for the Quadratic Association of Age With Each Groupa.
| Association | Estimate (SE) | P Value |
|---|---|---|
| Intercept | −5.947 (2.457) | .02 |
| Group | ||
| Aβ-negative ε4 noncarriers | −2.175 (0.441) | <.001 |
| Aβ-negative ε4 carriers | −2.008 (0.542) | <.001 |
| Aβ-positive ε4 noncarriers | −0.713 (0.66) | .28 |
| Aβ-positive ε4 carriers | NA | NA |
| Age | 0.211 (0.066) | .001 |
| Square of age | −0.002 (0.001) | <.001 |
| Square of age × group | ||
| Aβ-negative ε4 noncarriers | 0.0005 (0.00008) | <.001 |
| Aβ-negative ε4 carriers | 0.0005 (0.0001) | <.001 |
| Aβ-positive ε4 noncarriers | 0.0002 (0.0001) | .05 |
| Aβ-positive ε4 carriers | NA | NA |
Abbreviations: Aβ, β-amyloid; NA, not applicable.
The Aβ-positive ε4 carriers served as the reference group.
Table 3. Quadratic Equation for Each Group and Episodic Memory Estimates at Different Agesa.
| Group | β1 Value (SE) | β2 Value (SE) | c (Intercept) | Episodic Memory Estimates by Age, y | ||
|---|---|---|---|---|---|---|
| 65 | 75 | 85 | ||||
| Aβ-negative ε4 noncarriers | 0.211 (0.066) | −0.001 (0.0001) | −8.122 | −0.085 | 0.139 | 0.093 |
| Aβ-negative ε4 carriers | 0.211 (0.066) | −0.001 (0.0001) | −7.955 | −0.002 | 0.194 | 0.116 |
| Aβ-positive ε4 noncarriers | 0.211 (0.066) | −0.002 (0.0001) | −6.660 | 0.068 | −0.142 | −0.684 |
| Aβ-positive ε4 carriers | 0.211 (0.066) | −0.002 (0.0004) | −5.947 | −0.107 | −0.611 | −1.489 |
Abbreviation: Aβ, β-amyloid.
The quadratic equation used to calculate all estimates is given in the Association of Age With Memory Change subsection of Results section.
Discussion
The first hypothesis, that memory decline would accelerate with age in Aβ-positive adults and that this acceleration would be greater in ε4 carriers than ε4 noncarriers, was supported. Specifically, in Aβ-positive ε4 carriers, memory decline began at approximately 64.5 years of age and accelerated thereafter, such that by 85 years of age, Aβ-positive ε4 carriers performed approximately 1.5 SD units worse than matched Aβ-negative ε4 noncarriers and 0.8 SD units worse than Aβ-positive ε4 noncarriers (Table 2). Although memory decline also accelerated with increasing age in Aβ-positive ε4 noncarriers, this acceleration began later (76.5 years of age) and was not as great as that in Aβ-positive ε4 carriers. Thus, by 85 years of age, episodic memory in Aβ-positive ε4 noncarriers was approximately 0.70 SD units worse than that in Aβ-negative ε4 noncarriers. Previous studies of Aβ-positive adults have observed faster memory decline in ε4 carriers than ε4 noncarriers; however, in all studies to date, associations between time and memory decline have been modeled using linear methods, with age treated as a baseline covariate. Thus, although the nonlinear associations observed in the present study suggest that the presence of the ε4 allele is associated with increases in memory decline in Aβ-positive adults, they also extend understanding of this phenomena by showing that Aβ-related memory decline may accelerate with increasing age and may be increased further by ε4 carriage. The observations also demonstrate that in Aβ-positive ε4 carriers, memory decline may begin more than 10 years earlier than in Aβ-positive ε4 noncarriers (Figure). This acceleration of memory decline in Aβ-positive ε4 carriers is also consistent with data from epidemiologic studies showing that APOE ε4 hastens the age of onset of AD dementia by approximately 8 years.
The earlier presentation and faster acceleration of memory decline with increasing age in Aβ-positive ε4 carriers also provides a clinical expression of data from in vitro experiments, which demonstrate that APOE ε4 disrupts clearance of Aβ. Although ε4 carriers and noncarriers in the present sample showed equivalent levels of Aβ, the use of the single Aβ PET scan did not allow us to determine whether the deleterious association of ε4 with memory decline in Aβ-positive individuals was accompanied by accelerating Aβ accumulation. However, previous studies examining the association between memory decline and AD biomarkers in older adults without dementia suggest that this acceleration is likely. The results of our study are also consistent with those of studies that have used the same statistical modeling approach to demonstrate that memory decline accelerates with increasing age in APOE ε4 carriers, albeit in the absence of information about Aβ levels.
The results of the present study also support our second hypothesis, that in the absence of Aβ, APOE ε4 would not be associated with age-related memory decline. Despite the risk for AD and Aβ positivity associated with carriage of the APOE ε4 allele, APOE ε4 did not increase the risk for memory decline in Aβ-negative older adults in our study. Despite the long period of investigation and the large sample size of our study, we observed that episodic memory remained stable in Aβ-negative ε4 carriers even with increasing age, and the rate of episodic memory decline from 65 to 85 years of age was indistinguishable from that of Aβ-negative ε4 noncarriers (Figure). This finding is consistent with those of previous studies demonstrating that in the absence of Aβ, APOE ε4 is not associated with increased cognitive decline in cognitively healthy adults. Considered together, these results suggest that in the absence of occult disease, episodic memory does not deteriorate with increasing age, even in the presence of ε4.
Previously, age-related acceleration in memory decline was observed in APOE ε4 homozygotes compared with ε4 heterozygotes and noncarriers, albeit with unknown Aβ status. The age-related acceleration of memory decline observed in the present study was qualitatively similar to that observed for ε4 homozygotes in this previous study. However, in the current sample, only a small proportion (6 [8.3%]) of the Aβ-positive ε4 carriers, who showed the greatest acceleration of age-related memory decline, were ε4 homozygotes. Furthermore, Aβ-negative ε4 carriers showed no age-related decline in memory. Thus, the present data suggest that the memory decline observed in the older ε4 homozygotes in the previous studies reflected their risk of Aβ positivity rather than a specific effect of APOE ε4. Thus, in the presence of Aβ, even a single copy of the ε4 allele may be associated with memory decline that accelerates with increasing age.
When considered together, the findings of our study confirm initial observations that in preclinical AD, Aβ-positive ε4 carriers may have a faster rate of decline in episodic memory and that Aβ-related memory decline may also occur in ε4 noncarriers, albeit at a slower rate. Our present findings extend knowledge of the association among Aβ, APOE ε4, and memory decline because memory decline in Aβ-positive older adults accelerated with age and this acceleration was moderated by the APOE ε4 allele. Conversely, in Aβ-negative older adults, memory function did not change substantially with increasing age irrespective of the presence of the APOE ε4 allele. We have provided estimates (Table 3) to assist researchers and clinicians in determining the risk of memory decline given 3 known variables (ie, age, Aβ status, and APOE ε4 status). Although these estimations require further refinement and replication, they provide a foundation for predictions about the nature of the course of AD through its preclinical phase.
Limitations
Several points warrant consideration before generalizing the results of this study. First, the AIBL study is not a population-based sample. Strict inclusion and exclusion criteria were applied to the selection of participants, and consequently the sample is relatively free of cardiovascular risk factors and other medical and psychiatric illnesses. Most participants also had tertiary educational levels and high premorbid intelligence levels. Some or all of these factors may have limited the presence of memory decline in Aβ-negative ε4 carriers. Therefore, replication of these data in other cohorts of individuals at risk of developing AD will be important. Second, despite the relatively large sample, the study lacked sufficient statistical power to investigate any gene-dose effects of ε4 on Aβ-related memory decline. Previous investigators have reported that ε4 homozygotes show nearly double the magnitude of cognitive impairment compared with ε4 heterozygotes, although the extent to which this outcome occurs prospectively and in the presence of Aβ remains unclear. Finally, owing to limited samples with serial imaging measures, the extent to which memory decline in Aβ-positive ε4 carriers is associated with Aβ accumulation and brain volume loss could not be determined.
Conclusions
Prior work has shown that Aβ and ε4 combined are associated with memory decline in older adults without dementia. Results of this study suggest that increasing age may strengthen this association. The estimates provided can be used to determine the risk of memory decline due to Aβ and ε4 at each age.
References
- 1.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343-349. [DOI] [PubMed] [Google Scholar]
- 2.Lim YY, Maruff P, Pietrzak RH, et al. ; AIBL Research Group . Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain. 2014;137(pt 1):221-231. [DOI] [PubMed] [Google Scholar]
- 3.Doraiswamy PM, Sperling RA, Johnson K, et al. ; AV45-A11 Study Group; AV45-A11 Study Group . Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry. 2014;19(9):1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doré V, Villemagne VL, Bourgeat P, et al. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70(7):903-911. [DOI] [PubMed] [Google Scholar]
- 5.Villemagne VL, Burnham S, Bourgeat P, et al. ; Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group . Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357-367. [DOI] [PubMed] [Google Scholar]
- 6.Rowe CC, Bourgeat P, Ellis KA, et al. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian Imaging, Biomarkers, and Lifestyle study of ageing. Ann Neurol. 2013;74(6):905-913. [DOI] [PubMed] [Google Scholar]
- 7.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. ; Amyloid PET Study Group . Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921-923. [DOI] [PubMed] [Google Scholar]
- 9.Mormino EC, Betensky RA, Hedden T, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Harvard Aging Brain Study . Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim YY, Villemagne VL, Pietrzak RH, et al. ; Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group . APOE ε4 moderates amyloid-related memory decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2015;36(3):1239-1244. [DOI] [PubMed] [Google Scholar]
- 11.Lim YY, Laws SM, Villemagne VL, et al. Aβ-related memory decline in APOE ε4 noncarriers: implications for Alzheimer disease. Neurology. 2016;86(17):1635-1642. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 2015;72(5):511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE ε4 effect. N Engl J Med. 2009;361(3):255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caselli RJ, Dueck AC, Locke DE, et al. Longitudinal modeling of frontal cognition in APOE ε4 homozygotes, heterozygotes, and noncarriers. Neurology. 2011;76(16):1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis KA, Bush AI, Darby D, et al. ; AIBL Research Group . The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr. 2009;21(4):672-687. [DOI] [PubMed] [Google Scholar]
- 16.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275-1283. [DOI] [PubMed] [Google Scholar]
- 17.Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med. 2010;51(6):913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenberghe R, Van Laere K, Ivanoiu A, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68(3):319-329. [DOI] [PubMed] [Google Scholar]
- 19.Clark CM, Schneider JA, Bedell BJ, et al. ; AV45-A07 Study Group . Use of florbetapir-PET for imaging β-amyloid pathology. JAMA. 2011;305(3):275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villemagne VL, Doré V, Yates P, et al. En attendant centiloid. Adv Res. 2014;2(12):723-729. [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–Second Edition (CVLT-II). San Antonio, TX: Psychological Corp; 2000. [Google Scholar]
- 22.Wechsler D. WMS-R: Wechsler Memory Scale–Revised. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- 23.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial: Professional Manual. Lutz, FL: Psychological Assessment Resource Inc; 1995. [Google Scholar]
- 24.Cumming G. Inference by eye: reading the overlap of independent confidence intervals. Stat Med. 2009;28(2):205-220. [DOI] [PubMed] [Google Scholar]
- 25.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25(5):641-650. [DOI] [PubMed] [Google Scholar]
- 26.Kanekiyo T, Xu H, Bu G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81(4):740-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim YY, Pietrzak RH, Bourgeat P, et al. Relationships between performance on the CogState Brief Battery, neurodegeneration, and Aβ accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol. 2015;30(1):49-58. [DOI] [PubMed] [Google Scholar]
- 29.Jack CR Jr, Wiste HJ, Vemuri P, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Brain β-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133(11):3336-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim YY, Williamson R, Laws SM, et al. ; AIBL Research Group . Effect of APOE genotype on amyloid deposition, brain volume and memory in cognitively normal older individuals. J Alzheimers Dis. 2017;58(4):1293-1302. [DOI] [PubMed] [Google Scholar]